Abstract
Background
We evaluated the differential impact of levofloxacin administered for the prophylaxis of bloodstream infections compared with broad-spectrum beta-lactam (BSBL) antibiotics used for the treatment of neutropenic fever on the gut microbiome in patients with hematologic malignancy.
Methods
Stool specimens were collected from patients admitted for chemotherapy or stem cell transplant in the setting of the evaluation of diarrhea from February 2017 until November 2017. Microbiome characteristics were compared among those exposed to levofloxacin prophylaxis vs those who received BSBL antibiotics.
Results
Sixty patients were included, most with acute myeloid leukemia (42%) or multiple myeloma (37%). The gut microbiome of patients with BSBL exposure had significantly reduced Shannon’s alpha diversity compared with those without (median [interquartile range {IQR}], 3.28 [1.73 to 3.71] vs 3.73 [3.14 to 4.31]; P = .01). However, those with levofloxacin exposure had increased alpha diversity compared with those without (median [IQR], 3.83 [3.32 to 4.36] vs 3.32 [2.35 to 4.02]; P = .03). Levofloxacin exposure was also associated with a trend toward lower risk of dominance of non-Bacteroidetes genera compared with those without levofloxacin exposure (3 [14%] vs 15 [38%]; P = .051).
Conclusions
The impact of antibiotics on the gut microbiome varies by class, and levofloxacin may disrupt the gut microbiome less than BSBLs in this patient population.
Keywords: antibiotic prophylaxis, hematologic neoplasms, microbiota, levofloxacin
Patients receiving treatment for hematologic malignancy are at increased risk of infection due to their underlying malignancy, frequent hospitalizations, chemotherapy-induced immune suppression, and neutropenia [1]. In addition, patients often experience neutropenia of days to weeks, which, in combination with chemotherapy, may result in mucosal barrier injury of the gastrointestinal (GI) tract [2, 3]. This mucosal barrier injury may subsequently result in neutropenic fever or bloodstream infection (BSI) [2, 3]. Antibiotic prophylaxis with levofloxacin has been shown to reduce the incidence of neutropenic fever, BSI, and mortality [4–6].
Study of the gut microbiome, or the indigenous microbial communities within the GI tract [7], has revealed several important associations with clinical outcomes, including BSIs, in patients treated for hematologic malignancies [8]. The gut microbiome has also been linked to risk of graft-vs-host disease (GVHD), treatment failure, and mortality in allogeneic stem cell transplant (SCT) [9–11]. These findings suggest an important interaction between the gut microbiota and the greater immune system on risk for infection.
There has been limited study, however, of how antibiotics modulate the microbiome in patients with hematologic malignancy. In healthy populations, the impact of antibiotics varies by spectrum and class [12–16]. Although antibiotics have a profound impact on the gut microbiome and may play an important role in the mediation of both infectious and noninfectious clinical outcomes [15], there is a limited understanding of how prophylactic antibiotics modulate the gut microbiome in patients with hematologic malignancy. Thus, we aimed to compare the impact of levofloxacin used for the prophylaxis of BSI with that of broad-spectrum beta-lactam (BSBL) antibiotics commonly used for the treatment of neutropenic fever on the gut microbiome in patients admitted for the treatment of hematologic malignancy.
METHODS
Study Design and Setting
We performed a retrospective cohort study of patients admitted to the Hospital of the University of Pennsylvania (HUP) from February 13, 2017, to November 17, 2017. HUP is a 791-bed tertiary care medical center and a National Cancer Institute Comprehensive Cancer Care–designated center.
Study Population
Patients included those admitted for the treatment of hematologic malignancy. During the study period, residual stool was collected from routine specimens that were processed in the setting of the evaluation of diarrhea. All patients underwent testing for Clostridioides difficile using a commercial enzyme immunoassay (EIA) for toxin A, toxin B, and glutamate dehydrogenase (GDH; C Diff Quik Check Complete, Alere, Waltham, MA). Those negative for toxins A and B but positive for GDH were subsequently tested by polymerase chain reaction for toxin genes (BD MAX Cdiff Assay, Becton Dickinson, Franklin Lanes, NJ). Samples positive for C. difficile were excluded from analysis due to evidence that C. difficile infection has a strong independent impact on the gut microbiome [17]. Additionally, our analysis was limited to the first sample collected per patient due to significant correlation of microbiome measurements in serial stool samples [18, 19]. Patients who were receiving levofloxacin for indications other than prophylaxis (eg, treatment of infection) were excluded, although this was uncommon.
During this time frame, antibiotic prophylaxis regimens varied among the patient populations included. Levofloxacin prophylaxis was administered in patients undergoing autologous SCT for the duration of the study period, as previously described [20]. Beginning May 1, 2017, patients receiving induction chemotherapy for the treatment of acute myeloid leukemia (AML) received levofloxacin prophylaxis (500 mg oral daily) from day 8 after the beginning of chemotherapy until engraftment or neutropenic fever. Cefepime, piperacillin-tazobactam, or meropenem was administered for treatment of neutropenic fever, as per institutional neutropenic fever guidelines. Patients receiving allogeneic SCT received oral vancomycin prophylaxis, as previously described [21]. We compared patients exposed to levofloxacin for the prevention of BSI with those who received BSBL antibiotics (ie, cefepime, piperacillin-tazobactam, or meropenem) in the 7 days before sample collection. Therefore, there were 2 exposures of interest for this cohort study: levofloxacin exposure in the prior 7 days vs those who did not receive levofloxacin and BSBL antibiotic exposure vs those who did not receive BSBL antibiotics. Due to concurrent use of oral vancomycin for prophylaxis in some patients and prior evidence of its profound impact on the gut microbiome, oral vancomycin use was ascertained for adjustment in our analyses [18]. The study was reviewed and approved by the University of Pennsylvania Institutional Review Board.
Data Collection
Clinical data were collected via electronic medical record review, including demographics and comorbidities, type of malignancy, and the chemotherapy regimen received. Exposures to levofloxacin, oral vancomycin, and BSBL antibiotics in the 14 days before sample collection were measured in days of therapy. Microbiology data were also collected, including positive C. difficile test results at the time of sample collection and positive blood cultures within the 30 days after stool sample collection.
Sample Processing and Analysis
Sample processing including DNA extraction and 16S rRNA sequencing is described in the Supplementary Data. Formation of operational taxonomic units (OTUs) was performed using QIIME 1.9.1, and taxonomic assignment was performed via the GreenGenes database (13.8). Bacterial community diversity was described by calculation of Shannon’s alpha [7, 22]. The proportion of reads assigned to Bacteroidetes was evaluated in light of prior studies showing potential clinical significance of Bacteroidetes taxa for resistance to colonization with pathogens [9, 23, 24]. Domination of non-Bacteroidetes taxa was defined as representation of >30% of total bacterial diversity by a single non-Bacteroidetes genus [8]. To evaluate the difference in beta diversity, a Bray-Curtis dissimilarity matrix was created among OTU assignments. To compare beta diversity between categories of antibiotic exposure, principal coordinate analysis (PCoA) was performed and depicted graphically. Permutational analysis of variance (PERMANOVA) was performed to describe the ability of antibiotic category to account for sample variance within the Bray-Curtis dissimilarity matrix.
Statistical Analysis
Descriptive statistics were used to compare microbiome characteristics among those exposed to the antibiotics of interest in the prior 7 days. A multivariable linear regression model was created to assess the impact of malignancy, treatment regimen, sex, and dichotomous antibiotic exposures on Shannon’s alpha diversity. A manual stepwise selection procedure was performed including variables with P < .25 on bivariable analysis. Variables were maintained in the final model if inclusion was statistically significant on likelihood ratio testing.
To assess the impact of quantitative antibiotic exposures in the prior 7 days, multivariable regression models were created. Linear regression was used to model the impact of antibiotic exposure on Shannon’s alpha diversity and the logit-transformed proportion of Bacteroidetes, with calculation of a β coefficient and 95% confidence interval (CI). Logit-transformation of proportion of Bacteroidetes was performed due to a predicted and observed non-normal distribution of this variable. Logistic regression was used to model the impact of antibiotic exposure on dominance, with calculation of an odds ratio (OR) and 95% CI. Bivariable models were created to assess the impact of each antibiotic of interest on these microbiome measures. Additionally, multivariable models were created to assess the adjusted impact of antibiotic exposures, accounting for multiple classes of antibiotic exposure.
To explore the relative proportions of taxa that have been previously linked to clinical outcomes, we performed an analysis investigating the impact of antibiotic exposures in the prior 7 days on the abundance of these taxa. For these analyses, we compared patients with levofloxacin exposure with those without, and those with BSBL antibiotic exposure with those without. Taxa of interest were selected according to prior studies suggesting associations with clinical outcomes [8, 10, 23, 25, 26]. A subanalysis was also performed extending the antibiotic window of interest to 14 days before sample collection.
Calculation of microbiome measures was performed with R, version 3.4.2 (R Development Core Team, Vienna, Austria). Statistical analyses were performed using STATA, version 14.2 (StataCorp, College Station, TX).
RESULTS
Study Population
A total of 60 patients were included over the 9-month study period. The median age was 63 years, 47% were female, and 18% identified as nonwhite. Diagnoses included AML (42%), multiple myeloma (37%), or non-Hodgkin’s lymphoma (11%) (Table 1). Treatments received included autologous SCT (40%), allogeneic SCT (23%), induction chemotherapy for treatment of AML (17%), and other chemotherapy regimens (20%). The median time from initiation of cancer treatment to sample collection (interquartile range [IQR]) was 4.5 (2 to 7.5) days, and the median time from admission to sample collection (IQR) was 7.5 (5 to 11.5) days. Thirty-two (53%) patients received a proton pump inhibitor or histamine-2 antagonist in the 7 days before sample collection. All patients received a neutropenic diet, and no patients received enteral or parenteral nutritional support in the 7 days before sample collection.
Table 1.
Characteristics of Patients, Comparing Those who Received Levofloxacin Prophylaxis With Those With BSBL Exposure
| Levofloxacina | BSBL | Both | Neither | ||
|---|---|---|---|---|---|
| Characteristics | n = 17 | n = 17 | n = 4 | n = 22 | P a Value |
| Age, median (IQR), y | 65 (59 to 66) | 58 (49 to 67) | 56 (43 to 69) | 62 (52 to 67) | .69 |
| Male sex | 12 (71) | 5 (29) | 3 (75) | 12 (55) | .08 |
| Nonwhite race | 4 (24) | 2 (12) | 0 (0) | 5 (23) | .58 |
| Malignancy | <.001 | ||||
| Multiple myeloma | 14 (82) | 2 (12) | 3 (75) | 3 (14) | |
| Non-Hodgkin’s lymphoma | 2 (12) | 0 (0) | 0 (0) | 5 (23) | |
| Acute myeloid leukemia | 2 (12) | 13 (76) | 1 (25) | 10 (45) | |
| Other | 0 (0 | 2 (12) | 0 (0) | 4 (18) | |
| Treatment | <.001 | ||||
| Autologous SCT | 16 (94) | 2 (12 | 3 (75) | 3 (14) | |
| Allogeneic SCT | 0 (0) | 5 (29) | 0 (0) | 9 (41) | |
| Induction chemotherapyb | 1 (6) | 7 (41) | 1 (25) | 1 (5) | |
| Other chemotherapyc | 0 (0) | 3 (18) | 0 (0) | 9 (41) | |
| Oral vancomycind | 0 (0) | 6 (35) | 0 (0) | 7 (32) | .03 |
| Days from treatment, median (IQR) | 2 (2 to 2) | 4 (2 to 7) | 2 (1 to 2) | 3.5 (0 to 7) | .03 |
| Proton pump inhibitor used | 10 (59) | 8 (47) | 1 (25) | 8 (36) | .47 |
| Histamine-2 antagonist used | 1 (6) | 4 (24) | 2 (50) | 5 (23) | .21 |
| Enteric or parenteral feedinge | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .99 |
Unless noted otherwise, data are presented as No. (%).
Abbreviations: BSBL, broad-spectrum beta-lactam; IQR, interquartile range; SCT, stem cell transplantation.
aAnalysis of variance used to compare multiple categories.
bFor the treatment of acute myeloid leukemia.
cIncluding consolidation chemotherapy for acute myeloid leukemia and other chemotherapy regimens.
dReceipt within the 7 days before sample collection.
eIncluding total parenteral nutrition or tube feeding.
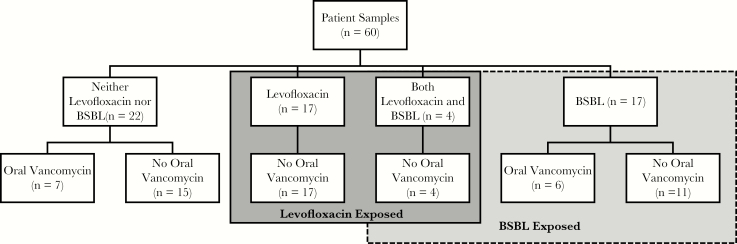
Of the 60 patients included, 17 (28%) received levofloxacin, 17 (28%) received a BSBL antibiotic, 4 (7%) received both levofloxacin and a BSBL antibiotic, and 22 (36%) received neither levofloxacin nor a BSBL antibiotic in the prior 7 days (Figure 1). Of the 21 patients who received a BSBL antibiotic, 18 (86%) received a BSBL antibiotic for treatment of neutropenic fever and 15 (71%) received only cefepime. Eight (38%) patients who received BSBL antibiotics were admitted for induction chemotherapy for AML, 5 (24%) for allogeneic SCT, and 5 (24%) for autologous SCT. Among the 21 patients receiving levofloxacin, 19 (90%) were admitted for autologous SCT and 2 (10%) for induction chemotherapy for AML. Thirteen (22%) patients received oral vancomycin, of whom 12 (92%) were admitted for allogeneic SCT.
Figure 1.
Flow diagram of patient samples and antibiotic exposure in the 7 days before collection. Abbreviation: BSBL, broad-spectrum beta-lactam.
Bacterial Community Diversity
In the total population of samples, the median Shannon’s diversity (IQR) was 3.46 (2.76 to 4.22). Graphical depiction of the distribution of bacterial phyla is shown in Figure 2, and a heatmap of bacterial taxa at the family level and among Proteobacteria at the genus level is shown in Supplementary Figure 1. Those exposed to levofloxacin in the prior 7 days had increased diversity compared with those who did not receive levofloxacin (median [IQR], 3.83 [3.32 to 4.36] vs 3.32 [2.35 to 4.02]; P = .03), whereas those with BSBL exposure showed reduced diversity compared with those who did not receive BSBL antibiotics (median [IQR], 3.28 [1.73 to 3.71] vs 3.73 [3.14 to 4.31]; P = .01) (Table 2). Comparing those patients who received levofloxacin prophylaxis only (n = 17) with those who did not receive levofloxacin, BSBL antibiotics, or oral vancomycin (n = 15), we found no difference in Shannon’s diversity (median [IQR], 4.02 [3.32 to 4.89] vs 3.95 [3.33 to 4.23]; P = .51). Comparing those who received levofloxacin only (n = 17) with those who received BSBL antibiotics but not oral vancomycin (n = 11), we found a trend toward increased Shannon’s diversity (median [IQR], 4.02 [3.32 to 4.89] vs 3.49 [2.60 to 4.67]; P = .10). On multivariable linear regression of Shannon’s alpha diversity, including malignancy, treatment, and dichotomous exposure to levofloxacin, BSBL, and oral vancomycin antibiotics in the prior 7 days, both BSBL exposure and oral vancomycin exposure were associated with reduction in alpha diversity, whereas levofloxacin was not (Supplementary Table 1).
Figure 2.
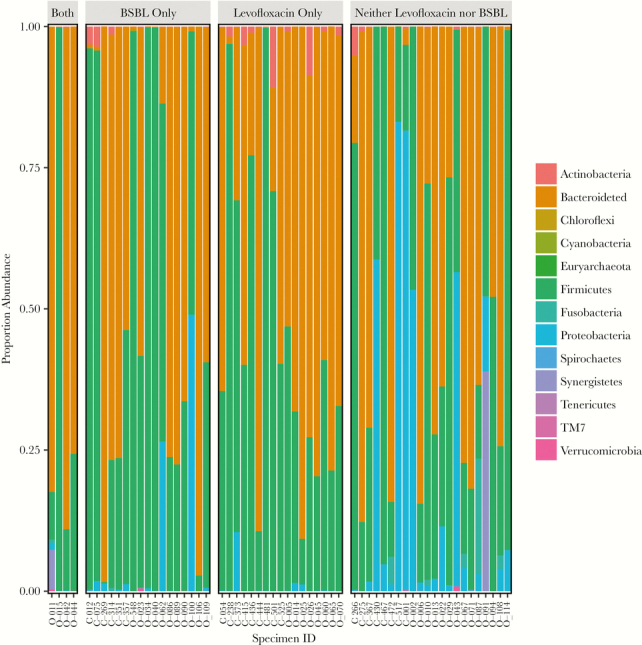
Proportion of bacterial phyla by antibiotic exposure. Abbreviation: BSBL, broad-spectrum beta-lactam.
Table 2.
Microbiome Characteristics by Dichotomous Antibiotic Exposure in the Prior 7 Days
| Variable | Unexposeda | Exposeda | P Value |
|---|---|---|---|
| Alpha diversitya | |||
| Levofloxacin | 3.32 (2.35 to 4.02) | 3.83 (3.32 to 4.36) | .03 |
| BSBL | 3.73 (3.14 to 4.31) | 3.28 (1.73 to 3.71) | .01 |
| Bacteroidetes proportional abundance | |||
| Levofloxacin | 0.54 (0.01 to 0.76) | 0.64 (0.31 to 0.79) | .19 |
| BSBL | 0.58 (0.03 to 0.74) | 0.59 (0.01 to 0.76) | .59 |
| Dominance by non-Bacteroidetes genus, No. (%) | |||
| Levofloxacin | 15 (38) | 3 (14) | .051 |
| BSBL | 10 (26) | 8 (38) | .32 |
Unless noted otherwise, data are presented as median (interquartile range).
Abbreviation: BSBL, broad-spectrum beta-lactam.
aShannon’s alpha diversity.
On bivariable analysis, we found an association of increased diversity with each additional day of levofloxacin and decreased diversity with each additional day for both BSBL antibiotics and oral vancomycin (Table 3). On multivariable analysis, we also found an association between both days of BSBL antibiotics and days of oral vancomycin exposure with a reduction in Shannon’s diversity (β [IQR], –0.13 [–0.24 to –0.02]; P = .02; null hypothesis β = 0; and β [IQR], –0.22 [–0.32 to –0.12]; P < .001) per day of therapy, respectively, without a significant impact from levofloxacin (β [IQR], 0.03 [–0.11 to 0.18]; P = .63).
Table 3.
Microbiome Characteristics by Continuous Antibiotic Exposure in the Prior 7 Days
| Multivariable Analysis β Coefficient | ||||
|---|---|---|---|---|
| Variable | Bivariable Analysis | P | (95% CI) | P Value |
| Alpha diversitya | ||||
| Levofloxacin | 0.18 (0.03 to 0.34) | .02 | 0.03 (–0.11 to 0.18) | .63 |
| BSBL | –0.17 (–0.29 to –0.05) | .007 | –0.13 (–0.24 to –0.02) | .02 |
| Oral vancomycin | –0.24 (–0.34 to –0.14) | <.001 | –0.22 (–0.32 to –0.12) | <.001 |
| Bacteroidetes proportional abundance | ||||
| Levofloxacin | 0.17 (–0.30 to 0.64) | .71 | ||
| BSBL | –0.06 (–0.44 to 0.32) | .77 | ||
| Oral vancomycin | –1.03 (–1.24 to –0.82) | <.001 | ||
| Dominance by non-Bacteroidetes genus | ||||
| Levofloxacin | 0.81 (0.57 to 1.14) | .23 | ||
| BSBL | 1.14 (0.91 to 1.41) | .25 | ||
| Oral vancomycin | 2.07 (1.43 to 3.00) | <.001 |
Unless noted otherwise, data are presented as median (interquartile range).
Abbreviations: BSBL, broad-spectrum beta-lactam; CI, confidence interval.
aShannon’s alpha diversity.
Proportion of Bacteroidetes Taxa
Across all specimens, the median proportion of Bacteroidetes taxa was 58%, with a bimodal distribution centered near 0% and 80%. There was no significant impact of either levofloxacin or BSBL antibiotics on proportion of Bacteroidetes when examined as a dichotomous exposure in the prior 7 days (0.54 vs 0.64; P = .19; and 0.59 vs 0.58; P = .59, respectively). Evaluating the logit-transformed proportion of Bacteroidetes as an outcome, there was no significant association with either levofloxacin or BSBL exposure (P = .59). Oral vancomycin was associated with a significant reduction in Bacteroidetes taxa (β [IQR], –1.03 [–0.08 to –0.82]; P < .001).
Dominance
Dominance with a non-Bacteroidetes taxa occurred in 18 (30%) of all samples, with the most common genus being Enterococcus (3/18, 17%). There was a trend toward decreased risk of dominance with levofloxacin exposure (3 [14%] vs 15 [38%]; P = .051) and no difference in the proportion of those who had dominance in those patients exposed to BSBL antibiotics (8 [38%] vs 10 [26%]; P = .32).
On bivariable analysis, only oral vancomycin exposure was associated with an increased risk of dominance (OR, 2.07; 95% CI, 1.43 to 3.00; P < .001). There was no significant association found with levofloxacin or BSBL exposure. As dominance has previously been associated with risk of BSI in the pre-engraftment period [8], we investigated rates of BSI in the 30-day period after sample collection. Among the 60 patients, there were 13 episodes of bacteremia, most (n = 12; 92%) occurring in the 14 days after sample collection. Among those with subsequent BSI, dominance with a non-Bacteroidetes taxa was found only in 2 cases: a patient with Lactobacillus dominance who developed Staphylococcus epidermidis BSI and a patient with Synergistes domination who developed an Escherichia coli BSI.
Subanalysis
Extending the window of antibiotic exposure to the 14 days before sample collection, levofloxacin was not associated with increased alpha diversity on bivariable analysis (β [IQR], 0.006 [–0.05 to 0.18]; P = .29) and there was a trend toward reduction in alpha diversity with receipt of BSBL antibiotics (β [IQR], –0.07 [–0.15 to 0.00]; P = .053) after adjusting for oral vancomycin exposure (Table 4). Neither BSBL antibiotics nor levofloxacin was associated with a difference in the proportion of Bacteroidetes or dominance with non-Bacteroidetes genera.
Table 4.
Microbiome Characteristics by Continuous Antibiotic Exposure in the Prior 14 Days
| Bivariable Analysis | P Value | Multivariable Analysis | ||
|---|---|---|---|---|
| β Coefficient | β Coefficient | |||
| Variable | (95% CI) | (95% CI) | P Value | |
| Alpha diversitya | ||||
| Levofloxacin | 0.06 (–0.05 to 0.18) | .29 | ||
| BSBL | –0.08 (–0.17 to 0.00) | .053 | –0.07 (–0.15 to 0.00) | .052 |
| Oral vancomycin | –0.14 (–0.20 to –0.08) | <.001 | –0.14 (–0.19 to –0.08) | <.001 |
| Bacteroidetes proportional abundance | ||||
| Levofloxacin | –0.01 (–0.35 to 0.34) | .98 | ||
| BSBL | –0.02 (–0.27 to 0.24) | .88 | ||
| Oral vancomycin | –0.48 (–0.72 to –0.33) | <.001 | ||
| Dominance by non-Bacteroidetes taxon | ||||
| Levofloxacin | 0.96 (0.78 to 1.20) | .76 | ||
| BSBL | 1.05 (0.91 to 1.22) | .73 | ||
| Oral vancomycin | 1.56 (1.21 to 2.00) | .001 |
Unless noted otherwise, data are presented as median (interquartile range).
Abbreviations: BSBL, broad-spectrum beta-lactam; CI, confidence interval.
aShannon’s alpha diversity.
Principal Coordinate Analysis
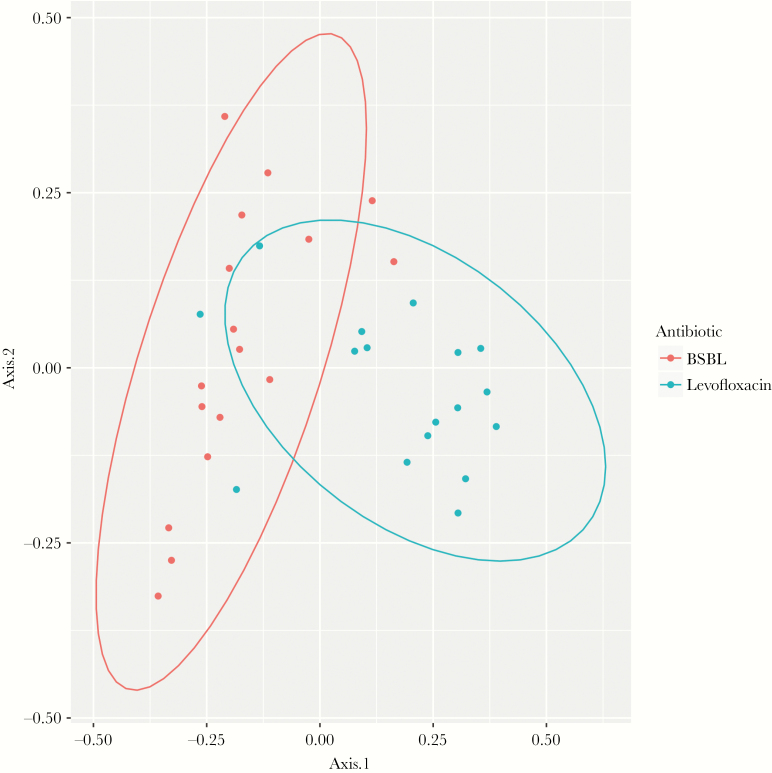
In the subset of 34 patients who had exposure to levofloxacin only or BSBL antibiotics only in the prior 7 days, we analyzed beta diversity using the Bray-Curtis dissimilarity index. Two-dimensional PCoA of pairwise Bray-Curtis distances is depicted in Figure 3 to evaluate clustering by levofloxacin or BSBL exposure. Using PERMANOVA, differential antibiotic exposure to levofloxacin vs BSBL antibiotics accounted for 12.4% of the overall variance within samples (R2 = .124; P < .001).
Figure 3.
Principal coordinate analysis. Abbreviation: BSBL, broad-spectrum beta-lactam.
Specific Taxa of Clinical Importance
Compared with no antibiotic exposure in the prior 7 days, levofloxacin exposure was not associated with a significant difference in the proportion of Enterococcus genus (2.2 × 10–5 with vs 3.9 × 10–5 without exposure; P = .28). BSBL exposure was associated with a trend toward increased proportional abundance of Enterococcus genus (2.2 × 10–4 vs 2.0 × 10–5; P = .08). Levofloxacin exposure was associated with a significant reduction in Proteobacteria (6.8 × 10–5 vs 1.3 × 10–2, P < .001), whereas BSBL exposure was not associated with a significant difference (1.1 × 10–2 vs 1.1 × 10–3; P = .22).
Levofloxacin exposure was associated with a greater proportion of Lachnospiraceae (8.3 × 10-2 vs 2.7 × 10-2; P = .04) and Ruminococcaceae (3.8 × 10-2 vs 9.8 × 10-3; P = .04). There was no significant difference in the class Clostridia (0.28 vs 0.23; P = .27), but levofloxacin exposure was associated with a greater proportion of Blautia genus (0.013 vs 1.1 × 10-3; P = .001). BSBL exposure was associated with no significant difference in Lachnospiraceae or Ruminococcaceae (8.3 × 10-2 vs 2.7 × 10-2; P = .34; and 8.7 × 10-3 vs 9.5 × 10-2; P = .26). However, BSBL exposure was associated with a reduction in Clostridia (0.10 vs 0.31; P < .001) and Blautia (1.1 × 10-3 vs 9.5 × 10-3; P = .005). Eubacterium limosum was not identified in our data set.
DISCUSSION
In this study, we demonstrated that levofloxacin prophylaxis is associated with less alteration of the gut microbiome compared with the receipt of BSBL antibiotics or oral vancomycin. Furthermore, receipt of levofloxacin was not associated with specific changes in the gut microbiome that have been previously associated with poor clinical outcomes, including reduction of alpha diversity, dominance of non-Bacteroidetes taxa, or reduction in taxa protective of C. difficile. In contrast, receipt of BSBL antibiotics was associated with a reduction in alpha diversity. These findings suggest that receipt of levofloxacin prophylaxis for the prevention of neutropenic fever and BSI in patients undergoing autologous SCT or induction chemotherapy for AML may have a potential protective effect on the gut microbiome by preventing exposure to BSBL antibiotics [20].
We showed that levofloxacin prophylaxis was not associated with a reduction in alpha diversity or dominance of non-Bacteroidetes taxa compared with patients who received BSBL antibiotics or a group of patients who received no antibiotics in the preceding 7 days. This contrasts with a prior study [27] that investigated the impact of the receipt of ciprofloxacin in 3 healthy individuals and found that ciprofloxacin decreased alpha diversity. Additionally, a study of colorectal surgery patients showed that ciprofloxacin suppressed both anaerobic and aerobic fecal flora [28]. In contrast, levofloxacin has been shown to have a minimal impact on anaerobic bacterial species, which have been associated with resistance to colonization with pathogenic bacteria [29–31]. However, these studies investigating the specific impact of fluoroquinolone antibiotics have primarily focused on small groups of healthy patients, and thus may not be applicable to patients receiving treatment for hematologic malignancy.
Patients admitted for chemotherapy and SCT have often been exposed to antibiotics during prior hospitalizations, which may have a lasting impact on their gut microbiome [13]. Further, chemotherapies may directly disrupt the gut microbiome through both antibiotic and immunologic effects [32–34]. These factors may potentially contribute to the differences that we observed compared with those in healthy hosts. Prior studies investigating the impact of antibiotic exposures on the gut microbiome in patients with hematologic malignancy have been limited, and few have investigated the impact of antibiotics given for the prophylaxis of BSI. A prior study showed that anti-anaerobic antibiotic prophylaxis with ciprofloxacin and metronidazole may increase transplant-related mortality and disrupt the gut microbiome [35, 36]. Our study of levofloxacin without metronidazole has greater relevance to current clinical practice, however, as levofloxacin is the most commonly prescribed agent for prophylaxis of BSI [37]. In another study of patients undergoing allogeneic SCT, receipt of levofloxacin was associated with reduction in domination of Proteobacteria and thus potentially lower risk of BSI with gram-negative bacteria [8]. However, this study did not explore the impact of levofloxacin on microbiome characteristics other than dominance.
In addition to the relatively limited impact of levofloxacin on the gut microbiome in this population, we have also shown that BSBL antibiotics are associated with a significant reduction in alpha diversity. This aligns with findings from a prior study in patients receiving treatment for AML, which showed that receipt of imipenem resulted in a decline in alpha diversity [38]. Furthermore, in a study of patients undergoing allogeneic SCT, early exposure to broad-spectrum beta-lactam antibiotics was associated with a higher transplant-related mortality and lower fecal abundance of commensal Clostridiales [36]. Use of broad-spectrum anti-anaerobic antibiotics in patients undergoing allogeneic SCT has also been associated with increased GVHD-associated mortality, potentially due to disruption of anaerobic taxa and microbiome disruption [39]. Additionally, broad-spectrum antibiotic-induced innate immune deficits have been associated with both vancomycin-resistant Enterococcus (VRE) acquisition and resulting BSI [8, 40, 41]. The results of our study further emphasize the important impact that BSBL antibiotics may have on the gut microbiome and the associated clinical outcomes in this population.
Our study has several potential limitations. First, patients had heterogenous antibiotic exposures, including exposure to multiple antibiotic classes. This may have led to misclassification of antibiotic effects, as patients who received BSBL antibiotics were more likely to receive oral vancomycin than those patients who received levofloxacin, potentially confounding the impact of BSBL antibiotics on the gut microbiome. However, by accounting for continuous antibiotic exposures though multivariable regression, we attempted to address this concern. Second, the significant window of antibiotic exposure to impact the gut microbiome remains unknown. We found that associations between antibiotic exposures and microbiome outcomes weakened as the antibiotic window was lengthened. Longitudinal prospective microbiome sampling would allow for better characterization of the impact of levofloxacin prophylaxis on the gut microbiome. Retrospective data also limited our ability to assess temporal changes to the gut microbiome, as it is possible that microbiome disruption may occur before neutropenic fever, rather than as a consequence of antibiotic treatment for neutropenic fever. Finally, residual patient samples were collected in the evaluation of diarrhea, which is associated with gut microbiome disruption [42]. However, chemotherapy-associated diarrhea occurs in the majority of patients after both transplant and inpatient chemotherapy treatment, with rates of up to 80% [43]. Thus, our sampling strategy represents the majority of patients after inpatient treatment of hematologic malignancy.
In conclusion, we found that receipt of levofloxacin for the prophylaxis of neutropenic fever and BSI in patients with hematologic malignancy is associated with lesser disruption of the gut microbiome as compared with receipt of BSBL antibiotics. Thus, by preventing exposure to BSBL antibiotics [20] and with relatively limited impact of this agent on the gut microbiome in this population, use of levofloxacin prophylaxis may be protective of the gut microbiome while preventing gram-negative BSIs. Given the association with gut microbiome disruption and adverse outcomes in this population, understanding the impact of this common antibiotic exposure may help guide the clinical decision for providing antibiotic prophylaxis in these patients. However, prospective studies are needed to replicate our findings, with rigorous methods to disentangle the impact of the timing and duration of antibiotic exposures.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by the National Institutes of Health (grant No. T32-AI055435 to M.Z. and grant No. K23 AI121485 to B.K.) and by a Centers for Disease Control and Prevention (CDC) Cooperative Agreement, FOA#CK16-004-Epicenters for the Prevention of Healthcare Associated Infections, and a contract award from the CDC (BAA 2016-N-17812: 200-2016-91937).
Disclaimer. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data availability. 16S rRNA sequence data have been made publicly available at the National Center for Biotechnology Information’s Sequence Read Archive (NCBI SRA): Bioproject ID: PRJNA542146.
Prior presentation. Interim results were presented as a poster presentation at the Infectious Diseases Society of America IDWeek conference; October 5, 2018; San Francisco, CA.
References
- 1. Tomblyn M, Chiller T, Einsele H, et al. ; Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 2010; 6:e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sonis ST. The pathobiology of mucositis. Nat Rev Cancer 2004; 4:277–84. [DOI] [PubMed] [Google Scholar]
- 4. Bucaneve G, Micozzi A, Menichetti F, et al. ; Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) Infection Program Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med 2005; 353:977–87. [DOI] [PubMed] [Google Scholar]
- 5. Cullen M, Steven N, Billingham L, et al. . Simple Investigation in Neutropenic Individuals of the Frequency of Infection after Chemotherapy +/- Antibiotic in a Number of Tumours (SIGNIFICANT) Trial Group Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med 2005; 353:988–98. [DOI] [PubMed] [Google Scholar]
- 6. Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med 2005; 142:979–95. [DOI] [PubMed] [Google Scholar]
- 7. Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ 2017; 356:j831. [DOI] [PubMed] [Google Scholar]
- 8. Taur Y, Xavier JB, Lipuma L, et al. . Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doki N, Suyama M, Sasajima S, et al. . Clinical impact of pre-transplant gut microbial diversity on outcomes of allogeneic hematopoietic stem cell transplantation. Ann Hematol 2017; 96:1517–23. [DOI] [PubMed] [Google Scholar]
- 10. Peled JU, Devlin SM, Staffas A, et al. . Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol 2017; 35:1650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taur Y, Jenq RR, Perales MA, et al. . The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abeles SR, Jones MB, Santiago-Rodriguez TM, et al. . Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome 2016; 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 2011; 108(Suppl 1):4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falony G, Joossens M, Vieira-Silva S, et al. . Population-level analysis of gut microbiome variation. Science 2016; 352:560–4. [DOI] [PubMed] [Google Scholar]
- 15. Shono Y, Docampo MD, Peled JU, et al. . Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8:339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donskey CJ, Helfand MS, Pultz NJ, Rice LB. Effect of parenteral fluoroquinolone administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. Antimicrob Agents Chemother 2004; 48:326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Revolinski SL, Munoz-Price LS. Clostridium difficile exposures, colonization, and the microbiome: implications for prevention. Infect Control Hosp Epidemiol 2018; 39:596–602. [DOI] [PubMed] [Google Scholar]
- 18. Isaac S, Scher JU, Djukovic A, et al. . Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother 2017; 72:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis BB, Buffie CG, Carter RA, et al. . Loss of microbiota-mediated colonization resistance to Clostridium difficile infection with oral vancomycin compared with metronidazole. J Infect Dis 2015; 212:1656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ziegler M, Landsburg D, Pegues D, et al. . Fluoroquinolone prophylaxis is highly effective for the prevention of central line-associated bloodstream infections in autologous stem cell transplant patients. Biol Blood Marrow Transplant 2019; 25:1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ganetsky A, Han JH, Hughes ME, et al. . Oral vancomycin prophylaxis is highly effective in preventing Clostridium difficile infection in allogeneic hematopoietic cell transplant recipients. Clin Infect Dis. 2019; 68:2003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgan XC, Huttenhower C. Chapter 12: human microbiome analysis. PLoS Comput Biol 2012; 8:e1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee YJ, Arguello ES, Jenq RR, et al. . Protective factors in the intestinal microbiome against Clostridium difficile infection in recipients of allogeneic hematopoietic stem cell transplantation. J Infect Dis 2017; 215:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wexler AG, Goodman AL. An insider’s perspective: bacteroides as a window into the microbiome. Nat Microbiol 2017; 2:17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jenq RR, Taur Y, Devlin SM, et al. . Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 2015; 21:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Narushima S, Sugiura Y, Oshima K, et al. . Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes 2014; 5:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brismar B, Edlund C, Malmborg AS, Nord CE. Ciprofloxacin concentrations and impact of the colon microflora in patients undergoing colorectal surgery. Antimicrob Agents Chemother 1990; 34:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stein GE, Goldstein EJ. Fluoroquinolones and anaerobes. Clin Infect Dis 2006; 42:1598–607. [DOI] [PubMed] [Google Scholar]
- 30. Holt HA, Lewis DA, White LO, et al. . Effect of oral ciprofloxacin on the faecal flora of healthy volunteers. Eur J Clin Microbiol 1986; 5:201–5. [DOI] [PubMed] [Google Scholar]
- 31. Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev 2017; 279:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viaud S, Daillère R, Yamazaki T, et al. . Why should we need the gut microbiota to respond to cancer therapies? Oncoimmunology 2014; 3:e27574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Viaud S, Saccheri F, Mignot G, et al. . The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bodet CA 3rd, Jorgensen JH, Drutz DJ. Antibacterial activities of antineoplastic agents. Antimicrob Agents Chemother 1985; 28:437–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weber D, Oefner PJ, Dettmer K, et al. . Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant 2016; 51:1087–92. [DOI] [PubMed] [Google Scholar]
- 36. Weber D, Jenq RR, Peled JU, et al. . Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2017; 23:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rashidi A, Wangjam T, Bhatt AS, et al. . BMT CTN Investigators Antibiotic practice patterns in hematopoietic cell transplantation: a survey of blood and marrow transplant clinical trials network centers. Am J Hematol 2018; 93:E348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galloway-Peña JR, Smith DP, Sahasrabhojane P, et al. . The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 2016; 122:2186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shono Y, Docampo MD, Peled JU, et al. . Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8:339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brandl K, Plitas G, Mihu CN, et al. . Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 2008; 455:804–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ubeda C, Taur Y, Jenq RR, et al. . Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 2010; 120:4332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Antharam VC, Li EC, Ishmael A, et al. . Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol 2013; 51:2884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol 2010; 2:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.