Abstract
Sertoli cells regulate male germ cell proliferation and differentiation and are a critical component of the spermatogonial stem cell (SSC) niche, where homeostasis is maintained by the interplay of several signaling pathways and growth factors. These factors are secreted by Sertoli cells located within the seminiferous epithelium, and by interstitial cells residing between the seminiferous tubules. Sertoli cells and peritubular myoid cells produce glial cell line-derived neurotrophic factor (GDNF), which binds to the RET/GFRA1 receptor complex at the surface of undifferentiated spermatogonia. GDNF is known for its ability to drive SSC self-renewal and proliferation of their direct cell progeny. Even though the effects of GDNF are well studied, our understanding of the regulation its expression is still limited. The purpose of this review is to discuss how GDNF expression in Sertoli cells is modulated within the niche, and how these mechanisms impact germ cell homeostasis.
Introduction:
Proper regulation of stem cell fate is critical to maintain adequate cell numbers in health and diseases. Evidence suggests that stem cell behavior is regulated by both extracellular signals from their microenvironment, or niche, and intrinsic signals within the cells (Li and Xie 2005). Much work has been recently done to understand how the niche controls stem cell self-renewal and differentiation and how, in turn, stem cells influence their environment (Chacon-Martinez, et al. 2018). The present review focuses on recent findings pertaining to glial cell-line derived neurotrophic factor (GDNF) as one of the major paracrine factors specifically responsible for self-renewal of spermatogonial stem cells (SSCs) within their niche, and proliferation of their direct progeny.
Mammalian sperm production occurs via a highly organized process called spermatogenesis, which is maintained throughout life by a small population of stem cells called spermatogonial stem cells (SSCs). Identifying SSCs and understanding their population dynamics has been a challenging task due to their low numbers (less than 0.03% of adult testicular cells)(Tegelenbosch and de Rooij 1993) and the lack of specific markers allowing the distinction between SSCs and subsets of undifferentiated progenitors (Grisanti, et al. 2009, Chan, et al. 2014, Hermann, et al. 2015). Therefore, over the past decades, several models have been proposed that describe the dynamics of the mammalian SSC population. Leblond and Clermont were first to describe in the rat the existence of rarely dividing type A spermatogonia, that they considered reserve stem cells (A0), coexisting with a population of renewing spermatogonia that they called A1-A4 (Clermont and Leblond 1953, Clermont and Bustos-Obregon 1968, Dym and Clermont 1970). The reserve stem cell would be able to repopulate the testis only after X-ray radiation or chemical injury (Dym and Clermont 1970). However, further investigations by Huckins and Oakberg demonstrated substantial radioactive thymidine incorporation in A0 spermatogonia, indicating their active proliferation (Huckins 1971a, b, Oakberg 1971). Precise cell cycle length evaluation and whole mount preparations subsequently led to the identification of different subsets of A spermatogonia with widely different cell kinetics properties, and to the proposition of a now accepted rodent model where SSCs, also named Asingle (or As) spermatogonia, either self-renew or differentiate to generate two Apaired (or Apr) spermatogonia connected by an intercellular bridge (De Rooij 1973, Huckins 1978). These cells further divide to generate chains of 4 Aaligned (or Aal) spermatogonia. Additional divisions amplify the germ cell population by generating chains of Aal8 to Aal16 cells. This step is considered an amplification step that increases the number of progenitors, and Asingle, Apaired and Aaligned are often referred to as undifferentiated spermatogonia (Huckins 1971a, Huckins and Oakberg 1978). Under the influence of retinoic acid, Aaligned cells differentiate into A1-A4 cells, or differentiating spermatogonia, which further divide to become Intermediate spermatogonia, B spermatogonia, and primary spermatocytes. Spermatocytes will undergo meiosis and give rise to haploid spermatids that will progress through spermiogenesis to become spermatozoa (Haneji, et al. 1983, Russell, et al. 1990, van Pelt and de Rooij 1991, Chen, et al. 2016b, Griswold 2016). In human and non-human primates, the SSC population consists in Adark and Apale spermatogonia, distinguished by their size, nuclear morphology, and different intensity of hematoxylin staining (Clermont and Leblond 1959). Incorporation of radioactive thymidine indicated that Apale spermatogonia were more active than Adark, and the latter were also considered reserve stem cells (Clermont 1969). In humans, each Apale divides into two type B spermatogonia, which in turn produce four spermatocytes (Clermont 1966). Recent investigations in the rhesus monkey, however, have shown that Adark and Apale shared similar molecular phenotypes and therefore might belong to the same population of Asingle cells, albeit at different stages of the cell cycle (Hermann, et al. 2009).
While the Asingle model of spermatogenesis in rodents and primates prevailed for decades, a novel “fragmentation” model was recently proposed in mice, whereby Apaired and Aaligned spermatogonia can detach from the cellular doublets and chains and revert from a transit amplifying mode to a self-renewal mode (Nakagawa, et al. 2007, Klein, et al. 2010, Nakagawa, et al. 2010). This latter model, devised following lineage tracing and live imaging, indicates that Apaired and Aaligned spermatogonia conserve some levels of plasticity, and are therefore not irreversibly committed to differentiation and meiosis, as previously thought. However, proliferation of undifferentiated spermatogonia after fragmentation is very slow (Hara, et al. 2014), and cannot produce the number of differentiating spermatogonia necessary to sustain the steady state of spermatogenesis. It might be because the in vivo imaging experiments used to observe chain fragmentation imposed stressful physiological conditions, or that only specific stages of the seminiferous epithelium cycle were visualized, where the proliferative activity of the cells is normally low (de Rooij 2017). It is now postulated that the Asingle model prevails in the steady state of spermatogenesis, while the plasticity of Apaired or Aaligned spermatogonia might allow them to restore spermatogenesis after external insult (Hara, et al. 2014, Lord and Oatley 2017). Kinetics of spermatogenesis regeneration after treatment with busulfan, combined with differential labeling of Asingle, Apaired and Aaligned spermatogonia confirmed that some Apaired and Aaligned spermatogonia may contribute to the stem cell pool (Zhang, et al. 2016).
Over the past few years, molecular tracing experiments coupled with single cell mRNA sequencing and transplantation assays uncovered the fact that the mammalian Asingle cell population is heterogeneous (Grisanti, et al. 2009, Chan, et al. 2014, Hermann, et al. 2015, Mutoji, et al. 2016, Guo, et al. 2017, Neuhaus, et al. 2017, Green, et al. 2018, Guo, et al. 2018, Hermann, et al. 2018), and that subsets of Asingle cells have different capacity for self-renewal (Aloisio, et al. 2014, Helsel, et al. 2017). According to these data, a new model is now emerging whereby in mice a subpopulation of Asingle cells, marked by high expression of both the transcription factor ID4 and the membrane receptor GFRA1, a co-receptor for glial cell line-derived neurotrophic factor (GDNF), is the purest functional SSC population described to date in immature and adult mice (Sun, et al. 2015, Lord and Oatley 2017, Hermann, et al. 2018, Lord and Oatley 2018). However, while ID4 expression is restricted to true SSCs (ID4high) and cells transitioning into Apaired spermatogonia (ID4low), expression of GFRA1 extends from SSCs to Apaired and some Aaligned spermatogonia (Ebata, et al. 2005, Hofmann, et al. 2005, Grasso, et al. 2012). Other molecules such as PAX7, BMI1, and SHISA6 also mark Asingle cell subsets, but their contribution to the “ultimate” stem cell pool is so far less well defined (Aloisio, et al. 2014, Komai, et al. 2014, Tokue, et al. 2017).
The process of spermatogenesis occurs within the seminiferous epithelium, which provides the specific microenvironment that will maintain SSC self-renewal and drive germ cell differentiation. The fate of the SSCs (i.e. the decision to self-renew or differentiate), depends on the complex interplay of various factors within their niche, which includes the neighboring somatic cells, the extracellular matrix and different soluble factors in their vicinity (Oatley and Brinster 2012).
Within the seminiferous epithelium, the somatic Sertoli cells are considered a critical component of the niche through their role in the maintenance of the stem cell pool and differentiation of the germ line. The factors produced by Sertoli cells that are critical for SSC self-renewal and maintenance include glial cell line-derived neurotrophic factor (GDNF)(Meng, et al. 2000), fibroblast growth factor (FGF2)(Kanatsu-Shinohara, et al. 2003, Kubota, et al. 2004), CSF1 (Oatley, et al. 2009), WNT family proteins (Yeh, et al. 2011, 2012), and leukemia inhibitory factor (LIF)(Kanatsu-Shinohara, et al. 2007). They all contribute to SSC expansion in vitro, as indicated by increased testes colonization after transplantation. But the factor that unequivocally regulates SSC self-renewal as well as proliferation of progenitors in vitro and in vivo is GDNF, a member of the transforming growth factor beta (TGF-b) superfamily that binds to the GFRA1/RET receptor complex (Meng, et al. 2000, Kubota, et al. 2004, Hofmann, et al. 2005, Naughton, et al. 2006, Oatley, et al. 2006, Jijiwa, et al. 2008, Chen, et al. 2016a). Understanding how GDNF expression is regulated within the niche is of paramount importance to understand the first steps of spermatogenesis.
GDNF Signaling
GDNF belongs to the GDNF family of ligands (GFLs), which consists of GDNF, Neurturin (NRTN), Artemin (ARTN) and Persephin (PSPN)(Airaksinen and Saarma 2002, Sariola and Saarma 2003). These proteins are members of the transforming growth beta (TGF-b) superfamily. GDNF was the first GFL discovered and was purified as a trophic factor from the supernatant of a rat glioma cell line (Lin, et al. 1993). GDNF protects and repairs dopaminergic neurons in animal models of Parkinson’s disease, and also rescues motor neurons in vivo (Drinkut, et al. 2016, Ruven, et al. 2018). GDNF is a glycosylated homodimer that binds specific GDNF-family-alpha receptors (GFRA1–4), which are bound to the plasma membrane of the cell by a glycosyl phosphatidylinositol (GPI) anchor (Figure 1)(Jing, et al. 1996, Baloh, et al. 1997, Jing, et al. 1997, Masure, et al. 2000). GFRA1–4 are co-receptors that upon GDNF binding activate the RET receptor tyrosine kinase present at the surface of the target cell (Trupp, et al. 1996). Binding of GDNF-GFRA to the extracellular domain of RET activates its intracellular tyrosine kinase domain, which triggers the activity of multiple pathways in responding cells (Manie, et al. 2001, Kawamoto, et al. 2004, Ibanez 2013).
Figure 1: GDNF/RET signaling.
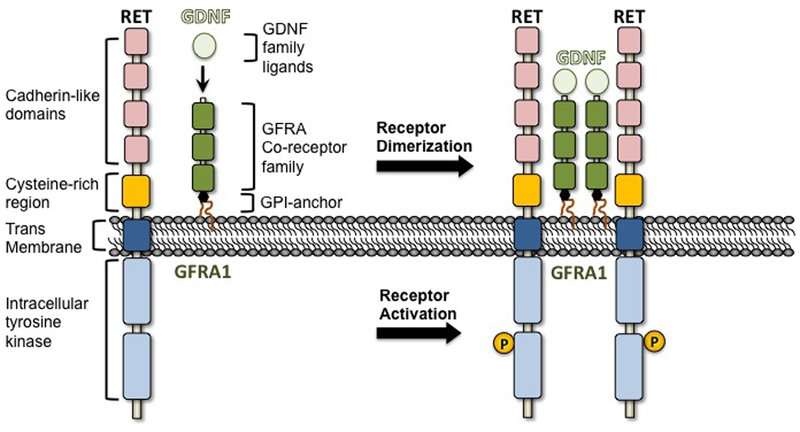
Diagram illustrating activation of the RET receptor, which is triggered through interaction with GDNF bound to the GFRA1 co-receptor. GDNF binding leads to auto-phosphorylation of several tyrosine residues in the cytoplasmic region of RET.
Global GDNF loss-of-function experiments in mice have demonstrated that its role is critical for the development of the kidneys, brain, and enteral nervous system. Mice lacking GDNF die at birth primarily from bilateral renal agenesis, but they also lack an enteric nervous system (Moore, et al. 1996, Pichel, et al. 1996, Sanchez, et al. 1996). These mice also have defects in the development of central and peripheral noradrenergic neurons (Granholm, et al. 1997). Similarly, ablations of the RET transmembrane receptor or its co-receptor GFRA1 lead to death shortly after birth, due to renal and neuronal abnormalities (Schuchardt, et al. 1994, Cacalano, et al. 1998). Studies using GDNF heterozygous (Gdnf +/−) mice have demonstrated that a partial reduction of GDNF leads to an age-related accelerated decline in the nigrostriatal dopaminergic system function and motor deficits (Littrell, et al. 2013). Therefore, GDNF is currently tested in clinical trials on Parkinson disease patients, but results are so far inconclusive. In the kidneys, the loss of one allele for Gdnf results in approximately 30% fewer but normal sized glomeruli in young mice (Cullen-McEwen, et al. 2003), and only mild reductions in enteric neuron size and neuronal fiber counts (Gianino, et al. 2003). All mice heterozygous for Gdnf, Gfra1 and Ret have problems with intestinal contractility and neurotransmitter release, demonstrating that this signaling pathway is critical for enteric nervous system structure and function (Gianino, et al. 2003). Disruption of GDNF/RET signaling in humans causes several distinct diseases. Notably, loss of RET is associated with thyroid C-cell reductions, and mutations leading to its constitutive activation leads to medullary thyroid cancer (Lindahl, et al. 2000, Lindfors, et al. 2006, Cote, et al. 2015). Alterations in the pattern of RET phosphorylation is associated with amyotrophic lateral sclerosis (Luesma, et al. 2014). Since GDNF/RET signaling also maintains the enteric nervous system, loss of its function in these neurons causes Hirschsprung’s disease, a condition characterized by the absence of enteric ganglia in the distal colon resulting in functional obstruction (Edery, et al. 1994).
Role of GDNF family ligands in the testis
The importance of GDNF for germ cell development was uncovered by the seminal work of Meng and colleagues (Meng, et al. 2000) who demonstrated that mice heterozygous for Gdnf, though fertile, exhibit increased numbers of seminiferous tubules lacking spermatogonia as the animals aged. Conversely, transgenic animals overexpressing Gdnf display an accumulation of undifferentiated spermatogonia. Thereafter, it was demonstrated that GFRA1 and RET proteins and mRNA are expressed in these cells (Viglietto, et al. 2000, Dettin, et al. 2003, Hofmann, et al. 2005), confirming that they are able to respond to GDNF influence. During development, Gdnf, Ret, and Gfra1 mRNAs are expressed at high levels in the male embryonic gonad as early as embryonic day 12.5 (E12.5), but their expression in the developing ovary is low (Nef, et al. 2005, Miles, et al. 2012). Gdnf is mainly detected in SF1-expressing somatic cells (Beverdam and Koopman 2006). In Ret−/− embryos, germ cells undergo apoptosis starting at E14.5 (Miles, et al. 2012), however ablation of Gdnf has little impact at this stage of germ cell development. Absence of the GDNF protein could be compensated by another GFL, Persephin (PSPN), which is also highly expressed between E12.5 and E15.5 (Milbrandt, et al. 1998, Miles, et al. 2012). Since prospermatogonia enter a period of mitotic arrest at E15.5, the role of GDNF from this time point until birth might be restricted to germ cell survival.
Shortly after birth, prospermatogonia migrate toward the basement membrane and become established SSCs (McGuinness and Orth 1992). GDNF then binds to the RET/GFRA1 receptor complex and induces their self-renewal (Meng, et al. 2000, Naughton, et al. 2006, Kanatsu-Shinohara and Shinohara 2013). As GFRA1 and RET are concomitantly expressed in Apaired and some Aaligned spermatogonia, it is likely that GDNF also induces and maintains the proliferation of these cells as SSCs embark on the path of differentiation (Viglietto, et al. 2000, Hofmann, et al. 2005, Sharma and Braun 2018). However, deciphering the functional importance of GDNF and its receptor complex in spermatogenesis has been difficult since global knockout mice die around birth, mainly from renal agenesis (Sanchez, et al. 1996). Further, GDNF is expressed not only by Sertoli cells but also by peritubular myoid cells (Chen, et al. 2014, Chen, et al. 2016a), which makes interpretation of knockout experiments challenging. By using xenograft transplantations of neonatal knockout testes, Naughton et al. demonstrated that the absence of GDNF or its receptors RET and GFRA1 after birth led to a lack of SSCs and failure of spermatogenesis (Naughton, et al. 2006). Similarly, inactivating the RET Y1062 phosphotyrosine docking site led to progressive loss of germ cells and their absence by day 21 after birth (Jain, et al. 2004, Jijiwa, et al. 2008). However, these mice were heterozygous, and although the penetrance of the mutation was high and recapitulated Hirschprung’s disease, some remaining GFRA1-positive cells with self-renewal ability were detected after more careful analysis (Takashima, et al. 2015). Therefore, at present, there is no satisfying model of a testis-specific Gdnf knockout. Additionally, it can be argued that GDNF is not the sole growth factor triggering SSC self-renewal and progenitor proliferation, as mentioned above. In particular, FGF2 can expand SSCs in cultures, maintain their stem cell activity and restore spermatogenesis in busulfan-treated mice as efficiently as GDNF (Takashima, et al. 2015). Therefore compensation by other self-renewal factors needs to be accounted for.
As previously mentioned, the GDNF family of ligands contains four related molecules that can putatively bind to the GFRA1/RET complex in the testis, albeit with different affinities (Viglietto, et al. 2000). Neurturin (NRTN) is expressed by Sertoli cells preferentially after puberty, and its expression overlaps with that of GFRA2 in spermatocytes and spermatids (Viglietto, et al. 2000, Meng, et al. 2001). Indeed, in other tissues, GFRA2 functions as a specific NRTN receptor, and mice deficient in Nrtn or Gfrα2 display similar phenotypes (Heuckeroth, et al. 1999, Rossi, et al. 1999, Wanigasekara, et al. 2004). According to Meng and colleagues, GFRA2 expression is not detectable in spermatogonia, and Nrtn overexpression in transgenic mice leads only to transient depletion of spermatocytes and spermatids (Meng, et al. 2001). Nrtn knockout testes are normal and mice are fertile (Heuckeroth, et al. 1999). Therefore, while NRTN and GDNF are both expressed by Sertoli cells (Viglietto, et al. 2000, Widenfalk, et al. 2000, Meng, et al. 2001, Johnston, et al. 2011), and RET is expressed by all undifferentiated spermatogonia (Yoshida, et al. 2004, Naughton, et al. 2006, Yoshida, et al. 2006), the phenotypes of Gdnf- and Nrtn-overexpressing testes are comparatively different, as are the respective knockouts. This demonstrates that the cellular distribution of the GFRA co-receptors, and not that of RET, determines the target cell population for GDNF and NRTN. Altogether, these data also indicate that a significant crosstalk between NRTN and GRFA1, or between GDNF and GFRA2, is unlikely in the testis. Further, recent data indicate that GFRA3 is expressed at low level by rodent spermatogonia (Green, et al. 2018), which explain why its ligand Artemin (ARTN) triggers the formation of short chains of Aaligned spermatogonia from cultured rat SSCs (Hamra, et al. 2007). An ARTN-like ligand has also been proposed as a homolog of GDNF in fish testes (Lucini, et al. 2004, Gautier, et al. 2014). However, ARTN does not appear to be expressed in the mammalian seminiferous epithelium, and its global ablation does not alter fertility (Honma, et al. 2002). Consequently, in vitro experiments using rodents and human SSCs cultured with rARTN may not reflect the in vivo situation. Finally, GFRA4, the receptor for Persephin (PSPN), is expressed by rat and mouse germ cells, in particular at the transition from spermatogonia to early spermatocytes (Masure, et al. 2000, Green, et al. 2018). The Gfra4 transcript appears to be truncated and produces a soluble protein in this organ (Lindahl, et al. 2000), while Pspn global knockout mice are fertile (Tomac, et al. 2002). Therefore, according to these studies, PSPN does not appear to exert a significant influence on mammalian spermatogenesis. PSPN, through GFRA4/RET, mainly participates in the development of thyroid parafollicular cells (C cells) and chromaffin cells of the adrenal medulla, which are destined to produce calcitonin and adrenaline/noradrenaline respectively in the adult (Lindahl, et al. 2000). The mining of recently published single-cell RNA-seq data confirmed that in mice and humans, Gfra1 is mostly found in undifferentiated spermatogonia. It is co-expressed with Id4, Nanos2/3 and Etv5, which are markers of SSCs (Green, et al. 2018, Guo, et al. 2018, Hermann, et al. 2018). Transcripts for Gfra2 are found in human undifferentiated spermatogonia, but are rare in the mouse equivalent germ cell population. Further, Gfra2, Gfra3 and Gfra4 are detected in spermatocytes and spermatids in both species. Additional studies will be required to understand the functional importance of GFRA2–4, in particular around meiosis. Altogether, these data demonstrate that in the testis, GDNF is the most critical GFL and that it exerts its influence on SSCs and progenitors probably exclusively through the GFRA1/RET receptor complex.
Two main signaling pathways triggered by the binding of GDNF to its receptor complex have been so far identified in undifferentiated spermatogonia in vitro and in vivo. In one of the pathways, RET phosphorylation induces SRC-family kinases (SFKs) and PI3K/AKT activation to allow spermatogonial proliferation (Braydich-Stolle, et al. 2007, Oatley, et al. 2007). Further analysis indicated that FYN kinase is the major SFK used by these cells, at least in vitro (Braydich-Stolle, et al. 2010). Recent studies demonstrated that only AKT3 is phosphorylated in response to GDNF in undifferentiated spermatogonia (Sharma and Braun 2018) and that the mTORC1 pathway is activated downstream of AKT, as spermatogonia differentiate from Aaligned to Adiff (Busada, et al. 2015). The other pathway activated by the binding of GDNF to the RET/GFRA1 complex is the canonical RAS/ERK1/2 (MAPK) pathway (He, et al. 2008, Lee, et al. 2009). RAS signaling in these cells has been demonstrated in vitro and in vivo, and ultimately upregulates the expression of the transcription factor c-FOS, a known inducer of different cyclins (Sunters, et al. 2004, He, et al. 2008, Wolgemuth, et al. 2013). Despite these advances, it is still not clear which one of these pathways, or both, specifically triggers self-renewal or differentiation. For example, Mycn is upregulated downstream of the SRC/PI3K/AKT/mTORC1 pathway, which might induce spermatogonial proliferation as they differentiate (Braydich-Stolle, et al. 2007, Lucas, et al. 2012). However, a study using transplantation of SSCs treated with Mycn shRNA demonstrated that MYC family transcription factors are in fact crucial for SSC self-renewal (Kanatsu-Shinohara, et al. 2014, Kanatsu-Shinohara, et al. 2016). Nonetheless, experiments using SSCs cultured with GDNF prior to testis transplantations have shown without doubt that GDNF increases the number of SSCs, and that downstream targets of the GDNF/RET signaling pathway such as ID4, BCL6, ETV5, and LHX1 are critical for SSC self-renewal (Kubota, et al. 2004, Oatley, et al. 2006, Wu, et al. 2011). Interestingly, a study by the group of Sada and colleagues demonstrated that GDNF signaling is essential to maintain expression of the RNA-binding protein NANOS2 in SSCs (Sada, et al. 2012). The authors propose that NANOS2 downstream of GDNF prevents spermatogonial differentiation in the postnatal testis.
Cyclic expression of GDNF
In the postnatal testis, GDNF is mainly expressed by Sertoli cells within the seminiferous epithelium (Tadokoro, et al. 2002, Johnston, et al. 2011). Recent data have demonstrated that peritubular myoid cells (PM cells) surrounding the seminiferous tubules also express this growth factor and contribute to germ cell maintenance (Chen, et al. 2014, Chen, et al. 2016a). In PM cells, GDNF expression is clearly under the influence of testosterone, while this has not been demonstrated in Sertoli cells. The interplay and relationship between GDNF from different sources is beyond the scope of this review and we will discuss here expression and regulation of GDNF in Sertoli cells only.
In the adult testis, within given segments of the seminiferous tubules, spermatogenesis proceeds in stages (I-XII in the mouse, I-XIV in the rat) characterized by defined germ cell associations (Leblond and Clermont 1952, Oakberg 1956, Russell, et al. 1990, Griswold 2016). The cyclical production of soluble factors by rat Sertoli cells according to the stages of the seminiferous epithelium has been demonstrated in 2008 by Johnston and colleagues, who separated the stages by transillumination-assisted microdissection, and assessed Sertoli cell mRNA expression in each stage by microarray analysis (Johnston, et al. 2008). They subsequently demonstrated that GDNF expression is highest at stages XIII-I, and lowest at stage VII (Johnston, et al. 2011). Similarly, in the mouse, GDNF expression is highest at stages IX-I and lowest at stages V-VIII (Caires, et al. 2012, Garcia, et al. 2017, Sharma and Braun 2018). However, discrepancies between studies are noted, which might be due to specific culture conditions of testicular tubules and to the species investigated (Sato, et al. 2011, Grasso, et al. 2012). Further, patch-like distribution of GDNF was detected by immunohistochemistry in the basal region of Sertoli cells in the seminiferous epithelium of mice and hamsters (Sato, et al. 2011), and these signals closely co-localized with a subpopulation of GFRA1-positive spermatogonia along the basement membrane. More recently, GDNF was ectopically overexpressed in Stages V-VIII by Sertoli cells in transgenic mice (Sharma and Braun 2018). This aberrant expression of GDNF significantly increased the number of GFRA1+/LIN28- germ cells, which are believed to be a subtype of As spermatogonia with enhanced self-renewal capacity (Chakraborty, et al. 2014, Sharma and Braun 2018). Further, the same study also demonstrated that GDNF maintains SSC self-renewal by blocking their differentiation into more mature spermatogonia, rather than actively promoting proliferation, strengthening previous data (Sada, et al. 2012, Garcia and Hofmann 2013).
Regulation of GDNF expression
While the influence of GDNF on SSCs and its progenitors is well established, little attention has been paid to the regulation of its production at the molecular level. The mechanisms responsible for its cyclic expression are so far not well understood, however it is evident that GDNF production must be modulated by positive or negative stimuli. For example, follicle-stimulating hormone (FSH) and cytokines might be positive regulators of GDNF expression in Sertoli cells (Tadokoro, et al. 2002, Lamberti and Vicini 2014), while NOTCH signaling provides for a novel mechanism of downregulation (Garcia, et al. 2017).
Regulation of GDNF expression by FSH
Gonadotropin-releasing hormone (GnRH) secreted by neurons in the hypothalamus is the master regulator of spermatogenesis. Cyclic production of GnRH induces the release of the glycoproteins follicle stimulating hormone (FSH) and luteinizing hormone (LH) by the pituitary, and these hormones will act on testicular somatic cells to regulate spermatogenesis. LH binds LH receptor (LHR) at the surface of Leydig cells, which ultimately induces the production of testosterone. FSH binds to its receptor (FSHR) at the surface of Sertoli cells and is responsible for the proliferation of these cells until around day 15 (P15) after birth in mice (Vergouwen, et al. 1991, Singh and Handelsman 1996, Baker and O’Shaughnessy 2001). Both FSH and LH (indirectly via testosterone and androgen receptor) exert their effects on spermatogenesis through the expression of Sertoli cell growth and differentiation factors. Upregulation of GDNF expression by FSH is supported by short-term in vitro studies using immature primary Sertoli cells cultured in presence of the hormone (Tadokoro, et al. 2002, Ding, et al. 2011, Lamberti and Vicini 2014), but the data obtained are not necessarily applicable to adult Sertoli cells. Tadokoro and colleagues also treated Sl/Sld mice, which lack A1-A4 differentiating spermatogonia, for 5 days with a GnRH antagonist (Nal-Glu) (Tadokoro, et al. 2002). They demonstrated a significant reduction of GDNF mRNA expression and a decreased rate of proliferation of undifferentiated spermatogonia in the testes of these mice, as measured with proliferating cell nuclear antigen (PCNA) expression. They attributed this decrease to a decrease in FSH, but were at the time unaware that the GnRH antagonist, which also suppresses LH and testosterone production, might also have reduced myoid cell-derived GDNF expression. In addition, the fact that Fshb knockout mice are fertile (Kumar, et al. 1997) indicates that this hormone is not an essential driver of GDNF production. Germ cell transplantation data recently revealed that SSC development in these mice is not significantly impaired (Tanaka, et al. 2016), although it is possible that GDNF provided by the peritubular myoid cells compensates for the loss of Fshb (Chen, et al. 2014, Chen, et al. 2016a).
Other mechanisms of GDNF upregulation
Other factors that induce GDNF expression in Sertoli cells include growth factors and chemokines/cytokines such as FGF2, TNF-α, and IL1-β (Simon, et al. 2007). Testicular macrophages located in the interstitial space between the tubules are thought to secrete some of these molecules (Bhushan and Meinhardt 2017). Sertoli cells express receptors for these factors, but because these studies have only been performed in vitro with a Sertoli cell line, TM4, which may not have conserved normal gene expression patterns, the results await confirmation from in vivo models. Constitutive activation of CTNNB1 (β-catenin) in Sertoli cells seems to induce high levels of GDNF expression in a transgenic mouse model (Tanwar, et al. 2010). This causes accumulation of undifferentiated spermatogonia and defective postnatal germ cell differentiation. Activation of CTNNB1 by treatment with LiCl causes a fivefold increase in Gdnf promoter activity, and the sequence of the proximal Gdnf promoter contains a TCF7 consensus-binding site. These results therefore suggest that Gdnf gene expression is directly regulated by CTNNB1 in vivo.
GDNF promoter analysis
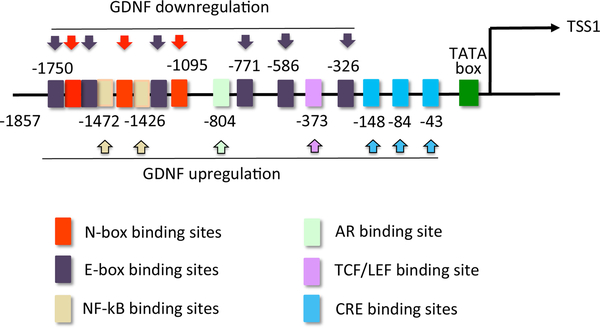
Lamberti and Vicini recently performed an in-silico analysis of evolutionarily conserved regions (ECRs) between human, rat, and mouse, followed by the identification of conserved DNA binding sites for known transcription factors (Lamberti and Vicini 2014). The human, rat and mouse genes share high similarities in intron lengths and coding exons, in particular in exon 1. The genomic 5’-flanking region of exon 1 shows very high sequence similarity among the three species, suggesting the presence of a conserved promoter. Conserved DNA binding sites among the three different species include a canonical TATA-box, several NF-kB binding sites, an androgen receptor (AR) binding site, and several cAMP-response elements (CRE) within the −2000 to +1 bp proximal promoter (Figure 2). Deletion of the CRE sites in a luciferase reporter plasmid reduced the response to dibutyryl-cAMP in transfected primary Sertoli cells in comparison to control. The presence of NFKB1 binding sites agrees with the observation that GDNF expression increases upon treatment of Sertoli cells with TNF-α and IL1-β (Simon, et al. 2007). The GDNF proximal promoter region also contains sequences bound by putative transcriptional repressors. A negative regulatory region containing 2 NRSE-like binding sites can be found within the 5’ UTR, but specific ablation of this region did not significantly increase reporter gene activity in immature primary Sertoli cells, and treatment with REST siRNA did not increase GDNF expression. Further analysis of the proximal promoter demonstrated the presence of several E-Boxes and N-boxes to allow the binding of basic helix-loop-helix proteins with possible repressor activity as explained below (Garcia, et al. 2017) (Figure 2).
Figure 2: Mouse GDNF proximal promoter.
Schematic representation of the mouse Gdnf proximal promoter, expanding on the data of Lamberti and Vicini, 2014. Potential regulatory elements are shown as boxes and are color-coded. HES1 and/or HEY1, together with co-repressors, will bind to N- or E-boxes within the Gdnf promoter and cause its downregulation. E- and N-boxes can also be occupied by bHLH transcriptional activators, which could putatively increase Gdnf expression. These activators, if any, are not known.
Regulation of GDNF via NOTCH signaling
In order to maintain germ cell homeostasis, pathways that negatively regulate growth factors essential for self-renewal or proliferation must exist. We recently demonstrated that NOTCH signaling in Sertoli cells modulates their expression of GDNF during embryonic development and after birth (Garcia, et al. 2013, Garcia, et al. 2014). NOTCH signaling is a highly conserved juxtacrine signaling pathway that mediates cell fate decisions during the development of multiple organs and tissues (Bray and Bernard 2010)(Figure 3). NOTCH1–4 are large cell-surface receptors that are activated by their ligands JAGGED (JAG1 and JAG2) and DELTA-like (DLL1, DLL3, and DLL4), which are transmembrane proteins displayed by neighboring cells (Wang 2011). Activation of the NOTCH receptor recruits ADAM10/TACE (Tumor necrosis factor (TNF)-α converting enzyme) and γ-secretase, which sequentially cleave and release the NOTCH intracellular domain (NICD) in the cytoplasm. NICD migrates to the nucleus where it forms a transcriptional complex with the DNA binding protein RBPJ (recombining binding protein suppressor of hairless) (Tanigaki and Honjo 2010), co-activators such as Mastermind (MAML) and histones acetyltransferases (Shao, et al. 2012)(Figure 3). The canonical targets of RBPJ include the HES and HEY families of transcriptional repressors, which are basic helix-loop-helix proteins (bHLH)(Iso, et al. 2003, Kageyama, et al. 2007, Bray and Bernard 2010). Transcriptional repressors of the HES family (HES1–7) bind to N-box promoter regions of their target genes, while repressors belonging to the HEY family (HEY1, HEY2, HEYL) bind to E-box promoter regions (Kageyama, et al. 2007). HES factors not only form homodimers, but they also form heterodimers with HEY1 or HEY2, which bind N-boxes with a higher affinity and repress transcription more efficiently (Iso, et al. 2003). HES/HEY proteins usually repress transcription by forming complexes with co-repressors of the Groucho/transducin-like Enhancer of split (Gro/TLE) family (Grbavec, et al. 1998). E- and N-boxes can also be bound by members of the MYC, MYOD, or NEUROG families of transcription factors, which drive cell proliferation and cell fate.
Figure 3: Schematic representation of the NOTCH signaling pathway.
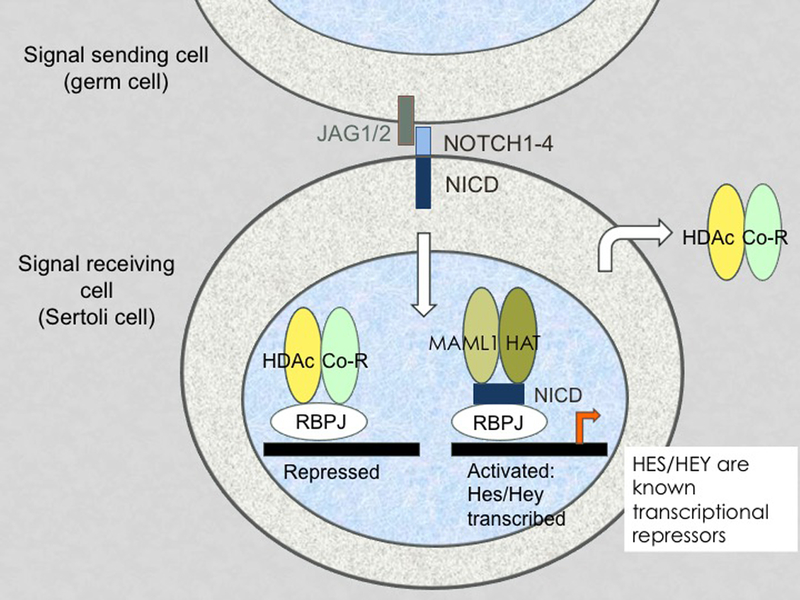
Ligands of the JAGGED (JAG) and DELTA-like families interact with NOTCH1–4 receptors on an adjacent cell. In the seminiferous epithelium, JAG1 at the surface of spermatogonia interacts with NOTCH1 at the surface of Sertoli cells. This releases the NOTCH intracellular domain (NICD), which translocates to the Sertoli cell nucleus. NICD forms a complex with RBPJ at the promoter of target genes. Binding of NICD releases co-repressors and allows the binding of co-activators. This leads to the transcriptional activation of the canonical NOTCH targets Hes1 and Hey1, which are known bHLH transcriptional repressors.
NOTCH pathway components are all expressed in germ cells and Sertoli cells in pre- and postnatal testes (Dirami, et al. 2001, Hahn, et al. 2009). However, the canonical pathway is not activated in male germ cells as demonstrated by the fact that fetal and post-natal germ cells are GFP negative in a transgenic NOTCH reporter mouse model called TNR-GFP. This model expresses GFP under the control of a promoter containing four RBPJ binding sites (Duncan, et al. 2005) (Garcia, et al. 2013). We recently established both a gain-of-function and loss-of-function mouse models of NOTCH signaling specifically in Sertoli cells. Constitutively activating NOTCH1 signaling in these cells led to infertility due to a complete lack of germ cells by P2 (Garcia, et al. 2013). In this model, E15.5 prospermatogonia, which normally do not proliferate at this stage of development, expressed the cyclins CCND1 and CCND3. They also expressed STRA8, KIT, and SYCP3 at the mRNA and protein levels, which are hallmarks of differentiating germ cells (Garcia, et al. 2013, Garcia and Hofmann 2013). Interestingly, expression of GDNF by Sertoli cells was significantly downregulated in the fetal testis and after birth, as well as in the adult mice. Overall these data indicated that GDNF expression was inversely correlated with NOTCH signaling activation, which drove early germ cell differentiation in the fetal testis. The loss of germ cells was attributed to a lack of niche molecules able to maintain differentiation at this stage of development (Garcia, et al. 2013, Garcia and Hofmann 2013).
Because the NOTCH receptors are redundant, functional studies relying on ablating expression of these proteins in mice, in particular NOTCH1, have been unsuccessful (unpublished data). Inhibiting gamma-secretase with a pharmacological inhibitor (DAPT) to prevent NICD cleavage and NOTCH activation was useful to demonstrate in vitro that NOTCH downregulation in isolated primary Sertoli cells promoted an increase of GDNF expression (Garcia, et al. 2013). However, treating cells or mice with this compound might lead to nonspecific results since the gamma-secretase complex is also critical for processing other integral membrane proteins, such as ERBB4, E- and N-cadherin (CDH1 and CDH2), ephrin-B2 (EFNB2) and CD44, a receptor for extracellular matrix proteins that functions as a transcription factor and also interacts with SRC family kinases within most cells (Ilangumaran, et al. 1999, Okamoto, et al. 2001). Therefore, since RBPJ is at the intersection of the NOTCH1–4 pathways, we disrupted the NICD- and DNA-binding exons of the Rbpj gene specifically in mouse Sertoli cells in vivo. Loss-of-function of Rbpj in these cells led to upregulation of Gdnf expression. We also observed an increase in the diameter of the seminiferous tubules, an increase in testis sizes and higher testis indexes. While the total number of Sertoli cells per testes remained the same, the number of GFRA1 and PLZF-positive cells (Aundiff) per Sertoli cell significantly increased and led to a 25–30% increase in the number of spermatocytes and elongated spermatids (hyperplasia)(Garcia, et al. 2014). The phenotype of the NOTCH signaling knockout was therefore the opposite to the phenotype of the NOTCH signaling overactivation mutant. Further, our data demonstrated that the canonical NOTCH pathway was active in Sertoli cells since expression of the transcriptional repressors Hes1 and Hey1 was upregulated in the overactivated NOTCH mutant, and downregulated in the knockout model. More recently, we demonstrated by ChIP-PCR that HES1 and HEY1 bind to the GDNF promoter, indicating that NOTCH signaling is a direct repressor of GDNF in Sertoli cells (Garcia, et al. 2017). Altogether, these results indicate that the canonical NOTCH signaling pathway is active in Sertoli cells and can be used —through the transcriptional repressors HES1 and HEY1— to inhibit GDNF production. Further, this mechanism may be used by Sertoli cells to dampen SSC self-renewal or progenitor proliferation when necessary.
Control of GDNF expression by germ cells
By producing specific growth factors, among them GDNF and FGF2, functional Sertoli cells create a unique physiological environment that directs SSC self-renewal and differentiation. In turn, different types of germ cells must influence Sertoli cells. These interactions between germ cells and Sertoli cells are facilitated by their direct contact within the seminiferous epithelium. To identify whether germ cells or Sertoli cells provide the ligand for NOTCH signaling in Sertoli cells, we specifically isolated populations of germ cells and Sertoli cells by FACS using transgenic mice expressing GFP specifically in Sertoli cells or germ cells. Germ cells highly expressed NOTCH ligands in comparison to Sertoli cells (Garcia, et al. 2014). Alternatively, RFP-expressing germ cell populations were isolated by FACS and further fractionated using fluorescence-labeled antibodies against GFRA1 or KIT. Spermatocytes were isolated through their high expression of GFP from a DAZL-GFP transgenic model (Nicholas, et al. 2009). We demonstrated that the NOTCH ligand JAG1 was highly expressed in GFRA1-positive spermatogonia, although some expression was also noted in KIT-positive differentiating spermatogonia, and early spermatocytes (Garcia, et al. 2017). Because there are few GFRA1-positive spermatogonia in comparison to differentiating spermatogonia and early spermatocytes, it is reasonable to expect that the accumulation of Aundiff and Adiff spermatogonia around stage VII will increase NOTCH activity through JAG1 and trigger a decrease in GDNF expression by Sertoli cells, leading to the cyclic expression of this growth factor, a hypothesis that we are currently further testing. Therefore, the dosage of JAG1 presented by germ cells might be critical for NOTCH activation in Sertoli cells. Type A spermatogonia, when in sufficient numbers, might regulate their own homeostasis through downregulation of GDNF. These data are compatible with the fact that in normal mice, the absence of germ cells after busulfan treatment induces higher expression of GDNF (Tadokoro, et al. 2002, Ryu, et al. 2006, Garcia, et al. 2017).
Conclusion
GDNF is a critical component of the SSC niche, whose roles include stimulation of stem cell self-renewal, proliferation of progenitors and maintenance of the undifferentiated state. Despite its critical effects on germ cell homeostasis, surprisingly little is known about its regulation. Unraveling molecular mechanisms has been hampered by the difficulty of working with adult primary Sertoli cells, which do not proliferate and are unsuitable for long-term cultures. Another challenge is the fact that Sertoli cells often lose their characteristics after isolation, and that their normal pattern of gene expression varies across the stages of the seminiferous epithelium cycle, which is hardly reproducible in two-dimensional cultures. While additional mouse models will be necessary to assess overall gene function, they are insufficient to understand regulation of gene expression at the molecular level. Improving ex vivo organ culture systems might be useful to conserve adult Sertoli cell functions while elucidating complex signaling pathways interactions. Indeed, cross-talks between GDNF signaling and other pathways are still poorly understood as are RET-independent mechanisms driven by GDNF/GFRA1, or GDNF-independent mechanisms driving RET in germ cells. In addition, how germ cells communicate with Sertoli cells needs to be further explored. We have highlighted JAG1/NOTCH signaling as one possible mechanism that fulfills this role, but other modes of germ cell to Sertoli cell communication assuredly exist that need to be unraveled. Elucidating these signaling events in their proper architectural context is key to understanding human idiopathic infertility.
Acknowledgments
Funding
This work was supported by the National Institutes of Health, grant number R01 HD081244
Footnotes
Declaration of interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported
References:
- Airaksinen MS, and Saarma M 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3 383–394. [DOI] [PubMed] [Google Scholar]
- Aloisio GM, Nakada Y, Saatcioglu HD, Pena CG, Baker MD, Tarnawa ED, Mukherjee J, Manjunath H, Bugde A, Sengupta AL, et al. 2014. PAX7 expression defines germline stem cells in the adult testis. J Clin Invest 124 3929–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PJ, and O’Shaughnessy PJ 2001. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction 122 227–234. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Tansey MG, Golden JP, Creedon DJ, Heuckeroth RO, Keck CL, Zimonjic DB, Popescu NC, Johnson EM Jr., and Milbrandt J 1997. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron 18 793–802. [DOI] [PubMed] [Google Scholar]
- Beverdam A, and Koopman P 2006. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet 15 417–431. [DOI] [PubMed] [Google Scholar]
- Bhushan S, and Meinhardt A 2017. The macrophages in testis function. J Reprod Immunol 119 107–112. [DOI] [PubMed] [Google Scholar]
- Bray S, and Bernard F 2010. Notch targets and their regulation. Curr Top Dev Biol 92 253–275. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, and Hofmann MC 2007. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol 304 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle LK, Lucas B, Schrand A, Murdock RC, Lee T, Schlager JJ, Hussain SM, and Hofmann MC 2010. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol Sci 116 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Niedenberger BA, Velte EK, Keiper BD, and Geyer CB 2015. Mammalian target of rapamycin complex 1 (mTORC1) Is required for mouse spermatogonial differentiation in vivo. Dev Biol 407 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, et al. 1998. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 21 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caires KC, de Avila J, and McLean DJ 2012. Endocrine regulation of spermatogonial stem cells in the seminiferous epithelium of adult mice. Biores Open Access 1 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Martinez CA, Koester J, and Wickstrom SA 2018. Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development 145. [DOI] [PubMed] [Google Scholar]
- Chakraborty P, Buaas FW, Sharma M, Snyder E, de Rooij DG, and Braun RE 2014. LIN28A marks the spermatogonial progenitor population and regulates its cyclic expansion. Stem Cells 32 860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan F, Oatley MJ, Kaucher AV, Yang QE, Bieberich CJ, Shashikant CS, and Oatley JM 2014. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev 28 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Brown PR, Willis WB, and Eddy EM 2014. Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance. Endocrinology 155 4964–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Willis WD, and Eddy EM 2016a. Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc Natl Acad Sci U S A 113 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ma L, Hogarth C, Wei G, Griswold MD, and Tong MH 2016b. Retinoid signaling controls spermatogonial differentiation by regulating expression of replication-dependent core histone genes. Development 143 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y 1966. Renewal of spermatogonia in man. Am J Anat 118 509–524. [DOI] [PubMed] [Google Scholar]
- Clermont Y 1969. Two classes of spermatogonial stem cells in the monkey (Cercopithecus aethiops). Am J Anat 126 57–71. [DOI] [PubMed] [Google Scholar]
- Clermont Y, and Bustos-Obregon E 1968. Re-examination of spermatogonial renewal in the rat by means of seminiferous tubules mounted “in toto”. Am J Anat 122 237–247. [DOI] [PubMed] [Google Scholar]
- Clermont Y, and Leblond CP 1953. Renewal of spermatogonia in the rat. Am J Anat 93 475–501. [DOI] [PubMed] [Google Scholar]
- Clermont Y, and Leblond CP 1959. Differentiation and renewal of spermatogonia in the monkey, Macacus rhesus. Am J Anat 104 237–273. [DOI] [PubMed] [Google Scholar]
- Cote GJ, Grubbs EG, and Hofmann MC 2015. Thyroid C-Cell Biology and Oncogenic Transformation. Recent Results Cancer Res 204 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, and Bertram JF 2003. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension 41 335–340. [DOI] [PubMed] [Google Scholar]
- De Rooij DG 1973. Spermatogonial stem cell renewal in the mouse: I. Normal situation. Cell Tissue Kinet 6 201–287. [DOI] [PubMed] [Google Scholar]
- de Rooij DG 2017. The nature and dynamics of spermatogonial stem cells. Development 144 3022–3030. [DOI] [PubMed] [Google Scholar]
- Dettin L, Ravindranath N, Hofmann MC, and Dym M 2003. Morphological characterization of the spermatogonial subtypes in the neonatal mouse testis. Biol Reprod 69 1565–1571. [DOI] [PubMed] [Google Scholar]
- Ding LJ, Yan GJ, Ge QY, Yu F, Zhao X, Diao ZY, Wang ZQ, Yang ZZ, Sun HX, and Hu YL 2011. FSH acts on the proliferation of type A spermatogonia via Nur77 that increases GDNF expression in the Sertoli cells. FEBS Lett 585 2437–2444. [DOI] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Achi MV, and Dym M 2001. Expression of Notch pathway components in spermatogonia and Sertoli cells of neonatal mice. J Androl 22 944–952. [DOI] [PubMed] [Google Scholar]
- Drinkut A, Tillack K, Meka DP, Schulz JB, Kugler S, and Kramer ER 2016. Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis 7 e2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. 2005. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 6 314–322. [DOI] [PubMed] [Google Scholar]
- Dym M, and Clermont Y 1970. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am J Anat 128 265–282. [DOI] [PubMed] [Google Scholar]
- Ebata KT, Zhang X, and Nagano MC 2005. Expression patterns of cell-surface molecules on male germ line stem cells during postnatal mouse development. Mol Reprod Dev 72 171–181. [DOI] [PubMed] [Google Scholar]
- Edery P, Lyonnet S, Mulligan LM, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fekete C, Ponder BA, and Munnich A 1994. Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature 367 378–380. [DOI] [PubMed] [Google Scholar]
- Garcia TX, DeFalco T, Capel B, and Hofmann MC 2013. Constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence. Dev Biol 377 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia TX, Farmaha JK, Kow S, and Hofmann MC 2014. RBPJ in mouse Sertoli cells is required for proper regulation of the testis stem cell niche. Development 141 4468–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia TX, and Hofmann MC 2013. NOTCH signaling in Sertoli cells regulates gonocyte fate. Cell Cycle 12 2538–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia TX, Parekh P, Gandhi P, Sinha K, and Hofmann MC 2017. The NOTCH Ligand JAG1 Regulates GDNF Expression in Sertoli Cells. Stem Cells Dev 26 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier A, Bosseboeuf A, Auvray P, and Sourdaine P 2014. Maintenance of potential spermatogonial stem cells in vitro by GDNF treatment in a chondrichthyan model (Scyliorhinus canicula L.). Biol Reprod 91 91. [DOI] [PubMed] [Google Scholar]
- Gianino S, Grider JR, Cresswell J, Enomoto H, and Heuckeroth RO 2003. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 130 2187–2198. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Srivastava N, Mott JL, Henry S, Henry M, Westphal H, Pichel JG, Shen L, and Hoffer BJ 1997. Morphological alterations in the peripheral and central nervous systems of mice lacking glial cell line-derived neurotrophic factor (GDNF): immunohistochemical studies. J Neurosci 17 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso M, Fuso A, Dovere L, de Rooij DG, Stefanini M, Boitani C, and Vicini E 2012. Distribution of GFRA1-expressing spermatogonia in adult mouse testis. Reproduction 143 325–332. [DOI] [PubMed] [Google Scholar]
- Grbavec D, Lo R, Liu Y, and Stifani S 1998. Transducin-like Enhancer of split 2, a mammalian homologue of Drosophila Groucho, acts as a transcriptional repressor, interacts with Hairy/Enhancer of split proteins, and is expressed during neuronal development. Eur J Biochem 258 339–349. [DOI] [PubMed] [Google Scholar]
- Green CD, Ma Q, Manske GL, Shami AN, Zheng X, Marini S, Moritz L, Sultan C, Gurczynski SJ, Moore BB, et al. 2018. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Dev Cell 46 651–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, Muciaccia B, Fuso A, Berno V, Boitani C, Stefanini M, et al. 2009. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells 27 3043–3052. [DOI] [PubMed] [Google Scholar]
- Griswold MD 2016. Spermatogenesis: The Commitment to Meiosis. Physiol Rev 96 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Grow EJ, Mlcochova H, Maher GJ, Lindskog C, Nie X, Guo Y, Takei Y, Yun J, Cai L, et al. 2018. The adult human testis transcriptional cell atlas. Cell Res 28 1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Grow EJ, Yi C, Mlcochova H, Maher GJ, Lindskog C, Murphy PJ, Wike CL, Carrell DT, Goriely A, et al. 2017. Chromatin and Single-Cell RNA-Seq Profiling Reveal Dynamic Signaling and Metabolic Transitions during Human Spermatogonial Stem Cell Development. Cell Stem Cell 21 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn KL, Beres B, Rowton MJ, Skinner MK, Chang Y, Rawls A, and Wilson-Rawls J 2009. A deficiency of lunatic fringe is associated with cystic dilation of the rete testis. Reproduction 137 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen D, and Garbers DL 2007. Identification of neuregulin as a factor required for formation of aligned spermatogonia. J Biol Chem 282 721–730. [DOI] [PubMed] [Google Scholar]
- Haneji T, Maekawa M, and Nishimune Y 1983. Retinoids induce differentiation of type A spermatogonia in vitro: organ culture of mouse cryptorchid testes. J Nutr 113 1119–1123. [DOI] [PubMed] [Google Scholar]
- Hara K, Nakagawa T, Enomoto H, Suzuki M, Yamamoto M, Simons BD, and Yoshida S 2014. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 14 658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, and Dym M 2008. Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 26 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel AR, Yang QE, Oatley MJ, Lord T, Sablitzky F, and Oatley JM 2017. ID4 levels dictate the stem cell state in mouse spermatogonia. Development 144 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Cheng K, Singh A, Roa-De La Cruz L, Mutoji KN, Chen IC, Gildersleeve H, Lehle JD, Mayo M, Westernstroer B, et al. 2018. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep 25 1650–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Mutoji KN, Velte EK, Ko D, Oatley JM, Geyer CB, and McCarrey JR 2015. Transcriptional and translational heterogeneity among neonatal mouse spermatogonia. Biol Reprod 92 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, and Orwig KE 2009. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod 24 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM Jr., et al. 1999. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 22 253–263. [DOI] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, and Dym M 2005. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol 279 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, and Milbrandt J 2002. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron 35 267–282. [DOI] [PubMed] [Google Scholar]
- Huckins C 1971a. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec 169 533–557. [DOI] [PubMed] [Google Scholar]
- Huckins C 1971b. The spermatogonial stem cell population in adult rats. II. A radioautographic analysis of their cell cycle properties. Cell Tissue Kinet 4 313–334. [DOI] [PubMed] [Google Scholar]
- Huckins C 1978. Spermatogonial intercellular bridges in whole-mounted seminiferous tubules from normal and irradiated rodent testes. Am J Anat 153 97–121. [DOI] [PubMed] [Google Scholar]
- Huckins C, and Oakberg EF 1978. Morphological and quantitative analysis of spermatogonia in mouse testes using whole mounted seminiferous tubules, I. The normal testes. Anat Rec 192 519–528. [DOI] [PubMed] [Google Scholar]
- Ibanez CF 2013. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilangumaran S, Borisch B, and Hoessli DC 1999. Signal transduction via CD44: role of plasma membrane microdomains. Leuk Lymphoma 35 455–469. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, and Hamamori Y 2003. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194 237–255. [DOI] [PubMed] [Google Scholar]
- Jain S, Naughton CK, Yang M, Strickland A, Vij K, Encinas M, Golden J, Gupta A, Heuckeroth R, Johnson EM Jr., et al. 2004. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development 131 5503–5513. [DOI] [PubMed] [Google Scholar]
- Jijiwa M, Kawai K, Fukihara J, Nakamura A, Hasegawa M, Suzuki C, Sato T, Enomoto A, Asai N, Murakumo Y, et al. 2008. GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells. Genes Cells 13 365–374. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, et al. 1996. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 85 1113–1124. [DOI] [PubMed] [Google Scholar]
- Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T, Delaney J, Schultz H, Zhou R, and Fox GM 1997. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J Biol Chem 272 33111–33117. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Olivas E, DiCandeloro P, and Wright WW 2011. Stage-specific changes in GDNF expression by rat Sertoli cells: a possible regulator of the replication and differentiation of stem spermatogonia. Biol Reprod 85 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DS, Wright WW, Dicandeloro P, Wilson E, Kopf GS, and Jelinsky SA 2008. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc Natl Acad Sci U S A 105 8315–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, and Kobayashi T 2007. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development 134 1243–1251. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Yoshida S, Toyokuni S, Lee J, Ogura A, and Shinohara T 2007. Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture. Biol Reprod 76 55–62. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, and Shinohara T 2003. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 69 612–616. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Onoyama I, Nakayama KI, and Shinohara T 2014. Skp1-Cullin-F-box (SCF)-type ubiquitin ligase FBXW7 negatively regulates spermatogonial stem cell self-renewal. Proc Natl Acad Sci U S A 111 8826–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, and Shinohara T 2013. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol 29 163–187. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Tanaka T, Ogonuki N, Ogura A, Morimoto H, Cheng PF, Eisenman RN, Trumpp A, and Shinohara T 2016. Myc/Mycn-mediated glycolysis enhances mouse spermatogonial stem cell self-renewal. Genes Dev 30 2637–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto Y, Takeda K, Okuno Y, Yamakawa Y, Ito Y, Taguchi R, Kato M, Suzuki H, Takahashi M, and Nakashima I 2004. Identification of RET autophosphorylation sites by mass spectrometry. J Biol Chem 279 14213–14224. [DOI] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, and Simons BD 2010. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7 214–224. [DOI] [PubMed] [Google Scholar]
- Komai Y, Tanaka T, Tokuyama Y, Yanai H, Ohe S, Omachi T, Atsumi N, Yoshida N, Kumano K, Hisha H, et al. 2014. Bmi1 expression in long-term germ stem cells. Sci Rep 4 6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, and Brinster RL 2004. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 101 16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, and Matzuk MM 1997. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15 201–204. [DOI] [PubMed] [Google Scholar]
- Lamberti D, and Vicini E 2014. Promoter analysis of the gene encoding GDNF in murine Sertoli cells. Mol Cell Endocrinol 394 105–114. [DOI] [PubMed] [Google Scholar]
- Leblond CP, and Clermont Y 1952. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci 55 548–573. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Morimoto H, Kazuki Y, Takashima S, Oshimura M, Toyokuni S, and Shinohara T 2009. Genetic reconstruction of mouse spermatogonial stem cell self-renewal in vitro by Ras-cyclin D2 activation. Cell Stem Cell 5 76–86. [DOI] [PubMed] [Google Scholar]
- Li L, and Xie T 2005. Stem cell niche: structure and function. Annu Rev Cell Dev Biol 21 605–631. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, and Collins F 1993. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260 1130–1132. [DOI] [PubMed] [Google Scholar]
- Lindahl M, Timmusk T, Rossi J, Saarma M, and Airaksinen MS 2000. Expression and alternative splicing of mouse Gfra4 suggest roles in endocrine cell development. Mol Cell Neurosci 15 522–533. [DOI] [PubMed] [Google Scholar]
- Lindfors PH, Lindahl M, Rossi J, Saarma M, and Airaksinen MS 2006. Ablation of persephin receptor glial cell line-derived neurotrophic factor family receptor alpha4 impairs thyroid calcitonin production in young mice. Endocrinology 147 2237–2244. [DOI] [PubMed] [Google Scholar]
- Littrell OM, Granholm AC, Gerhardt GA, and Boger HA 2013. Glial cell-line derived neurotrophic factor (GDNF) replacement attenuates motor impairments and nigrostriatal dopamine deficits in 12-month-old mice with a partial deletion of GDNF. Pharmacol Biochem Behav 104 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord T, and Oatley JM 2017. A revised Asingle model to explain stem cell dynamics in the mouse male germline. Reproduction 154 R55–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord T, and Oatley JM 2018. Functional assessment of spermatogonial stem cell purity in experimental cell populations. Stem Cell Res 29 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas BE, Fields C, Joshi N, and Hofmann MC 2012. Mono-(2-ethylhexyl)-phthalate (MEHP) affects ERK-dependent GDNF signalling in mouse stem-progenitor spermatogonia. Toxicology 299 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucini C, Maruccio L, Tafuri S, Staiano N, and Castaldo L 2004. Artemin-like immunoreactivity in the zebrafish, Danio rerio. Anat Embryol (Berl) 208 403–410. [DOI] [PubMed] [Google Scholar]
- Luesma MJ, Cantarero I, Alvarez-Dotu JM, Santander S, and Junquera C 2014. New insights into c-Ret signalling pathway in the enteric nervous system and its relationship with ALS. Biomed Res Int 2014 328348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manie S, Santoro M, Fusco A, and Billaud M 2001. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet 17 580–589. [DOI] [PubMed] [Google Scholar]
- Masure S, Cik M, Hoefnagel E, Nosrat CA, Van der Linden I, Scott R, Van Gompel P, Lesage AS, Verhasselt P, Ibanez CF, et al. 2000. Mammalian GFRalpha −4, a divergent member of the GFRalpha family of coreceptors for glial cell line-derived neurotrophic factor family ligands, is a receptor for the neurotrophic factor persephin. J Biol Chem 275 39427–39434. [DOI] [PubMed] [Google Scholar]
- McGuinness MP, and Orth JM 1992. Reinitiation of gonocyte mitosis and movement of gonocytes to the basement membrane in testes of newborn rats in vivo and in vitro. Anat Rec 233 527–537. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, et al. 2000. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287 1489–1493. [DOI] [PubMed] [Google Scholar]
- Meng X, Pata I, Pedrono E, Popsueva A, de Rooij DG, Janne M, Rauvala H, and Sariola H 2001. Transient disruption of spermatogenesis by deregulated expression of neurturin in testis. Mol Cell Endocrinol 184 33–39. [DOI] [PubMed] [Google Scholar]
- Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, et al. 1998. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 20 245–253. [DOI] [PubMed] [Google Scholar]
- Miles DC, van den Bergen JA, Wakeling SI, Anderson RB, Sinclair AH, and Western PS 2012. The proto-oncogene Ret is required for male foetal germ cell survival. Dev Biol 365 101–109. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, and Rosenthal A 1996. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382 76–79. [DOI] [PubMed] [Google Scholar]
- Mutoji K, Singh A, Nguyen T, Gildersleeve H, Kaucher AV, Oatley MJ, Oatley JM, Velte EK, Geyer CB, Cheng K, et al. 2016. TSPAN8 Expression Distinguishes Spermatogonial Stem Cells in the Prepubertal Mouse Testis. Biol Reprod 95 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, and Yoshida S 2007. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 12 195–206. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, and Yoshida S 2010. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, and Milbrandt J 2006. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod 74 314–321. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. 2005. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol 287 361–377. [DOI] [PubMed] [Google Scholar]
- Neuhaus N, Yoon J, Terwort N, Kliesch S, Seggewiss J, Huge A, Voss R, Schlatt S, Grindberg RV, and Scholer HR 2017. Single-cell gene expression analysis reveals diversity among human spermatogonia. Mol Hum Reprod 23 79–90. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Xu EY, Banani SF, Hammer RE, Hamra FK, and Reijo Pera RA 2009. Characterization of a Dazl-GFP germ cell-specific reporter. Genesis 47 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakberg EF 1956. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat 99 507–516. [DOI] [PubMed] [Google Scholar]
- Oakberg EF 1971. Spermatogonial stem-cell renewal in the mouse. Anat Rec 169 515–531. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, and Brinster RL 2007. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem 282 25842–25851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, and Brinster RL 2006. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A 103 9524–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, and Brinster RL 2012. The germline stem cell niche unit in mammalian testes. Physiol Rev 92 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, and Brinster RL 2009. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 136 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, Wong AJ, and Saya H 2001. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol 155 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. 1996. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382 73–76. [DOI] [PubMed] [Google Scholar]
- Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, et al. 1999. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron 22 243–252. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Hikim APS, and Clegg ED 1990. Histological and Histopathological Evaluation of the Testis Clearwater, Fl: Cache River Press. [Google Scholar]
- Ruven C, Badea SR, Wong WM, and Wu W 2018. Combination Treatment With Exogenous GDNF and Fetal Spinal Cord Cells Results in Better Motoneuron Survival and Functional Recovery After Avulsion Injury With Delayed Root Reimplantation. J Neuropathol Exp Neurol 77 325–343. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, and Brinster RL 2006. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24 1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A, Hasegawa K, Pin PH, and Saga Y 2012. NANOS2 acts downstream of glial cell line-derived neurotrophic factor signaling to suppress differentiation of spermatogonial stem cells. Stem Cells 30 280–291. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, and Barbacid M 1996. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382 70–73. [DOI] [PubMed] [Google Scholar]
- Sariola H, and Saarma M 2003. Novel functions and signalling pathways for GDNF. J Cell Sci 116 3855–3862. [DOI] [PubMed] [Google Scholar]
- Sato T, Aiyama Y, Ishii-Inagaki M, Hara K, Tsunekawa N, Harikae K, Uemura-Kamata M, Shinomura M, Zhu XB, Maeda S, et al. 2011. Cyclical and patch-like GDNF distribution along the basal surface of Sertoli cells in mouse and hamster testes. PLoS One 6 e28367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, and Pachnis V 1994. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367 380–383. [DOI] [PubMed] [Google Scholar]
- Shao H, Huang Q, and Liu ZJ 2012. Targeting Notch signaling for cancer therapeutic intervention. Adv Pharmacol 65 191–234. [DOI] [PubMed] [Google Scholar]
- Sharma M, and Braun RE 2018. Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Ekman GC, Tyagi G, Hess RA, Murphy KM, and Cooke PS 2007. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res 313 3090–3099. [DOI] [PubMed] [Google Scholar]
- Singh J, and Handelsman DJ 1996. Neonatal administration of FSH increases Sertoli cell numbers and spermatogenesis in gonadotropin-deficient (hpg) mice. J Endocrinol 151 37–48. [DOI] [PubMed] [Google Scholar]
- Sun F, Xu Q, Zhao D, and Degui Chen C 2015. Id4 Marks Spermatogonial Stem Cells in the Mouse Testis. Sci Rep 5 17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunters A, Thomas DP, Yeudall WA, and Grigoriadis AE 2004. Accelerated cell cycle progression in osteoblasts overexpressing the c-fos proto-oncogene: induction of cyclin A and enhanced CDK2 activity. J Biol Chem 279 9882–9891. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, and Nishimune Y 2002. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 113 29–39. [DOI] [PubMed] [Google Scholar]
- Takashima S, Kanatsu-Shinohara M, Tanaka T, Morimoto H, Inoue K, Ogonuki N, Jijiwa M, Takahashi M, Ogura A, and Shinohara T 2015. Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Reports 4 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kanatsu-Shinohara M, Lei Z, Rao CV, and Shinohara T 2016. The Luteinizing Hormone-Testosterone Pathway Regulates Mouse Spermatogonial Stem Cell Self-Renewal by Suppressing WNT5A Expression in Sertoli Cells. Stem Cell Reports 7 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki K, and Honjo T 2010. Two opposing roles of RBP-J in Notch signaling. Curr Top Dev Biol 92 231–252. [DOI] [PubMed] [Google Scholar]
- Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, and Teixeira J 2010. Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod 82 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelenbosch RA, and de Rooij DG 1993. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res 290 193–200. [DOI] [PubMed] [Google Scholar]
- Tokue M, Ikami K, Mizuno S, Takagi C, Miyagi A, Takada R, Noda C, Kitadate Y, Hara K, Mizuguchi H, et al. 2017. SHISA6 Confers Resistance to Differentiation-Promoting Wnt/beta-Catenin Signaling in Mouse Spermatogenic Stem Cells. Stem Cell Reports 8 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac AC, Agulnick AD, Haughey N, Chang CF, Zhang Y, Backman C, Morales M, Mattson MP, Wang Y, Westphal H, et al. 2002. Effects of cerebral ischemia in mice deficient in Persephin. Proc Natl Acad Sci U S A 99 9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, and Arumae U 1996. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature 381 785–789. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, and de Rooij DG 1991. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology 128 697–704. [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, and de Rooij DG 1991. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil 93 233–243. [DOI] [PubMed] [Google Scholar]
- Viglietto G, Dolci S, Bruni P, Baldassarre G, Chiariotti L, Melillo RM, Salvatore G, Chiappetta G, Sferratore F, Fusco A, et al. 2000. Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int J Oncol 16 689–694. [DOI] [PubMed] [Google Scholar]
- Wang MM 2011. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol 43 1550–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekara Y, Airaksinen MS, Heuckeroth RO, Milbrandt J, and Keast JR 2004. Neurturin signalling via GFRalpha2 is essential for innervation of glandular but not muscle targets of sacral parasympathetic ganglion neurons. Mol Cell Neurosci 25 288–300. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Parvinen M, Lindqvist E, and Olson L 2000. Neurturin, RET, GFRalpha-1 and GFRalpha-2, but not GFRalpha-3, mRNA are expressed in mice gonads. Cell Tissue Res 299 409–415. [DOI] [PubMed] [Google Scholar]
- Wolgemuth DJ, Manterola M, and Vasileva A 2013. Role of cyclins in controlling progression of mammalian spermatogenesis. Int J Dev Biol 57 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Goodyear SM, Tobias JW, Avarbock MR, and Brinster RL 2011. Spermatogonial stem cell self-renewal requires ETV5-mediated downstream activation of Brachyury in mice. Biol Reprod 85 1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JR, Zhang X, and Nagano MC 2011. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci 124 2357–2366. [DOI] [PubMed] [Google Scholar]
- Yeh JR, Zhang X, and Nagano MC 2012. Indirect effects of Wnt3a/beta-catenin signalling support mouse spermatogonial stem cells in vitro. PLoS One 7 e40002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, and Nabeshima Y 2006. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133 1495–1505. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, and Nabeshima Y 2004. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol 269 447–458. [DOI] [PubMed] [Google Scholar]
- Zhang T, Oatley J, Bardwell VJ, and Zarkower D 2016. DMRT1 Is Required for Mouse Spermatogonial Stem Cell Maintenance and Replenishment. PLoS Genet 12 e1006293. [DOI] [PMC free article] [PubMed] [Google Scholar]