Abstract
Background
Treatment of serious infections due to multidrug-resistant (MDR) Pseudomonas aeruginosa remains a challenge, despite the introduction of novel therapeutics. In this study, we report 2 extensively drug-resistant clinical isolates of sequence type (ST) 309 P aeruginosa resistant to all β-lactams, including the novel combinations ceftolozane/tazobactam, ceftazidime/avibactam, and meropenem/vaborbactam.
Methods
Isolates were sequenced using both short-read (Illumina) and long-read technology to identify resistance determinants, polymorphisms (compared with P aeruginosa PAO1), and reconstruct a phylogenetic tree. A pair of β-lactamases, Guiana extended spectrum β-lactamase (GES)-19 and GES-26, were cloned and expressed in a laboratory strain of Escherichia coli to examine their relative impact on resistance. Using cell lysates from E coli expressing the GES genes individually and in tandem, we determined relative rates of hydrolysis for nitrocefin and ceftazidime.
Results
Two ST309 P aeruginosa clinical isolates were found to harbor the extended spectrum β-lactamases GES-19 and GES-26 clustered in tandem on a chromosomal class 1 integron. The presence of both enzymes in E coli was associated with significantly elevated minimum inhibitory concentrations to aztreonam, cefepime, meropenem, ceftazidime/avibactam, and ceftolozane/tazobactam, compared with those expressed individually. The combination of ceftazidime/avibactam plus aztreonam was active in vitro and used to achieve cure in one patient. Phylogenetic analysis revealed ST309 P aeruginosa are closely related to MDR strains from Mexico also carrying tandem GES.
Conclusions
The presence of tandem GES-19 and GES-26 is associated with resistance to all β-lactams, including ceftolozane/tazobactam. Phylogenetic analysis suggests that ST309 P aeruginosa may be an emerging threat in the United States.
Keywords: carbapenem-resistant Pseudomonas aeruginosa, ceftolozane/tazobactam, combination therapy, GES beta-lactamase
The Centers for Disease Control and Prevention has identified multidrug-resistant (MDR) Pseudomonas aeruginosa as a serious threat, and treatment of such isolates often requires the use of drugs with significant toxicities [1]. Carbapenem resistance in P aeruginosa in the United States is mostly mediated by noncarbapenemase mechanisms [2]. In response, novel therapeutics such as ceftolozane/tazobactam (C/T), which is stable to the pseudomonal AmpC β-lactamase and less susceptible to porin loss and drug efflux, have entered the clinic to combat this threat [3]. Although C/T remains broadly active against most clinical isolates of carbapenem-resistant P aeruginosa, resistance associated with mutations in AmpC or the expression of acquired β-lactamases has been described [4, 5].
The Guiana extended spectrum β-lactamase (GES) enzyme was first isolated from a Klebsiella pneumoniae obtained from a rectal swab of an infant in Cayenne, French Guiana [6] and, since then, 32 variants have been identified. In general, these enzymes confer resistance to penicillins, including ureidopenicillins, and oxyimino-cephalosporins, but they show less activity against aztreonam and imipenem [6, 7]. Nonetheless, specific substitutions can significantly alter this susceptibility profile, including G243A, which improves activity against aztreonam, and G170S, conferring increased carbapenem hydrolyzing activity [8]. These enzymes are found in association with class 1 integrons, a gene cassette acquisition system known to harbor multiple antimicrobial resistance determinants associated with mobile genetic elements [9]. A study of isolates from Mexico City identified ST309 as a potential high-risk clone associated with acquired β-lactamases, and a large percentage of carbapenem-resistant P aeruginosa from Mexico have been associated with GES enzymes carried by class 1 integrons [10, 11]. In this study, we report the identification of 2 isolates of extensively drug-resistant P aeruginosa ST309 causing bloodstream infections in unrelated patients and carrying simultaneously 2 variants of blaGES within a class 1 integron. The isolates exhibited resistance to all β-lactams including novel β-lactam/β-lactamase inhibitor combinations. Phylogenetic analyses suggested that this MDR lineage is closely related to ST309 isolates found in Mexico with the potential to disseminate.
METHODS
Bacterial Strains and Growth Conditions
Clinical P aeruginosa isolates PA_HTX1 and PA_HTX2 were purified on MacConkey agar. Single colonies were tested to ensure they retained the resistance phenotype, and stocks were frozen in Brucella broth plus 15% glycerol and stored at −80°C. Escherichia coli TG1 was grown on Lysogeny broth (LB) or LB agar supplemented with 100 µg/mL ampicillin or 25 µg/mL chloramphenicol when needed. All bacteria were grown at 37°C and with gentle agitation for liquid media.
Genetic Manipulation of bla Genes
The genes blaOXA-2, blaGES-19, blaGES-26, and both blaGES genes in combination were cloned into the isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible E coli expression vector pBA169 [12] and transformed into E coli TG1. Genomic deoxyribonucleic acid (DNA) isolated from PA_HTX1 was used as a template, and primers are listed in Supplementary Table 1. Insert DNA and plasmid pBA169 were digested with EcoRI and BamHI (New England Biolabs), ligated, and transformed into E coli TG1. Transformants were screened on LB agar containing chloramphenicol plus ampicillin and then verified by polymerase chain reaction and Sanger sequencing.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) of the clinical isolates PA_HTX1 and PA_HTX2 was performed in the clinical laboratory using a Microscan Walk-Away and E-test (for colistin, C/T and ceftazidime/avibactam [CZA]). Synergy testing was performed by applying an aztreonam (ATM) or meropenem (MEM) E-test strip to Mueller-Hinton agar plates containing either ceftazidime plus avibactam (Allergan), at a final concentration of 2.2 µg/mL of the avibactam component, or vaborbactam alone (The Medicines Co.) at 2 µg/mL. This concentration was selected to mimic the serum nadir of avibactam and vaborbactam from human pharmacokinetic/pharmacodynamic data [13–17]. The AST for the E coli mutants was performed in triplicate by normalizing strains to an optical density (OD)600 of 0.08 in Mueller-Hinton II broth, inducing with 1 mM IPTG for 2 hours and normalizing again to a 0.5 McFarland standard (OD600nm 0.08–0.1), before plating on Mueller-Hinton agar plates containing 40 µL of 100 mM IPTG. E-test strips (bioMérieux) were applied for all antibiotics tested, and MICs were read after 24 hours of incubation at 37°C.
Whole-Genome Sequencing and Pseudomonas aeruginosa Phylogenetics
Genomic DNA was extracted using the DNeasy Blood and Tissue kit (QIAGEN). Genome sequencing of the 2 isolates was performed on a Miseq (Illumina) and with MinION (Oxford Nanopore Technologies) for long reads. Sequence data have been deposited in the National Center for Biotechnology Information (NCBI) database (Bioproject: PRJNA414583). Resistance detection, polymorphism analysis, and reconstruction of the phylogenetic tree were performed using a custom pipeline. To study the phylogenetic relationships between the isolates with other P aeruginosa genomes, all assembled genomes of P aeruginosa available at the NCBI genome database were downloaded, and the MLST was obtained using the mlst tool. Nine additional ST309 genomes were identified, and PAO1 (GCA_000006765.1), PA_D1 (GCA_001721745.1), L10 (GCA_002223805.1), M18 (GCA_000226155.1), and FRD1 (GCA_000829885.1) from STs 539, 1971, 253, 1239, and 111, respectively, were used as references (Supplementary Figure 1). Genome annotation of the 16 genomes was carried out with RAST [18], a core genome was determined with Roary [19], and multiple sequence alignment of the orthogroups belonging to the core genome was done with Muscle [20] and later concatenated to be used as the matrix for phylogenetic reconstruction with RAxML [21]. The best tree of 20 runs was selected with a General Time Reversible evolution model and a Gamma model of rate heterogeneity with 100 bootstrap resampling. The tree was plotted with iTol [22] after rooting at the split between ST309 and the other references, and modification of the distance between the root and the branches of ST309 was changed to a value near zero to allow the visualization of ST309 branching.
Expression of Guiana Extended Spectrum β-Lactamase Proteins and β-Lactamase Assay
Overnight cultures of E coli cells transformed with GES-19, GES-26, or GES-19/GES-26 in the IPTG-inducible pBA169 expression plasmid were diluted 1:100 in 50 mL LB medium containing 25 µg/mL chloramphenicol (to maintain the plasmid). The diluted cultures were incubated with shaking at 37oC to OD600 of 0.8. The IPTG was added to a final concentration of 0.5 mM, and cells were incubated with shaking at 20oC for another 20 hours to induce the expression of GES proteins. Cells were then pelleted by centrifugation at 6000 rpm for 20 minutes, and the cell pellet was resuspended in 2 mL B-PER (Thermo Fisher) containing 100 µg/mL lysozyme and 20 µg/mL DnaseI and incubated at room temperature for 15 minutes. Samples were then centrifuged again at 13000 rpm for 5 minutes to collect cell lysate in the supernatant. To ensure the expression of GES enzymes, 50 µM nitrocefin diluted in 50 mM HEPES (pH7.4) was incubated with 1 µL cell lysate, and absorbance at 482 nm (A482) was monitored on Beckman DU800 spectrometer using a 1-cm cuvette. To determine hydrolysis of ceftazidime by the GES enzymes, 50 µM ceftazidime diluted in 50 mM HEPES (pH7.4) was incubated with 1 µL cell lysate, and absorbance at 260 nm (A260) was monitored on Beckman DU800 spectrometer using a 1-cm cuvette.
RESULTS
Two patients admitted to separate hospitals in a large urban hospital network developed bacteremia with MDR P aeruginosa. In the first case, the patient had 2 weeks of prior exposure to cefepime (FEP), metronidazole, and vancomycin. Subsequently, the patient received MEM for a complicated intra-abdominal infection before isolation of MDR P aeruginosa (PA_HTX1) (Table 1, Supplementary Table 2) resistant to all β-lactams, including novel β-lactam/β-lactamase inhibitor combinations. Despite therapy with polymyxin B, MEM, and amikacin, the patient’s clinical condition worsened. On the suspicion that the isolate may harbor a metallo-β-lactamase, synergy testing with the combination of CZA plus ATM was performed, with an observed reduction in the ATM MIC from >256 µg/mL alone to 8 µg/mL in the presence of avibactam. The patient was started on 1.5 grams of CZA given after hemodialysis with ATM (2 grams IV daily) and was ultimately cured after 105 days of therapy. In the second case, the patient had bronchoalveolar lavage cultures positive for E coli, and received 5 days of MEM, before developing MDR P aeruginosa pneumonia with a similar antimicrobial susceptibility profile to PA_HTX1 (PA_HTX2) (Table 1, Supplementary Table 2). The patient received colistin, MEM, and 3 doses of C/T, but developed bacteremia despite antibiotics, and ultimately died due to the infection. An epidemiologic investigation within the hospital system did not reveal a link between the 2 patients, and there was no shared intensive care unit staff or equipment between the 2 hospitals during the period of patient admissions.
Table 1.
Minimum Inhibitory Concentrations for β-Lactams and β-Lactam/β-Lactam Inhibitor Combinations
| MIC (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | AMP | ATM | FEP | CZAa | C/T | MEM | CZA + ATMb | MEM + VABc | ATM + VABc |
| PA_HTX1 | ND | >256 | >256 | 128 | >256 | >32 | 8 | >32 | >256 |
| PA_HTX2 | ND | >256 | >16 | >256 | >256 | >32 | 4 | >32 | >256 |
| TG1 + pBA169 | 3 | 0.032 | 0.064 | 0.25 | 0.064 | 0.016 | ND | ND | ND |
| TG1 + pBA169::blaGES-19 | >256 | 1.5 | 1 | 4 | 0.125 | 0.012 | ND | ND | ND |
| TG1 + pBA169::blaGES-26 | >256 | 0.25 | 0.5 | 2 | 0.38 | 0.016 | ND | ND | ND |
| TG1 + pBA169::blaGES-19–26 | >256 | >256 | >256 | >256 | 48 | 2 | 2 | ND | 0.25 |
| TG1 + pBA169::blaOXA-2 | >256 | 0.094 | 0.5 | 0.125 | 0.125 | 0.064 | ND | ND | ND |
Abbreviations: AMP, ampicillin; ATM, aztreonam; C/T, ceftolozane/tazobactam; CZA, ceftazidime/avibactam; FEP, cefepime; MEM, meropenem; MIC, minimum inhibitory concentration; ND, not done; VAB, vaborbactam.
aPerformed by broth microdilution, all others performed by E-test.
bAvibactam 2.2 µg/mL, to mimic the mean steady-state nadir in human plasma.
cVaborbactam 2 µg/mL, to mimic the mean steady-state nadir in human plasma.
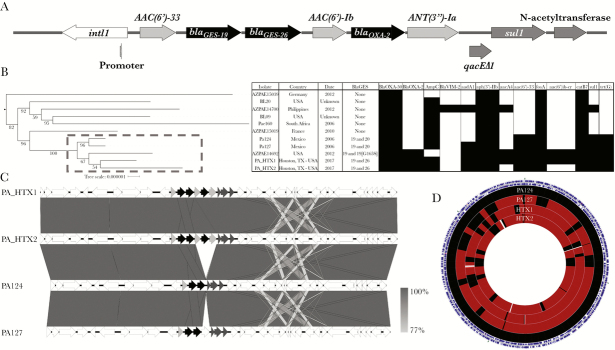
We performed whole-genome sequencing on PA_HTX1 and PA_HTX2. Both isolates were identified as sequence type 309 and carried a chromosomal class 1 integron with multiple resistance determinants, including blaGES-19 and blaGES-26 (Figure 1A, Supplementary Table 3). To determine whether this tandem carriage of GES β-lactamases was present in other ST309 P aeruginosa, we performed a search of the assembled P aeruginosa genomes available in the NCBI database. Eleven ST309 P aeruginosa (including the 2 patient isolates in this study) were identified. It is interesting to note that a cluster of 5 ST309 isolates of clinical origin harboring tandem blaGES genes from the United States and Mexico, isolated from 2006 to 2017, appear to form a distinct group (Figure 1B). An analysis of the integron and surrounding genomic context revealed that the Houston isolates possessed an OXA-2 β-lactamase and aminoglycoside-modifying enzymes not present in the Mexican isolates (Figure 1C). These strains possess a high degree of shared gene content, suggesting a close relationship between the isolates, although several gaps in the genomic alignment of the Houston strains are likely driven by phage and mobile genetic elements (Figure 1D, Supplementary Table 4).
Figure 1.
Integron structure, phylogenetics, and resistome of ST309 Pseudomonas aeruginosa isolates available in the National Center for Biotechnology Information (NCBI) database. (A) Structure of the class 1 integron in PA_HTX1. Gene names are listed next to the predicted open reading frames ([ORF] arrows). The integrase (white) and internal promoter, aminoglycoside-modifying enzymes ([AME] light gray), beta-lactamases (black), and other predicted resistance genes (dark gray) are shown. The ORF of qacEΔ1 overlaps the ORF of sul1 and is shifted down for clarity. (B) A core genome-based tree (RAST annotations) of ST309 using the reference genomes PAO1, PA_D1, L10, M18, and FDR1. The root of the tree was defined before the split of ST309, the clade of the references was removed, and distances were set to allow resolution of the ST309 branch lengths (shown to the left) (see Supplemental Figure 1 for complete tree). Isolate, origin, year, Guiana extended spectrum β-lactamase (GES) enzyme type, and presence (black box) or absence (white box) of resistance genes is shown to the right. A gray box with dashed line indicates the GES-positive isolates. (C) Comparison of the genetic context of blaGES between the Houston and Mexican isolates. In addition to the acquisition of OXA-2 and AMEs, there is a downstream region of variability associated with an IS6 transposon mobile genetic element. Grayscale gradient bar denotes nucleotide sequence identity. (D) Sequence-based alignment of the genomes of the Houston and Mexican isolates using PA124 as the reference. Outer ring of blue arrows represents predicted ORFs. Regions in black represent 100% nucleotide identity on BLAST hits, and regions colored maroon represent at least 98% identity. Areas represented in white show gaps in the alignment, associated with presence or absence of phage or mobile genetic elements.
To investigate the genetic bases of the MDR phenotype, we compared genes associated with antimicrobial resistance using P aeruginosa PAO1 as the reference strain (Supplementary Table 5). The ampC gene for both PA_HTX strains codes for the PDC-19a variant, and polymorphisms in ampR, ampD, and ampG have been previously reported in both sensitive and resistant strains. The sequences of the ampD homologs ampDh2 and ampDh3, as well as dacB, which encodes a low molecular weight penicillin-binding protein associated with AmpC expression, were identical to PAO1. The oprD gene was disrupted by insertion of ISPa1328, an IS256 family element that truncated the first 126 base pairs of oprD including the start codon. This is predicted to result in loss of oprD expression and likely contributes to the carbapenem resistance phenotype seen in these isolates. In addition, both HTX isolates possessed a mutation predicted to result in a deletion of 6 amino acids (residues 189–194) near the C-terminal end of MexZ, a repressor of the MexXY efflux pump. This resistance-nodulation-cell division family efflux pump is the primary determinant of aminoglycoside resistance in P aeruginosa, especially in cystic fibrosis lung isolates [23, 24]. The region with the mutation in the HTX isolates lies at the dimerization interface of MexZ, and we hypothesize that this change may lead to upregulation of the MexXY efflux pump [25]. Mutations in gyrA and parC seen in these isolates have been previously linked to decreased susceptibility to fluoroquinolone antibiotics [2].
The PA_HTX isolates were resistant to all available β-lactams, including the novel combinations of C/T, CZA, and MEM/vaborbactam (Table 1). In the presence of CZA, the MIC of ATM decreased from >256 µg/mL in PA_HTX1 and PA_HTX2 to 8 and 4 µg/mL, respectively. No change in MIC was found with either MEM or ATM in combination with vaborbactam. To evaluate the spectrum of the integron-encoded β-lactamases, blaGES-19, blaGES-26, and blaOXA-2 from PA_HTX1 were expressed individually, and blaGES-19 and blaGES-26 in tandem, in E coli TG1 [12] (Table 1). The presence of blaGES-19, blaGES-26, and blaOXA-2 alone led to modest increases in MIC for ATM, FEP, CZA (GES only), and C/T, but not MEM, compared with TG1 carrying empty pBA169. In contrast, the presence of both blaGES-19 and blaGES-26 resulted in a marked increase of MICs of ATM, FEP, CZA, C/T, and MEM. This effect was reversed by addition of the β-lactamase inhibitors avibactam and vaborbactam. Although the IPTG-induced levels of enzyme are not physiologic, the results suggest that the presence of both enzymes, rather than one alone, leads to high-level resistance.
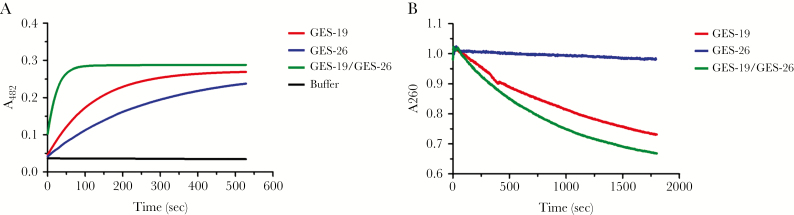
To support that the combination of the GES enzymes is responsible for the phenotype, cell lysates of induced E coli TG1 with each of the GES constructs were tested for hydrolysis of nitrocefin and ceftazidime (Figure 2). Nitrocefin hydrolysis by the cell lysate of GES-19, GES-26, and GES-19/GES-26 was 0.34 ± 0.03, 0.19 ± 0.006, and 1.34 ± 0.02 μM s−1 per μL of cell lysate, respectively. It is interesting to note that the cell lysate of the combined GES-19/GES-26 had 4- and 7-fold higher hydrolytic activity than that of GES-19 or GES-26 alone, respectively. Minimal hydrolysis of ceftazidime was seen in the GES-26 cell lysate, whereas hydrolysis by GES-19 was 0.029 ± 0.0015 μM s−1 per μL. With the GES-19/GES-26 lysate, ceftazidime hydrolysis was 0.048 ± 0.0084 μM s−1 per μL, a 1.7-fold increase. Although we were not able to quantify absolute differences in expression of GES enzymes in each lysate, which could account for differences in nitrocefin and ceftazidime hydrolysis, these data suggest that expression of both β-lactamases, as opposed to a single enzyme, provides increased hydrolysis of β-lactams. Thus, the tandem acquisition of GES enzymes in combination with efflux and decreased permeability in P aeruginosa has the potential to further compromise β-lactam activity.
Figure 2.
Rates of hydrolysis of nitrocefin and ceftazidime from Escherichia coli cell lysates. Escherichia coli lysates containing Guiana extended spectrum β-lactamase (GES)-19, GES-26, and GES-19/GES-26 were prepared from cultures grown in the presence of 0.5 mM isopropyl β-d-1-thiogalactopyranoside for 20 hours to induce protein expression. (A) Hydrolysis of 50 µM nitrocefin in 50 mM HEPES pH7.4 monitored by absorbance at 482 nm. Cell lysate with both enzymes was more efficient than that of GES-19 or GES-26 individually by 4-fold and 7-fold, respectively. (B) Hydrolysis of 50 µM ceftazidime in 50 mM HEPES pH7.4 monitored by absorbance at 260 nm. Hydrolysis of ceftazidime in the presence of cell lysate containing both enzymes was 1.7-fold higher than GES-19 alone.
DISCUSSION
Infections due to MDR P aeruginosa are associated with increased morbidity, mortality, and higher healthcare costs [26, 27]. This burden of resistance has largely been driven by the success of the epidemic clones ST 111, 175, and 235, which possess a variety of virulence factors and have spread globally [28]. The MDR phenotype in these strains stems from the propensity to develop mutational mechanisms of resistance, such as loss or inactivation of the porin gene oprD (leading to carbapenem resistance) or overexpression of an array of intrinsic efflux pumps [2]. The introduction of newer therapeutics such as C/T, which circumvents these common resistance mechanisms [3, 29], has offered clinicians additional options for treating MDR strains. However, the emergence of resistance to C/T associated with mutations in the intrinsic AmpC cephalosporinase is increasingly reported [4]. In addition, the acquisition of β-lactam resistance arising from exogenous β-lactamase enzymes is another growing concern, especially in the high-risk clones. These resistance determinants are often associated with class 1 integrons in the context of integrative conjugative elements or pathogenicity islands, increasing the potential for dissemination [30]. Most frequently reported are metallo-β-lactamases, specifically VIM and IMP, although a variety of class D OXA enzymes and class A enzymes, including KPC and GES, among others, have been described [5, 31].
The cases reported here offer several important insights into the evolving landscape of MDR P aeruginosa. Both were serious infections due to ST309 P aeruginosa with an extensive antibiotic resistance phenotype. By phylogenetic analysis, these isolates were linked to strains reported from several clusters of ST309 P aeruginosa infections in Mexico City, which also displayed an MDR phenotype [11]. Indeed, GES enzymes seem to be an important contributor to carbapenem resistance in P aeruginosa from Mexico, with a prevalence of 30.6% [10]. Furthermore, these enzymes are also present as plasmid-borne resistance determinants among Enterobacteriaceae from this region, suggesting a potential for spread across species [32]. Neither of our patients had a reported history of travel to Mexico, suggesting that the geographic distribution of ST309, or the mobile genetic element carrying the tandem GES genes, may be more widespread. It is important to understand the circulating resistance mechanisms to detect the potential emergence of new epidemic clones.
Another interesting feature of the study was that high-level β-lactam resistance was associated with the 2 GES variants, present in tandem, in our isolates. In general, the GES enzymes are extended spectrum β-lactamases, although amino acid substitutions leading to increased carbapenemase activity (G170S) are reported. In a recent study, the G170S substitution (in the GES-6 variant) was linked to both increased ceftolozane hydrolysis and decreased inhibition of the enzyme by clavulanate and tazobactam, although avibactam appears to retain activity [33]. The GES enzymes mimic the phenotype of class B metallo-β-lactamases in that they efficiently hydrolyze all β-lactams with the exception of monobactams, which is likely the basis for the observed efficacy of the CZA plus ATM combination. Avibactam, a novel β-lactamase inhibitor, can bind reversibly and inhibit multiple β-lactamases simultaneously without being susceptible to hydrolysis [34]. It inhibits class A β-lactamases and is effective against AmpC-mediated resistance in P aerugonisa [35]. This effect likely “protects” and allows ATM to be effective against our isolates harboring GES enzymes.
Tandem carriage of GES (GES-1 and GES-5) has been previously reported in a P aeruginosa isolate from Spain [36]. In this report, the entire integron was transferred to a susceptible PAO1, and the individual contributions of each enzyme were not assessed. Guiana extended spectrum β-lactamase-5 is known to have carbapenemase activity due to the presence of serine at position 170. Of note, both GES-19 and GES-26 have glycine at position 170, and we hypothesize that tandem expression, rather than an amino acid change, influences the resistance phenotype. This is supported by the genetic and hydrolysis studies showing a greater than additive effect with tandem versus single expression in E coli. Furthermore, gene dosage has been linked to β-lactam resistance associated with mobile elements in Enterobacteriaecae [37], and this is a potential new mechanism for resistance to C/T in P aeruginosa. It is interesting to note that the P aeruginosa isolates were not susceptible to MEM or ATM in combination with vaborbactam. In the case of MEM, disruption of the OprD porin and the resulting decrease in permeability to MEM would not be overcome with addition of a β-lactamase inhibitor. The lack of efficacy when paired with ATM is likely multifactorial, because vaborbactam is a less potent inhibitor of class C β-lactamases, does not inhibit class D enzymes, and may possibly be impacted by porin mutations (vaborbactam uses OmpK36 and OmpK35 to cross the membrane in K pneumoniae), although data regarding vaborbactam entry in Pseudomonas are lacking [38].
CONCLUSIONS
In summary, we report the occurrence of serious infections caused by ST309 P aeruginosa carrying blaGES-19 and blaGES-26 in Houston, Texas. Tandem expression of the 2 GES enzymes under IPTG induction in E coli resulted in resistance to all β-lactams including novel combinations. Ceftazidime/avibactam plus ATM was active in vitro against these 2 isolates and was successfully used in one patient failing polymyxin B-based therapy. The prevalence of GES enzymes reported in Mexico, and the close relation of the strains identified here to P aeruginosa Mexican isolates, suggests that ST309 may be a newly emerging high-risk lineage in the United States.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Christophe Herman for providing the pBA169-cm plasmid.
Financial support. This work was funded by the National Institutes of Health (NIH) Grants R01 AI134637, R21 AI143229, and K24 AI121296 (to C. A. A.); UTHealth Presidential Award (to C. A. A.); University of Texas System STARS Award (to C. A. A.); NIH Grant K08 AI113317 (to T. T. T.); NIH Grant K08 AI135093 (to W. R. M.); NIH Grant R01 AI32956 (to T. P.); and UTHealth Center for Antimicrobial Resistance and Microbial Genomics (CARMiG) seed funds (to W. R. M.).
Disclaimer. The funding agency had no role in experimental design, data collection or interpretation of this work.
Potential conflicts of interest. C. A. A. has received grants from Merck, MeMed Diagnostics, and Entasis Pharmaceuticals. L. O.-Z. has received grants and/or speaking and consulting honoraria from Merck, Astellas, Pfizer, Gilead, The Medicines Company, Cidara, Scynexis, Aradigm, and Bayer. W. R. M. has received grants and/or honoraria from Merck, Entasis, Achaogen, and Shionogi. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: ASM Microbe 2018, Atlanta, GA.
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. ANTIBIOTIC RESISTANCE THREATS in the United States, 2013. [online] CDC, pp. 11–23. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed March 3, 2019.
- 2. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duin D van, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 2016; 63:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haidar G, Philips NJ, Shields RK, et al. . Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Livermore DM, Mushtaq S, Meunier D, et al. . Activity of ceftolozane/tazobactam against surveillance and ‘problem’ Enterobacteriaceae, Pseudomonas aeruginosa and non-fermenters from the British Isles. J Antimicrob Chemother 2017; 72:2278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poirel L, Le Thomas I, Naas T, et al. . Biochemical sequence analyses of GES-1, a novel class A extended-spectrum beta-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob Agents Chemother 2000; 44:622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poirel L, Weldhagen GF, Naas T, et al. . GES-2, a class A beta-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob Agents Chemother 2001; 45:2598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delbrück H, Bogaerts P, Kupper MB, et al. . Kinetic and crystallographic studies of extended-spectrum GES-11, GES-12, and GES-14 β-lactamases. Antimicrob Agents Chemother 2012; 56:5618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gillings M, Boucher Y, Labbate M, et al. . The evolution of class 1 integrons and the rise of antibiotic resistance. J Bacteriol 2008; 190:5095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garza-Ramos U, Barrios H, Reyna-Flores F, et al. . Widespread of ESBL- and carbapenemase GES-type genes on carbapenem-resistant Pseudomonas aeruginosa clinical isolates: a multicenter study in Mexican hospitals. Diagn Microbiol Infect Dis 2015; 81:135–7. [DOI] [PubMed] [Google Scholar]
- 11. Morales-Espinosa R, Delgado G, Espinosa LF, et al. . Fingerprint analysis and identification of strains ST309 as a potential high risk clone in a Pseudomonas aeruginosa population isolated from children with bacteremia in Mexico City. Front Microbiol 2017; 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh NP, Alba BM, Bose B, et al. . OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 2003; 113:61–71. [DOI] [PubMed] [Google Scholar]
- 13. Nicolau DP, Siew L, Armstrong J, et al. . Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother 2015; 70:2862–9. [DOI] [PubMed] [Google Scholar]
- 14. Dimelow R, Wright JG, MacPherson M, et al. . Population pharmacokinetic modelling of ceftazidime and avibactam in the plasma and epithelial lining fluid of healthy volunteers. Drugs R D 2018; 18:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith DC, Sabet M, Tarazi Z, Lomovskaya O, Dudley MN. Pharmacokinetics/pharmacodynamics of vaborbactam, a novel beta-lactamase inhibitor, in combination with meropenem. Antimicrob Agents Chemother United States 2019;63: e01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vabomere [package insert]. Melinta Therapeutics, New Haven, CT, USA; 2017.
- 17.Ceftazidime/Avibactam Avycaz [package insert]. Allergan, Dublin, Ireland; 2015.
- 18. Overbeek R, Olson R, Pusch GD, et al. . The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 2014; 42:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page AJ, Cummins CA, Hunt M, et al. . Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015; 31:3691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004; 32:1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 2016; 44:W242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morita Y, Tomida J, Kawamura Y. MexXY multidrug efflux system of Pseudomonas aeruginosa. Front Microbiol 2012; 3:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2011; 2:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guénard S, Muller C, Monlezun L, et al. . Multiple mutations lead to MexXY-OprM-dependent aminoglycoside resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2014; 58:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peña C, Suarez C, Gozalo M, et al. . Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother 2012; 56:1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mauldin PD, Salgado CD, Hansen IS, et al. . Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant Gram-negative bacteria. Antimicrob Agents Chemother 2010; 54:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliver A, Mulet X, López-Causapé C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 2015; 21-22:41–59. [DOI] [PubMed] [Google Scholar]
- 29. Castanheira M, Mills JC, Farrell DJ, Jones RN. Mutation-driven β-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother 2014; 58:6844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Botelho J, Grosso F, Peixe L. Unravelling the genome of a Pseudomonas aeruginosa isolate belonging to the high-risk clone ST235 reveals an integrative conjugative element housing a blaGES-6 carbapenemase. J Antimicrob Chemother 2018; 73:77–83. [DOI] [PubMed] [Google Scholar]
- 31. Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 2015; 45:568–85. [DOI] [PubMed] [Google Scholar]
- 32. Barrios H, Garza-Ramos U, Ochoa-Sanchez LE, et al. . A plasmid-encoded class 1 integron contains GES-type extended-spectrum β-lactamases in Enterobacteriaceae clinical isolates in Mexico. Antimicrob Agents Chemother 2012; 56:4032–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poirel L, Ortiz De La Rosa JM, Kieffer N, Dubois V, Jayol A, Nordmann P. Acquisition of extended-spectrum β-lactamase GES-6 leading to resistance to ceftolozane-tazobactam combination in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2018; 63: pii: e01809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ehmann DE, Jahić H, Ross PL, et al. . Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A 2012; 109:11663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lahiri SD, Johnstone MR, Ross PL, et al. . Avibactam and class C β-lactamases: mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob Agents Chemother 2014; 58:5704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viedma E, Juan C, Acosta J, et al. . Nosocomial spread of colistin-only-sensitive sequence type 235 Pseudomonas aeruginosa isolates producing the extended-spectrum beta-lactamases GES-1 and GES-5 in Spain. Antimicrob Agents Chemother 2009; 53:4930–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandegren L, Andersson DI. Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nat Rev Microbiol 2009; 7:578–88. [DOI] [PubMed] [Google Scholar]
- 38.Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 2017; 6: pii: e01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.