Abstract
Background
Pediatric differentiated thyroid cancer (DTC) rates have increased over time in the United States and worldwide. Improvements in imaging for the diagnosis of DTC have been hypothesized as a potential driver of these increases. Here, we stratify temporal trends in pediatric DTC by stage and tumor size to assess whether rates of large, late stage cancers, which are likely to be clinically-meaningful, are increasing over time.
Methods
Age-standardized incidence rates (ASR) of DTC and annual percent change (APC) in primary DTC rates were estimated among 0–19 year-olds, using data from 39 U.S. cancer registries during 1998–2013.
Results
During 1998–2013, 7,296 cases of DTC were diagnosed (6,652 papillary; 644 follicular). ASRs of pediatric DTCs significantly increased by 4.43%/year (95% confidence interval [CI]:3.74, 5.13), primarily due to increases in papillary histologies. Increasing trends were observed for children aged 10–19 years old, for both genders and for non-Hispanic whites, non-Hispanic blacks and Hispanics. Rates increased significantly over the time period for all tumors stages [APClocalized= +4.06%/year (95%CI: 2.84,5.29), APCregional= +5.68%/year (95%CI: 4.64,6.73) and APCdistant= +8.55%/year (95%CI: 5.03,12.19)], and across tumor sizes [APC<1cm = +9.46%/year (95%CI: 6.13,12.90), APC1–2cm= +6.92%/year (95%CI: 4.31,9.60) and APC>2cm= +4.69%/year; (95%CI: 2.75,6.67)].
Conclusion
Significant increasing rates of DTC over time among 10–19-year-olds in the U.S. are unlikely to be entirely explained by increases in medical surveillance during childhood as rates of large and late stage DTCs are increasing over time. Future studies should examine environmental and other factors that may be contributing to rising DTC rates.
Keywords: thyroid cancer, pediatrics, epidemiology, registries, incidence
Condensed abstract:
Significant increasing rates of pediatric DTC between 1998 and 2013 were reported in the U.S. They are unlikely to be entirely explained by increases in medical surveillance during childhood as rates of large and late stage DTCs are increasing over time.
Introduction
Differentiated thyroid carcinoma (DTC) is rare among children and adolescents aged 0–19 years old, representing 2–4% of all pediatric malignancies (1,2). Pediatric thyroid cancer rates are also much lower than rates in adults, representing only 2.3% of all thyroid cancer diagnoses (3). As observed in thyroid cancer occurring during adulthood, increased incidence of pediatric thyroid cancer over the last decades has been reported in the United States and worldwide (2,4). In the U.S., the incidence rate for the period 2001–2009 was 6.83 per million with a significant annual percent change (APC) of 4.9%/year (2). A similar increase has been observed when restricted to papillary thyroid cancers, which represent the vast majority of DTCs (5).
In adult DTC, it has been debated whether observed increases in DTC rates are driven by over diagnosis from screening practices and/or improved imaging, or possibly due to changes in the prevalence of environmental risk factors (3,6). Incidentally-detected DTCs are likely to be smaller, early stage tumors, while larger, late stage tumors are more likely to be symptomatic and clinically-detected. Thus, tumor size and stage can be used as a proxy to differentiate increases due to incidental detection during medical surveillance versus increases due to clinical work-up of patients with specific symptoms or palpable neck lesions (3). However, overdiagnosis is less likely to occur in children than in adults as thyroid nodule screening would be rarely required (7) for this age group and medical imaging of the neck would not be usually performed for other clinical purposes (8).
The rarity of pediatric DTC and/or the lack of information on histologic features have prevented prior studies from analyzing trends by tumor size or cancer stage at diagnosis (2,9–12). The only prior study to examine DTC trends during 1984–2010 by tumor size in children and young adults included patients ≤30 years old. As 85% of DTC cases occurred among 20–30 year-olds, conclusions about the pediatric population were limited (13).
The present study utilizes 39 U.S. population-based state and metropolitan cancer registries (covering approximatively 80% of the U.S. population) to assess pediatric DTC incidence rates and calendar trends during 1998–2013, overall and by age-group, gender, histologic type, race/ethnicity, tumor stage and size.
Methods
Study population
The data were obtained from the North American Association of Central Cancer Registries (NAACCR) which provides cancer incidence data since 1995 from cancer registries that participate in the Centers for Disease Control and Prevention National Program of Cancer Registries (NPCR) and the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program. To maximize both the number of years of follow-up and the number of registries included in the analysis, data spanning 1998 to 2013 from 39 registries were utilized in this analysis: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Atlanta, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Missouri, Montana, Nebraska, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Washington, West Virginia, Wisconsin and Wyoming.
Case definition
DTC cases were classified according to the International Classification of Diseases for Oncology, 3rd ed. [ICD-O-3] by topography (C73) and histology: papillary (histologic codes 8050, 8052, 8130, 8260, 8340–8344, 8450, and 8452), and follicular (ICD-O-3 8290, 8330–8332, and 8335). Cases were restricted to children (aged 0–19 years) who were diagnosed with a primary DTC. Second primary DTCs were excluded because of the known impact of cancer treatment, especially radiotherapy, on thyroid cancer risk (14).
Tumor size was collected routinely between 2004 and 2013 with the introduction of collaborative stage; thus, trends by tumor size are restricted to this time period. Tumor size has been categorized in <1 cm, 1–2 cm, and >2cm – 93% of cases had size reported. Tumor stage at diagnosis had high rates of missingness in certain included registries, so for stage-specific analyses we restricted to the 25 registries that had at least 50% completeness - 97% had known stage in these registries. Stage at diagnosis was defined as localized (limited to the thyroid gland), regional (tumor extension beyond the limits of the thyroid gland or spread by more than 1 lymphatic or vascular supply route), distant (extracervical metastasis) and unknown (15).
Statistical analyses
All incidence rates were age-standardized to the 2000 U.S. standard population and expressed per million person-years of follow-up, according to gender, age at diagnosis (0–9; 10–14; 15–19 years), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic other), histologic subtype (papillary, follicular), tumor size and stage at diagnosis as calculated using the National Cancer Institute’s SEER*Stat software package (version 8.2.1; National Cancer Institute, Bethesda, MD). Trends in DTC incidence rates during 1998–2013 were estimated with annual percent changes (APCs) and corresponding confidence intervals (CIs) with Joinpoint software (Version 4.5.0.1). Graphically, trends are presented using a three-year moving average for each calendar year. Statistical significance was determined based on p value ≤0.05.
Results
Between 1998 and 2013, a total of 7,296 childhood DTC (age-standardized incidence rate [ASR]=6.66/million) occurred in 39 U.S. cancer registries, including 6,652 (91%) cases of papillary (ASR=6.07/million) and 644 (9%) cases of follicular thyroid cancer (ASR=0.59/million). Girls (n=6,028, 83%; ASR=11.3/million), 15–19-year-olds (n=5,578, 76%; ASR=20.1/million), and non-Hispanic whites (n=4,990, 68%; ASR=7.67/million) represented the majority of the cases. About half of the cases were reported in the last 6 years of the 16 years studied period, with 599 cases in 2013. Frequencies, ASRs and APCs for all DTC by age-group categories are presented in Table 1. ASRs calculated for the beginning (1998–2001) and the end (2011–2013) of the studied period are presented in the supplemental Table 1 for gender, histology type and race and ethnicity and in Table 2 for tumor size and stage at diagnosis.
Table 1:
ASR and APC of differentiated thyroid cancer in children and adolescents, 1998–2013 according to category of age
| All | 0–9 years | 10–14 years | 15–19 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | N | ASR (95 % CI) | APC (95 % CI) | N | ASR (95 % CI) | APC (95 % CI) | N | ASR (95 % CI) | APC (95 % CI) | N | ASR (95 % CI) | APC (95 % CI) |
| Total | 7 296 | 6.66 (6.51,6.81) | 4.43 (3.74,5.13) | 267 | 0.52 (0.46,0.58) | 0.81 (−2.15,3.86) | 1 451 | 5.32 (5.05,5.6) | 5.44 (4.19,6.70) | 5 578 | 20.06 (19.54,20.60) | 4.33 (3.52,5.15) |
| Gender | ||||||||||||
| Female | 6 028 | 11.3 (11.02,11.59) | 4.34 (3.39,5.30) | 168 | 0.67 (0.57,0.78) | 2.15 (−1.90,6.37) | 1 141 | 8.57 (8.08,9.08) | 5.27 (3.95,6.61) | 4 719 | 34.93 (33.94,35.94) | 4.18 (3.16,5.22) |
| Male | 1 268 | 2.26 (2.14,2.39) | 4.94 (3.23,6.67) | 99 | 0.38 (0.31,0.46) | -0.10 (−5.31,5.40) | 310 | 2.22 (1.98,2.48) | 5.67 (2.77,8.64) | 859 | 6.01 (5.61,6.42) | 5.18 (3.47,6.92) |
| Histologic type | ||||||||||||
| Papillary | 6 652 | 6.07 (5.92,6.22) | 4.65 (3.87,5.43) | 245 | 0.48 (0.42,0.54) | −0.05 (−3.30,3.32) | 1 279 | 4.69 (4.44,4.95) | 5.67 (4.48,6.88) | 5 128 | 18.44 (17.94,18.96) | 4.61 (3.73,5.51) |
| Follicular | 644 | 0.59 (0.54,0.64) | 2.08 (0.41,3.78) | 22 | 0.04 (0.03,0.06) | ~ | 172 | 0.63 (0.54,0.73) | 4.15 (0.51,7.93) | 450 | 1.62 (1.47,1.78) | 0.88 (−1.49,3.30) |
| Race and Ethnicity | ||||||||||||
| Hispanic, all races | 1414 | 6.21 (5.89,6.54) | 5.10 (3.50,6.73) | 65 | 0.54 (0.42,0.69) | −1.68 (−8.48,5.62) | 311 | 5.33 (4.75,5.95) | 4.97 (1.94,8.09) | 1038 | 18.24 (17.14,19.38) | 5.58 (3.90,7.28) |
| Non-Hispanic, white | 4990 | 7.67 (7.45,7.88) | 4.45 (3.76,5.15) | 172 | 0.59 (0.50,0.68) | 1.70 (−1.55, 5.07) | 951 | 5.94 (5.57,6.33) | 5.72 (4.16,7.31) | 3 867 | 23.31 (22.58,24.05) | 4.21 (3.40,5.03) |
| Non-Hispanic, black | 350 | 2.31 (2.07,2.57) | 3.78 (1.34–6.27) | 14 | 0.20 (0.11,0.33) | ~ | 79 | 2.07 (1.64,2.58) | ~ | 257 | 6.70 (5.91,7.57) | 4.25 (1.90,6.66) |
| Non-Hispanic, other | 419 | 6.35 (5.75,6.99) | 4.61 (1.89,7.41) | 12 | 0.38 (0.20,0.67) | ~ | 89 | 5.54 (4.45,6.81) | 3.98 (−0.16,8.30) | 318 | 18.87 (16.85,21.06) | 4.88 (1.65,8.22) |
ASR: Age standardized incidence rate; APC: annual percentage change; CI: confidence interval; ~: Too few cases for annual percentage change to be estimated. ASRs are expressed as cases/million person-years and APCs are expressed as %/year.
Table 2:
ASR and APC of differentiated thyroid cancer in children and adolescents, 1998–2013
| All patients |
|||||
|---|---|---|---|---|---|
| Variables | N | ASR (95 % CI) | APC (95 % CI) | ||
| Period of available data | Beginning | End | Whole period | ||
| 2004–2006 | 2011–2013 | 2004–2013 | |||
| Size | |||||
| <1 cm | 751 | 0.75 (0.63,0.88) | 1.44 (1.28,1.62) | 1.08 (1.00,1.16) | 9.46 (6.13,12.90) |
| 1–2 cm | 1 298 | 1.41 (1.25,1.58) | 2.37 (2.16,2.58) | 1.87 (1.76,1.97) | 6.92 (4.31,9.60) |
| > 2 cm | 2 695 | 3.26 (3.02,3.52) | 4.5 (4.22,4.8) | 3.88 (3.74,4.03) | 4.69 (2.75,6.67) |
| Unknown | 367 | 0.56 (0.46,0.67) | 0.54 (0.45,0.65) | 0.53 (0.48,0.59) | −0.79 (−4.47,3.03) |
| Period of available data | Beginning | End | Whole period | ||
| 1998–2000 | 2011–2013 | 1998–2013 | |||
| Stage at diagnosis* | |||||
| Local | 1751 | 2.58 (2.27,2.93) | 4.49 (4.07,4.94) | 3.44 (3.28,3.60) | 4.06 (2.84,5.29) |
| Regional | 1493 | 2.16 (1.88,2.48) | 4.23 (3.82,4.66) | 2.94 (2.79,3.10) | 5.68 (4.64,6.73) |
| Distant | 118 | 0.14 (0.07,0.24) | 0.43 (0.31,0.59) | 0.24 (0.19,0.28) | 8.55 (5.03,12.19) |
| Unknown | 98 | 0.21 (0.13,0.33) | 0.11 (0.05,0.2) | 0.19 (0.16,0.24) | −4.09 (−9.06,1.15) |
Analysis on stage at diagnosis was restricted to a subset of 25 registries that had greater than 50% completeness for the time period under study.
ASR: Age standardized incidence rate; APC: annual percentage change; ASRs are expressed as cases/million person-years and APCs are expressed as %/year.
Overall, pediatric DTC incidence rates significantly increased 4.43%/year (95% CI:3.74, 5.13), from 4.77/million (95% CI: 4.26, 5.33) in 1998 to 8.82/million (95% CI: 8.13, 9.56) in 2013 (Figure 1). The trends reported for all DTCs were largely driven by papillary histologic types (Figure 1), but significant increasing trends were also reported for follicular histologic subtypes (APC: +2.08%/year, 95% CI: 0.41,3.78).
Figure 1.
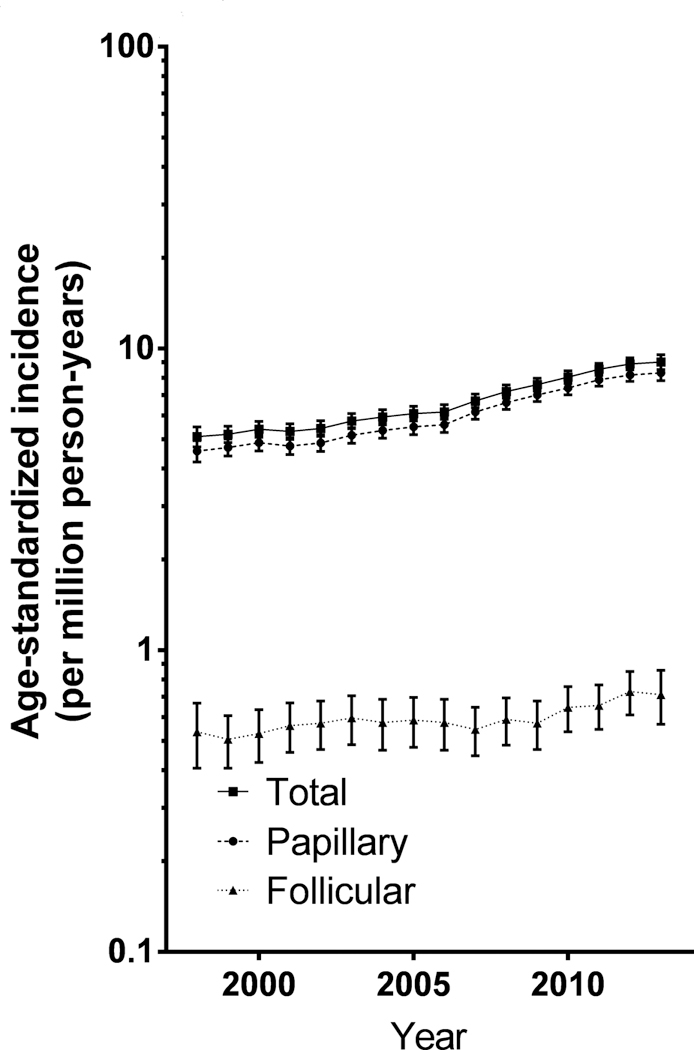
Trends in differentiated thyroid cancer incidence by histology, 3-year moving average.
Age-specific incidence rates for all DTC by sex are presented in Figure 2. From 1998 to 2013, significantly increasing trends were observed only for 10–19 year-olds (Table 1, Figure 2). Significant increasing trends were observed in both sexes (Figure 2), and among all race/ethnicities (Figure 5).
Figure 2. Trends in differentiated thyroid cancer incidence by age and sex, 3-year moving average.
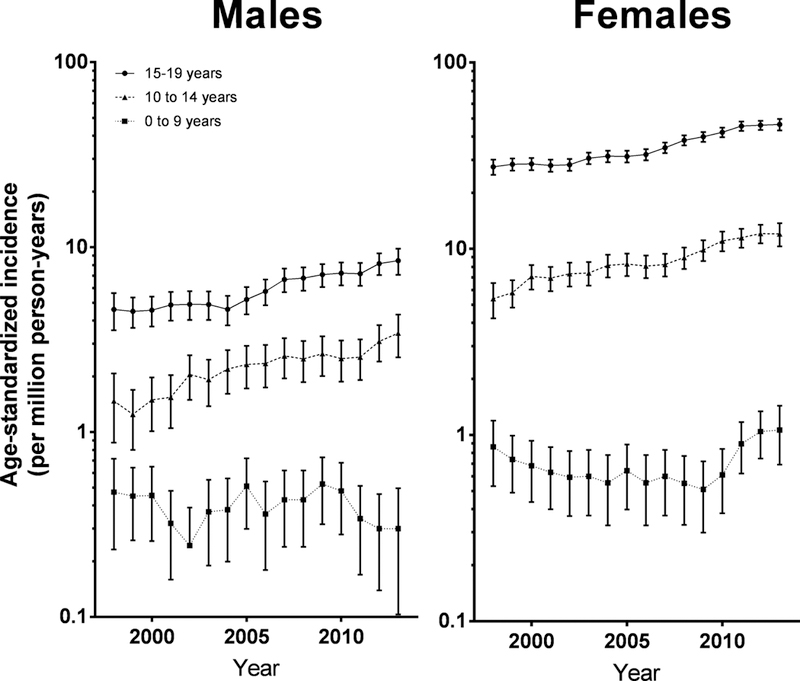
The error bars that would have crossed the x-axis has been truncated at 2013.
Figure 5.
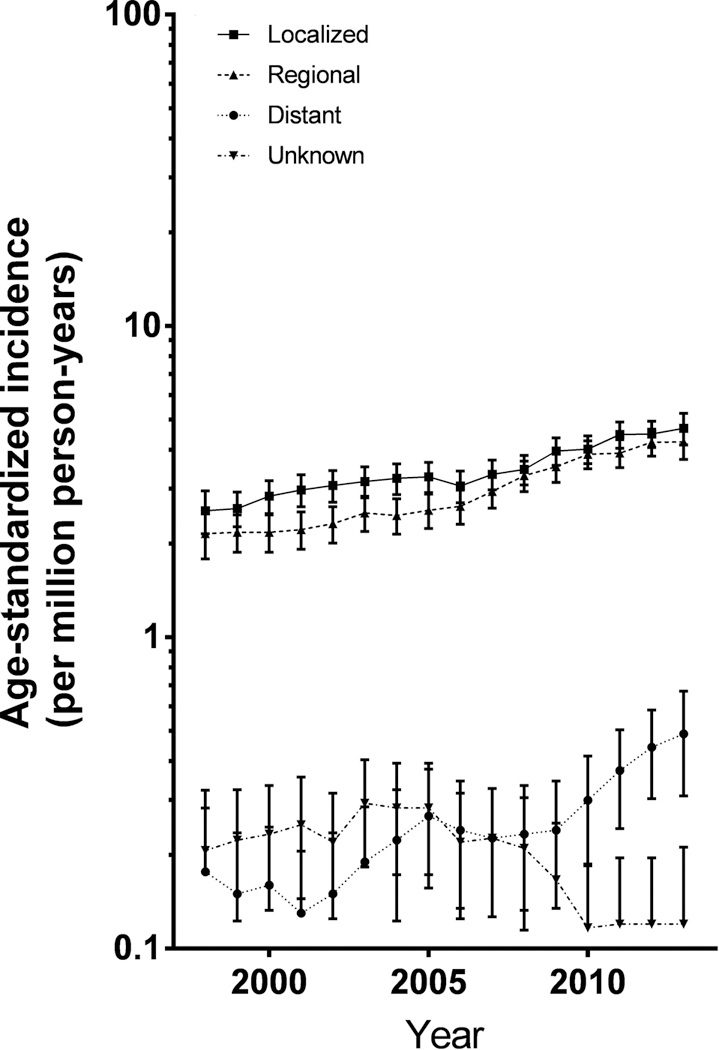
Trends in differentiated thyroid cancer incidence by race and ethnicity
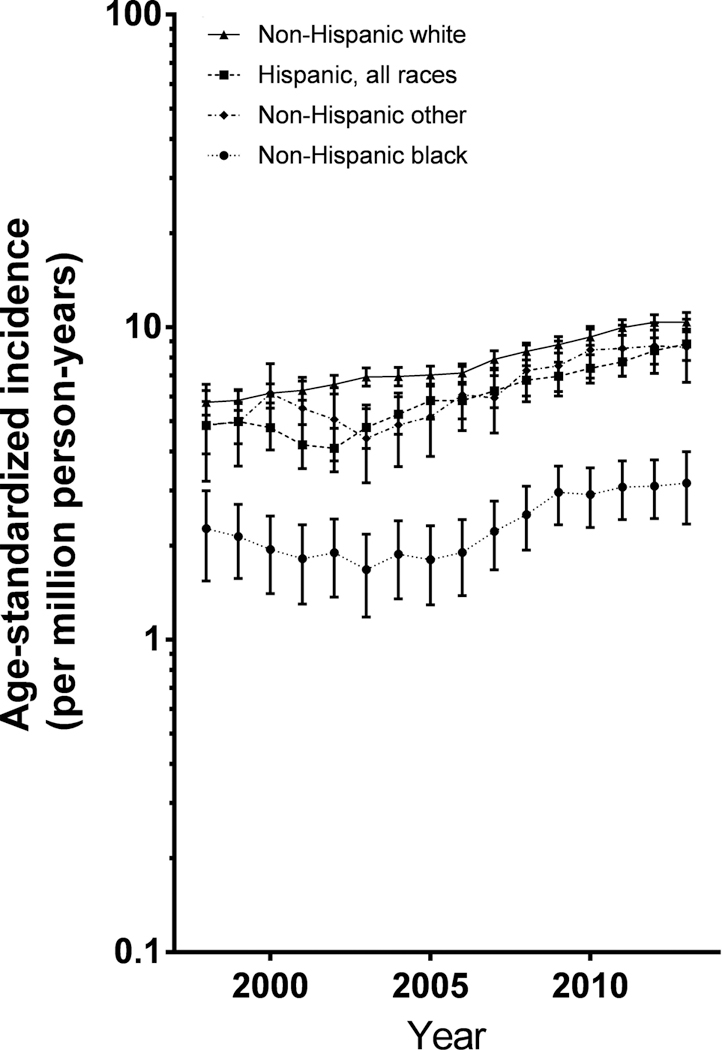
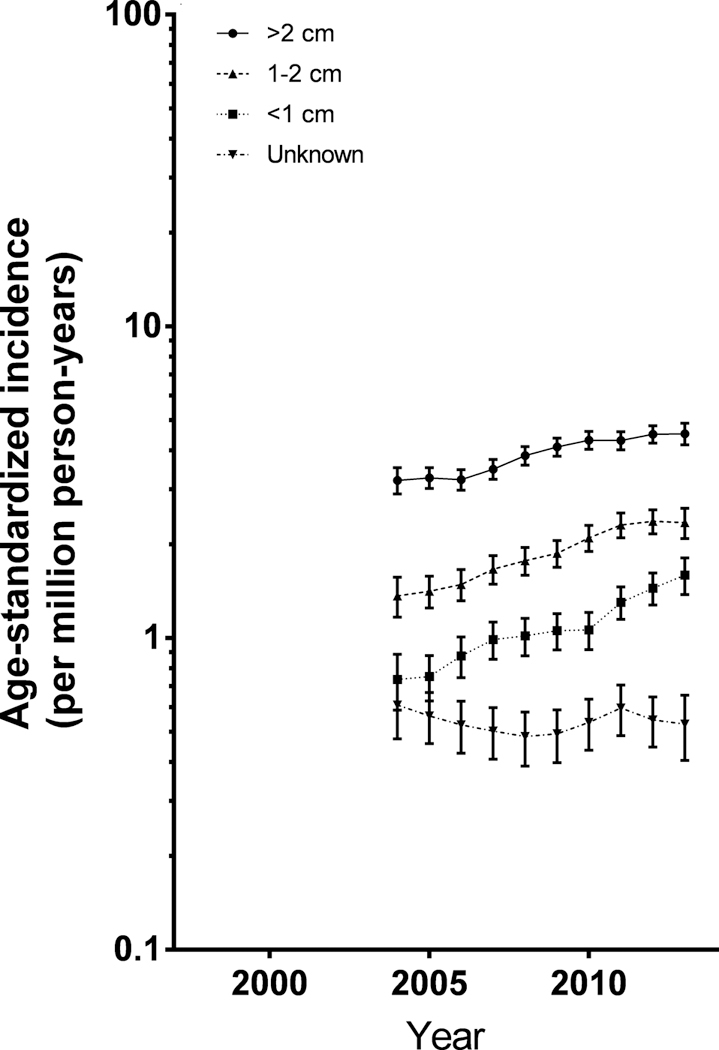
Rates increased significantly over the time period for both localized tumors [APClocalized= +4.06%/year (95% CI: 2.84, 5.29)] and more aggressive tumors [APCregional= +5.68%/year (95% CI: 4.64, 6.73) and APCdistant= +8.55%/year (95% CI: 5.03, 12.19)]. Rates for tumors <1cm in size (APC<1 cm= +9.46%/year; 95% CI: 6.13,12.90) increased more rapidly than tumors larger than 2cm (APC>2 cm= +4.69%/year; 95% CI: 2.75,6.67) (Figures 3 and 4, Table 2).
Figure 3. Trends in differentiated thyroid cancer incidence by tumor size, 3-year moving average.
Study period limited to 2004–2013, when tumor size was routinely collected
Figure 4. Trends in differentiated thyroid cancer incidence by stage at diagnosis, 3-year moving average.
Analysis limited to the 25 registries that had at least 50% completeness for this variable - 97% known stages. Error bars that would cross the x-axis have been omitted.
Discussion
With 7,296 available cases from 39 US cancer registries covering approximatively 80% of the U.S. population, this study included 1.5-times more cases than the next largest study to date (2). During 1998–2013, rates of DTC increased significantly among children and adolescents aged 10–19 years, and across gender and all race/ethnicities. Given that the rates for both smaller, early stage and larger, later stage tumors increased significantly over time, rising rates are unlikely to be entirely explained by increases in follow-up of thyroid conditions or other conditions that involve imaging of the neck or thyroid palpation and more sensitive diagnostic technology – environmental and individual factors may also have impacted rising trends.
The incidence of DTC is known to vary widely according to age, gender and race/ethnicity. Very low in the first years of life, the incidence rates increase dramatically in adolescence, and are generally higher in girls than boys (5). This study is consistent with prior findings, with rates increasing from 0.52 to 20.06 per 1,000,000 person-years in the 0–9 years and 15–19 years age-groups respectively. The observed highest rates of incidence in girls, non-Hispanic whites and lowest rates in non-Hispanic blacks are consistent with previous reports (2,5,10,12).
We observed a significant increase of pediatric DTC incidence of 4.43%/year during the period 1998–2013, consistent with the increasing trend of 4.9%/year reported in a previous analysis in the U.S. on 4,812 cases collected in 47 population-based state cancer registries during 2001–2009 (2). In our study, the observed increasing rates over time in all DTC incidence were driven by papillary histologies (91% of all cases). However, rates of follicular DTC also increased significantly 2.08%/year.
The main objective of our study was to investigate whether trends in DTC in children and adolescents were consistent with increased medical surveillance and subsequent incidental detection of small and indolent DTC. In adulthood, the observed increase in DTC rates has been suspected to be linked to the introduction and increasing widespread use of diagnostic ultrasonography and fine needle aspirations biopsies starting in the 1980s. Indeed, these technologies are able to detect incidental cancers, mostly localized and/or small tumors that are unlikely to have presented clinically (6,16–18). However, growing evidence of a true increase of DTC in adults is now supported by increasing rates of larger and advanced stage DTCs over the past several decades (3). We have shown for the first time that similar patterns have occurred among children and adolescents with rates of DTC increasing across stage and tumor size. Moreover, we observed that the annual increase in rates of distant DTC are higher than those observed for localized or regionally extended DTC. A prior study reported significant increases in DTC rates for all tumor sizes in girls and for tumors >1 cm for boys; however, only 15% of patients were <20 years old, precluding comparison with our study (13). From a clinical point of view, the likelihood of an over detection effect in children and adolescents is smaller than in adults as this population is not usually subjected to thyroid screening or medical imaging of the neck for other indications (8). However, the significant annual increases in the rates of small and local stage DTCs suggest that we might not be able to rule out a contribution of over detection to rising pediatric DTC rates.
Age-specific trends of thyroid cancer rates in the pediatric population have seldom been studied because of the rarity of thyroid cancer during childhood, especially before age 10. The large number of cases available in our study, due to a substantially longer period and larger population studied, allowed us to conduct such analyses in contrast to previous studies (2,9,13). No significant increase in DTC rates among 0–9-year-olds was observed, although only 267 DTC cases occurred in this age group. For older age groups, our results confirmed the increased trends reported previously among 10–14-year-olds (13) and 15–19-year-olds (2,11,13). Consistent with prior studies, we observed significant increases in DTC rates among non-Hispanic white and black children (2,11).
Observed differences in trends across demographic subgroups may result from one or more factors. These might include socioeconomic disparities and associated differential access to medical care. Another possibility could be differences in environmental or exogenous exposures. Host factors (obesity, hormonal or other physiological factors) may be important. Genetic variation or gene-environment interaction may also play a role. In our analysis, increasing rates were observed mostly after the age of 10 years and not restricted to one race/ethnicity or gender. Accordingly, global environmental or behavioral factors rather than socioeconomic status or genetic background are more likely to explain this increase.
Studies examining etiological factors associated with thyroid cancer have general focused on adult thyroid cancers (19). Large epidemiological studies of DTC risk factors among children are needed to provide insight into etiology. However, known risk factors for thyroid carcinogenesis among adults may also be associated with childhood DTC. Ionizing radiation is the best-known risk factor for thyroid cancer. Elevated rates of DTC were observed after the Chernobyl accident in 1986 (20) and during the follow-up of atomic bomb survivors (21), and also after medical exposure (22). Indeed, diagnostic imaging during childhood has been associated with a 7-fold increase of thyroid cancer per Gy among adults (22). Increased risks associated with computed tomography (CT) scan exposure have also been reported (23), but the association between diagnostic imaging with lower radiation doses (i.e. conventional radiology procedures or dental X-rays) are still unclear (24). It should be noted that while the studies highlighted here focus on childhood exposure to radiation, thyroid cancers were diagnosed any time including during adulthood. The increased radiation exposure of children resulting from the large increase of medical diagnostic imaging use over time in pediatrics, especially diagnostic procedures associated with high radiation doses (e.g., CT scans) (25), might have contributed to rising pediatric DTC rates. Indeed, between 1996 and 2005, the use of CT scans tripled for children 5 to 14 years of age in the U.S. and approximately 4.25 million pediatric CTs are performed annually (26). Some previous analyses of increasing pediatric DTC trends have hypothesized that radiotherapy for prior malignancies may contribute to rising rates (10,11); however, this could not be the case in our study, as we restricted the analysis to first primary thyroid cancers.
Rates of pediatric DTC are higher in girls than boys – these differences are even greater after the beginning of puberty (2,13). The predominance in girls is hypothesized to be related to sex hormone changes during puberty influencing thyroid carcinogenesis. Indeed, estrogen is known to be a potent growth factor for both benign and malignant thyroid cells. It exerts its growth-promoting effect through a classical genomic and a non-genomic pathway, mediated via a membrane-bound estrogen receptor (27). Obesity, through estrogen-related pathways and insulin-resistance mechanisms, is also likely to impact thyroid carcinogenesis, as suggested by the positive association of obesity in childhood and adulthood with adult DTC reported in epidemiological studies (28–30). Thus, the epidemic of obesity reported in the U.S. (31) and in other developed countries might explain part of the observed increase in pediatric DTC rates, though studies focused on examining associations between obesity and childhood DTC risk are needed. Other environmental risk factors, such as endocrine-disrupting chemicals (eg, pesticides, bisphenol A, perchlorate and polybrominated diphenyl ethers) have been hypothesized as potential risk factors for DTC because of their effect on thyroid hormone metabolism (32,33), though these associations have not been consistently observed (34).
The strengths of this study include the large number of cases available, even for the youngest age group, the quality standards of NAACCR registries and the coverage of about 80% of U.S. population. Information on tumor size and stage allowed us to study a potential overdiagnosis effect on rising rates of pediatric DTC. Though information on tumor stage was incomplete, once we restricted the analyses to the 25 registries with more complete stage data, stage information was available in 97% of the cases. Variation of completeness of these variables over the studied period might have a small impact on size-specific incidence trends. However, the consistency of the results for both tumor size and cancer stage is not in favor of such a bias. Other limitations included a relatively short study period (15 years), and the possible increase in quality of cases ascertainment over time that might influence increasing trends. Last, cancer registries do not collect information on risk factors, such as medical radiation exposure and obesity, preventing the evaluation of the role of these potential risk factors in increasing rates of DTC.
Rates of pediatric DTC have increased significantly between 1998 and 2013 among both boys and girls and across racial and ethnic groups. This trend is unlikely to be explained solely by increased medical surveillance or improved detection, as small, early stage and larger, late stage tumors have increased over the study period. Additional studies are needed to explore environmental, dietary, and genetic factors that might be involved in the physiopathology of pediatric thyroid cancer, although these studies will be challenging given the rarity of these cancers.
Supplementary Material
Acknowledgements
These data are based on the NAACCR December 2015 data submission. Support for cancer registries is provided by the state, province or territory in which the registry is located. In the U.S., registries also participate in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program or the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) or both. In Canada, all registries submit data to the Canadian Cancer Registry maintained by Statistics Canada.
Funding sources:
This research was supported by the Intramural Research Program of the National Cancer Institute.
Footnotes
No conflict of interest disclosures
Contributor Information
Diana Withrow, Email: diana.withrow@nih.gov.
Amy Berrington de Gonzalez, Email: berringtona@mail.nih.gov.
Clara Lam, Email: clara.lam@nih.gov.
Martha Linet, Email: linetm@exchange.nih.gov.
Cari M. Kitahara, Email: meinholdc@mail.nih.gov.
Meredith S. Shiels, Email: shielsms@mail.nih.gov.
References
- 1.Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: Diagnosis and management. Curr Opin Oncol 2008;20:59–65. [DOI] [PubMed] [Google Scholar]
- 2.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics 2014;134:945–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim H, Devesa SS, Sosa JA, Check D, Kitahara C. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017; 317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaatsch P, Steliarova-Foucher E, Crocetti E, Magnani C, Spix C, Zambon P. Time trends of cancer incidence in European children (1978–1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:1961–71. [DOI] [PubMed] [Google Scholar]
- 5.Golpanian S, Perez EA, Tashiro J, Lew JI, Sola JE, Hogan AR. Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int 2016;32:201–8. [DOI] [PubMed] [Google Scholar]
- 6.Lubitz CC, Kong CY, Mcmahon PM, et al. Annual financial impact of well-differentiated thyroid cancer care in the United States. Cancer 2014; 120: 1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer 2006;13:427–53. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Ly S, Castroneves LA, et al. How are childhood thyroid nodules discovered: opportunities for improving early detection. J Pediatr 2014;164:658–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer 2008;113:2575–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan AR, Zhuge Y, Perez EA et al. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res 2009;156:167–72. [DOI] [PubMed] [Google Scholar]
- 11.Holmes L Jr, Hossain J, Opara F. Pediatric thyroid carcinoma incidence and temporal trends in the USA (1973–2007): race or shifting diagnostic paradigm? ISRN Oncol 2012;2012:906197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dermody S, Walls A, Harley EH. 2016 Pediatric thyroid cancer: An update from the SEER database. International Journal of Pediatric Otorhinolaryngology 2016; 89:121–26. [DOI] [PubMed] [Google Scholar]
- 13.Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr 2014;164:1481–85. [DOI] [PubMed] [Google Scholar]
- 14.Sigurdson AJ, Ronckers CM, Mertens, et al. Primary thyroid cancer after a first tumor in childhood (the Childhood Cancer Survivor Study): A nested case-control study. Lancet 2005; 365:2014–23. [DOI] [PubMed] [Google Scholar]
- 15.Young JL, Roffers SD, Ries LAG, Fritz AG, Hurbut AA, eds. SEER Summary Staging Manual-2000: Codes and coding instructions, National Cancer Institute, NIH pub N°. 01–04969, Bethesda, MD, 2001. [Google Scholar]
- 16.Leenhardt L, Bernier MO, Boin-Pineau MH, Conte Devolx B, Marechaud R, Niccoli-Sire P, et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol 2004;150:133–9. [DOI] [PubMed] [Google Scholar]
- 17.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295: 2164–67. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980–2008. Thyroid 2013;23:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Su L, Xiao H. Review of factors related to the thyroid cancer epidemic. Int J Endocrinol 2017;2017:ID5308635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst 2005;97:724–32. [DOI] [PubMed] [Google Scholar]
- 21.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007;168:1–64. [DOI] [PubMed] [Google Scholar]
- 22.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 1995;141:259–77. [PubMed] [Google Scholar]
- 23.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, Giles GG, Wallace AB, Anderson PR, Guiver TA, McGale P, Cain TM, Dowty JG, Bickerstaffe AC, Darby SC. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memon A, Godward S, Williams D, Siddique I, Al-Saleh K. Dental x-rays and the risk of thyroid cancer: a case-control study. Acta Oncol 2010;49:447–53. [DOI] [PubMed] [Google Scholar]
- 25.Mettler FA Jr, Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources e 1950e2007. Radiology 2009;253:520–31. [DOI] [PubMed] [Google Scholar]
- 26.Miglioretti DL, Johnson E, Williams A. The use of computed tomography in pediatrics and the associated radiation exposure and estimated risk cancer. JAMA Pediatr 2013;167:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer 2014;21:T273–83. [DOI] [PubMed] [Google Scholar]
- 28.Peterson E, De P, Nuttall R. BMI, diet and female reproductive factors as risks for thyroid cancer. PLoSOne 2012;7:e29177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitahara CM, Gamborg M, Berrington de Gonza¡lez A, Sarensen TI, Baker JL.Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res 2014;;74:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitahara CM, McCullough ML, Franceschi S, et al. Anthropometric factors and thyroid cancer risk by histological Subtype: pooled analysis of 22 prospective studies. Thyroid 2016;26:306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016; 315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Guo GL, Han X, Zhu C, Kilfoy BA, Zhu Y, Boyle P, Zheng T. Do Polybrominated Diphenyl Ethers (PBDEs) Increase the Risk of Thyroid Cancer? Biosci Hypotheses 2008;1:195–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarone RE, Lipworth L, McLaughlin JK. The epidemiology of environmental perchlorate exposure and thyroid function: a comprehensive review. J Occup Environ Med 2010. June;52:653–60. [DOI] [PubMed] [Google Scholar]
- 34.Aschebrook-Kilfoy B, DellaValle CT, Purdue M, Kim C, Zhang Y, Sjodin A, Ward MH. Polybrominated Diphenyl Ethers and Thyroid Cancer Risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial Cohort. Am J Epidemiol 2015;181:883–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


