ABSTRACT
Background
After meal ingestion, a series of coordinated hormone responses occur concomitantly with changes in perceived appetite. It is not known whether interindividual variability in appetite exists in response to a meal.
Objectives
The aim of this study was to 1) assess the reproducibility of appetite responses to a meal; 2) quantify individual differences in responses; and 3) explore any moderating influence of the fat mass and obesity associated (FTO) gene.
Methods
Using a replicated crossover design, 18 healthy men (mean ± SD age: 28.5 ± 9.8 y; BMI: 27.0 ± 5.0 kg/m2) recruited according to FTO genotype (9 AA, 9 TT) completed 2 identical control and 2 identical standardized meal conditions (5025 kJ) in randomized sequences. Perceived appetite and plasma acylated ghrelin, total peptide YY (PYY), insulin, and glucose concentrations were measured before and after interventions as primary outcomes. Interindividual differences were explored using Pearson's product-moment correlations between the first and second replicates of the control-adjusted meal response. Within-participant covariate-adjusted linear mixed models were used to quantify participant-by-condition and genotype-by-condition interactions.
Results
The meal suppressed acylated ghrelin and appetite perceptions [standardized effect size (ES): 0.18–4.26] and elevated total PYY, insulin, and glucose (ES: 1.96–21.60). For all variables, SD of change scores was greater in the meal than in the control conditions. Moderate-to-large positive correlations were observed between the 2 replicates of control-adjusted meal responses for all variables (r = 0.44–0.86, P ≤ 0.070). Participant-by-condition interactions were present for all variables (P ≤ 0.056). FTO genotype-by-condition interactions were nonsignificant (P ≥ 0.19) and treatment effect differences between genotype groups were small (ES ≤ 0.27) for all appetite parameters.
Conclusions
Reproducibility of postprandial appetite responses is generally good. True interindividual variability is present beyond any random within-subject variation in healthy men but we detected no moderation by the FTO genotype. These findings highlight the importance of exploring individual differences in appetite for the prevention and treatment of obesity. This trial was registered at clinicaltrials.gov as NCT03771690.
Keywords: appetite, individual variability, ghrelin, PYY, hunger, FTO, replicated crossover design
Introduction
Interindividual variability in response to an intervention, including manipulations of dietary intake and exercise energy expenditure, has received considerable interest from the scientific community in recent years (1–3). The notion that individuals may differ in the magnitude of response to an identical stimulus has widespread implications, and the potential to engender tailored strategies that optimize health outcomes for individuals is undoubtedly appealing (4). Within this sphere, an increasing number of studies have been undertaken to quantify individual differences in appetite and energy intake responses to an intervention (5, 6). Specifically, marked individual variability in ad libitum energy intake has been reported in response to a single bout of cycling (5); however, recent evidence from a large pooled data set suggests that such variability can be explained by normal day-to-day variation in most cases (6). This line of enquiry has direct implications for energy balance and weight control.
The consumption of a meal suppresses the gut hormone acylated ghrelin and perceived hunger, and increases peptide YY (PYY) and perceived satisfaction (7). Standardized meals, as opposed to ad libitum buffet meals, provide a fixed amount of preselected food items and can be used to compare meal-related outcomes when the exact same stimulus is given. Previous studies have suggested that individuals exhibit reproducible ad libitum energy intake, as well as changes in subjective appetite perceptions, glucose, and appetite-related hormones, in response to a standardized meal provided on separate occasions (8–11). Although such efforts to explore reproducibility and individual responses alongside the mean effects of an intervention should be encouraged, there are significant methodological and analytical challenges to adequately quantifying interindividual variability (1). In the context of a crossover study, these challenges include replicating each intervention and control condition and partitioning true response heterogeneity from within-subjects random measurement variability with an appropriate statistical model (1).
Current evidence is often limited by the absence of a control group or condition, which has been highlighted as an imperative study design feature to account for random within-subject variability over time (1, 3, 12). A recent approach proposed to quantify individual differences in the intervention response involves quantifying the participant-by-response interaction from replicated intervention and control groups or conditions (3, 13, 14). We have recently adopted this framework to highlight the presence of individual variability in subjective and hormonal appetite responses to acute exercise (15); however, it is not known whether such variability in appetite responses exists in response to a standardized meal.
A further consideration, if true individual differences are present, involves identifying potential moderators that could explain the individual variability (1). The fat mass and obesity associated (FTO) gene represents the most extensively studied gene associated with obesity, with individuals homozygous for the obesity risk A allele (AA) of FTO rs9939609 weighing a mean 3 kg more and having a 1.7-fold higher obesity risk than those homozygous for the low-risk T allele (TT) (16). Although the physiological mechanisms underlying this heightened risk are not fully understood, it has been demonstrated that AA individuals exhibit a blunted postprandial suppression of acylated ghrelin and hunger compared with adiposity-matched TT individuals (17). Given that interindividual variability in the responses of appetite to repeated meal intake is suspected, it is possible that groups with different risk variants of the FTO gene may moderate these responses.
Therefore, the aims of this study were 1) to investigate whether the perceived appetite and appetite-related hormone responses to a standardized meal are reproducible on repeated occasions; 2) to examine whether there is true individual variability in appetite responses to a standardized meal; and 3) to determine whether the FTO genotype moderates the magnitude of appetite responses to a standardized meal.
Methods
Ethical approval
All procedures included in this study (NCT03771690) were approved by Loughborough University Ethics Advisory Committee. All participants provided written informed consent before taking part in any aspect of the study.
Participants
Participants were selected from 2 previous study databases where participants were genotyped for the rs9939609 allele of the FTO gene. Genomic DNA was extracted from the whole blood samples using the QIAamp DNA Mini kit (QIAGEN). The samples were genotyped using the Applied Biosystems TaqMan (Roche Molecular Systems) genotyping assay and real-time PCR system. Eighteen healthy white European men were recruited for this study between January and April, 2018 according to their FTO genotype: 9 homozygous minor allele (AA) and 9 homozygous major allele (TT) (refer to the participant flowchart supplied in Supplemental Figure 1). Participants were informed about the study purpose; however, their genotype was not disclosed until the end of the study in order to avoid any potential effect on the outcomes of interest. Participants were body-mass stable (≤3 kg change in the previous 3 mo), nonsmokers, had no history of cardiovascular or metabolic disease, and were not dieting or taking any medications. Participants were habitual breakfast eaters and “moderately active” according to the International Physical Activity Questionnaire (18), which was used to ensure homogeneity in physical activity levels across the study sample.
Anthropometry
Height was measured to the nearest 0.1 cm and body mass to the nearest 0.1 kg using an electronic measuring station (Seca). BMI was calculated as kg/m2. Skinfold thickness was measured at 3 sites (chest, abdomen, and thigh) and body fat percentage was estimated using the equations of Jackson and Pollock (19) and Siri (20).
Experimental design
Using a replicated crossover experimental design (3), participants completed 4 visits in a randomized order, each separated by an interval of ≥3 d: 2 identical fasting control and 2 identical standardized meal conditions. The block randomization plan (21) was obtained by the main investigator (FRG), who also enrolled participants and assigned participants to interventions. Participants completed a weighed food record in the 24 h preceding the first visit and were instructed to replicate this feeding pattern before each subsequent visit. Participants refrained from alcohol, caffeine, and strenuous physical activity during the same period. A standardized meal was consumed in the evening before the laboratory visits consisting of a pizza (3054 kJ, 44% carbohydrate, 22% protein, 34% fat). Participants were instructed to consume the whole meal without any additional food or drink items except plain water, and compliance was confirmed from the food record completed before the first visit, and verbally on the remaining visits. After this meal, participants consumed no food or drink except plain water before arriving at the laboratory the next day.
Main conditions
Participants arrived at the laboratory at 0900 after a 13-h overnight fast. A cannula (Venflon; Becton Dickinson) was inserted into an antecubital vein 60 min before the collection of venous blood samples to eliminate any stress effects associated with cannula insertion (22). A fasting venous blood sample and rating of perceived appetite were taken at ∼1000 (0 h). Participants rested throughout all 4 conditions but were provided with a standardized breakfast meal after the fasting measurements during the 2 meal conditions. Breakfast was consumed within 15 min and consisted of croissants, butter, chocolate spread, cereal biscuits, and milkshake which provided 5025 kJ energy (47% carbohydrate, 9% protein, 44% fat). Subsequent venous blood samples were taken at 0.5 and 1 h, and appetite perceptions were assessed at 1 h. Environmental temperature and humidity were monitored and kept constant throughout all main experimental conditions using a wireless weather station (Opes).
Subjective appetite ratings
Subjective appetite ratings [hunger, satisfaction, fullness, and prospective food consumption (PFC)] were assessed at 0 and 1 h using 100-mm visual analog scales (8) as primary study outcomes. The scales were anchored by a descriptor at each end defining the extremes of the appetite perception being measured.
Blood sampling and biochemical analysis
Venous blood samples were collected in the semisupine position for the measurement of plasma acylated ghrelin, total PYY, insulin, and glucose concentrations as primary study outcomes. Plasma acylated ghrelin and total PYY concentrations were quantified from samples at 0 and 1 h, and plasma insulin and glucose concentrations were measured at 0 and 0.5 h to capture the peak change in concentration after the meal.
Venous blood samples were collected into prechilled 4.9-mL EDTA-coated monovettes (Sarstedt) for the determination of plasma acylated ghrelin concentrations, and into 9-mL EDTA-coated monovettes (Sarstedt) for the determination of total PYY, insulin, and glucose plasma concentrations. The blood processing methods have been detailed previously elsewhere (23, 24). Duplicate hemoglobin and hematocrit measurements were taken at each blood sampling time point to calculate the acute change in plasma volume (25). Commercially available enzyme immunoassays were used to determine the plasma concentrations of acylated ghrelin (Bertin Pharma), total PYY (Millipore), and insulin (Mercodia). Plasma glucose concentrations were analyzed by enzymatic, colorimetric methods using a benchtop analyzer (Pentra 400, HORIBA Medical). All samples were analyzed in duplicate and, in order to eliminate interassay variation, samples for each participant were analyzed in the same run. The within-batch CV values for acylated ghrelin, total PYY, insulin, and glucose concentrations were 4.0%, 4.6%, 5.9%, and 0.4%, respectively.
Statistical analyses
In our previous replicated crossover study (15), we detected statistically significant participant-by-treatment interactions with a sample size of 15 participants. Based on information from this study, we assumed a correlation between trials of 0.7. Using G*Power version 3.1.9.2 (University of Kiel), it was estimated that a total sample size of 16 participants would provide 80% statistical power to detect a statistically significant interaction between our 2-level between-subjects factor of genotype and within-subjects factor of treatment effect when this interaction amounted to a standardized effect size (ES) of 0.2 (α = 0.05). The 4 measurements of each outcome associated with our replicated crossover design increase statistical power over a conventional 2-level crossover study for detection of this group-by-treatment interaction.
Between-genotype differences in participant characteristics were quantified using linear mixed models with group (AA compared with TT) modelled as a fixed factor. The presence of interindividual differences in responses of appetite-related blood parameters and perceived appetite to a standardized meal was examined according to 3 analytical approaches (1, 3, 13). The 3 approaches, detailed recently by Goltz et al. (15), were as follows:
The association between the first and second replicate of the control-adjusted treatment effect was quantified for each outcome using Pearson's product-moment correlation coefficients (3). The first meal condition in any participant's sequence was paired to the first control condition in the same individual's sequence. Thresholds of 0.1, 0.3, and 0.5 were used to label correlation coefficients as small, moderate, and large, respectively (26). This correlation coefficient quantifies the consistency of meal effect across the replicated experimental conditions.
-
The following equation (1) was used to provide an overall estimate of the true (control condition–adjusted) between-subject differences in treatment response:
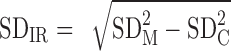
(1) SDIR represents the true interindividual variation in treatment effect. SDM and SDC are the SDs of the pre-to-post change scores for the meal and fasting control conditions [averaged over the 2 replicates using the relevant equation for pooling SDs (27)].
Although the equation in 2) estimates response variance adjusted for control condition change variance, the associated SEs and CIs are not appropriate for our within-subjects crossover study design, hence our adjunct approach of within-subjects general linear modelling. Using the MIXED procedure in SAS OnDemand for Academics (SAS Institute) (28), a within-participant linear mixed model was formulated to quantify any participant-by-condition interaction for each outcome. Condition and period (sequence), and their interaction effects, were modelled as fixed effects, and participant and participant-by-condition terms were modelled as random effects (refer to the SAS code supplied in Supplemental Methods). Standard residual diagnostics were undertaken to assess the “influence diagnostics” of a potential set of observations on the adequacy and the stability of the modelled covariance parameter estimates (29–31).
The grand mean differences between conditions and associated 95% CIs were quantified with a within-subjects linear mixed model run in SPSS version 23 (IBM Corporation) without the participant-by-condition random effect, but with a covariate of baseline values. The FTO genotype was included in this model as a fixed between-subjects effect, and the genotype-by-condition interaction was quantified.
Correction of hormone and glucose concentrations for acute changes in plasma volume had a negligible influence on our findings and, therefore, the unadjusted plasma concentrations are displayed for simplicity. In the absence of a robust and precise prognostic anchor for an important difference in our appetite-related outcomes, we calculated distribution-based standardized ESs (32). An ES of 0.2 denoted the minimum important mean difference for all outcomes, with an ES of 0.5 being moderate and an ES of 0.8 being large (26). To calculate the minimal clinically important difference (MCID) for individual responses, the threshold of 0.2 for interpreting standardized mean changes (26) was halved, i.e., 0.1, and multiplied by the baseline between-subject SD (1, 33). Pearson's product-moment correlation coefficients were quantified between the mean control-adjusted meal response for each of the appetite measures and body adiposity measurements. Correlation coefficients were also quantified between the pooled mean pre-to-post change in concentrations of plasma constituents and the pooled mean pre-to-post change in appetite perceptions across the 4 conditions.
Data are presented as mean ± SD. Mean differences or changes and correlation coefficients are presented along with their respective 95% CIs. Statistical significance was accepted as P < 0.05 and P values are expressed in exact terms apart from for very low values, which are expressed as P < 0.001.
Results
Participant characteristics
Participant characteristics are presented in Table 1. All 95% CIs for the difference between AAs and TTs overlapped zero (P ≥ 0.41), although these 95% CIs were relatively wide. All standardized ESs were very small, except for the small-to-moderate ESs found for body fat percentage and fat-free mass.
TABLE 1.
Participant characteristics1
| All (n = 18) | FTO homozygous minor allele (AA) (n = 9) | FTO homozygous major allele (TT) (n = 9) | AA vs. TT mean difference (95% CI) | ES | |
|---|---|---|---|---|---|
| Age, y | 28.5 ± 9.8 | 28.5 ± 9.6 | 28.4 ± 10.5 | 0.1 (−9.9, 10.2) | 0.01 |
| Height, m | 1.78 ± 0.06 | 1.78 ± 0.07 | 1.78 ± 0.05 | −0.002 (−0.06, 0.06) | 0.03 |
| Body mass, kg | 85.5 ± 16.0 | 85.7 ± 14.2 | 85.3 ± 18.5 | 0.4 (−16.1, 16.9) | 0.03 |
| BMI, kg/m2 | 27.0 ± 5.0 | 27.1 ± 4.7 | 26.8 ± 5.6 | 0.2 (−4.9, 5.4) | 0.05 |
| Body fat percentage, % | 20.2 ± 9.1 | 18.9 ± 9.1 | 21.4 ± 9.5 | −2.6 (−11.8, 6.7) | 0.27 |
| Fat-free mass, kg | 67.1 ± 7.8 | 68.7 ± 8.8 | 65.5 ± 6.8 | 3.1 (−4.7, 11.0) | 0.40 |
Values are means ± SDs, n = 18 healthy men (9 AA, 9 TT). ES, standardized effect size (mean difference); FTO, fat mass and obesity associated gene.
Plasma hormone and metabolite concentrations
Acylated ghrelin
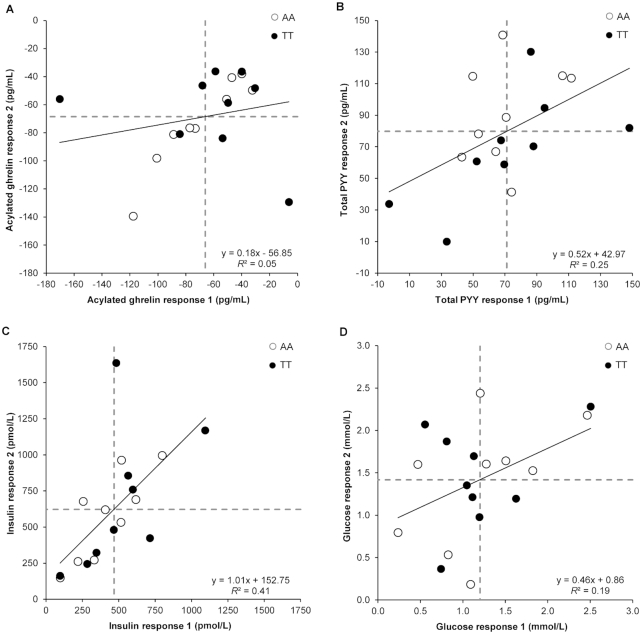
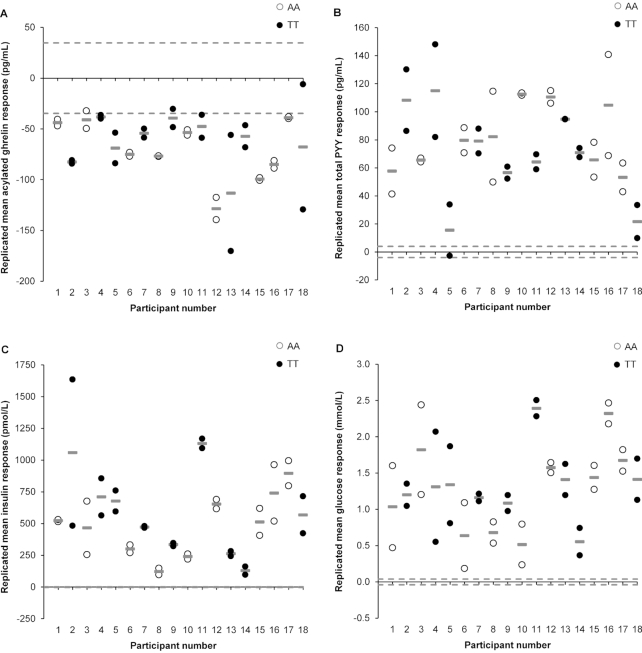
A weak correlation was observed between the 2 replicates of control-adjusted meal responses for acylated ghrelin (r = 0.22, 95% CI: −0.27, 0.62, P = 0.38, Figure 1A). In agreement with our postestimation residuals diagnostics, 2 distinct outliers can be seen in Figure 1A, which were >3 times higher or lower than the sample SD. Although we explored several data transformations, these were not successful in improving the nonnormal distribution of the ghrelin data. We could not identify any systematic protocol variation or measurement issues that would explain these 2 outliers. The removal of the 2 outliers in a sensitivity analysis improved the correlation coefficient of treatment effect between replicates to 0.86 (95% CI: 0.64, 0.95, P < 0.001). The SD of within-trial change was substantially greater for the meal than for the control conditions and remained so after removal of the 2 outliers (Table 2). After adjustment for period (sequence) influences, the estimated marginal mean acylated ghrelin concentration was 62 pg/mL lower (95% CI: −69, −54 pg/mL, P < 0.001, ES = 0.18) in the meal than in the control conditions. The P value for the participant-by-condition interaction was just above the threshold for statistical significance after the removal of the 2 outliers (Table 2). The magnitude of change in individual replicated mean responses after the meal for acylated ghrelin ranged from −128 to −38 pg/mL, with all participants demonstrating a meal-mediated suppression of ghrelin beyond the MCID (±34.8 pg/mL) (Figure 2A).
FIGURE 1.
Correlation between meal (standardized meal providing 5025 kJ) and control (no intervention) pre-to-post change scores on the 2 occasions for plasma acylated ghrelin (A), plasma total PYY (B), plasma insulin (C), and plasma glucose (D) in 18 healthy men genotyped for the rs9939609 allele of the fat mass and obesity associated (FTO) gene (n = 9 AA, n = 9 TT). “Response 1” corresponds to the first pair of conditions (meal 1 minus control 1) and “response 2” to the second pair of conditions (meal 2 minus control 2). Dashed lines represent the mean responses. PYY, peptide YY.
TABLE 2.
Means and SDs of the pre-to-post change scores for the meal (standardized meal providing 5025 kJ) and control (no intervention) conditions and the true individual differences SD1
| Estimate 12 | Estimate 23 | ||||
|---|---|---|---|---|---|
| Variable | Meal change, mean ± SD | Control change, mean ± SD | Individual differences, SD | Individual differences, SD (SE) | P value |
| Plasma acylated ghrelin, pg/mL | −61.1 ± 36.2 | 6.2 ± 27.7 | 23.3 | 4.9 (16.1) | 0.93 |
| −57.0 ± 20.44 | 7.4 ± 12.54 | 16.24 | 18.0 (13.0)4 | 0.0564 | |
| Plasma total PYY, pg/mL | 61.6 ± 35.1 | −13.9 ± 11.1 | 33.3 | 31.8 (20.6) | 0.020 |
| Plasma insulin, pmol/L | 545 ± 324 | 0.1 ± 11.5 | 324 | 502 (300) | 0.005 |
| 515 ± 2874 | 0.0 ± 11.84 | 2864 | 349 (208)4 | 0.0054 | |
| Plasma glucose, mmol/L | 1.22 ± 0.62 | −0.09 ± 0.17 | 0.60 | 0.58 (0.37) | 0.012 |
| Hunger, mm | −40.4 ± 30.0 | 8.7 ± 15.2 | 25.9 | 22.7 (15.4) | 0.031 |
| Satisfaction, mm | 48.3 ± 24.0 | −3.0 ± 9.4 | 22.0 | 19.5 (12.7) | 0.018 |
| Fullness, mm | 52.8 ± 20.2 | 1.4 ± 12.3 | 16.0 | 13.6 (9.8) | 0.054 |
| Prospective food consumption, mm | −44.1 ± 27.8 | 2.2 ± 9.8 | 26.0 | 23.9 (15.3) | 0.015 |
Data are presented for n = 18 healthy men. PYY, peptide YY.
Estimate 1: individual differences SD estimated using 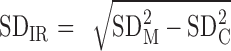 where SDIR is the SD of the true individual response, and SDM and SDC are the SDs of the pre-to-post change scores for the meal and the control condition (averaged over both replicates), respectively (1).
where SDIR is the SD of the true individual response, and SDM and SDC are the SDs of the pre-to-post change scores for the meal and the control condition (averaged over both replicates), respectively (1).
Estimate 2: period-adjusted individual differences SD estimated using a random-effects statistical model (13). The SD was derived from the SAS model participant-by-condition interaction term (as a random effect) (refer to the SAS code supplied in the Supplemental Methods). The P value shown is also for this interaction term.
After the removal of outliers.
FIGURE 2.
Individual changes in hormone and glucose concentrations between the meal (standardized meal providing 5025 kJ) and control (no intervention) conditions (meal minus control): plasma acylated ghrelin (A), plasma total PYY (B), plasma insulin (C), and plasma glucose (D) in 18 healthy men genotyped for the rs9939609 allele of the fat mass and obesity associated (FTO) gene (n = 9 AA, n = 9 TT). Pre-to-post change scores for “response 1” and “response 2” are indicated by white and black circles. Gray lines (—) represent each participant's replicated mean response. Dashed lines indicate the standardized minimal clinically important difference, calculated as 0.1 × the baseline between-subject SD. PYY, peptide YY.
Total PYY
A large positive correlation of 0.50 (95% CI: 0.04, 0.78, P = 0.034) was observed between the 2 replicates of control-adjusted meal responses for total PYY (Figure 1B). The within-trial SD for total PYY was substantially greater for the meal than for the control conditions (Table 2). The period-adjusted mean total PYY concentration was 78 pg/mL higher (95% CI: 70, 87 pg/mL, P < 0.001, ES = 1.96) in the meal than in the control conditions. A statistically significant participant-by-condition interaction was found (Table 2). The magnitude of change in individual replicated mean responses after the meal for total PYY ranged from 15 to 115 pg/mL, with all participants demonstrating an increase beyond the MCID (±3.99 pg/mL) (Figure 2B).
Insulin
A large positive correlation of 0.64 (95% CI: 0.25, 0.85, P = 0.004) was observed between the 2 replicates of control-adjusted meal responses for insulin (Figure 1C). After our residuals diagnostics, we undertook a sensitivity analysis where we removed 1 outlier, which was >4 times higher than the sample SD. This improved the correlation to 0.82 (95% CI: 0.56, 0.93, P < 0.001). The within-trial SD for insulin was substantially greater for the meal than for the control conditions (Table 2). The period-adjusted mean insulin concentration was 526 pmol/L higher (95% CI: 442, 610 pmol/L, P < 0.001, ES = 21.60) in the meal than in the control conditions. The participant-by-condition interaction was statistically significant both with and without inclusion of the outlier (Table 2). The magnitude of change in individual replicated mean responses after the meal for insulin ranged from 123 to 1130 pmol/L, with all participants demonstrating an increase beyond the MCID (±2.43 pmol/L) (Figure 2C).
Glucose
A moderate positive correlation of 0.44 (95% CI: −0.03, 0.75, P = 0.07) was observed between the 2 sets of control-adjusted meal responses for glucose (Figure 1D). The within-trial SD for glucose was substantially greater for the meal than for the control conditions (Table 2). The period-adjusted mean glucose concentration was 1.30 mmol/L higher (95% CI: 1.14, 1.46 mmol/L, P < 0.001, ES = 3.61) in the meal than in the control conditions. The participant-by-condition interaction was statistically significant (Table 2). The magnitude of change in individual replicated mean responses after the meal for glucose ranged from 0.52 to 2.39 mmol/L, with all participants demonstrating an increase beyond the MCID (±0.04 mmol/L) (Figure 2D).
Subjective appetite ratings
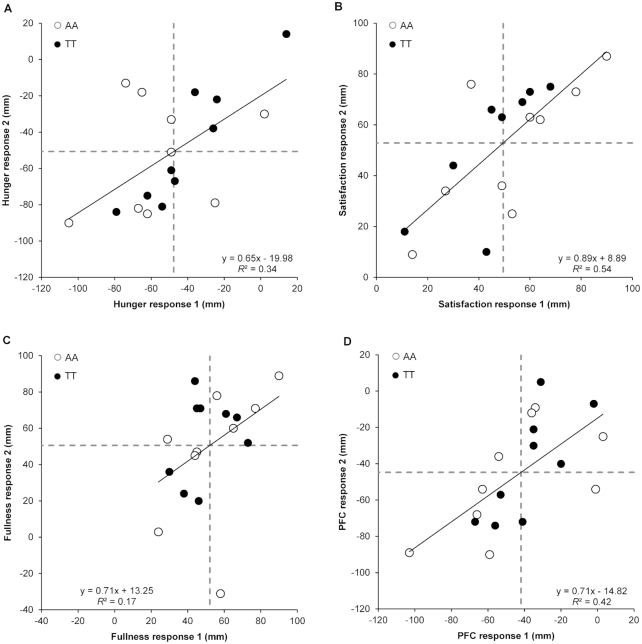
Moderate-to-large positive correlations were observed between the 2 sets of control-adjusted meal responses for hunger (r = 0.59, 95% CI: 0.17, 0.83, P = 0.010), satisfaction (r = 0.74, 95% CI: 0.42, 0.90, P < 0.001), fullness (r = 0.41, 95% CI: −0.07, 0.73, P = 0.09), and PFC (r = 0.65, 95% CI: 0.26, 0.86, P = 0.003) (Figure 3). Removal of 1 outlier for fullness improved the correlation coefficient to 0.62 (95% CI: 0.22, 0.84, P = 0.008). The within-trial SD was substantially greater for the meal than for the control conditions for hunger, satisfaction, fullness, and PFC (Table 2).
FIGURE 3.
Correlation between meal (standardized meal providing 5025 kJ) and control (no intervention) pre-to-post change scores on the 2 occasions for hunger (A), satisfaction (B), fullness (C), and PFC (D) in 18 healthy men genotyped for the rs9939609 allele of the fat mass and obesity associated (FTO) gene (n = 9 AA, n = 9 TT). “Response 1” corresponds to the first pair of conditions (meal 1 minus control 1) and “response 2” to the second pair of conditions (meal 2 minus control 2). Dashed lines represent the mean responses. PFC, prospective food consumption.
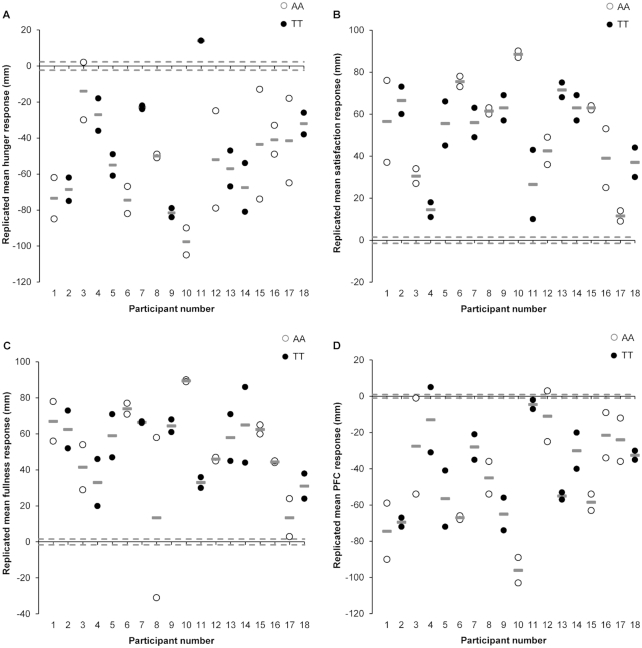
For all perceived appetite variables, the participant-by-condition interaction was statistically significant (Table 2). Exclusion of the previously mentioned outlier for fullness did not affect the significance of the participant-by-condition interaction or the estimated individual differences SD and therefore the data for fullness are presented with the outlier. The period-adjusted mean ratings of hunger and PFC were 49 mm (95% CI: −53, −44 mm, P < 0.001, ES = 2.16) and 43 mm (95% CI: −48, −38 mm, P < 0.001, ES = 4.26) lower, respectively, in the meal than in the control conditions. The period-adjusted mean ratings of satisfaction and fullness were 52 mm (95% CI: 47, 56 mm, P < 0.001, ES = 3.46) and 51 mm (95% CI: 45, 57 mm, P < 0.001, ES = 3.23) higher, respectively, in the meal than in the control conditions. The magnitude of change in individual replicated mean responses after the meal ranged from −97 to 14 mm for hunger, 11 to 88 mm for satisfaction, 13 to 89 mm for fullness, and −96 to −4 mm for PFC. All participants demonstrated a response beyond the MCID for hunger (±2.27 mm), satisfaction (±1.49 mm), fullness (±1.59 mm), and PFC (±1.59 mm) (Figure 4).
FIGURE 4.
Individual changes in each appetite perception between the meal (standardized meal providing 5025 kJ) and control (no intervention) conditions (meal minus control): hunger (A), satisfaction (B), fullness (C), and PFC (D) in 18 healthy men genotyped for the rs9939609 allele of the fat mass and obesity associated (FTO) gene (n = 9 AA, n = 9 TT). Pre-to-post change scores for “response 1” and “response 2” are indicated by white and black circles. Gray lines (—) represent each participant's replicated mean response. Dashed lines indicate the standardized minimal clinically important difference, calculated as 0.1 × the baseline between-subject SD. PFC, prospective food consumption.
Moderating effect of FTO genotype on individual variability
The FTO genotype-by-condition interaction was statistically nonsignificant for acylated ghrelin (P = 0.42), total PYY (P = 0.19), insulin (P = 0.54), glucose (P = 0.70), and all of the perceived appetite ratings (P ≥ 0.47). The differences in the mean treatment effects observed for all appetite parameters between genotype groups were small and statistically nonsignificant (ES ≤ 0.27, P ≥ 0.17). The upper confidence limit of the FTO gene difference was also only small to moderate.
Correlations between appetite outcomes and individual characteristics
Large positive correlations were observed between mean acylated ghrelin control-adjusted meal responses and body mass (r = 0.55, P = 0.019), BMI (r = 0.56, P = 0.015), and body fat percentage (r = 0.56, P = 0.016). Large positive correlations were also observed between hunger mean responses and BMI (r = 0.53, P = 0.023) and body fat percentage (r = 0.55, P = 0.018). None of the remaining appetite parameter mean responses were significantly correlated with the adiposity parameters assessed in this study (r = −0.46 to 0.41, P ≥ 0.06). No significant correlations were observed between fat-free mass and any of the mean appetite parameter responses to the meal (r = −0.14 to 0.36, P ≥ 0.15).
Correlations between changes in study outcome variables
A large positive correlation was observed between the pre-to-post change in acylated ghrelin and the change in both hunger and PFC, whereas a large negative correlation was observed between the pre-to-post change in acylated ghrelin and the change in both satisfaction and fullness. A large negative correlation was observed between the pre-to-post change in total PYY and the change in both hunger and PFC, whereas a large positive correlation was observed between the pre-to-post change in total PYY and the change in both satisfaction and fullness. A moderate negative correlation was observed between the pre-to-post change in insulin and the change in both hunger and PFC, whereas moderate-to-large positive correlations were observed between the pre-to-post change in insulin and the change in both satisfaction and fullness. A large negative correlation was observed between the pre-to-post change in glucose and the change in both hunger and PFC, whereas a large positive correlation was observed between the pre-to-post change in glucose and the change in both satisfaction and fullness (refer to results presented in Supplemental Table 1).
Discussion
The main findings from our study are that control-adjusted appetite-related blood parameters and perceived appetite responses to a standardized meal are reproducible when measured on 2 separate occasions. True interindividual variability exists in the postmeal responses of all studied outcomes, but we did not detect any worthwhile or statistically significant moderating influence of the FTO genotype on the magnitude of postmeal responses.
As expected, meal intake after an overnight fast resulted in mean suppressions of acylated ghrelin, hunger, and PFC, concomitantly with increases in total PYY, insulin, glucose, fullness, and satisfaction. Correlation coefficients between the 2 replicates of control condition–adjusted responses were positive, significant, and large for total PYY, insulin, hunger, satisfaction, and PFC. The correlation for acylated ghrelin was positive, but small and nonsignificant. However, the exclusion of 2 outliers improved the correlation markedly and we could not identify any methodological factors that could explain the one-off large or small values. Correlation coefficients for glucose and fullness were positive and moderate, although nonsignificant. Removal of 1 outlier for fullness improved the correlation. Overall, the postprandial changes in appetite parameters were similar between the 2 experiment replicates, suggesting good reproducibility of appetite responses to meal intake.
Previous studies have also reported good reproducibility of ad libitum energy intake, cholecystokinin, glucose, insulin (9, 11), and appetite perceptions after repeated fixed test meals (8, 10, 11). Although Nair et al. (9) observed good reproducibility of blood glucose AUC after a glucose preload on 3 occasions, the time taken for glucose to peak varied between visits. In our study, the pre-to-post change score was calculated between the fasting state and a single postprandial time point when the peak postmeal change was expected. It is possible that the relatively small correlation for pre-to-post changes in glucose on 2 occasions reflects inconsistency in the time taken for glucose to peak after a meal for some participants, rather than a lack of reproducibility of the response magnitude per se.
Recently, Gonzalez et al. (34) observed poor reproducibility at the individual level in perceived appetite after the consumption of liquid meals. Data were pooled from 2 previous studies comparing low- and high-energy liquid meals, but no control condition was included. The inclusion of a condition where no intervention takes place is essential so that the natural oscillation in the outcomes can be quantified and, therefore, the “true” effect of the intervention can be assessed (1, 3). Our results indicate that, besides the good reproducibility of appetite responses to meal intake, the magnitude of change varied considerably between individuals, supported by the participant-by-condition interactions. No previous studies have examined the interindividual variability in appetite responses to a meal including control conditions in a replicated crossover design. Therefore, our study adds to the literature by using a novel and appropriate study design and statistical analysis approach (1, 3).
The SD of the pre-to-post change scores was substantially larger in the meal than in the control conditions, indicating that the individual differences could not be explained by random within-subject variation or measurement errors (1). All participants exhibited perceived appetite and appetite-related blood parameter responses beyond our defined MCIDs, but a few participants were “small responders,” whereas others were “very large responders” according to the degree of change in the appetite parameters after meal intake. Of note, there are no clinically relevant target differences established for appetite parameters and the MCID thresholds chosen were based on the statistical threshold of 0.1 SD for judging clinical importance of individual differences. Clinically relevant differences are most appropriately defined using “hard” anchors to changes in morbidity and/or mortality (32), but information is lacking on this at present. All participants exhibited the expected direction of meal-induced change in the various outcomes, i.e., suppression of acylated ghrelin, hunger, and PFC and increase in total PYY, insulin, glucose, fullness, and satisfaction, except for 1 participant who exhibited an increase in hunger after meal intake on both occasions.
The FTO genotype-by-condition interaction was statistically nonsignificant for all of the appetite parameters. Although statistical power can be lower for the detection of subgroup-by-treatment interactions than for the overall treatment effect, ESs were low for all FTO gene subgroup interaction terms. It is well established that the homozygous AA variant of the FTO genotype confers a higher obesity risk (16). Although the mechanisms are not fully elucidated, it has been suggested that AAs demonstrate attenuated postprandial suppression of acylated ghrelin and hunger (17). In contrast, a recent study did not identify differences in hunger or total ghrelin between FTO genotype groups in individuals with overweight or obesity after standardized and buffet meals, even though AAs had higher energy intake at the buffet meal (35). Of note, the assessment of total ghrelin in this study could have influenced the results as the active part of the hormone only represents ∼5–10% of total ghrelin (36, 37). Longer-term studies are needed to confirm whether differences in appetite-related outcomes are observed between FTO genotype groups which may culminate in continuous differences in energy intake, and consequently, body mass alterations.
Exploratory analyses of our data indicated that higher adiposity was associated with smaller changes in the mean postprandial acylated ghrelin and hunger responses, supporting previous evidence suggesting that individuals with obesity exhibit a reduced postprandial suppression of ghrelin (38). However, our study was not designed to answer this question and participant recruitment aimed to match the 2 FTO-genotype groups for age and adiposity. Besides adiposity levels, individual differences in rates of stomach distention and gastric emptying (39), as well as differences in gut microbiota, could potentially explain the interindividual variability in postprandial appetite responses (40). Indeed, direct associations between insulin and glucose responses to a glucose preload and rates of gastric emptying have been observed (9), and a growing body of evidence points to the important role of the gut microbiota in nutrient sensing and appetite regulation (40, 41). Future research is required to determine moderators of appetite responses to meal intake that may explain the individual variability.
All the correlations between the changes in perceived appetite and appetite-related blood parameters were significant apart from the correlations between insulin and hunger, satisfaction, and PFC. Although the exact pathways are unclear, insulin has been associated previously with short-term appetite control in healthy individuals, with evidence demonstrating that higher postprandial insulin concentrations were associated with greater sensations of satiety and lower sensations of hunger (42). Overall, our results provide evidence of very strong associations between perturbations in appetite-related blood parameters and perceived sensations of hunger, satisfaction, fullness, and PFC. This supports previous evidence showing that changes in glucose, insulin, acylated ghrelin, PYY3–36, and glucagon-like peptide-1 concentrations occur synchronously with changes in perceived appetite after the consumption of test meals (43).
The strengths of our study include the novel study design and statistical approaches employed, which have been advocated to quantify interindividual variability in responses to an intervention appropriately (1, 3). Furthermore, the combination of circulating blood parameters with perceived appetite ratings known to respond episodically to meal intake represents a further strength. Care should be taken when generalizing the findings because alternative blood processing or analysis methods, as well as inclusion of females, older individuals, and individuals with obesity, may result in different findings.
In conclusion, the reproducibility of appetite responses to standardized meals is generally good. True interindividual variability is present in appetite-related blood parameter responses and perceived appetite responses to meal intake beyond any random within-subject variation over time in healthy men, but the magnitude of change in postprandial appetite responses was not influenced by the FTO gene. Our study supports the existence of true interindividual variability in postprandial appetite changes between individuals, which should be considered in future research as well as for interpreting group mean results from intervention studies. Furthermore, these findings highlight the importance of exploring individual differences in appetite response in the context of the prevention and treatment of obesity. Further studies with longer-term interventions using appropriate study designs and statistical analyses are needed to identify potential moderators responsible for the individual variability in postprandial appetite responses and to confirm the exact clinical relevance of our findings.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—FRG, AET, GA, and DJS: designed the research; FRG, AET, JAK, JLD, SM, MD, and DJS: conducted the research; JLD, SM, MD, and DJS: provided essential reagents and/or provided essential materials; FRG, AET, GA, and LL: analyzed data and performed statistical analysis; FRG, AET, GA, LL, and DJS: wrote the manuscript and had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre.
Author disclosures: FRG, AET, GA, LL, JAK, JLD, MD, SM, and DJS, no conflicts of interest.
The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care.
Supplemental Figure 1, Supplemental Methods, and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ES, effect size; FTO, fat mass and obesity associated gene; MCID, minimal clinically important difference; PFC, prospective food consumption; PYY, peptide YY.
References
- 1. Atkinson G, Batterham AM. True and false interindividual differences in the physiological response to an intervention. Exp Physiol. 2015;100(6):577–88. [DOI] [PubMed] [Google Scholar]
- 2. Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, Theisen D, Senn S, Meyer T. Individual response to exercise training – a statistical perspective. J Appl Physiol. 2015;118(12):1450–9. [DOI] [PubMed] [Google Scholar]
- 3. Senn S. Mastering variation: variance components and personalised medicine. Stat Med. 2016;35:966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Betts JA, Gonzalez JT. Personalised nutrition: what makes you so special?. Nutr Bull. 2016;41(4):353–9. [Google Scholar]
- 5. Hopkins M, Blundell JE, King NA. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br J Sports Med. 2014;48(20):1472–6. [DOI] [PubMed] [Google Scholar]
- 6. King JA, Deighton K, Broom DR, Wasse LK, Douglas JA, Burns SF, Cordery PA, Petherick ES, Batterham RL, Goltz FR et al.. Individual variation in hunger, energy intake, and ghrelin responses to acute exercise. Med Sci Sports Exerc. 2017;49(6):1219–28. [DOI] [PubMed] [Google Scholar]
- 7. Neary MT, Batterham RL. Gut hormones: implications for the treatment of obesity. Pharmacol Ther. 2009;124(1): 44–56. [DOI] [PubMed] [Google Scholar]
- 8. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 9. Nair NS, Brennan IM, Little TJ, Gentilcore D, Hausken T, Jones KL, Wishart JM, Horowitz M, Feinle-Bisset C. Reproducibility of energy intake, gastric emptying, blood glucose, plasma insulin and cholecystokinin responses in healthy young males. Br J Nutr. 2009;101(7):1094–102. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez JT, Veasey RC, Rumbold PL, Stevenson EJ. Consistency of metabolic responses and appetite sensations under postabsorptive and postprandial conditions. Appetite. 2012;59(2):228–33. [DOI] [PubMed] [Google Scholar]
- 11. Horner KM, Byrne NM, King NA. Reproducibility of subjective appetite ratings and ad libitum test meal energy intake in overweight and obese males. Appetite. 2014;81:116–22. [DOI] [PubMed] [Google Scholar]
- 12. Williamson PJ, Atkinson G, Batterham AM. Inter-individual responses of maximal oxygen uptake to exercise training: a critical review. Sports Med. 2017;47(8):1501–13. [DOI] [PubMed] [Google Scholar]
- 13. Senn S, Rolfe K, Julious SA. Investigating variability in patient response to treatment – a case study from a replicate cross-over study. Stat Methods Med Res. 2011;20(6):657–66. [DOI] [PubMed] [Google Scholar]
- 14. Atkinson G, Williamson P, Batterham AM. Exercise training response heterogeneity: statistical insights. Diabetologia. 2018;61(2):496–7. [DOI] [PubMed] [Google Scholar]
- 15. Goltz FR, Thackray AE, King JA, Dorling JL, Atkinson G, Stensel DJ. Interindividual responses of appetite to acute exercise: a replicated crossover study. Med Sci Sports Exerc. 2018;50(4):758–68. [DOI] [PubMed] [Google Scholar]
- 16. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW et al.. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karra E, O'Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, Scott WR, Chandarana K, Manning S, Hess ME et al.. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest. 2013;123(8):3539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al.. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:381–95. [DOI] [PubMed] [Google Scholar]
- 19. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. [DOI] [PubMed] [Google Scholar]
- 20. Siri WE. Body composition from fluid space and density. In: Brozek J, , Hanschel A, editors. Techniques for measuring body composition. Washington (DC): National Academy of Science; 1961. pp. 223–44. [Google Scholar]
- 21. Randomization.com. [Internet] 2007. [cited 2017 April 12]. Available from: www.randomization.com. [Google Scholar]
- 22. Chandarana K, Drew ME, Emmanuel J, Karra E, Gelegen C, Chan P, Cron NJ, Batterham RL. Subject standardization, acclimatization, and sample processing affect gut hormone levels and appetite in humans. Gastroenterology. 2009;136(7):2115–26. [DOI] [PubMed] [Google Scholar]
- 23. Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol. 2007;102(6):2165–71. [DOI] [PubMed] [Google Scholar]
- 24. Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R29–35. [DOI] [PubMed] [Google Scholar]
- 25. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–8. [DOI] [PubMed] [Google Scholar]
- 26. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Hillsdale (NJ): Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 27. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. [Internet]. Version 5.1.0. The Cochrane Collaboration; 2011. [cited 2018 Nov 20]. Available from: http://handbook-5-1.cochrane.org/. [Google Scholar]
- 28. SAS® OnDemand for Academics. [Internet]. SAS Institute; 2018. [cited 2018 April 23]. Available from: https://www.sas.com/en_us/software/on-demand-for-academics.html. [Google Scholar]
- 29. Oman SD. Checking the assumptions in mixed-model analysis of variance: a residual analysis approach. Comput Stat Data An. 1995;20(3):309–30. [Google Scholar]
- 30. Schabenberger O. Mixed model influence diagnostics. In: Proceedings of the twenty-ninth annual SAS Users Group International Conference. Cary (NC): SAS Institute; 2004. Paper 189–29. [Google Scholar]
- 31. West BT, Galecki AT. An overview of current software procedures for fitting linear mixed models. Am Stat. 2012;65(4):274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cook JA, Julious SA, Sones W, Hampson LV, Hewitt C, Berlin JA, Ashby D, Emsley R, Fergusson DA, Walters SJ et al.. DELTA2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a randomised controlled trial. BMJ. 2018;363:k3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williamson PJ, Atkinson G, Batterham AM. Inter-individual differences in weight change following exercise interventions: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2018;19(7):960–75. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez JT, Frampton J, Deighton K. Postprandial suppression of appetite is more reproducible at a group than an individual level: implications for assessing inter-individual variability. Appetite. 2017;108:375–82. [DOI] [PubMed] [Google Scholar]
- 35. Melhorn SJ, Askren MK, Chung WK, Kratz M, Bosch TA, Tyagi V, Webb MF, De Leon MRB, Grabowski TJ, Leibel RL et al.. FTO genotype impacts food intake and corticolimbic activation. Am J Clin Nutr. 2018;107(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279(3):909–13. [DOI] [PubMed] [Google Scholar]
- 37. Yoshimoto A, Mori K, Sugawara A, Mukoyama M, Yahata K, Suganami T, Takaya K, Hosoda H, Kojima M, Kangawa K et al.. Plasma ghrelin and desacyl ghrelin concentrations in renal failure. J Am Soc Nephrol. 2002;13(11):2748–52. [DOI] [PubMed] [Google Scholar]
- 38. Le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90(2):1068–71. [DOI] [PubMed] [Google Scholar]
- 39. Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33(8):880–94. [DOI] [PubMed] [Google Scholar]
- 40. van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-gut-brain axis: modulator of host metabolism and appetite. J Nutr. 2017;147(5):727–45. [DOI] [PubMed] [Google Scholar]
- 41. Lam YY, Maguire S, Palacios T, Caterson ID. Are the gut bacteria telling us to eat or not to eat? Reviewing the role of gut microbiota in the etiology, disease progression and treatment of eating disorders. Nutrients. 2017;9(6):602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flint A, Gregersen NT, Gluud LL, Møller BK, Raben A, Tetens I, Verdich C, Astrup A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br J Nutr. 2007;98(1):17–25. [DOI] [PubMed] [Google Scholar]
- 43. Lemmens SG, Martens EA, Kester AD, Westerterp-Plantenga MS. Changes in gut hormone and glucose concentrations in relation to hunger and fullness. Am J Clin Nutr. 2011;94(3):717–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.