Abstract
Background
There are limited nationally representative data on correlates of cytomegalovirus (CMV) shedding among children and adolescents. In addition, the genotype distribution of CMV infections has not been well characterized among general populations in the United States
Methods
This study characterized urinary CMV shedding among CMV immunoglobulin G-positive 6- to 19-year-olds in the US household population using data from the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Multivariable Poisson regression was used to estimate adjusted prevalence ratios (aPR) and corresponding 95% confidence intervals (CIs). Analyses were weighted and multiple imputation was performed to handle missing data (with the exception of CMV genotypes).
Results
Prevalence of urinary CMV shedding was significantly lower among 9- to 11-year-olds (20.6%; aPR = 0.61; 95% CI, 0.44–0.83) and 12- to 19-year-olds (7.0%; aPR = 0.21; 95% CI, 0.14–0.30) compared with 6- to 8-year-olds (34.4%). Among CMV shedders, the youngest age group also had the highest urinary CMV viral loads. The prevalence of urinary CMV shedding among obese individuals was significantly lower compared with lean individuals (aPR = 0.68; 95% CI, 0.47–0.99). Among CMV shedders, glycoprotein B (gB)1 (51%) was the most prevalent gB variant, followed by gB2 (29%), gB3 (21%), and gB4 (13%); glycoprotein H (gH)2 (60%) was more prevalent than gH1 (48%). Multiple (≥2) gB (14%) and multiple gH (7%) infections were detected among CMV shedders.
Conclusions
This study underscores the importance of young children even above the age of 5 years as a potential source of CMV transmission. The detection of multiple CMV strains among CMV shedders may have implications for the transmission of viral diversity as well as vaccine development.
Keywords: cytomegalovirus (CMV), genotype, National Health and Nutrition Examination Survey (NHANES), shedding
Cytomegalovirus (CMV) is a globally ubiquitous herpesvirus that establishes a lifelong latent reservoir after infection [1]. Congenital CMV infection causes permanent disability and birth defects [1–3]. In individuals with weakened immune systems, such as transplant patients or persons living with human immunodeficiency virus, CMV infection can cause severe disease [1, 4, 5]. Although CMV infection is typically asymptomatic in healthy individuals, it has been associated with inflammation and immunosenescence as well as cognitive decline, cancer, and all-cause mortality [1, 6].
The presence of CMV immunoglobulin (Ig)G antibodies indicates prior exposure to CMV [1]. Cytomegalovirus transmission typically occurs by direct or indirect contact with the bodily fluids of a person who is actively shedding the virus. Cytomegalovirus infects many tissue types; consequently, CMV shedding occurs via multiple fluids, such as saliva, urine, and genital secretions [1, 7–9]. Cytomegalovirus shedding is common after initial infection, reinfection with a new strain, or reactivation of latent virus [1]. Different CMV strains are identified by gene variants such as those found in viral envelope glycoprotein B (gB) and glycoprotein H (gH) [10]. The genotypic distribution of CMV is largely unknown in general populations.
The National Health and Nutrition Examination Survey (NHANES) is the largest population-based study to monitor the epidemiology of CMV infection in the United States. In the 1999–2004 NHANES, CMV seroprevalence was estimated to be 50.4% among persons aged 6–49 years [11]. Using the same data source, Amin et al [12] showed that the prevalence of urinary CMV shedding was 9.7% among CMV-seropositive persons aged 6–49 years. Although CMV IgG seroprevalence increased with age into adulthood [11], prevalence of urinary CMV shedding decreased with age among CMV-seropositive individuals [12]. In age-adjusted analyses of CMV-seropositive 6- to 49-year-olds, Amin et al [12] showed that the detection of urinary CMV shedding was associated with female sex and household income level. However, the cited study did not examine the correlates of urinary CMV shedding among CMV-seropositive children and adolescents—the population that has the highest probability of urinary CMV shedding. The increased prevalence of urinary CMV shedding in pediatric populations is likely reflective of recent primary infection [2, 12]. Thus, we hypothesized that the determinants of urinary CMV shedding are likely different in pediatric and adult populations. In this study, data from the 1999–2004 NHANES were used to examine the prevalence, magnitude, genotype distribution, and correlates of urinary CMV shedding among CMV-seropositive children and adolescents aged 6–19 years.
METHODS
Study Design and Population
A cross-sectional analysis was performed on data from the 1999–2004 NHANES, which was conducted by the National Center for Health Statistics (NCHS) of the Center for Disease Control and Prevention. The NHANES uses a stratified, multistage probability sampling design to select study participants that are representative of the US noninstitutionalized civilian population. Respondents participated in a household interview and a follow-up physical examination at a local mobile examination center (MEC) [13]. At the MEC, participants had venous blood samples drawn and provided self-administered urine samples. Individuals aged 6–49 years with available stored serum were eligible to have their serum tested for CMV IgG antibody. Individuals who were seropositive for CMV IgG, had available stored urine samples, and identified as non-Hispanic white, non-Hispanic black, or Mexican American were eligible to be tested for urinary viral shedding.
Of the 12 072 6- to 19-year-olds that were screened to enroll in NHANES between 1999 and 2004, 10 235 (84.8%) participated in the medical examination component of NHANES (Supplementary Figure S1). The analytic sample for the present study consisted of 9546 examinees aged 6–19 years who identified as non-Hispanic white, non-Hispanic black, or Mexican American.
Ethics Statement
Data collection and study procedures were approved by the NCHS Research Ethics Board. This analysis of deidentified and publicly available data was deemed exempt from review by the Johns Hopkins University School of Medicine Institutional Review Board.
Laboratory Testing
Measures of CMV IgG antibody status and urinary CMV shedding were conducted on surplus serum and urine samples. These samples were stored at the NHANES surplus biorepository at −80°C [14]. Cytomegalovirus IgG status was measured on serum samples by enzyme-linked immunosorbent assay (ELISA) (Quest International, Inc., Miami, FL) and confirmed with a second ELISA (bioMerieux, Inc., Durham, NC) and/or immunofluorescence assay (Bion International, Inc., Woodburn, MA) [11]. Deoxyribonucleic acid (DNA) was extracted from urine samples of CMV IgG-seropositive persons (QIAGEN, Valencia, CA) [12, 15]. Urinary CMV DNA was detected by real-time polymerase chain reaction (PCR) of the immediate early-2 region of the viral genome [15, 16]. Viral loads were quantified using commercially standardized CMV DNA from Advanced Biotechnologies, Inc. (Eldersburg, MD) [12]. All CMV PCR testing was performed in duplicate, and both tests were required to be positive for an individual to be categorized as shedding the virus [12]. Urine samples that had sufficient CMV DNA were genotyped for 4 gB and 2 gH variants [17, 18].
Blood hemoglobin was measured using a single-beam photometer, and lymphocyte count was derived using the Beckman-Coulter method on the Beckman Coulter MAXM Hematology Analyzer. C-reactive protein levels were estimated in sera by latex-enhanced nephelometry using the Dade Behring Nephelometer II Analyzer System. Urinary albumin was measured using solid-phase fluorescent immunoassay on a Sequoia-Turner Fluorometer, model 450. Urinary creatinine was estimated by the colorimetric Jaffe method on the Beckman Synchron CX3 Clinical Analyzer.
Statistical Analysis
The analysis was weighted to account for the complex survey design, unless specified otherwise. The medical examination weights provided by NCHS were pooled across the 3 cycles and used to adjust for differential probabilities of selection, unit nonresponse to the medical exam component, and noncoverage of the noninstitutionalized US civilian population. Taylor series linearization was used to estimate design-corrected (robust) standard errors.
Cytomegalovirus IgG antibody prevalence was estimated among all eligible participants who attended the medical examination component. Urinary CMV shedding prevalence was estimated among CMV IgG antibody-positive individuals. Geometric mean urinary CMV viral loads were estimated among those with detectable urinary CMV shedding. Poisson regression was used to examine associations of sociodemographic, behavioral, and clinical variables with prevalence of urinary CMV shedding among CMV IgG antibody-positive individuals. Variables associated with prevalent CMV shedding using a design-adjusted Wald F-test (P < .15) and/or deemed to be important a priori (eg, age, sex, and race/ethnicity) were included in multivariable analysis.
There were missing data on key variables (Supplementary Table S1). For instance, in the analytic sample (n = 9546), 17.2% of participants were missing data on CMV IgG antibody status. Urinary CMV shedding data was missing for 17.1% of the 4032 participants who were identified to be CMV IgG-antibody positive. Variables outside of age, race/ethnicity, and sex were associated with missing data on CMV IgG antibody and urinary CMV shedding, such as household crowding (Supplementary Table S1). Multiple imputation with chained equations was performed to handle missing data, assuming data were missing at random (ie, missing data could be explained by observed characteristics). The imputation model included variables related to the complex survey design (eg, survey weights), all variables in the analysis, and auxiliary variables associated with missing data. The imputation process was conducted in a stepwise manner for CMV outcomes; for example, CMV shedding status was only imputed if the participant was identified as CMV IgG seropositive in a given imputed data set. Forty complete data sets were generated and results were combined using Rubin’s rules [19]. All weighted analyses were conducted using -mi est- and -svy- commands in Stata/MP, version 15.2 (StataCorp LP, College Station, TX).
In a separate analysis, the genotype distribution of urinary CMV shedders was examined in the unweighted observed data set (ie, those who had sufficient DNA for genotyping). We used an available-case analytic approach when examining genotype data, because there was poor information in the dataset to inform an imputation.
RESULTS
Prevalence and Magnitude of Urinary Cytomegalovirus Shedding
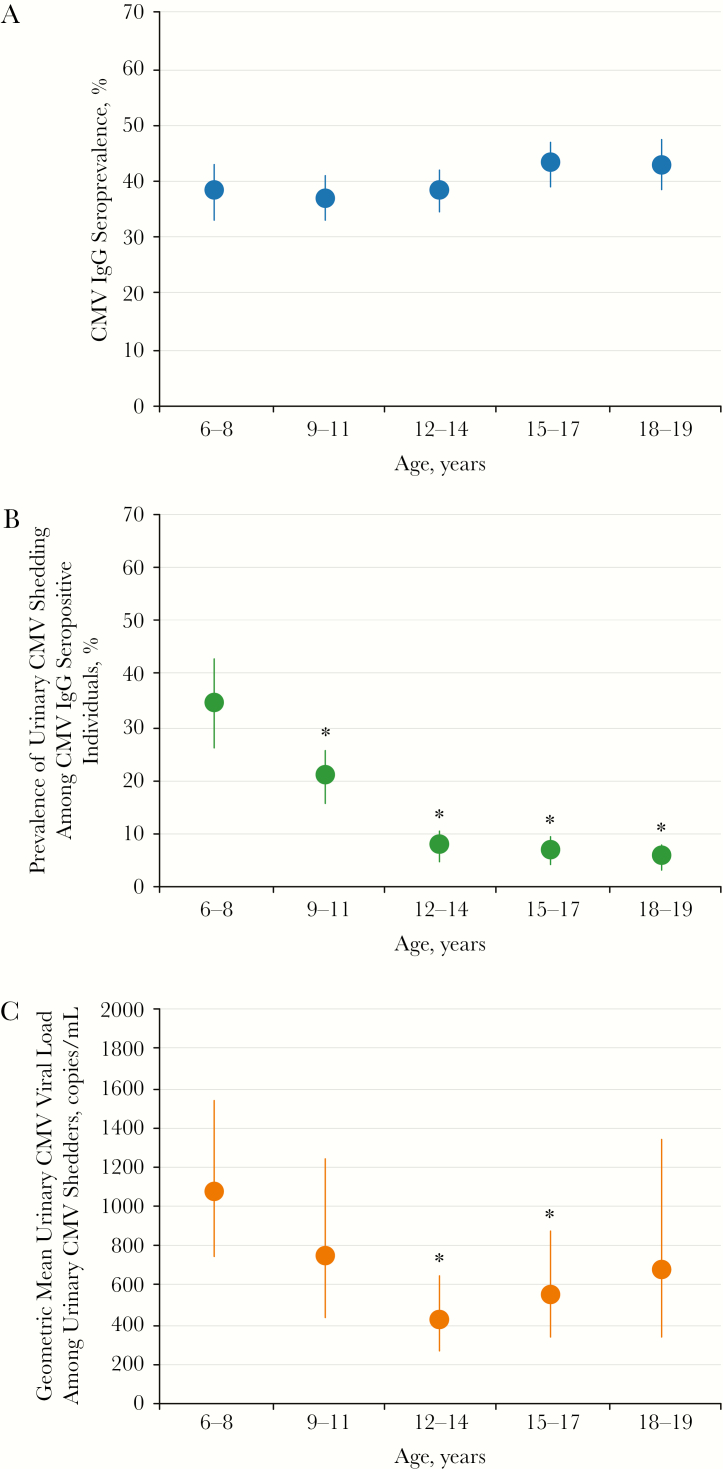
In the study population, the prevalence of CMV IgG antibody was 39.6% (95% confidence interval [CI], 36.9%–42.3%). Among CMV IgG-seropositive individuals, the prevalence of urinary shedding was 15.5% (95% CI, 13.1%–17.9%). The prevalence of CMV IgG antibody increased overall with older age (Figure 1A). In contrast, the prevalence of urinary CMV shedding among CMV IgG-seropositive individuals decreased overall with older age (Figure 1B). Children aged 6–8 years had a higher prevalence of urinary CMV shedding (34.4%) compared to 9- to 11-year-olds (20.6%, P = .003), 12- to 14-year-olds (7.8%, P < .001), 15- to 17-year-olds (6.9%, P < .001), and 18- to 19-year-olds (5.8%, P < .001). There was a nonlinear relationship between age and urinary CMV viral loads among urinary CMV shedders (Figure 1C).
Figure 1.
Weighted age-specific prevalence of cytomegalovirus (CMV) outcomes in the pediatric population aged 6–19 years, National Health and Nutrition Examination Survey, 1999–2004 (n = 9564). (A) Prevalence of CMV immunoglobulin (Ig)G antibody, (B) prevalence of urinary CMV shedding among the IgG-seropositive individuals, and (C) geometric mean urinary CMV viral loads among urinary CMV shedders were estimated accounting for the complex weighted survey design. Error bars reflect 95% confidence intervals estimated using Taylor series linearization and Rubin’s rules. Asterisks represent a P value less than .05, indicating a statistically significant difference compared with 6- to 8-year-olds. P values were calculated from a t test.
Correlates of Urinary Cytomegalovirus Shedding
Factors associated with the prevalence of urinary CMV shedding in the CMV IgG-seropositive population are shown in Table 1. Multivariable analysis included age, race/ethnicity, sex, annual family income, body mass index (BMI), lymphocyte count, and anemia. Older age and higher BMIs were independent factors negatively associated with the prevalence of urinary CMV shedding (P < .05). For instance, the prevalence of urinary CMV shedding was significantly lower in obese individuals compared with persons who were of lean weight (adjusted prevalence ratio = 0.68; 95% CI, 0.47–0.99). No other variables were independently associated with urinary CMV shedding in the CMV IgG-seropositive population.
Table 1.
Factors Associated With Urinary CMV Shedding Among CMV IgG-Seropositive 6- to 19-Year-Olds, National Health and Nutrition Examination Survey, 1999–2004 (n = 9546)
| Characteristic | CMV Shedding Prevalence (95% CI) | Univariable PR (95% CI) | P Value | Multivariable PR (95% CI) | P Value |
|---|---|---|---|---|---|
| Age, Years | |||||
| 6–8 | 34.4 (26.2–42.6) | Ref. | Ref. | ||
| 9–11 | 20.6 (15.6–25.5) | 0.60 (0.43–0.83) | .003 | 0.61 (0.44–0.83) | .003 |
| 12–19 | 7.0 (5.4–8.6) | 0.20 (0.14–0.29) | <.001 | 0.21 (0.14–0.30) | <.001 |
| Sex | |||||
| Male | 15.1 (11.7–18.6) | Ref. | Ref. | ||
| Female | 15.8 (12.9–18.8) | 1.04 (0.79–1.38) | .745 | 1.07 (0.82–1.40) | .586 |
| Race/Ethnicity | |||||
| Non-Hispanic White | 14.9 (10.8–19.0) | Ref. | Ref. | ||
| Non-Hispanic Black | 18.1 (15.4–20.7) | 1.22 (0.89–1.66) | .210 | 1.22 (0.89–1.67) | .204 |
| Mexican American | 14.3 (11.7–16.9) | 0.96 (0.68–1.37) | .816 | 0.96 (0.68–1.34) | .784 |
| Annual Family Income | |||||
| <$35 000 | 17.1 (14.0–20.1) | Ref. | Ref. | ||
| ≥$35 000 | 13.5 (10.2–16.9) | 0.79 (0.59–1.06) | .108 | 0.85 (0.64–1.12) | .226 |
| Household Crowdinga | |||||
| <0.75 people per room | 14.7 (10.8–18.6) | Ref. | |||
| 0.75–0.9 people per room | 13.9 (9.0–18.8) | 0.94 (0.59–1.50) | .785 | ||
| ≥1 person per room | 17.8 (14.3–21.3) | 1.21 (0.87–1.68) | .240 | ||
| Recent Health in Past 30 Daysb | |||||
| Not sick | 15.2 (12.4–18.0) | Ref. | |||
| Sick | 16.2 (12.3–20.0) | 1.06 (0.80–1.42) | .672 | ||
| Maximum Household Educationc | |||||
| Less than high school | 16.6 (12.9–20.3) | Ref. | |||
| High school or GED | 17.4 (12.3–22.5) | 1.05 (0.73–1.50) | .799 | ||
| Some college or more | 13.9 (10.8–17.0) | 0.84 (0.61–1.15) | .256 | ||
| Body Mass Indexd | |||||
| Lean | 17.0 (13.9–20.2) | Ref. | Ref. | ||
| Overweight | 13.5 (9.2–17.7) | 0.79 (0.55–1.14) | .196 | 0.82 (0.58–1.14) | .227 |
| Obese | 11.3 (7.3–15.3) | 0.66 (0.45–0.98) | .038 | 0.68 (0.47–0.99) | .043 |
| Log-lymphocyte count (1000 cells/μL) | 1.93 (1.23–3.03) | .006 | 1.03 (0.66–1.62) | .889 | |
| Albuminuria (Albumin/Creatinine) | |||||
| Normal (<30 mg/g) | 15.2 (12.9–17.5) | Ref. | |||
| Albuminuria (≥30 mg/g) | 18.1 (9.9–26.3) | 1.19 (0.76–1.86) | .430 | ||
| C-Reactive Protein Quartiles (mg/dL) | |||||
| 1 (≤0.02) | 17.5 (12.7–22.2) | Ref. | |||
| 2 (0.03–0.05) | 15.7 (11.2–20.1) | 0.90 (0.63–1.29) | .543 | ||
| 3 (0.06–0.16) | 13.8 (9.8–17.8) | 0.79 (0.53–1.17) | .225 | ||
| 4 (>0.16) | 13.6 (9.5–17.7) | 0.78 (0.51–1.19) | .236 | ||
| Anemiae | |||||
| Normal | 14.1 (11.6–16.7) | Ref. | Ref. | ||
| Anemic | 21.5 (16.1–27.0) | 1.52 (1.13–2.06) | .009 | 1.01 (0.74–1.39) | .946 |
Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; CMV, cytomegalovirus; GED, General Education Diploma; IgG, immunoglobulin G; MEC, mobile examination center; PR, prevalence ratio; Ref., reference group.
NOTE: Data are weighted prevalence estimates and PRs calculated by Poisson regression. Taylor series linearization was used to estimate standard errors. Estimates were also calculated using Rubin’s rules. Bolded numbers indicate statistically significant estimates (P < .05).
aHousehold crowding was calculated by dividing the number of persons living in the home by the number of rooms in the home, excluding the kitchen.
bRecent health in the past 30 days was defined as a response of “yes” to having a head cold, chest cold, stomach or intestinal illness with vomiting or diarrhea, the flu, pneumonia, or an ear infection in the 30 days before the MEC examination.
cMaximum household education is the highest level of education achieved by either the head of household or their spouse.
dBody mass index (BMI) categories were defined by the CDC pediatric BMI-for-age weight status categories, where individuals were considered lean at <85th percentile, overweight at ≥85th to <95th percentile, and obese at ≥95th percentile.
eAnemia was defined as hemoglobin levels ≤12g/dL for females and ≤13.5g/dL for males.
Genotype Distribution of Urinary Cytomegalovirus Shedding
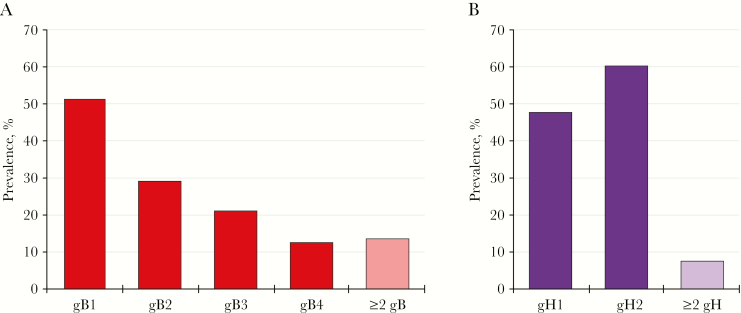
Figure 2 shows the unweighted prevalence of 4 gB and 2 gH variants among urinary CMV shedders with sufficient CMV DNA for genotyping in the observed data set. A gB variant was detected among 71.6% (174 of 243) of urinary CMV shedders who had sufficient CMV DNA for gB genotyping. Among those with a detectable gB variant (n = 174), 150 (86.2%) had a single gB variant detected, whereas 24 (13.8%) had multiple (≥2) gB variants detected; the most commonly detected gB variant was gB1 (n = 89; 51.1%) followed by gB2 (n = 51; 29.3%), gB3 (n = 37; 21.3%), and gB4 (n = 22; 12.6%). A gH variant was detected among 88.1% (214 of 243) of urinary CMV shedders who had sufficient CMV DNA for gH genotyping. Among those with a detectable gH variant (n = 214), 198 (92.5%) had a single gH variant detected, whereas 16 (7.5%) had both gH1 and gH2 variants detected. Detection of gH2 (n = 128; 59.8%) was more common than gH1 (n = 102; 47.7%).
Figure 2.
Unweighted prevalence of cytomegalovirus (CMV) genotype-specific variants among urinary CMV shedders in the pediatric sample aged 6–19 years, National Health and Nutrition Examination Survey, 1999–2004. (A) Prevalence of each glycoprotein B (gB) variant was estimated among 174 participants with data on gB. (B) Prevalence of each glycoprotein H (gH) variant was estimated among 214 participants with data on gH.
DISCUSSION
To the best of our knowledge, this is the largest population-based study to characterize urinary CMV shedding and the genotype distribution of CMV infections among 6- to 19-year-olds in the United States. In this population, the prevalence and magnitude of urinary CMV shedding among CMV IgG-seropositive individuals was significantly associated with age. Younger children had a higher prevalence of urinary CMV shedding and higher viral loads compared with adolescents. Although Amin et al [12] assumed uniform prevalence of urinary CMV shedding among 6- to 11-year-olds, the smaller age ranges examined in the present analysis demonstrate that the prevalence of urinary CMV shedding is significantly different between children aged 6–8 and 9–11 years. The results from the previous study indicated that urinary CMV shedding prevalence was associated with sex and household income level among 6- to 49-year-olds [12]; however, these associations were not observed in this analysis restricted to 6- to 19-year-olds. The present study suggests that age is the predominant demographic factor associated with urinary CMV shedding among children and adolescents in the United States.
The strong association between age and CMV shedding seen in this study is consistent with the current literature [2, 3, 8, 12, 20]. Because the lower age limit of the study was 6 years, it was not possible to characterize urinary CMV shedding in very young children [20, 21]. A previous study showed that 21% of 1- to 5-year-olds in the United States were CMV seropositive [21]. Other studies have shown that the prevalence of urinary CMV shedding in postnatally infected children who are not attending daycare and are less than 6 years of age ranges from 1% [22] to 36% [23]. As expected, we found a higher prevalence of CMV IgG antibody among 6- to 8-year-olds (38%); however, among these individuals, the prevalence of urinary CMV shedding was 34%. Given the similar prevalence of urinary CMV shedding between 1- to 5-year-olds and 6- to 8-year-olds, children over the age of 5 should also be considered an important risk group for CMV transmission. This is particularly important in the context of potential CMV transmission to seronegative pregnant women who have CMV-infected children, which could have implications regarding the incidence of congenital infections [24, 25].
It is interesting to note that higher BMI was independently associated with a lower prevalence of urinary CMV shedding among CMV IgG-seropositive individuals. Previous work has shown that obese 4- and 6-year-olds are more likely to be CMV IgG seropositive [26], but, to our knowledge, the association between BMI and CMV shedding has not been studied in pediatric populations. The lower prevalence of urinary CMV shedding observed among obese CMV IgG-seropositive persons in this study may be explained by several factors. The natural history of CMV infections has been shown to be influenced by the gut microbiome [27, 28], which differs between obese and nonobese individuals [29]. It is also possible that obese individuals acquire infection earlier than nonobese individuals and therefore had time to control their infection before participating in this study. This hypothesis cannot be tested using cross-sectional data. The potential link between obesity and the natural history of CMV requires further investigation.
Given that these data are cross-sectional, it was unclear whether participants were shedding CMV due to primary infections, reactivation of latent infections, and/or reinfection with a different strain [1]. In addition, because CMV shedding was only examined in urine at a single point in time and CMV shedding can occur dynamically in most bodily fluids [2–4, 20, 30, 31], this study likely underestimates the true prevalence of CMV shedding. In addition, this study did not measure several factors that have previously been shown to be associated with urinary CMV shedding in pediatric populations, such as current daycare attendance [2, 9, 32]. This study was also limited due to missing data among examined participants, particularly on CMV outcomes. Previous analyses of CMV data in this database have largely assumed missing data on CMV outcomes were missing at random within age-sex-race strata [11, 12]. However, we found that missing data on CMV IgG antibody and urinary CMV shedding status were associated with variables beyond age, sex, and race/ethnicity. Given that these data were not missing by chance alone, we handled missing data by multiple imputation using chained equations [19]. Finally, these data represent non-Hispanic whites, non-Hispanic blacks, and Mexican Americans in 1999–2004 and may not be generalizable to the current US population of children and adolescents.
Data on the genotype distribution of CMV in general populations are limited. This study shows the most prevalent CMV strains observed in 6- to 19-year-old urinary CMV shedders were gB1 and gH2, which is consistent with reported genotype distributions in transplant patients and congenitally infected infants [7, 17, 33]. Multiple CMV strains, defined by the detection of 1 or more variants of a single glycoprotein, have been documented in both immunocompromised and healthy populations [7, 10, 33–37]. Although not all CMV gB variants (eg, gB5, gB6, gB7) were genotyped in this study [37–39], 14% of the pediatric sample had multiple infections based on the 4 gB genotypes that were examined. These data may have implications for transmission of viral diversity as well as vaccine development. It is important to note that this study provides a national perspective, and the distribution of CMV genotypes may vary based on geographic location, owing to global variation in CMV genotypes and geographic differences in migrant populations in the United States [7, 17, 40].
CONCLUSIONS
In summary, young children above the age of 5 are potentially a high-risk source of CMV transmission given their high prevalence and magnitude of urinary CMV shedding. This study also suggests that obesity may influence the natural history of CMV infection among children and adolescents, but this hypothesis warrants further investigation.
Supplementary Material
Acknowledgments
Financial support. This work was funded in part by extramural funding from the National Institutes of Health (Grants 1R01AI120938 and 1R01AI128779 [to A. A. R. T] and K01AI125086 [to M. K. G.]) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Mocarski ES, Shenk T, Griffiths PD, Pass RF. Cytomegaloviruses. In: Fields Virology. 6th ed. Vol. 2 Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013: pp 1961–2014. [Google Scholar]
- 2. Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21:240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenthal LS, Fowler KB, Boppana SB, et al. . Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J 2009; 28:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deayton JR, Prof Sabin CA, Johnson MA, et al. . Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet 2004; 363:2116–21. [DOI] [PubMed] [Google Scholar]
- 5. Patel R, Paya CV. Infections in solid-organ transplant recipients. Clin Microbiol Rev 1997; 10:86–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simanek AM, Dowd JB, Pawelec G, et al. . Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 2011; 6:e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coaquette A, Bourgeois A, Dirand C, et al. . Mixed cytomegalovirus glycoprotein B genotypes in immunocompromised patients. Clin Infect Dis 2004; 39:155–61. [DOI] [PubMed] [Google Scholar]
- 8. Stowell JD, Mask K, Amin M, et al. . Cross-sectional study of cytomegalovirus shedding and immunological markers among seropositive children and their mothers. BMC Infect Dis 2014; 14:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dollard SC, Keyserling H, Radford K, et al. . Cytomegalovirus viral and antibody correlates in young children. BMC Res Notes 2014; 7:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arav-Boger R. Strain variation and disease severity in congenital cytomegalovirus infection: in search of a viral marker. Infect Dis Clin North Am 2015; 29:401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin Infect Dis 2010; 50:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amin MM, Bialek SR, Dollard SC, Wang C. Urinary cytomegalovirus shedding in the United States: the National Health and Nutrition Examination Surveys, 1999–2004. Clin Infect Dis 2018; 67:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curtin LR, Mohadjer L, Dohrmann S, et al. . The National Health and Nutrition Examination Survey: sample design, 1999–2006. Vital Health Stat 2 2012; 1–39. [PubMed] [Google Scholar]
- 14. McQuillan G, McLean J, Chiappa M, Lukacs S. National Health and Nutrition Examination Survey Biospecimen Program: NHANES III (1988–1994) and NHANES 1999–2014. Vital Health Stat 2 2015; 1–14. [PubMed] [Google Scholar]
- 15. National Center for Health and Vital Statistics. National Health and Nutrition Examination Survey. 1999–2004 Data Documentation, Codebook, and Frequencies Data File: SSUCSH_A.xpt. Available at: https://www.cdc.gov/Nchs/Nhanes/1999–2000/SSUCSH_A.htm. Accessed 10 April 2019.
- 16. Boppana SB, Ross SA, Novak Z, et al. . Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 2010; 303:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Vries JJ, Wessels E, Korver AM, et al. . Rapid genotyping of cytomegalovirus in dried blood spots by multiplex real-time PCR assays targeting the envelope glycoprotein gB and gH genes. J Clin Microbiol 2012; 50:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health and Vital Statistics. National Health and Nutrition Examination Survey. 1999-2004 Data Documentation, Codebook, and Frequencies. Data File: SSCMVG_A.xpt Available at: https://www.cdc.gov/Nchs/Nhanes/1999-2000/SSCMVG_A.htm. Accessed 10 April 2019. [Google Scholar]
- 19. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley, 1987. [Google Scholar]
- 20. Cannon MJ, Stowell JD, Clark R, et al. . Repeated measures study of weekly and daily cytomegalovirus shedding patterns in saliva and urine of healthy cytomegalovirus-seropositive children. BMC Infect Dis 2014; 14:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lanzieri TM, Kruszon-Moran D, Amin MM, et al. . Seroprevalence of cytomegalovirus among children 1 to 5 years of age in the United States from the National Health and Nutrition Examination Survey of 2011 to 2012. Clin Vaccin Immunol 2015; 22:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanshaw JB, Betts RF, Simon G, Boynton RC. Acquired cytomegalovirus infection: association with hepatomegaly and abnormal liver-function tests. N Engl J Med 1965; 272:602–9. [DOI] [PubMed] [Google Scholar]
- 23. Li F, Hanshaw JB. Cytomegalovirus infection among migrant children. Am J Epidemiol 1967; 86:137–41. [DOI] [PubMed] [Google Scholar]
- 24. Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 2003; 289:1008–11. [DOI] [PubMed] [Google Scholar]
- 25. Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 2010; 20:311–26. [DOI] [PubMed] [Google Scholar]
- 26. Karachaliou M, de Sanjose S, Waterboer T, et al. . Is early life exposure to polyomaviruses and herpesviruses associated with obesity indices and metabolic traits in childhood? Int J Obes (Lond) 2018; 42:1590–601. [DOI] [PubMed] [Google Scholar]
- 27. Carvalho-Queiroz C, Johansson MA, Persson JO, et al. . Associations between EBV and CMV seropositivity, early exposures, and gut microbiota in a prospective birth cohort: a 10-year follow-up. Front Pediatr 2016; 4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gianella S, Chaillon A, Mutlu EA, et al. . Effect of cytomegalovirus and Epstein-Barr virus replication on intestinal mucosal gene expression and microbiome composition of HIV-infected and uninfected individuals. AIDS 2017; 31:2059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature 2006; 444:1022. [DOI] [PubMed] [Google Scholar]
- 30. Forman MS, Vaidya D, Bolorunduro O, et al. . Cytomegalovirus kinetics following primary infection in healthy women. J Infect Dis 2017; 215:1523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amin MM, Stowell JD, Hendley W, et al. . CMV on surfaces in homes with young children: results of PCR and viral culture testing. BMC Infect Dis 2018; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pass RF, Hutto SC, Reynolds DW, Polhill RB. Increased frequency of cytomegalovirus infection in children in group day care. Pediatrics 1984; 74:121–6. [PubMed] [Google Scholar]
- 33. Ross SA, Novak Z, Pati S, et al. . Mixed infection and strain diversity in congenital cytomegalovirus infection. J Infect Dis 2011; 204:1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lisboa LF, Tong Y, Kumar D, et al. . Analysis and clinical correlation of genetic variation in cytomegalovirus. Transpl Infect Dis 2012; 14:132–40. [DOI] [PubMed] [Google Scholar]
- 35. Pati SK, Pinninti S, Novak Z, et al. . Genotypic diversity and mixed infection in newborn disease and hearing loss in congenital cytomegalovirus infection. Pediatr Infect Dis J 2013; 32:1050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Novak Z, Ross SA, Patro RK, et al. . Cytomegalovirus strain diversity in seropositive women. J Clin Microbiol 2008; 46:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murthy S, Hayward GS, Wheelan S, et al. . Detection of a single identical cytomegalovirus (CMV) strain in recently seroconverted young women. PLoS One 2011; 6:e15949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shepp DH, Match ME, Lipson SM, Pergolizzi RG. A fifth human cytomegalovirus glycoprotein B genotype. Res Virol 1998; 149:109–14. [DOI] [PubMed] [Google Scholar]
- 39. Trincado DE, Scott GM, White PA, et al. . Human cytomegalovirus strains associated with congenital and perinatal infections. J Med Virol 2000; 61:481–7. [DOI] [PubMed] [Google Scholar]
- 40. Pignatelli S, Dal Monte P, Rossini G, et al. . Human cytomegalovirus glycoprotein N (gpUL73-gN) genomic variants: identification of a novel subgroup, geographical distribution and evidence of positive selective pressure. J Gen Virol 2003; 84:647–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.