Summary
Encoding the temporal properties of external signals that comprise multimodal events is a major factor guiding everyday experience. However, during the natural aging process, impairments to sensory processing can profoundly multimodal temporal perception. Various mechanisms can contribute to temporal perception, thus it is imperative to understand how each can be affected by age. In the current study, using 3 different temporal order judgment tasks (unisensory, multisensory and sensorimotor), we investigated the effects of age on two separate temporal processes: synchronization and integration of multiple signals. These two processes rely on different aspects of temporal information, either the temporal alignment of processed signals or the integration/segregation of signals arising from different modalities, respectively. Results showed that the ability to integrate/segregate multiple signals decreased with age regardless of the task, and that the magnitude of such impairment correlated across tasks, suggesting a widespread mechanism affected by age. In contrast, perceptual synchrony remained stable with age, revealing a distinct intact mechanism. Overall, results from this study suggest that aging has differential effects on temporal processing, and general impairments with aging may impact global temporal sensitivity while context-dependent processes remain unaffected.
Keywords: Multisensory integration, Aging, Time perception, Sensorimotor integration
Introduction
As we go about our everyday routines, we seldom notice the temporal discrepancies between sensory signals that constitute the events we experience. For instance, speech provides both auditory and visual cues (accompanying mouth movements of the speaker) that are processed within the brain at different speeds, yet speech is perceived as a simultaneous multisensory event (Conrey and Pisoni, 2006; van Wassenhove et al., 2007; Eg and Behne, 2015). Compensation for small temporal differences is beneficial for coherent multisensory experiences. However, just as important is segregation of asynchronous sensory signals that belong to different sources. Unfortunately temporal sensitivity, i.e. the ability to discriminate the timing of multiple sensory signals, is not stable across the lifespan and deteriorates with age, leading to incoherent and unreliable global percepts (Setti et al., 2011; Baum and Stevenson, 2017; Brooks et al., 2018; Stevenson et al., 2018).
Reduced temporal sensitivity in the aging population manifests in an impaired ability to determine the temporal separation, and likewise the temporal order, between two asynchronous stimuli (Setti et al., 2011; Chan et al., 2014; Bedard and Barnett-Cowan, 2016). This deficit is also related to the widening of the temporal binding window, the time span over which sensory signals arising from different modalities appear integrated into a global percept (Bedard and Barnett-Cowan, 2016; Baum and Stevenson, 2017). With poor temporal sensitivity, elderly individuals are inclined to bind together stimuli even when they are separated by large delays (Setti et al., 2011; Bedard and Barnett-Cowan, 2016; Stevenson et al., 2018). As a result, the aging process leads to a deficit in integrating cross-modal information and segregating unrelated sensory signals, sensory overload and increased susceptibility to multisensory illusions (Stevenson et al., 2012; Setti et al., 2014).
Temporal perception impairments in older individuals are not limited to multisensory interactions. Older adults also show deficits in unisensory temporal processing, such as in auditory duration discrimination (P J Fitzgibbons & Gordon-Salant, 1995; Peter J. Fitzgibbons & Gordon-Salant, 1994) and auditory temporal order judgements (Ulbrich, Churan, Fink, & Wittmann, 2009), and in visual gap detection (Humes et al., 2009) and visual temporal order judgements (Ulbrich et al., 2009; Busey et al., 2010). In the sensorimotor domain, temporal sensitivity (Vercillo et al., 2017), accuracy in predicting the time-course of observed actions (Diersch et al., 2012), and precise coordination and control of voluntary actions (Heuninckx et al., 2005; Seidler et al., 2010) are impaired with aging. Moreover, there is an increased visual reliance on balance in older adults (Jeka et al., 2010; Franz et al., 2015) and delays in visual feedback during postural control results in poorer performance in older compared to younger adults (Yeh et al., 2014). While these findings are not unexpected, since the sensory and motor systems each exhibit global structural and perceptual declines with natural aging (Howarth and Shone, 2006; Seidler et al., 2010; Werner et al., 2010; Owsley, 2011; Andersen, 2012), it is still important to identify specific effects of aging on the temporal binding of multiple sensory signals and of sensory and motor signals. Further, using the visual system in multisensory and sensorimotor combinations is of particular interest since healthy older adults become more visually dominant when performing multisensory detection tasks and postural control tasks (Jeka et al., 2010; Diaconescu et al., 2013; Yeh et al., 2014; Franz et al., 2015; Murray et al., 2018).
Age-related temporal deficits may reflect the impairments of a general all-inclusive mechanism that applies to all aspects of temporal perception or the deficits may reflect discrete mechanisms that apply to different aspects of temporal perception. On one side, perceptual studies support the idea of a shared timing mechanism, showing transfer of perceptual training from unisensory to multisensory temporal perception (Stevenson et al., 2013) and vice versa (Alais and Cass, 2010), as well as transfer of temporal adaptation from the audio-motor to the visuo-motor domain (Heron et al., 2009; Sugano et al., 2010). Additional evidence for a central timing mechanism comes from studies on neurological disorders, such as Parkinson’s disease, and animal models of depleted dopaminergic circuits that reveal temporal processing impairments (Meck, 2006b, 2006a; Allman and Meck, 2012; Jones and Jahanshahi, 2014). Specifically, the dopaminergic cortico-striatal system that supports time perception as well as motor and executive functions (Meck and Benson, 2002; Meck, 2006a; Jahanshahi et al., 2010; Agostino and Cheng, 2016; Matthews and Meck, 2016), is one of the most sensitive to age-related declines (Li and Backman, 2010; Turgeon et al., 2016).
Alternatively, temporal deficits may result from impairments to specific mechanisms guiding different aspects of time perception. Studies using transcranial magnetic stimulation have revealed distinct cortical regions necessary for proper performance in auditory and visual temporal tasks (Bueti, Bahrami, et al., 2008; Bueti, van Dongen, et al., 2008). Duration perception studies also provide evidence for modality-specific clocks rather than a singular central clock for interval estimation (Morrone et al., 2005; Burr et al., 2007; Klink et al., 2011).
Age-related changes in temporal processing not only may reduce temporal sensitivity but could similarly affect perceptual synchrony. Perceptual synchrony has been previously quantified through the point of subjective simultaneity (PSS), i.e. the physical temporal delay between two signals at which an observer is unsure about their temporal order (Stone et al., 2001). In other words, the PSS is the physical asynchrony between two signals that induces perceptual synchrony. The percept of synchrony is often guided by prior experiences wherein naturally occurring time differences are learned reflecting the unique properties of sensory stimuli as well as the temporal properties of neural processing. Prior studies comparing perceptual synchrony between older and younger adults do not reveal any effect of age for both audiovisual (de Boer-Schellekens and Vroomen, 2014; Bedard and Barnett-Cowan, 2016) and visual only tasks (de Boer-Schellekens and Vroomen, 2014; Norman et al., 2014). Possibly, compensatory mechanisms take place in the aging brain to account for changes in sensory processing and prevent extreme variations in the perception of unity between multimodal signals.
In the current study we investigated the effects of aging on two distinct aspects of temporal processing, 1) temporal sensitivity and 2) perceptual synchrony, across multiple modalities: visual (unisensory), audiovisual, and visuomotor temporal order judgment tasks. While sensitivity has previously been shown to decline with age, as discussed above, little attention has been given to how perceptual synchrony may be affected with age. Perceptual synchrony is a foundation of multimodal processing and guides subjective experience, thus it is extremely important to assess whether such a mechansism is preserved in older adults. Elucidating how these two processes vary across conditions and between different age groups, can shed light on the temporal mechanisms affected by aging.
Material and methods
Participants
Fifteen young adults (mean age: 22.7 ± 0.60 years, 10 females), 15 middle-aged adults (mean age: 45.7 ± .87 years, 8 females), and 15 older adults (mean age: 67.9 ± 0.75 years, 12 females) were recruited from the University of Nevada, Reno and the surrounding community to participate in the study. The middle-aged group was included to track gradual impairments in temporal processing induced by aging. All subjects completed each of the three tasks except for 1 young subject that did not complete the audiovisual temporal order judgment (TOJ) task. All subjects reported normal or corrected to normal vision and normal hearing. Participants were verbally screened for any history of neurological or psychiatric disorders as well as cognitive decline. All participants were right handed. Older adults were additionally screened for any hearing loss and were required to have a pure tone threshold lower than 40 dB for 1 and 2 kHz. Participants provided signed informed consent before any experimentation and were financially compensated for their time. Protocol was reviewed and approved by the Institutional Review Board at the University of Nevada, Reno.
Stimuli
Stimuli were generated using MATLAB (Mathworks, Natick, MA) and Psychtoolbox extensions (Brainard, 1997; Pelli, 1997). The visual stimulus was a stationary white circle with a diameter of 3.5° presented on a grey background for 30 ms.In the audiovisual TOJ task, the auditory stimulus was a 30 ms pure tone of 1000 Hz created in MATLAB and presented at 75 dB via a speaker that was positioned in front of the computer screen. Figure 1A shows a graphical representation of the experimental setup. The visual and auditory stimuli were delivered through a Display ++ system with a refresh rate of 120 Hz and an AudioFile stimulus processor (Cambridge Research Systems).
Figure 1. Experimental designs.
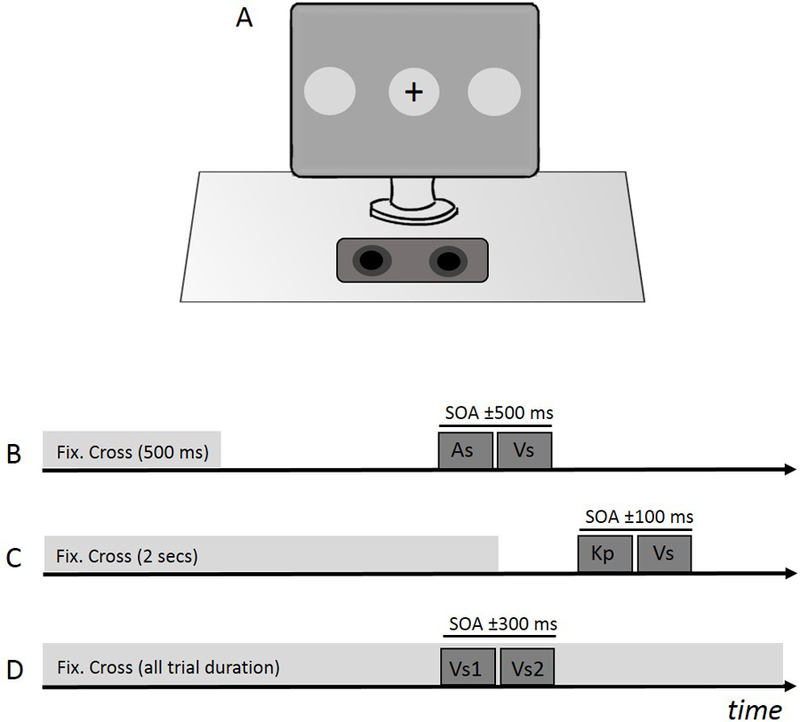
Panel A shows the experimental set-up used for all three tasks. The speaker used to present auditory cues was located in a central location relative to the observer and display. The fixation cross and visual stimulus were presented in the center of the screen for the audiovisual and visuomotor tasks while the two visual stimuli were presented peripheral to the fixation cross in the visual task. The time-course of the audiovisual TOJ task is shown in panel B. After 500 ms, the fixation cross disappeared to signal the start of the trial. During each trial a puretone auditory stimulus (As) and a visual stimulus (Vs) appeared on the screen at variable temporal delays (SOAs). Panel C shows the time-course of the visuomotor task. Participants were asked to make a keypress (Kp) immediately following the fixation cross’s disappearance. A visual stimulus (Vs) also appeared on the screen following the cross at variable temporal delays and participants then judged the temporal order. For the visual task (panel D), a visual stimulus flashed on the far right and on the far left of the fixation cross at variable asynchronies.. The fixation cross remained on the screen throughout the trial.
Audiovisual TOJ task
Participants were asked to determine the temporal order between an auditory and a visual stimulus, both presented centrally. During the temporal order judgment (TOJ) task, participants sat 57 cm from the computer screen. Each trial began with participants focusing attention on a central fixation cross for 500 ms (Fig. 1B). The cross then disappeared from the screen indicating the stimuli were about to be presented. The temporal difference between the two stimuli was defined by a method of constant stimuli algorithm. Stimulus onset asynchrony (SOA) values were selected from a uniform distribution between −500 to + 500 ms, with 50 ms steps, where negative SOAs indicated the visual stimulus leading and positive SOAs indicated auditory stimulus leading. Each SOA value was repeated 5 times in random order. At the end of each trial, participants were asked to report whether the visual stimulus was displayed before or after the sound by pressing a computer key. Participants performed a total of 105 trials (see Fig. 1B for a schematic representation of the experimental procedure).
Visuomotor TOJ task
To assess sensorimotor timing, we employed a TOJ task adapted from Vercillo et al. (2017). The motor action was a voluntary button press recorded via a CB6 response box that interfaced with the Display++. To prevent any auditory feedback that could result from the button press, participants wore headphones that played white noise throughout the experiment.
At the start of each trial, participants fixated a black cross in the center of the monitor for 2 seconds. Once the cross disappeared, participants were instructed to press a button on the response box. The latency of the visual stimulus was calculated from the disappearance of the fixation cross. Subjects were asked to verbally report whether they perceived the visual stimulus before or after their button press (see Fig. 1C for a schematic representation of the experimental procedure).
The latency of the visual stimulus, and consequently the SOA values, were partially determined by the participant’s observed reaction time. For this reason, prior to experimentation, participants performed a practice block of 30 trials to calculate individual reaction times and modify the latency of the visual stimulus during the experimental block. Latencies were calculated from the timing of the disappearance of the fixation cross and were selected to ensure that the visual stimulus was presented either before or after participants’ button presses. The protocol attempted to present the visual stimulus before and after the individual’s button press at the following “ideal” SOA values: 0 ms, ±20 ms, ±40 ms, ±60 ms, ±80 ms, and ±100 ms. Specifically, to obtain a SOA value equal to 0, the latency of the visual stimulus was set to the participant’s average reaction time. Other SOA values were obtained by adding/subtracting the ideal SOA value to the average reaction time. It should be noted that as the participant’s reaction time varied from their average on each trial, these SOA values are relative and not exact. However, using this paradigm, we were able to present stimuli with SOAs as great as ±300 ms. Stimuli were delivered with a method of constant stimuli algorithm where each ideal SOA value was repeated 10 times in random order for a total of 110 trials.
Visual TOJ task
This unisensory task measured the ability to discriminate the temporal order of two visual stimuli that were presented at the right and the left side of the screen (Figs 1A, 1D). A fixation cross was always displayed in the center of the screen for the participant to focus on. Each trial, two visual stimuli flashed on the screen, one 18° to the right and the other 18° to the left of the fixation cross. The temporal asynchrony between the two stimuli randomly varied between −300 to +300 ms with 25 ms increments, with negative SOAs representing left-leading trials and positive SOAs representing right-leading trials. At the end of each trial, participants were instructed to determine which stimulus (the left or the right) appeared on the screen first and respond via a keyboard press. Each SOA value was repeated 5 times in random order for a total of 125 trials (see Fig. 1D for a schematic representation of the experimental procedure).
Data analysis
For each task, the individual’s perceptual responses were plotted as a function of SOA values. The characterization of the response depended on the task: visual TOJ – proportion of “left first”; audiovisual TOJ – proportion of “flash first”; sensorimotor TOJ – proportion of “flash first”. Individual data were then fit with a psychometric function and two parameters, the mean and the standard deviation, were estimated from the cumulative distribution (Weber, 1834; Fechner, 1860; van Eijk et al., 2008; Burr et al., 2009; Vercillo et al., 2017). The mean represented the point of subjective simultaneity (PSS), a measure of perceptual synchrony, and of the participant’s bias in determining temporal judgments. The standard deviation represented the sensitivity, or just noticeable difference (JND), which is the smallest temporal differences between the two stimuli (or the motor and the sensory signal) that a participant could reliably detect (1 SD = 1 JND). A bootstrap procedure (Efron and Tibshirani, 1994) was used to determine the standard errors of both estimates. JND and PSS values were then averaged across subjects within each age group. To assess statistical differences between age groups and associations between estimates, repeated measure ANOVAs and linear regression were performed. To further quantify and interpret non-statistical results (Dienes, 2014; Morey et al., 2016), we calculated Bayes Factors using the BayesFactor package in the statistical software R (Richard Morey, 2018). Default priors were used for both ANOVA and linear regression designs (Rouder and Morey, 2012; Rouder et al., 2012).
Results
Figure 2A shows average JND values for each age group for all three temporal tasks. Black bars represent young adults, dark grey bars represent middle-aged adults and light grey bars represent older adults. Overall, older adults showed higher JNDs than young adults, while neither group differed from middle-aged adults. Average JNDs in the audiovisual task were equal to 179.1 ± 25.0 ms for young adults, 216.1 ± 28. 5 ms for middle-aged adults, and 260.3 ± 27.3 ms for older adults. Similarly, in the visuomotor task, average JND values were 69.9 ± 8.2 ms for young adults, 69.1 ± 6.3 ms for middle-aged adults, and 99.8 ± 9.6 ms for older adults. In the visual task the average JND for young adults was 45.6 ± 12.4 ms, 44.3 ± 11.1 ms for middle-aged adults and 75.3 ± 11.4 ms for older adults. A repeated measure ANOVA (within factor: task, between factor: age) revealed a main effect of task (F(2,82) = 102.93; p < 0.001; partial ƞ2 = 0.72) and a main effect of age (F(2, 41) = 3.84; p < 0.05; partial ƞ2 = 0.16) but no interaction (F(4,82) = 0.951; p = 0.439; partial ƞ2 = 0.04). Post-hoc comparisons with Bonferroni adjustments showed that audiovisual JND estimates were significantly larger than both visuomotor (p < 0.001) and visual JND measures (p < 0.001). In addition, visuomotor JND values were significantly larger than visual JND values (p < 0.001). To determine differences between age groups, post-hoc Tukey HSD analyses were used. Older adults had significantly larger JND values than younger adults (p < 0.05) but not compared to middle-aged adults (p = .128). Moreover, middle-aged adults did not significantly differ from young adults in their JND values (p = .762). To quantify the relation between age and temporal acuity, individual JND data points were fit with a linear regression model (data are not shown in the figures). A significant regression was found for the visuorimotor TOJ (R2 = 0.21, p < 0.01), audiovisual TOJ (R2 = 0.17, p < 0.01), and visual TOJ (R2 = .16, p < 0.01) tasks suggesting reduced temporal sensitivity with increasing age for all TOJ contexts.
Figure 2. Increased JND, not PSS, values for older adults across tasks.
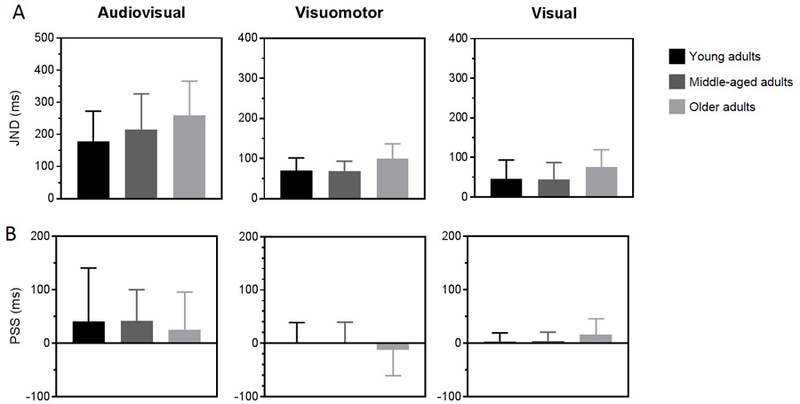
Older adults (light grey bars) demonstrated significantly higher temporal order thresholds (top row) for the audiovisual (left panel), visuomotor (middle panel), and visual (right panel) TOJ tasks as compared to young (black bars) and middle-aged adults (dark grey bars). There was no difference in PSS measures (bottom row) across age groups.
Figure 2B shows the effect of age on perceptual synchrony for each TOJ task. PSS values were first estimated for each individual and then averaged across individuals for each group. In the audiovisual task, group average PSS values were 40.5 ± 26.8 ms for young adults, 41.3 ± 15.1 ms for middle-aged adults, and 25.0 ± 18.2 ms for older adults. In the visuomotor task, PSS values were 2.2 ± 9.5 ms for young adults, 1.3 ± 9.9 ms for middle-aged adults, and −12.3 ± 12.5 ms for older adults. In the visual task, average PSS values were 3.2 ± 4.1 ms for young adults, 4.2 ± 4.1 ms for middle-aged adults, and 16.2 ± 7.6 ms for older adults. A repeated measure ANOVA (within factor: task, between factor: age) showed a significant main effect of task (F(2,82) = 6.253, p < 0.01; partial ƞ2 = 0.13) but no significant main effect of age (F(2,41) = 0.174, p = .841; partial ƞ2 = 0.01) or any significant interaction between task and age (F(4,82) = 0.488, p = .744; partial ƞ2 = 0.02). Post-hoc comparisons with Bonferroni adjustments revealed that audiovisual PSS estimates were significantly larger than visuomotor (p <0.05) but not visual PSS values (p = 0.071) and that there was no difference between PSS values from visuomotor and visual tasks (p = 0.503). We computed a Bayes Factor to assess the likelihood of the null hypothesis that age did not have an effect on PSS given the data. A Bayes Factor of 8.80 provided substantial evidence in support of the null hypothesis that age did not have an effect on perceptual synchrony.
In addition, a linear regression model was applied to the data to analyze changes in the PSS based on age (data not shown). No significant correlation occurred for the visuomotor TOJ (R2 = 0.031, p = .248), audiovisual TOJ (R2 = 0.005, p = .655), or the visual TOJ (R2 = 0.057, p = .116), indicating that PSS estimates remain stable across the age groups tested. However, Bayes factors were estimated from the data comparing the null hypothesis (age cannot predict PSS) to the alternative (age can predict PSS) for the visuomotor (BF = 1.94), audiovisual (BF =3.08) and visual (BF = 1.21) tasks. This subsequent analysis confers weak support for both the null and alternative models indicating that the current assessment may not be sensitive enough to examine the relationship between age and PSS, especially for the visuomotor and visual only tasks.
In Figure 3 we reported associations between JND measures estimated from the three different tasks. Individual data were fitted using a linear regression model. A significant, though weak, positive linear relationship was found between the audiovisual and the visuomotor JND values (R2 = 0.114, p < 0.05, Fig. 3A), suggesting that individuals who show a large window of audiovisual integration also tend to show a large window of visuomotor integration. Similarly, we found a significant, positive linear relation between visual and audiovisual individual JNDs (R2 = 0.348, p < 0.0001, Fig. 3B) and between visual and visuomotor JND values (R2 = 0.111, p < 0.05, Fig. 3C), indicating that poor temporal sensitivity correlates at least weakly across all sensory conditions.
Figure 3. Significant relationships between JND values from all 3 tasks.
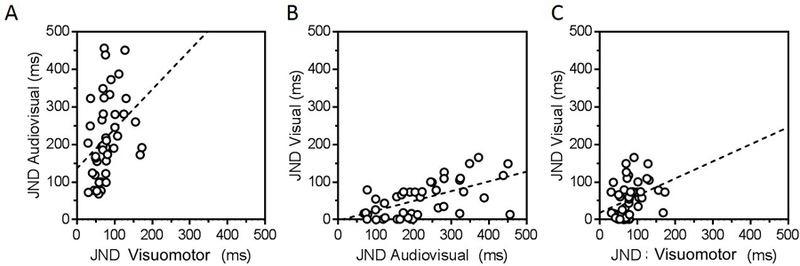
Simple linear regression models were fit to individual JND data from the audiovisual and visuomotor (left panel), the visual and audiovisual (middle panel), and the visual and visuomotor (right panel) conditions revealing positive associations between all tasks tested.
Figure 4 shows the relationships between perceptual synchrony, measured through the PSS in the audiovisual, visuomotor and visual tasks. A linear regression model did not reveal any significant relationship between the audiovisual and visuomotor PSS (R2 = 0.001, p = .867, Fig. 4A), between the visual and audiovisual PSS (R2 < 0.001, p = .998, Fig. 4B), or between the visual and visuomotor PSS (R2 = 0.005, p = .639, Fig. 4C), suggesting that perceptual synchrony is context-dependent. The data were also examined by estimating a Bayes factor comparing the data under the null hypothesis, wherein the PSS from one task did not predict the PSS from another task, and the alternative hypothesis, where the PSS from one task did predict the PSS from another task. Estimated Bayes factors (null/alternative) suggest that the data favors the null hypothesis for audiovisual and visuomotor (BF = 3.32), for visual and audiovisual (BF = 3.37), and for visual and visuomotor (BF = 3.10) PSS estimates. In other words, under the current data PSS estimates are more than 3 times more likely to not correlate between any two of the tasks we tested.
Figure 4. Distinct synchronization processes for unisensory, multisensory, and sensorimotor frameworks.
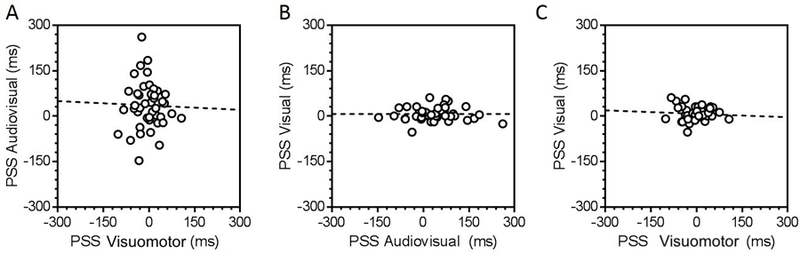
Simple linear regression models were fit to individual PSS data from the audiovisual and visuomotor (left panel), the visual and audiovisual (middle panel), and the visual and visuomotor (right panel) conditions revealing no significant associations.
Discussion
Reliable multisensory processing is important for several tasks such as speech recognition and comprehension, and illustrates the importance of multisensory integration in everyday functions (Stevenson et al., 2015; Gordon-Salant et al., 2017). Heightened temporal sensitivity increases the likelihood of accurately binding and segregating information from the same or different sources, respectively. Conversely, reduced sensitivity in temporal integration can lead to significant distortions in global perceptual estimates and greatly impact quality of life. Findings from this study show that the ability to temporally segregate/integrate sensory and motor signals significantly declines with age. In contrast, perceptual synchrony is not affected by age in any task assessed, indicating selective sparing of context-specific processes. These results suggest that while integration likely represents a global mechanism guiding general sensitivity in temporal perception, perceptual synchrony reflects context-dependent biases based on unique properties of the stimulus that remain intact throughout the lifespan.
Since age significantly impacts sensory perception (Howarth and Shone, 2006; Werner et al., 2010; Owsley, 2011; Andersen, 2012), the reported deficits may be partially due to declines in unisensory processing (Ostroff et al., 2003; Čeponienė et al., 2008). With increasing age, the sensory organs responsible for encoding auditory and visual information deteriorate as do structures within the central nervous system that process sensory events (Goodin et al., 1978; Celesia et al., 1987; Ostroff et al., 2003; Lemaître et al., 2005; Werner et al., 2010; Kraus and Anderson, 2013). In order to compensate for slower processing times, the temporal constraints guiding the integration or segregation of information must be more flexible. Therefore, stimuli separated by large temporal delays may become perceptually bound in an older adult, leading to poorer temporal sensitivity (Diederich et al., 2008; Mozolic et al., 2012). In addition, structural and functional alterations associated with aging result in noisy neural signals and degraded perceptual estimates. This may facilitate an increased benefit of multisensory information, as predicted by the principle of inverse effectiveness where the strength of multisensory integration increases when unisensory signals are less reliable (Meredith and Stein, 1983, 1986). Indeed, older adults have exhibited greater multisensory behavioral gains than young adults (Laurienti et al., 2006; Peiffer et al., 2007). However, greater reliance on multiple sensory signals instead of degraded unitary signals could make older adults more susceptible to sensory integration despite extreme temporal delays, resulting in reduced sensitivity.
Because the age-related sensitivity deficits shown here are irrespective of task-specific contexts, there is likely a general impairment affecting an all-inclusive process rather than selective targeting of multiple mechanisms (Mozolic et al., 2012). A likely candidate is the cortico-striatal dopaminergic system that enables general time perception (Meck, 2006b, 2006a; Agostino and Cheng, 2016; Turgeon et al., 2016). Neurological disorders affecting dopaminergic activity cause deficits in time perception. For example, individuals affected with Parkinson’s disease show poor temporal estimation in both audition and vision (Pastor et al., 1992; Smith et al., 2007; Allman and Meck, 2012), supporting the involvement of the dopaminergic system in a global timing mechanism. Moreover, the striatum, a subcortical nucleus that receives dopaminergic inputs from the midbrain, also exhibits heightened susceptibility to age as older adults show significant dopamine depletion in this region (Li and Backman, 2010). Therefore, aging may impact the dopaminergic circuity and have consequent global effects on temporal sensitivity. The impairment in this central timing mechanism might be a plausible explanation for the widespread discrepancy reported here and for the significant correlations between JND values from all 3 conditions observed in the current study.
Another possible cause of global impairments in older adults is a reduction in GABA concentration, the main inhibitory neurotransmitter, as previously reported for this population (Leventhal et al., 2003; Betts et al., 2005; Pinto et al., 2010; Porges et al., 2017). Altered lateral inhibition can lead to a noisy neural network that produces unreliable signals, impacting resolution and impairing perceptual sensitivity. Indeed, decreased GABA levels and imbalanced excitatory/inhibitory (E/I) connections can result in general cognitive slowing and less efficient temporal integration (Leventhal et al., 2003; Hoshino, 2014; van Atteveldt et al., 2014; Porges et al., 2017). GABAergic activity and E/I balance also contribute to the generation and synchronization of gamma band oscillations (Bartos et al., 2007; Atallah and Scanziani, 2009; Isaacson and Scanziani, 2011; Balz et al., 2016). This process facilitates integration through phase coherence of groups of neurons as described in both multisensory and sensorimotor contexts (Senkowski et al., 2008; Atallah and Scanziani, 2009). However gamma band synchronization is reduced in older adults (Goossens et al., 2016). Therefore, altered GABA-mediated transmission and imbalanced E/I connections can diminish the efficiency of multisensory integration (Hoshino, 2014) and may play a role in the poor sensitivity found in older adults from the present study. In addition, reductions to GABAergic activity are shown to gradually occur over the lifespan (Pinto et al., 2010), beginning around 30 years of age. This parallels the gradual reduction of temporal sensitivities shown across the 3 age groups in the current study.
So far we have discussed how changes in low level processing may be responsible for the temporal impairments we found in the older population. However, changes in higher cognitive function might have similarly induced these temporal deficits. For example, the ability to divide attention across multiple modalities is a crucial factor for efficient and reliable integration (Alsius et al., 2005; Talsma et al., 2006; Mozolic et al., 2007; Vercillo and Gori, 2015; Macaluso et al., 2016). Yet older adults show diminished top-down attentional control and increased susceptibility to distracting information (Dywan et al., 1998; Alain and Woods, 1999; Andrés et al., 2006; Glisky, 2007). Unlike young adults, older adults also show deficits in selective attention during the presentation of audiovisual stimuli (Hugenschmidt et al., 2009). While young adults are able to selectively attend to one modality leading to diminished integration, older adults continue to integrate the two signals due to a reduced ability to control and direct attention toward specific stimuli (Hugenschmidt et al., 2009). Another cognitive factor that may affect temporal sensitivity is fatigue. Previous studies show that older adults are more susceptible to mental fatigue with increased time on an experimental task leading to reduced attentional control and increased variability in responses (Boksem et al., 2006; Boksem and Tops, 2008; Wascher and Getzmann, 2014). Age-related degeneration of working memory capacity (Craik and Salthouse, 2000) may also account for the temporal impairments reported here as working memory capacity is associated with enhanced abilities to recall temporal relations between events and with greater temporal resolution (Unsworth and Engle, 2007; Broadway and Engle, 2011; Bartholomew et al., 2015).
Temporal judgments are subjective, with estimates based on prior experiences and individual percepts reflected in an internal decision criteria (Treisman, 1984; Sperling, 2008; Yarrow et al., 2011). For instance, a detected temporal delay must be compared to an internal criterion (i.e. a sound is synchronous to a flash) to determine if the delay surpassed the criterion and a response of flash first can be made or vice versa (Yarrow et al., 2011, 2016). Therefore, a possible explanation of our results may be a change in the response criterion inherent to TOJ tasks. Older adults often adopt a more conservative decision strategy (Ratcliff et al., 2006) and don’t update their response criterion in a similar manner as young adults due to reduced perceptual reliability (Brown and Steyvers, 2005; Rakitin and Malapani, 2008; Solomon et al., 2012). However, changes to decision criteria cannot be the main driver of age-related changes in temporal processing reported in this study. A shift in response criterion would likely induce a shift in the PSS, not necessarily a reduction of JND values as reported (Di Luca et al., 2009; van Eijk et al., 2010). Future studies are necessary to understand the contributions from change in neural processing times and change in response criteria that can decrease temporal precision over the course of aging.
The variety of global deleterious effects resulting from aging likely also have consequences on the sensitivity for temporal integration, as discussed above. In contrast, there was no evidence for an age-related effect on perceptual synchrony providing support for distinct mechanisms. One hypothesis predicting how the brain adjusts for natural temporal discrepancies in order to perceive synchrony is temporal renormalization. Under this concept, the timing of an event is defined as the average across multiple neural timings from different modalities and stimuli (Freeman et al., 2013). If a particular neural timing changes, for instance a dramatic reduction in processing speed in one modality, then the average timing reflects this change with a resultant perceptual shift in observer bias. For example, in a unique case study of a patient presenting with a brain lesion along the olivo-collicular pathway likely affecting early processing of auditory information, the individual’s perceptual synchrony showed a change toward audio-leading bias however audiovisual integration was unaffected (Freeman et al., 2013). Assuming that normal aging affects all sensory and motor systems fairly equally (Eckert, 2011; Harris et al., 2011), the average neural timing across different modalities and stimuli would remain relatively stable leading to preservation of observer bias.
Unlike the presumed supramodal processes underlying integration, perceptual synchrony appears to depend on rather specific contextual factors. The concept of synchrony is inherent to an individual’s experience of the world as coherent and is heavily biased toward the natural asynchronies of the stimuli themselves (Aschersleben and Prinz, 1995). For instance, the consistent visual-lead bias in terms of audiovisual events reflects the natural difference in propagation times between sound and light (Keetels and Vroomen, 2012). In addition, we often assume voluntary motor actions directly produce consequential sensory events leading to a perceptual anticipation of sensory signals for sensorimotor binding. The properties of the stimuli also significantly impact observer bias of simultaneity, for example semantically congruent audiovisual speech stimuli shifts PSS estimates in temporal judgments (Vatakis et al., 2008). These variable scenarios reflect biases developed specifically to the unique properties of those events, not some shared property that can be reflected across stimuli, supporting the notion that observer bias is derived from stimulus-specific and content-dependent properties.
Despite the specific deficits acquired with aging, the brain has a unique capacity to adjust and recalibrate in order to stabilize perception. For instance, color perception remains stable across the lifespan despite a brunescent lens and functional changes along the various cone pathways (Webster et al., 2005; Webster, 2015), similar to maintenance of audiovisual synchrony perception in older adults with hearing loss (Tye-Murray et al., 2007). Following this explanation, maintenance of PSS measures across age groups may reflect a general ability to recalibrate for relative delays between sensory systems. While various perceptual functions or modalities may be affected by aging to different degrees resulting in a nosier, less sensitive system, a long-term adaptive process may be responsible for the perceptual constancy found for PSS estimates reflecting differential effects of age on temporal integration. However, while the visual system was a major interest in the present study, the constant use of visual stimuli in all 3 tasks may present a confound. In addition, visual latencies in the sensorimotor task were not constant across experimental blocks or participants due to the method of delivering a visual latency based on participant’s reaction time measured prior to experimentation. With these various limitations, our results should be interpreted with some caution.
Understanding the specific alterations to multisensory and sensorimotor integration that occur with aging is necessary for the development and application of non-invasive strategies benefitting overall daily function of the older population. Indeed, deficits in both the audiovisual (Setti et al., 2011; Merriman et al., 2015) and sensorimotor domain (Tinetti et al., 1988; Maki and McIlroy, 1996) have been related to balance impairments and increased risk for falls in older adults. Results reported here show that aging effects integration but not perceptual synchrony, suggesting discreet processes guiding these two aspects of temporal perception. While various global mechanisms are impacted by aging leading to greater variability in processing external information and reduced sensitivity, the subjective judgments of these events remain intact in order to maintain consistency in synchronous perception. Future studies are necessary to parse out the specific mechanisms underlying each process to further elucidate how the aging process affects these variable aspects and develop more targeted approaches to enhance daily function in the older adult.
Acknowledgements
We thank Dr. A. Grant Schissler for his assistance with performing and interpreting Bayesian analysis. This research was supported by EY023268 to FJ, EY10834 to MW, 5U54GM104944, and P20 GM103650. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Agostino PV and Cheng R-K (2016). Contributions of dopaminergic signaling to timing accuracy and precision. Current Opinion in Behavioral Sciences, 8, 153–160. [Google Scholar]
- Alain C and Woods DL (1999). Age-related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychology and aging, 14(3), 507–19. [DOI] [PubMed] [Google Scholar]
- Alais D and Cass J (2010). Multisensory perceptual learning of temporal order: audiovisual learning transfers to vision but not audition. PloS one, 5(6), e11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ and Meck WH (2012). Pathophysiological distortions in time perception and timed performance. Brain : a journal of neurology, 135(Pt 3), 656–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsius A, Navarra J, Campbell R and Soto-Faraco S (2005). Audiovisual Integration of Speech Falters under High Attention Demands. Current Biology, 15(9), 839–843. [DOI] [PubMed] [Google Scholar]
- Andersen GJ (2012). Aging and vision: Changes in function and performance from optics to perception. Wiley Interdisciplinary Reviews: Cognitive Science, 3(3), 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés P, Parmentier FBR and Escera C (2006). The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia, 44(12), 2564–2568. [DOI] [PubMed] [Google Scholar]
- Aschersleben G and Prinz W (1995). Synchronizing actions with events: The role of sensory information. Perception & Psychophysics, 57(3), 305–317. [DOI] [PubMed] [Google Scholar]
- Atallah BV and Scanziani M (2009). Instantaneous Modulation of Gamma Oscillation Frequency by Balancing Excitation with Inhibition. Neuron, 62(4), 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balz J et al. (2016). GABA concentration in superior temporal sulcus predicts gamma power and perception in the sound-induced flash illusion. NeuroImage, 125, 724–730. [DOI] [PubMed] [Google Scholar]
- Bartholomew AJ, Meck WH and Cirulli ET (2015). Analysis of Genetic and Non-Genetic Factors Influencing Timing and Time Perception. PLOS ONE Edited by van Wassenhove V, 10(12), e0143873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I and Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience, 8(1), 45–56. [DOI] [PubMed] [Google Scholar]
- Baum SH and Stevenson RA (2017). Shifts in Audiovisual Processing in Healthy Aging. Current Behavioral Neuroscience Reports, 4(3), 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard G and Barnett-Cowan M (2016). Impaired timing of audiovisual events in the elderly. Experimental Brain Research, 234(1), 331–340. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB and Bennett PJ (2005). Aging reduces center-surround antagonism in visual motion processing. Neuron, 45(3), 361–6. [DOI] [PubMed] [Google Scholar]
- de Boer-Schellekens L and Vroomen J (2014). Multisensory integration compensates loss of sensitivity of visual temporal order in the elderly. Experimental Brain Research, 232(1), 253–262. [DOI] [PubMed] [Google Scholar]
- Boksem MAS, Meijman TF and Lorist MM (2006). Mental fatigue, motivation and action monitoring. Biological Psychology, 72(2), 123–132. [DOI] [PubMed] [Google Scholar]
- Boksem MAS and Tops M (2008). Mental fatigue: Costs and benefits. Brain Research Reviews, 59(1), 125–139. [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Broadway JM and Engle RW (2011). Individual differences in working memory capacity and temporal discrimination. PloS one, 6(10), e25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CJ, Chan YM, Anderson AJ and McKendrick AM (2018). Audiovisual Temporal Perception in Aging: The Role of Multisensory Integration and Age-Related Sensory Loss. Frontiers in Human Neuroscience, 12, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S and Steyvers M (2005). The dynamics of experimentally induced criterion shifts. Journal of experimental psychology. Learning, memory, and cognition, 31(4), 587–99. [DOI] [PubMed] [Google Scholar]
- Bueti D, Bahrami B and Walsh V (2008). Sensory and Association Cortex in Time Perception. Journal of Cognitive Neuroscience, 20(6), 1054–1062. [DOI] [PubMed] [Google Scholar]
- Bueti D, van Dongen EV and Walsh V (2008). The Role of Superior Temporal Cortex in Auditory Timing. PLoS ONE Edited by Greene E, 3(6), e2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D, Banks MS and Morrone MC (2009). Auditory dominance over vision in the perception of interval duration. Experimental Brain Research, 198(1), 49–57. [DOI] [PubMed] [Google Scholar]
- Burr D, Tozzi A and Morrone MC (2007). Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nature Neuroscience, 10(4), 423–425. [DOI] [PubMed] [Google Scholar]
- Busey T, Craig J, Clark C and Humes L (2010). Age-related changes in visual temporal order judgment performance: Relation to sensory and cognitive capacities. Vision research, 50(17), 1628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesia GG, Kaufman D and Cone S (1987). Effects of age and sex on pattern electroretinograms and visual evoked potentials. Electroencephalography and clinical neurophysiology, 68(3), 161–71. [DOI] [PubMed] [Google Scholar]
- Čeponienė R, Westerfield M, Torki M and Townsend J (2008). Modality-specificity of sensory aging in vision and audition: Evidence from event-related potentials. Brain Research, 1215, 53–68. [DOI] [PubMed] [Google Scholar]
- Chan YM, Pianta MJ and McKendrick AM (2014). Older age results in difficulties separating auditory and visual signals in time. Journal of Vision, 14(11), 1–11. [DOI] [PubMed] [Google Scholar]
- Conrey B and Pisoni DB (2006). Auditory-visual speech perception and synchrony detection for speech and nonspeech signals. The Journal of the Acoustical Society of America, 119(6), 4065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM and Salthouse TA (eds) (2000). The handbook of aging and cognition [Google Scholar]
- Diaconescu AO, Hasher L and McIntosh AR (2013). Visual dominance and multisensory integration changes with age. NeuroImage, 65, 152–166. [DOI] [PubMed] [Google Scholar]
- Diederich A, Colonius H and Schomburg A (2008). Assessing age-related multisensory enhancement with the time-window-of-integration model. Neuropsychologia, 46(10), 2556–62. [DOI] [PubMed] [Google Scholar]
- Dienes Z (2014). Using Bayes to get the most out of non-significant results. Frontiers in psychology, 5, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diersch N, Cross ES, Stadler W, Schütz-Bosbach S and Rieger M (2012). Representing others’ actions: the role of expertise in the aging mind. Psychological Research, 76(4), 525–541. [DOI] [PubMed] [Google Scholar]
- Dywan J, Segalowitz SJ and Webster L (1998). Source Monitoring: ERP Evidence for Greater Reactivity to Nontarget Information in Older Adults. Brain and Cognition, 36(3), 390–430. [DOI] [PubMed] [Google Scholar]
- Eckert MA (2011). Slowing down: age-related neurobiological predictors of processing speed. Frontiers in neuroscience, 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B and Tibshirani R (1994). An introduction to the bootstrap
- Eg R and Behne DM (2015). Perceived synchrony for realistic and dynamic audiovisual events. Frontiers in psychology, 6, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk RLJ, Kohlrausch A, Juola JF and van de Par S (2008). Audiovisual synchrony and temporal order judgments: Effects of experimental method and stimulus type. Perception & Psychophysics, 70(6), 955–968. [DOI] [PubMed] [Google Scholar]
- van Eijk RLJ, Kohlrausch A, Juola JF and van de Par S (2010). Temporal order judgment criteria are affected by synchrony judgment sensitivity. Attention, Perception & Psychophysics, 72(8), 2227–2235. [DOI] [PubMed] [Google Scholar]
- Fechner GT (1860). Elements of Psychophysics
- Fitzgibbons PJ and Gordon-Salant S (1994). Age Effects on Measures of Auditory Duration Discrimination. Journal of Speech Language and Hearing Research, 37(3), 662. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ and Gordon-Salant S (1995). Age effects on duration discrimination with simple and complex stimuli. The Journal of the Acoustical Society of America, 98(6), 3140–5. [DOI] [PubMed] [Google Scholar]
- Franz JR, Francis CA, Allen MS, O’Connor SM and Thelen DG (2015). Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Human movement science, 40, 381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ED et al. (2013). Sight and sound out of synch: Fragmentation and renormalisation of audiovisual integration and subjective timing. Cortex, 49(10), 2875–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL (2007). Changes in Cognitive Function in Human Aging Brain Aging: Models, Methods, and Mechanisms. [PubMed] [Google Scholar]
- Goodin DS, Squires KC, Henderson BH and Starr A (1978). Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalography and clinical neurophysiology, 44(4), 447–58. [DOI] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J and van Wieringen A (2016). Aging Affects Neural Synchronization to Speech-Related Acoustic Modulations. Frontiers in aging neuroscience, 8, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S, Yeni-Komshian GH, Fitzgibbons PJ, Willison HM and Freund MS (2017). Recognition of asynchronous auditory-visual speech by younger and older listeners: A preliminary study. The Journal of the Acoustical Society of America, 142(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Eckert MA, Ahlstrom JB and Dubno JR (2011). Age-related differences in gap detection: Effects of task difficulty and cognitive ability Kelly, 264(843), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron J, Hanson JVM and Whitaker D (2009). Effect before cause: supramodal recalibration of sensorimotor timing. PloS one, 4(11), e7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R and Swinnen SP (2005). Neural basis of aging: the penetration of cognition into action control. The Journal of neuroscience : the official journal of the Society for Neuroscience, 25(29), 6787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino O (2014). Balanced crossmodal excitation and inhibition essential for maximizing multisensory gain. Neural computation, 26(7), 1362–85. [DOI] [PubMed] [Google Scholar]
- Howarth A and Shone GR (2006). Ageing and the auditory system. Postgraduate medical journal, 82(965), 166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt CE, Mozolic JL and Laurienti PJ (2009). Suppression of multisensory integration by modality-specific attention in aging. Neuroreport, 20(4), 349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Busey TA, Craig JC and Kewley-port D (2009). The effects of age on sensory thresholds and temporal gap detection in hearing, vision, and touch. Atten Percept Psychophys, 71(4), 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS and Scanziani M (2011). How Inhibition Shapes Cortical Activity. Neuron, 72(2), 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M et al. (2010). Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain, 133(3), 727–745. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Allison LK and Kiemel T (2010). The Dynamics of Visual Reweighting in Healthy and Fall-Prone Older Adults. Journal of Motor Behavior, 42(4), 197–208. [DOI] [PubMed] [Google Scholar]
- Jones CRG and Jahanshahi M (2014). Contributions of the Basal Ganglia to Temporal Processing: Evidence from Parkinson’s Disease. Timing & Time Perception, 2(1), 87–127. [Google Scholar]
- Keetels M and Vroomen J (2012). Perception of Synchrony between the Senses The Neural Bases of Multisensory Processes. [Google Scholar]
- Klink PC, Montijn JS and van Wezel RJA (2011). Crossmodal duration perception involves perceptual grouping, temporal ventriloquism, and variable internal clock rates. Attention, Perception, and Psychophysics, 73(1), 219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N and Anderson S (2013). The Effects of Aging on Auditory Processing. The Hearing journal, 66(1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Maldjian JA and Wallace MT (2006). Enhanced multisensory integration in older adults. Neurobiology of aging, 27(8), 1155–63. [DOI] [PubMed] [Google Scholar]
- Lemaître H, Crivello F, Grassiot B, Alpérovitch A, Tzourio C and Mazoyer B (2005). Age- and sex-related effects on the neuroanatomy of healthy elderly. NeuroImage, 26(3), 900–911. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y and Ma Y (2003). GABA and Its Agonists Improved Visual Cortical Function in Senescent Monkeys. Science, 300(5620), 812–815. [DOI] [PubMed] [Google Scholar]
- Li S-C and Backman L (2010). Dopaminergic modulation of cognition across the life span. Neuroscience and Biobehavioral Reviews, 34, 625–630. [DOI] [PubMed] [Google Scholar]
- Di Luca M, Machulla TK and Ernst MO (2009). Recalibration of multisensory simultaneity: Cross-modal transfer coincides with a change in perceptual latency. Journal of Vision, 9(12), 7–7. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Noppeney U, Talsma D, Vercillo T, Hartcher-O’Brien J and Adam R (2016). The Curious Incident of Attention in Multisensory Integration: Bottom-up vs. Top-down. Multisensory Research, 29(6–7), 557–583. [Google Scholar]
- Maki BE and McIlroy WE (1996). Postural control in the older adult. Clinics in geriatric medicine, 12(4), 635–58. [PubMed] [Google Scholar]
- Matthews WJ and Meck WH (2016). Temporal Cognition: Connecting Subjective Time to Perception, Attention, and Memory. [DOI] [PubMed] [Google Scholar]
- Meck WH (2006a). Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Research, 1108(1), 157–167. [DOI] [PubMed] [Google Scholar]
- Meck WH (2006b). Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research, 1109(1), 93–107. [DOI] [PubMed] [Google Scholar]
- Meck WH and Benson AM (2002). Dissecting the Brain’s Internal Clock: How Frontal–Striatal Circuitry Keeps Time and Shifts Attention. Brain and Cognition, 48(1), 195–211. [DOI] [PubMed] [Google Scholar]
- Meredith MA and Stein BE (1983). Interactions among converging sensory inputs in the superior colliculus. Science (New York, N.Y.), 221(4608), 389–91. [DOI] [PubMed] [Google Scholar]
- Meredith MA and Stein BE (1986). Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. Journal of Neurophysiology, 56(3), 640–662. [DOI] [PubMed] [Google Scholar]
- Merriman NA, Whyatt C, Setti A, Craig C and Newell FN (2015). Successful balance training is associated with improved multisensory function in fall-prone older adults. Computers in Human Behavior, 45, 192–203. [Google Scholar]
- Morey RD, Romeijn J-W and Rouder JN (2016). The philosophy of Bayes factors and the quantification of statistical evidence. Journal of Mathematical Psychology, 72, 6–18. [Google Scholar]
- Morrone MC, Ross J and Burr D (2005). Saccadic eye movements cause compression of time as well as space. Nature Neuroscience, 8(7), 950–954. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hugenschmidt CE, Peiffer AM and Laurienti PJ (2007). Modality-specific selective attention attenuates multisensory integration. Experimental Brain Research, 184(1), 39–52. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hugenschmidt CE, Peiffer AM and Laurienti PJ (2012). Multisensory Integration and Aging [PubMed]
- Murray MM, Eardley AF, Edginton T, Oyekan R, Smyth E and Matusz PJ (2018). Sensory dominance and multisensory integration as screening tools in aging. Scientific Reports, 8(1), 8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JF, Cheeseman JR, Baxter MW, Thomason KE, Adkins OC and Rogers CE (2014). Aging and visual length discrimination: Sequential dependencies, biases, and the effects of multiple implicit standards. Vision Research, 98, 89–98. [DOI] [PubMed] [Google Scholar]
- Ostroff JM, McDonald KL, Schneider BA and Alain C (2003). Aging and the processing of sound duration in human auditory cortex. Hearing Research, 181(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Owsley C (2011). Aging and vision. Vision research, 51(13), 1610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Jahanshahi M and Obeso JA (1992). Time estimation and reproduction is abnormal in Parkinson’s disease. Brain: A journal of neurology, 115 Pt 1, 211–25. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Mozolic JL, Hugenschmidt CE and Laurienti PJ (2007). Age-related multisensory enhancement in a simple audiovisual detection task. Neuroreport, 18(10), 1077–1081. [DOI] [PubMed] [Google Scholar]
- Pelli DG (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442. [PubMed] [Google Scholar]
- Pinto JGA, Hornby KR, Jones DG and Murphy KM (2010). Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Frontiers in cellular neuroscience, 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC et al. (2017). Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(1), 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakitin BC and Malapani C (2008). Effects of feedback on time production errors in aging participants. Brain Research Bulletin, 75(1), 23–33. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A and McKoon G (2006). Aging and individual differences in rapid two-choice decisions. Psychonomic bulletin & review, 13(4), 626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard Morey MD (2018). Package ‘BayesFactor’ Title Computation of Bayes Factors for Common Designs
- Rouder JN, Morey RD, Speckman PL and Province JM (2012). Default Bayes factors for ANOVA designs. Journal of Mathematical Psychology, 56(5), 356–374. [Google Scholar]
- Rouder JN and Morey RD (2012). Default Bayes Factors for Model Selection in Regression. Multivariate Behavioral Research, 47(6), 877–903. [DOI] [PubMed] [Google Scholar]
- Seidler RD et al. (2010). Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neuroscience and biobehavioral reviews, 34(5), 721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D et al. (2008). Crossmodal binding through neural coherence: implications for multisensory processing. Trends in Neurosciences, 31(8), 401–409. [DOI] [PubMed] [Google Scholar]
- Setti A, Finnigan S, et al. (2011). Audiovisual temporal discrimination is less efficient with aging: an event-related potential study. Neuroreport, 22(11), 554–8. [DOI] [PubMed] [Google Scholar]
- Setti A, Burke KE, Kenny RA and Newell FN (2011). Is inefficient multisensory processing associated with falls in older people? Experimental Brain Research, 209(3), 375–384. [DOI] [PubMed] [Google Scholar]
- Setti A, Stapleton J, Leahy D, Walsh C, Kenny RA and Newell FN (2014). Improving the efficiency of multisensory integration in older adults: Audio-visual temporal discrimination training reduces susceptibility to the sound-induced flash illusion. Neuropsychologia, 61, 259–268. [DOI] [PubMed] [Google Scholar]
- Smith JG, Harper DN, Gittings D and Abernethy D (2007). The effect of Parkinson’s disease on time estimation as a function of stimulus duration range and modality. Brain and Cognition, 64(2), 130–143. [DOI] [PubMed] [Google Scholar]
- Solomon JA, Cavanagh P and Gorea A (2012). Recognition criteria vary with fluctuating uncertainty. Journal of Vision, 12(8), 2–2. [DOI] [PubMed] [Google Scholar]
- Sperling G (2008). Type 1 and Type 2 Experiments
- Stevenson RA, Wilson MM, Powers AR and Wallace MT (2013). The effects of visual training on multisensory temporal processing. Experimental Brain Research, 225(4), 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA et al. (2015). Deficits in audiovisual speech perception in normal aging emerge at the level of whole-word recognition. Neurobiology of aging, 36(1), 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Baum SH, Krueger J, Newhouse PA and Wallace MT (2018). Links between temporal acuity and multisensory integration across life span. Journal of Experimental Psychology: Human Perception and Performance, 44(1), 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Zemtsov RK and Wallace MT (2012). Individual differences in the multisensory temporal binding window predict susceptibility to audiovisual illusions. Journal of experimental psychology. Human perception and performance, 38(6), 1517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JV et al. (2001). When is now? Perception of simultaneity. Proceedings of the Royal Society B: Biological Sciences, 268(1462), 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano Y, Keetels M and Vroomen J (2010). Adaptation to motor-visual and motor-auditory temporal lags transfer across modalities. Experimental brain research, 201(3), 393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma D, Doty TJ and Woldorff MG (2006). Selective Attention and Audiovisual Integration: Is Attending to Both Modalities a Prerequisite for Early Integration? Cerebral Cortex, 17(3), 679–690. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M and Ginter SF (1988). Risk Factors for Falls among Elderly Persons Living in the Community. New England Journal of Medicine, 319(26), 1701–1707. [DOI] [PubMed] [Google Scholar]
- Treisman M (1984). A theory of criterion setting: an alternative to the attention band and response ratio hypotheses in magnitude estimation and cross-modality matching. Journal of experimental psychology. General, 113(3), 443–63. [DOI] [PubMed] [Google Scholar]
- Turgeon M, Lustig C and Meck WH (2016). Cognitive Aging and Time Perception: Roles of Bayesian Optimization and Degeneracy. Frontiers in Aging Neuroscience, 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye-Murray N, Sommers MS and Spehar B (2007). Audiovisual Integration and Lipreading Abilities of Older Adults with Normal and Impaired Hearing. Ear and Hearing, 28(5), 656–668. [DOI] [PubMed] [Google Scholar]
- Ulbrich P, Churan J, Fink M and Wittmann M (2009). Perception of Temporal Order: The Effects of Age, Sex, and Cognitive Factors. Aging, Neuropsychology, and Cognition, 16(2), 183–202. [DOI] [PubMed] [Google Scholar]
- Unsworth N and Engle RW (2007). The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review, 114(1), 104–132. [DOI] [PubMed] [Google Scholar]
- van Atteveldt N, Murray MM, Thut G and Schroeder CE (2014). Multisensory Integration: Flexible Use of General Operations. Neuron, 81(6), 1240–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatakis A, Ghazanfar AA and Spence C (2008). Facilitation of multisensory integration by the "unity effect" reveals that speech is special. Journal of Vision, 8(9), 14–14. [DOI] [PubMed] [Google Scholar]
- Vercillo T, Carrasco C and Jiang F (2017). Age-Related Changes in Sensorimotor Temporal Binding. Frontiers in Human Neuroscience, 11, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercillo T and Gori M (2015). Attention to sound improves auditory reliability in audio-tactile spatial optimal integration. Frontiers in integrative neuroscience, 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher E and Getzmann S (2014). Rapid Mental Fatigue Amplifies Age-Related Attentional Deficits. Journal of Psychophysiology, 28(3), 215–224. [Google Scholar]
- van Wassenhove V, Grant KW and Poeppel D (2007). Temporal window of integration in auditory-visual speech perception. Neuropsychologia, 45(3), 598–607. [DOI] [PubMed] [Google Scholar]
- Weber EH (1834). De Tactu
- Webster MA (2015). Visual Adaptation. Annual review of vision science, 1, 547–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MA, Werner JS and Field DJ (2005). Adaptation and the Phenomenology of Perception. in Fitting the Mind to the World: Adaptation and Aftereffects in High Level Visions: Advances in Visual Cognition Series [Google Scholar]
- Werner JS, Schefrin BE and Bradley A (2010). Optics and vision of the aging eye. in Handbook of Optics 3rd edn, 14.11–14.38. [Google Scholar]
- Yarrow K, Jahn N, Durant S and Arnold DH (2011). Shifts of criteria or neural timing? The assumptions underlying timing perception studies. Consciousness and Cognition, 20(4), 1518–1531. [DOI] [PubMed] [Google Scholar]
- Yarrow K, Martin SE, Di Costa S, Solomon JA and Arnold DH (2016). A Roving Dual-Presentation Simultaneity-Judgment Task to Estimate the Point of Subjective Simultaneity. Frontiers in Psychology, 7, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TT, Cluff T and Balasubramaniam R (2014). Visual reliance for balance control in older adults persists when visual information is disrupted by artificial feedback delays. PloS one, 9(3), e91554. [DOI] [PMC free article] [PubMed] [Google Scholar]


