Abstract
Melanocortin 2 receptor (MC2R) is the receptor for the pituitary hormone adrenocorticotropin (ACTH). When activated, MC2R stimulates cAMP production and adrenal steroidogenesis. The functional expression of the receptor requires Melanocortin 2 receptor accessory protein (MRAP) a single transmembrane domain protein involved in the trafficking of MC2R from the endoplasmic reticulum to the cell surface. Mutations in both MC2R and MRAP cause the inherited disease Familial Glucocorticoid Deficiency. At present, little is known regarding the mechanism of MRAP in MC2R functional expression. Here we report the characterisation of MRAP in the trafficking of MC2R to the cell surface and the formation of a functional receptor. We identify the transmembrane domain of MRAP as the MC2R interaction domain, and a conserved N-terminal tyrosine rich domain of MRAP that is required for trafficking MC2R to the cell surface.
Keywords: GPCR, trafficking, Melanocortin receptors, ACTH
Introduction
The Melanocortin 2 receptor (MC2R) is the smallest member of the G-protein coupled receptor (GPCR) superfamily and belongs to the Melanocortin (MC) subfamily of receptors (1, 2). The pituitary hormone adrenocorticotropin (ACTH) acts through MC2R to induce intracellular production of cAMP and the stimulation of steroidogenesis in the adrenal cortex. Mutations in MC2R are responsible for around 25% of the cases of autosomal recessive ACTH insensitivity syndrome, Familial Glucocorticoid Deficiency (FGD) (3, 4, 5). Analysis of MC2R function, using heterologous expression in cultured cells has been difficult, as, unlike the other MC receptors, it is retained in the endoplasmic reticulum and due to lack of cell surface expression is unable to form a functional ACTH responsive receptor (6, 7). This characteristic of MC2R led to the hypothesis that it requires a specific accessory factor, present in adrenal cell types, in order to traffic efficiently to the cell surface.
In an effort to find causative genes mutated for FGD, a disease locus was mapped to chromosome 21, identifying mutations in a gene encoding a small single transmembrane domain protein that showed high adrenal expression, which was subsequently named MRAP (Melanocortin 2 Receptor Accessory Protein) (8). MRAP interacts with MC2R and facilitates MC2R cell surface expression, thereby producing an ACTH responsive receptor. In addition, knockdown of endogenous mouse MRAP in Y1 adrenocortical cells, which express a functional endogenous MC2R, leads to insensitivity to ACTH (9). Interestingly, recent work suggests that MRAP exists as a unique antiparallel homodimer where one copy of MRAP has a “C-terminus out” orientation and the other an “N-terminus out” orientation (10). It also appears that this MRAP homodimer is in a stable complex with MC2R, although it remains unclear how this complex forms.
Despite the recent advances in the understanding of MRAP, little is known regarding the mechanism of how it traffics MC2R to the cell surface. Superficially, MRAP is similar to a number of other GPCR accessory proteins that have been identified in the past decade. Like MRAP, the RAMPs (receptor activity modifying proteins) which are required for the expression of the calcitonin and calcitonin-like receptors (11, 12, 13), and the RTPs (Receptor Trafficking Proteins) and related REEPs (Receptor Expression Enhancing Proteins), which traffic a number of odorant receptors (14), are small single-transmembrane domain proteins. However, notwithstanding the similar basic structure and parallel roles of these GPCR accessory proteins, there is no sequence identity and no indication that they function by a common mechanism.
Here we report the identification of the transmembrane domain as the site for MRAP interaction with MC2R. In addition, we identify a requirement for a conserved tyrosine rich region in the N-terminus of MRAP for the cell-surface expression of MC2R and the consequent formation of a functional ACTH responsive receptor.
Materials and Methods
DNA Constructs
MRAP-3XFLAG was constructed by directional cloning into the HindIII and EcoRI restriction sites of the p3xFLAG-CMV-14 expression vector (Sigma) following PCR amplification of human MRAPα (Forward primer; ACGCTGAAA GCTTAGTGCCACAGACATG, Reverse primer; ACCAGTTGAATTCGCTATGGC CACGAT). Each of the MRAP deletion constructs was directionally cloned into p3xFLAG-CMV-14 using the HindIII and EcoRI restriction sites (NT2 Forward; ATTGCCAAGCTTACCAACGC CATGGCCCCA. Reverse; As MRAPα. NT3 Forward; CTGGACAAGCTTGA CCTCATGCCCGTGGAC. Reverse; As MRAPα. NT4; AAGCTGAAGCTTATGC ATTCCATCGCA; Reverse; As MRAPα. CT2 Forward; As MRAPα. Reverse; CGGGGAGGAATTCCAGGACATGTAG AGCAA. TMT Forward; As NT4. Reverse; As CT2). The cDNA clone for Hemagglutinin tagged human MC2R (HA-MC2R) was obtained from the Missouri S&T cDNA Resource Center (www.cdna.org).
Cell Culture and Transfection
CHO cells were grown in DMEM:F12 (1:1) supplemented with 10% FCS and 1% penicillin/streptomycin. Plasmids were transiently transfected using Lipofectamine 2000 (Invitrogen). Plasmid DNA amounts were kept constant in transfection with pCDNA3.1.
Immunoprecipitation and Western Blotting
Lysates were prepared 24 hours post-transfection. Cells were washed (2x PBS, 10 mins), and lysates generated using lysis buffer (0.1% n-dodecyl-β-maltoside in PBS with protease inhibitors). Lysates were centrifuged, passed through a 0.22μM filter and either SDS loading buffer added for Western blotting or immunoprecipitation performed. For immunoprecipitation, lysates were incubated for 2 hours at 4°C with either anti-FLAG beads (Sigma) or anti-HA beads (Sigma). Following incubation, beads were washed 5 times in lysis buffer, supernatant removed and beads were resuspended in SDS loading buffer. To detect the FLAG epitope, beads were heated for 5 minutes at 90°C, to detect HA-MC2R, beads were heated at 70°C for 2 minutes. Proteins were resolved by SDS-PAGE and Western blotting performed as described previously (8).
Immunofluorescence
24 hours post-transfection, coverslips were washed (PBS 2x5mins) and fixed in 4% formaldehyde (10 minutes), cells were permeabilized by washing with PBS-Tween 20 (0.1%) (3x5 minutes). Cells were blocked for 1 hour with blocking buffer (PBS, 1% BSA, 10% Donkey serum), incubated with primary antibody (anti-FLAG, Sigma, at 1:200 or anti-HA, Sigma, 1:200) in blocking buffer, washed (PBS-Tween 20, 3x 10 minutes), incubated with secondary antibody (FITC or CY3, both Sigma, 1:200), and washed (PBS-Tween 20, 3x10min). Coverslips were mounted in Fluorescent mounting medium (Dako). Cells were imaged using a laser scanning confocal microscope (Zeiss LSM 510).
Fluorescent Cell Surface Assay
To measure cell surface expression of HA-MC2R, cells were grown in 24-well plates. Transfections were performed in triplicate in parallel on 2X24-well plates. 24 hours post transfection one plate was used for the detection of cell-surface HA-MC2R and the other used as a control for total cellular HA-MC2R. Cell-surface HA-MC2R was detected by staining live cells with anti-HA antibody (1:500) for 20 minutes at 37°C in cell growth media. Cells were then washed in PBS (3x 5minutes), fixed in 4% formaldehyde (10 minutes), washed in PBS (2x5minutes) and incubated with a infrared secondary antibody (goat anti-mouse, Licor, 1:1500) in blocking buffer (Licor). After further washing (PBS, 3x5min) plates were scanned using an infrared scanner (Licor Odyssey). To control for total protein, cells were washed twice in PBS (5min) fixed in 10% formaldehyde (10 min), washed (PBS 2x5min), permeabilized (0.025% triton 10 min), washed (PBS 2x5min), blocked for 1 hour (Licor blocking buffer), incubated with anti-HA (1hour), washed (PBS 3x5min), incubated with infrared secondary antibody (Licor), washed (3x5min PBS), and quantitated on the infrared plate reader (as above). Results are presented as the percentage of total protein detected at the cell surface.
cAMP Reporter Assay
cAMP production was assessed using a cAMP luciferase reporter construct and assayed using the Dual Glo Luciferase Reporter System (Promega), as reported previously (9). Briefly, 24 hours after co-transfection of HA-MC2R and MRAP constructs with α-GSU-846 luciferase and pRL-CMV Renilla luciferase, cells were incubated with 10-6M ACTH for 6 hours. Following stimulation cells were harvested and Dual Glo Luciferase assay performed. Luciferase activity was measured using a multiplate reader (Wallac Victor 2 PerkinElmer), and values normalized to the pRL-CMV Renilla luciferase activity.
Statistical Analysis
Statistical analysis was performed using the t test. Results are expressed as mean ± SEM.
Results
Human MRAPα interacts with MC2R and is required for receptor cell surface expression and ACTH responsiveness
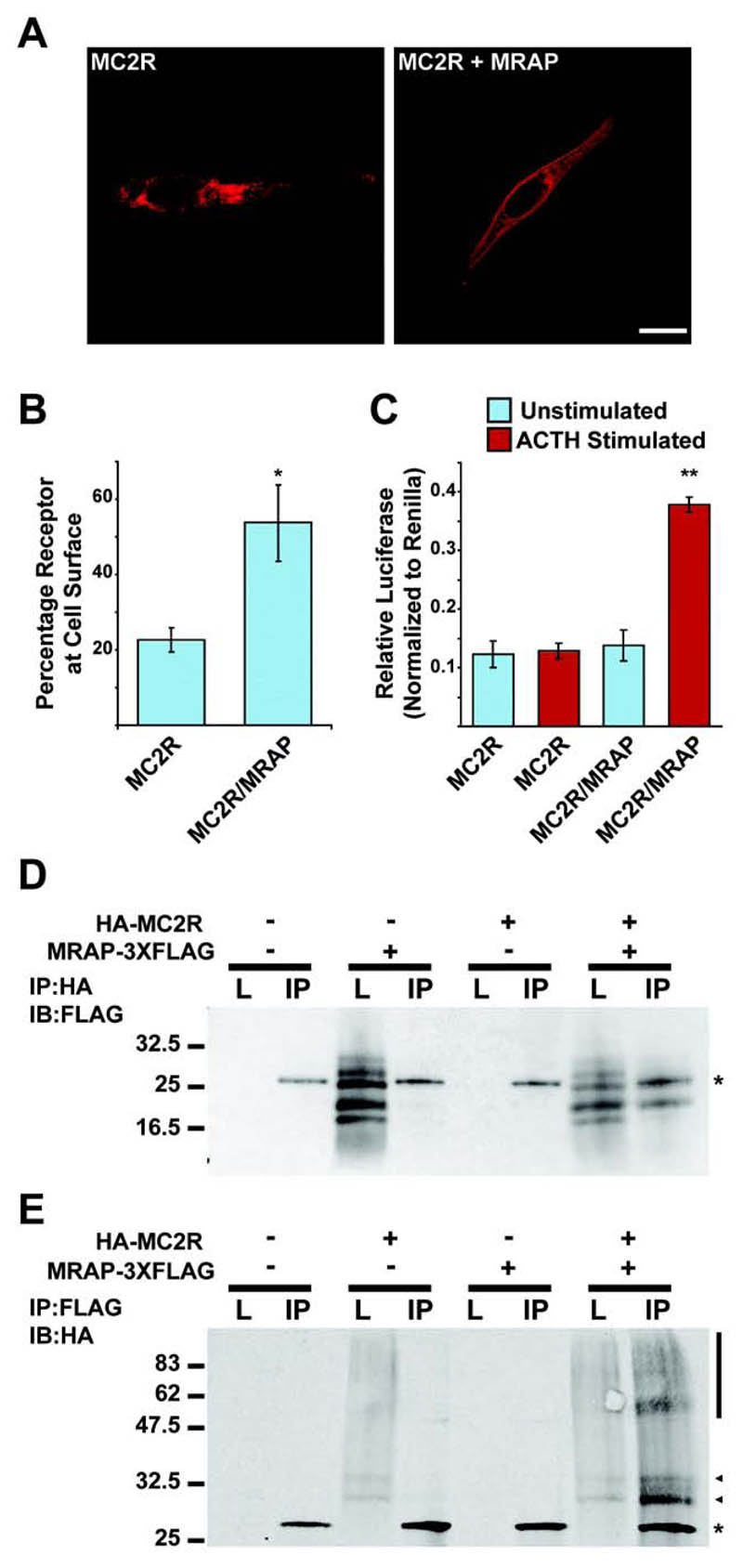
We have previously shown that mouse MRAP is able to interact with MC2R and is required for the cell surface expression of MC2R (8). To investigate how MRAP directs the functional expression of MC2R we first tested whether human MRAPα has the same effect as mouse MRAP. Human MC2R epitope tagged at the N-terminus with Hemagglutanin (HA-MC2R) was only expressed at the cell surface of permeabilized CHO cells, as visualised by immunofluorescence, when co-expressed with MRAPα (epitope tagged at the C-terminus with FLAG, subsequently referred to as MRAP-FLAG) (Fig. 1A). Using a fluorescent cell surface assay, where live cells are incubated with HA-antibody, and therefore only cell surface HA epitopes are detected and normalized against total HA-MC2R in fixed permeabilized cells, there was a significant increase in HA-MC2R at the cell surface in the presence of MRAP-FLAG (Fig. 1B). Furthermore, using a cAMP luciferase reporter assay, a cAMP response to ACTH stimulation was only detected when HA-MC2R was expressed in the presence of MRAP-FLAG, demonstrating that MRAP is required for functional MC2R cell surface expression (Figure 1C). Coimmunoprecipitation confirmed that human MRAP-FLAG and HA-MC2R interact (Fig. 1D,E). Human MRAP-FLAG resolved as several bands between 16kDa and 32 kDa on SDS-PAGE (Fig. 1D). MRAP-FLAG has a predicted molecular weight of ~ 24kDa, and the major band may correspond to an unmodified MRAP. Like mouse MRAP, human MRAP contains a predicted N-glycosylation site, and glycosylated MRAP corresponds to the slowest migrating band detected (data not shown). The other bands may correspond to post-translational modifications or, as some of the bands are smaller than the predicted molecular weight, cleavage products. Immunoprecipitation using HA-conjugated agarose beads and immunoblotting using FLAG antibody specifically detected MRAP-FLAG. HA-MC2R resolved as a series of bands, typical of the migration pattern of other GPCRs on SDS-PAGE. HA-MC2R bands were specifically detected following immunoprecipitation with FLAG-conjugated agarose beads and immunoblotting using an HA antibody (Fig. 1E). Therefore, human MRAPα interacts with MC2R and promotes its trafficking to the cell surface, where it forms a functional receptor.
Figure. 1. Human MRAPα interacts with and promotes trafficking of MC2R to the cell surface producing an ACTH responsive receptor.
(A) HA-MC2R was detected at the cell-surface of transiently transfected CHO cells by immunofluorescent staining only when co-expressed with MRAP (Bar, 10μm). (B) Cell culture plate based fluorescent cell surface assay showing surface anti-HA staining of live cells as a percentage of total anti-HA staining in permeabilized cells. There was a significant increase in HA-MC2R detected at the cell surface when co-expressed with MRAP-FLAG. Mean ± SEM; n=3; *, P < 0.05. (C) Luciferase assay to measure cAMP response following 6 hour 10-6 M ACTH stimulation. There is a significant increase in cAMP following ACTH stimulation when HA-MC2R is co-expressed with MRAP-FLAG. Mean ± SEM; n=3; **, P < 0.005. (D and E) Co-immunoprecipitation between HA-MC2R and MRAP-FLAG. L = Lysate, IP = Immunoprecipitation. * represents Light Chain. (E) Arrow heads and vertical bar represent MC2R.
Identification of functional MRAP domains
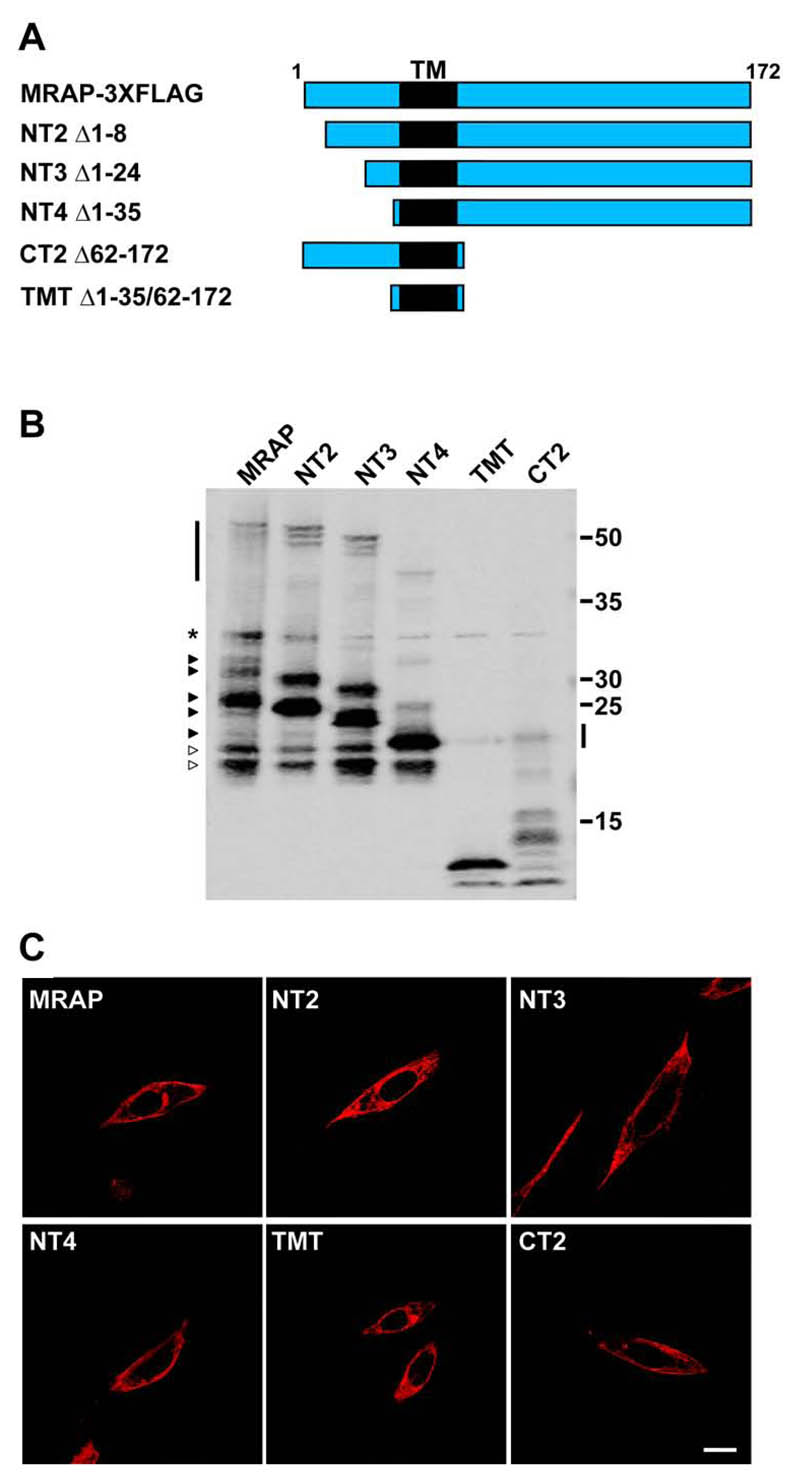
In an effort to identify the MRAP domains involved in the interaction with and functional expression of MC2R we produced a series of human MRAP truncation constructs that are FLAG tagged at their C-terminus (Fig. 2A and Supplemental Figure 1). Each of the constructs was detectable by Western blotting and immunofluorescence following transient transfection into CHO cells (Fig. 2B,C). We also produced constructs encoding the MRAP N-terminus and C-terminus alone, however, these were not detected by either Western blot or immunofluorescence, suggesting that these constructs either do not express or that the proteins are rapidly degraded (data not shown). As with the full length MRAP-FLAG construct, the three N-terminal truncation constructs, NT2, NT3, and NT4, were detected as multiple bands by Western blot, with the major band, corresponding to unmodified MRAP (Fig. 2B). Interestingly, as reported previously with mouse MRAP (9), specific immunoreactive species migrating at ~50kDa were detected, suggesting the presence of SDS resistant homodimers. Curiously, lower molecular weight bands observed with full length MRAP-FLAG were also present at the same molecular weight with each of the N-terminal truncation constructs. This implies that MRAP undergoes degradation at specific sites, or it might be that the N-terminus of MRAP undergoes specific processing events. A similar phenomenon was observed with the TMT and CT2 constructs by Western blot. The transmembrane domain construct was detected as two bands at ~10kDa, and the same bands were also detected with the CT2 construct. As with the N-terminal truncations, a faint band was detected at ~20kDa that might represent an SDS resistant dimer.
Figure. 2. Expression of MRAP truncation constructs.
(A) Schematic showing MRAP truncation constructs in relation to full-length MRAPα. TM = transmembrane domain. Each construct is epitope tagged at the C-terminus with FLAG. Numbers represent deleted residues, inclusive. (B) Western blot showing the expression of each of the constructs when co-expressed with HA-MC2R. * represents non-specific band. Arrowheads on left hand side represent wild-type MRAP bands. White arrowheads represent putative cleavage products. Vertical bars represent SDS resistant homodimers. (C) Each of the MRAP constructs can be seen at the cell surface of permeabilized CHO cells by immunofluorescent staining. Bar, 10μm.
Each of the MRAP truncation constructs could be detected at the cell-surface by immunofluorescence using the FLAG antibody on permeabilized cells (Fig. 2C), signifying that the MRAP transmembrane domain alone is sufficient for the cell surface expression of MRAP.
The transmembrane domain of human MRAP is sufficient for MRAP/MC2R interaction
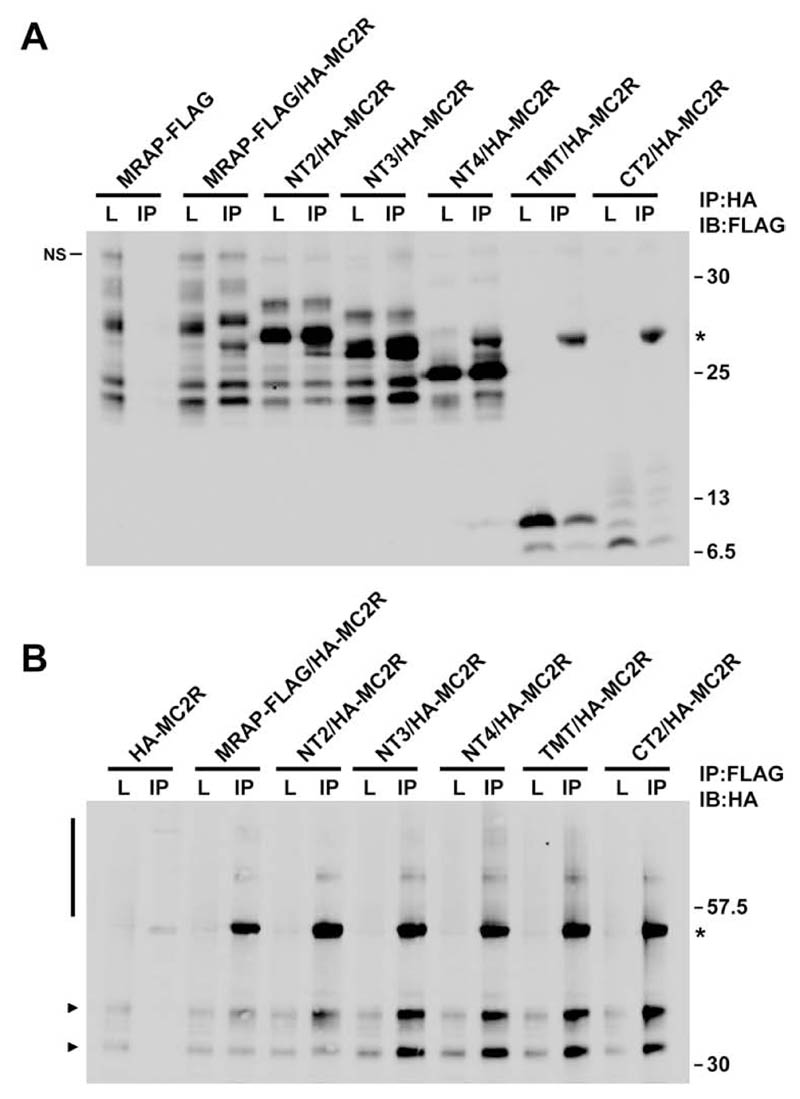
To identify the MRAP domain required for its interaction with MC2R, HA-MC2R was coexpressed with each of the MRAP truncation constructs and coimmunoprecipitated. Immunoblotting using FLAG identified each of the MRAP truncation proteins following immunoprecipitation with HA-conjugated beads (Fig. 3A). In the reverse experiment, immunoprecipitation using FLAG-conjugated agarose specifically precipitated HA-MC2R with each of the FLAG tagged MRAP truncation proteins (Fig. 3B). A concern with immunoprecipitation of membrane proteins is that if lysates are incompletely solubilized, two proteins that are not in physical contact, may be present in a membrane fragment and produce spurious interaction data (15). These coimmunoprecipitations have therefore been performed using lysates that have been passed through a 0.22μM filter to ensure that membrane fragments have been removed. Using unfiltered lyastes and different detergent conditions the same results were obtained (data not shown). We have, therefore, mapped the MRAP/MC2R interaction domain to a 27 aa region between residues 36 and 62 of MRAP that consists almost entirely of the transmembrane domain. It is thus likely that MC2R interacts with MRAP via one or more of its transmembrane domains.
Figure. 3. The MRAP transmembrane domain is required for receptor interaction.
(A and B) Co-immunoprecipitation between HA-MC2R and each of the FLAG tagged MRAP constructs. Each of the MRAP constructs interact with HA-MC2R as seen by immunoblotting with FLAG following immunoprecipitation with HA and vice-versa. L = Lysate, IP = Immunoprecipitation. * = Light chain in A and Heavy chain in B. NS = non-specific in A. Bar and arrows in B show HA-MC2R.
The N-terminus of MRAP is required for MC2R cell surface expression and ACTH responsiveness
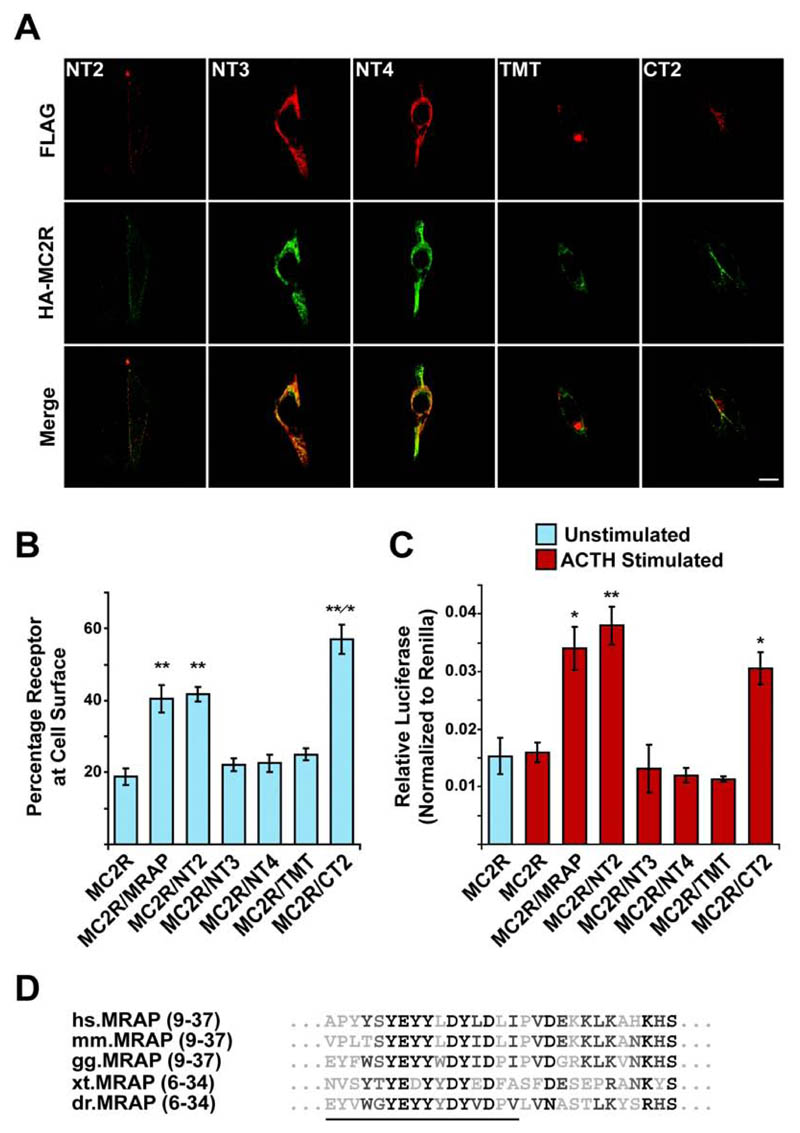
We then tested if the interaction between MRAP and MC2R was sufficient to mediate the trafficking of MC2R to the cell surface. HA-MC2R was co-transfected with each of the MRAP truncation constructs and cell surface expression monitored using immunofluorescence and the fluorescent cell-surface assay. HA-MC2R was detected at the cell surface of permeabilized cells in the presence of both the NT2 and CT2 constructs as detected by immunofluorescence staining (Fig. 4A). However, HA-MC2R remained intracellular when cotransfected with each of the NT3, NT4 and TMT constructs. As with full-length MRAP-FLAG the amount of HA-MC2R detected at the cell-surface was around 2-fold higher when coexpressed with the NT2 construct, indicating that the N-terminal 8 amino acids of MRAP, which, contain the glycosylation site, are not required for MRAP to traffic MC2R to the cell-surface (Fig. 4B). In the presence of CT2 the amount of HA-MC2R at the cell-surface was significantly higher compared to coexpression with either MRAP-FLAG or NT2. No difference in the total levels of HA-MC2R was seen (data not shown). The increase in HA-MC2R at the cell-surface seen with the CT2 construct is in contrast to previously published data using a mouse MRAP construct with a C-terminal deletion (10). This mouse construct supported functional MC2R expression; however, this was not greater than that seen with wild type MRAP. This could be explained either by species differences or may be due to the presence of both N- and C-terminal epitope tags on the mouse MRAP fusion, which could impair or alter its function.
Figure. 4. MC2R cell surface trafficking requires the N-terminus of MRAP and is regulated by the C-terminus of MRAP.
(A) Immunofluorescent staining showing localization of HA-MC2R with each of the FLAG tagged MRAP constructs in permeabilized transiently transfected CHO cells. Top panels, anti-FLAG staining of MRAP constructs (red). Middle panels, anti-HA staining of HA-MC2R (green). Bottom panels, merge. HA-MC2R co-localizes with each of the MRAP constructs, and is detected at the cell surface in the presence of the NT2 and CT2 constructs. With NT3, NT4, and TMT, HA-MC2R is not seen at the cell surface. Bar, 10μm. (B) Fluorescent cell surface assay showing the percentage of MC2R at the cell surface when co-transfected with each of the MRAP constructs. Significant cell surface expression is seen in the presence of MRAP, NT2, and CT2 (**, P < 0.005) compared to HA-MC2R alone. There is no increase seen with the remaining constructs. HA-MC2R cell surface expression is significantly higher when co-expressed with CT2 compared to MRAP or NT2 (*, P < 0.05). Mean ± SEM; n=4. (C) Luciferase assay to measure cAMP response following 6 hour 10-6 M ACTH stimulation. There is a significant increase in cAMP following ACTH stimulation when HA-MC2R is co-expressed with MRAP, NT2, and CT2. Mean ± SEM; n=3; * P < 0.05, **, P < 0.005. (D) Alignment of amino acids 9 to 37. Residues deleted in the NT3 construct are underlined. hs, Homo sapiens. mm, Mus musculus. gg, Gallus gallus. xt, Xenopus tropicalis. dr, Danio rerio.
When co-transfected with NT3, NT4 or TMT, there was no significant increase in HA-MC2R detected at the cell-surface compared to HA-MC2R alone using the fluorescent cell surface assay, demonstrating that an interaction between MC2R and MRAP alone is not sufficient for trafficking but that the MRAP N-terminal domain between residues 9 and 24 is also required.
To investigate whether the MRAP truncation constructs affect the function of MRAP we performed a cAMP luciferase reporter assay (Fig. 4C). In line with the cell surface assay, there was only a cAMP response when HA-MC2R was coexpressed with either full length MRAP-FLAG, NT2 or CT2, although, there was no increase in signalling in the presence of CT2 to match the increase in HA-MC2R cell surface expression. Therefore, the N-terminus of MRAP is required for the cell-surface expression of MC2R and the resultant formation of an ACTH responsive receptor. The key region is a conserved tyrosine rich region that begins between residues 9 and 24 (Fig. 4D) as the NT3 construct, which has the first 24 amino acids deleted, is unable to support MC2R cell surface expression.
Discussion
MRAP is a small single transmembrane domain protein, which is required for the proper function of the ACTH receptor MC2R. It has previously been reported that MRAP interacts with MC2R and promotes its trafficking to the cell surface. However, the means of the MRAP/MC2R interaction and the role of MRAP in receptor trafficking have so far been elusive. Here we have studied the role of MRAP, and have identified that the transmembrane domain of MRAP as the site of MC2R interaction, while a conserved 15 residue region in the N-terminus of MRAP is required for trafficking. In addition MRAPs C-terminus, which shows little sequence conservation between species, may have a role in the control of MC2R cell surface expression.
The identification of the MRAP transmembrane domain as the site of MRAP/MC2R interaction highlights differences between the mechanisms of MRAP and the RAMPs. For RAMP1 it is the large extracellular N-terminal domain that has been shown to be sufficient for its interaction with the calcitonin-like receptor (13, 16, 17, 18).
Although, the MRAP transmembrane domain is sufficient for interaction with MC2R it is not able to support MC2R trafficking. The key region controlling trafficking is a 15 aa tyrosine rich region between residues 9 and 24 in the N-terminus of MRAP, as the NT3 MRAP construct is unable to promote MC2R trafficking. The role of this 15 amino acid domain in the cell-surface expression of MC2R is not yet known. It is not required for the MRAP/MC2R interaction and it does not appear to be needed for MRAP to reach the cell surface. It is possible that it plays a role in the stabilisation of the MRAP/MC2R interaction during trafficking to the cell surface. Alternatively, it might interact with another protein that regulates MRAP/MC2R cell-surface expression. Recently, the mouse MRAP protein has been shown to function as an antiparallel homodimer. The N-terminus of MRAP could be involved in establishing this unique structural confirmation either intrinsically or by interacting with an unidentified partner, however, the presence of an apparent SDS resistant dimer, as seen by Western blot when this region is deleted (Fig. 2B), suggests that the loss of MC2R trafficking is not through a failure of MRAP homodimerization. At present it is unclear whether this novel 15 amino acid tyrosine rich domain is unique to MRAP or if it is a general feature of proteins involved in the trafficking of GPCRs. Although sequence homology is not readily identifiable between this domain and other proteins, it does resemble a region within another family of GPCR trafficking proteins. REEP1 was first identified as a transmembrane protein that is able to promote the cell surface expression of odorant receptors (14). Like MRAP, the REEPs have a tyrosine rich region in their N-termini. It is therefore possible that a tyrosine rich domain may be a more common feature of proteins involved in GPCR trafficking.
The C-terminus of MRAP is not required for MRAP to interact with MC2R or for MRAP to traffic MC2R to the cell surface. Instead, the C-terminus may play a role in the amount of MC2R that reaches the cell-surface as deletion of MRAPs C-terminus resulted in significantly higher MC2R cell surface expression compared to MC2R with wild type MRAP. A possible role for the C-terminus of MRAP in regulating MC2R cell surface expression is supported by the different effects the two human MRAP isoforms, which differ in their C-terminus, have been shown to have on MC2R (19). When coexpressed with MRAPβ, MC2R cell surface expression was significantly higher than when coexpressed with MRAPα.
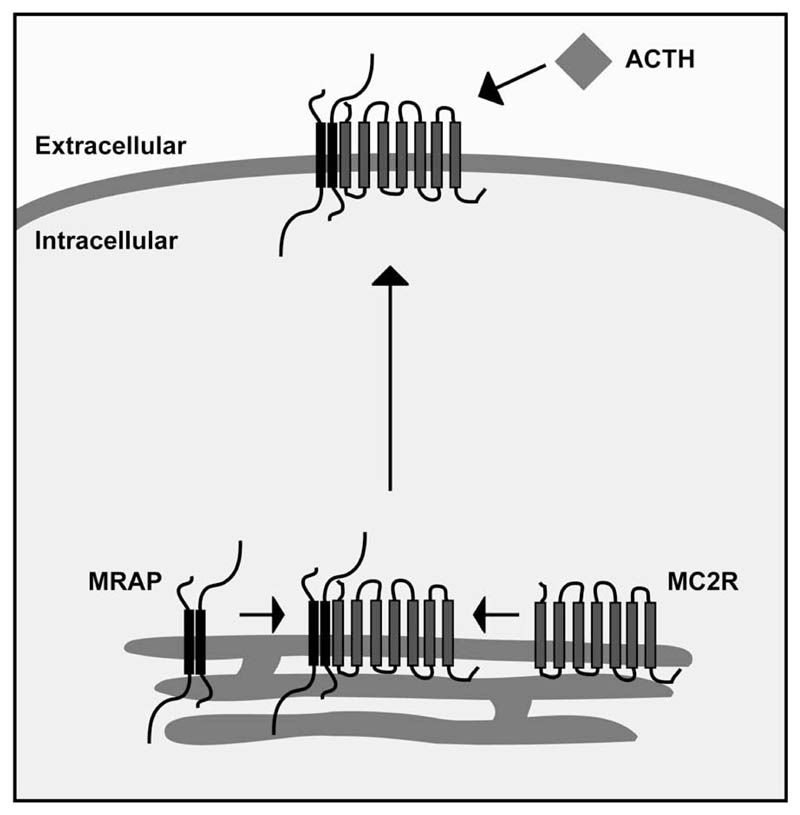
In summary, MRAP is a small single transmembrane domain protein that is required for the cell-surface expression of the G-protein coupled MC2R (Fig. 5). We have previously reported that MRAP interacts with MC2R and enhances its trafficking to the cell surface, producing a functional ACTH responsive receptor. However, both the mechanism of the interaction and the means of trafficking have been elusive. The N-terminus and transmembrane domain of MRAP are conserved throughout vertebrates, whilst its C-terminus is highly divergent. In this study, we have shown that the C-terminus of MRAP is not necessary for either MC2R interaction or trafficking, but instead may have a regulatory role in MC2R cell surface expression. Furthermore, we have shown a requirement for a tyrosine rich region of the N-terminus of MRAP in trafficking MC2R to the cell surface and demonstrated that the MRAP transmembrane domain is sufficient for the MRAP/MC2R interaction.
Figure 5. Hypothetical model of MRAP/MC2R interaction and receptor trafficking.
MRAP (black), which acts as an anti-parallel homodimer (10), interacts with MC2R (grey) within the endoplasmic reticulum via its transmembrane domain. This heterotrimeric structure then traffics to the cell surface through a process dependent upon a 15 amino acid tyrosine rich region of the N-terminus of MRAP, where it is capable of recognising and responding to the ACTH peptide.
Acknowledgements
The authors are grateful to Professor J. Burrin for the α-GSU luciferase reporter.
Grants: This work was supported by the Wellcome Trust and a MRC Clinical Training Fellowship (Li Chan).
Footnotes
This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
Disclosure Statement: The authors have nothing to disclose
References
- 1.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 2.Cone RD, Mountjoy KG, Robbins LS, Nadeau JH, Johnson KR, Roselli-Rehfuss L, Mortrud MT. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann N Y Acad Sci. 1993;680:342–363. doi: 10.1111/j.1749-6632.1993.tb19694.x. [DOI] [PubMed] [Google Scholar]
- 3.Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;341:461–462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- 4.Weber A, Kapas S, Hinson J, Grant DB, Grossman A, Clark AJ. Functional characterization of the cloned human ACTH receptor: impaired responsiveness of a mutant receptor in familial glucocorticoid deficiency. Biochem Biophys Res Commun. 1993;197:172–178. doi: 10.1006/bbrc.1993.2456. [DOI] [PubMed] [Google Scholar]
- 5.Clark AJ, Cammas FM, Watt A, Kapas S, Weber A. Familial glucocorticoid deficiency: one syndrome, but more than one gene. J Mol Med. 1997;75:394–399. doi: 10.1007/s001090050124. [DOI] [PubMed] [Google Scholar]
- 6.Noon LA, Franklin JM, King PJ, Goulding NJ, Hunyady L, Clark AJ. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J Endocrinol. 2002;174:17–25. doi: 10.1677/joe.0.1740017. [DOI] [PubMed] [Google Scholar]
- 7.Rached M, El Mourabit H, Buronfosse A, Blondet A, Naville D, Begeot M, Penhoat A. Expression of the human melanocortin-2 receptor in different eukaryotic cells. Peptides. 2005;26:1842–1847. doi: 10.1016/j.peptides.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, Huebner A, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 9.Cooray SN, Almiro Do Vale I, Leung KY, Webb TR, Chapple JP, Egertova M, Cheetham ME, Elphick MR, Clark AJ. The Melanocortin 2 Receptor Accessory protein exists as a homodimer and is essential for the function of the Melanocortin 2 Receptor in the mouse Y1 cell line. Endocrinol. 2008;149:1935–1941. doi: 10.1210/en.2007-1463. [DOI] [PubMed] [Google Scholar]
- 10.Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci U S A. 2007;104:20244–20249. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 12.Morfis M, Christopoulos A, Sexton PM. RAMPs: 5 years on, where to now? Trends Pharmacol Sci. 2003;24:596–601. doi: 10.1016/j.tips.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Milligan G, Bouvier M. Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J. 2005;272:2914–2925. doi: 10.1111/j.1742-4658.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- 16.Steiner S, Muff R, Gujer R, Fischer JA, Born W. The transmembrane domain of receptor-activity-modifying protein 1 is essential for the functional expression of a calcitonin gene-related peptide receptor. Biochemistry. 2002;41:11398–11404. doi: 10.1021/bi020279r. [DOI] [PubMed] [Google Scholar]
- 17.Fitzsimmons TJ, Zhao X, Wank SA. The extracellular domain of receptor activity-modifying protein 1 is sufficient for calcitonin receptor-like receptor function. J Biol Chem. 2003;278:14313–14320. doi: 10.1074/jbc.M211946200. [DOI] [PubMed] [Google Scholar]
- 18.Ittner LM, Luessi F, Koller D, Born W, Fischer JA, Muff R. Aspartate(69) of the calcitonin-like receptor is required for its functional expression together with receptor-activity-modifying proteins 1 and -2. Biochem Biophys Res Commun. 2004;319:1203–1209. doi: 10.1016/j.bbrc.2004.05.103. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21:1656–1669. doi: 10.1210/me.2007-0041. [DOI] [PubMed] [Google Scholar]