Abstract
Background
Herpes zoster (HZ) develops in up to 50% of unvaccinated individuals, accounting for >1 million cases annually in the United States. A live attenuated HZ vaccine (LAV) is Food and Drug Administration approved for those age 50 years or older, though Advisory Committee on Immunization Practices recommendations are only for those age 60 years or older. LAV efficacy is ~70% for persons 50–59 years of age, with lower efficacy in older adults. A new 2-dose adjuvanted subunit vaccine (SUV) has >95% efficacy in persons 50–69 years of age and remains ~90% efficacious in persons vaccinated at age 70 years.
Methods
To estimate the relative cost-effectiveness of SUV, LAV, and no vaccination (NoV) strategies, a Markov model was developed based on published data on vaccine efficacy, durability of protection, quality of life, resource utilization, costs, and disease epidemiology. The perspective was US societal, and the cycle length was 1 year with a lifelong time horizon. SUV efficacy was estimated to wane at the same rate as LAV. Outcomes evaluated included lifetime costs, discounted life expectancy, and incremental cost-effectiveness ratios (ICERs).
Results
For individuals vaccinated at age 50 years, the ICER for LAV vs NoV was $118 535 per quality-adjusted life-year (QALY); at age 60 years, the ICER dropped to $42 712/QALY. SUV was more expensive but had better ICERs than LAV. At age 50, the ICER was $91 156/QALY, and it dropped to $19 300/QALY at age 60.
Conclusions
Vaccination with SUV was more cost-effective than LAV in all age groups studied. Vaccination with SUV at age 50 years appears cost-effective, with an ICER <$100 000/QALY.
Keywords: cost-effectiveness, herpes zoster, prevention, vaccine
This manuscript describes a cost-effectiveness analysis utilizing a Markov decision model that demonstrates vaccination with the two-dose adjuvanted subunit herpes zoster vaccine, given current pricing, offers very good value in comparison to the live attenuated herpes zoster vaccine or no vaccination strategy. Even at higher prices, faster waning rates, and poorer adherence to the second dose, our model suggests the subunit vaccine remains cost-effective at a willingness-to-pay threshold of $100,000 per quality-adjusted life-year.
During primary infection with varicella-zoster virus (VZV), a diffuse vesicular rash develops as VZV enters the bloodstream, leading to seeding of sensory nerve ganglia and establishment of a reservoir of latent VZV [1, 2]. From this reservoir, reactivation can occur in the form of herpes zoster (HZ) [1, 2]. HZ typically manifests as a painful rash following the distribution of 1 or more sensory nerves, usually developing decades after primary infection [1, 2]. Although the pain of acute HZ itself can be severe and result in hospitalization, a dreaded complication of HZ is post-herpetic neuralgia (PHN), which can persist for months to years, potentially resulting in marked debilitation and reduced quality of life [1, 2].
The risk of HZ and PHN increases with age as the level of T-cell immunity declines with immunosenescence [1, 2]. This risk also increases in immunocompromised persons with impaired T-cell immunity, including those infected with HIV, recipients of immunosuppressive therapy after organ or stem cell transplant, recipients of other forms of immunosuppressive therapy, and those with lymphoma or leukemia [2]. HZ develops in up to 50% of unvaccinated individuals who live to 85 years of age, and there are more than 1 million cases of HZ in the United States each year [1, 2]. The direct medical cost burden of HZ infection in the United States has been estimated to possibly exceed $1 billion annually [3].
A live attenuated Oka strain varicella-zoster vaccine (LAV; Zostavax, Merck & Co., inc., Whitehouse Station, NJ [Merck]) was approved by the Food and Drug Administration (FDA) in 2006 for the prevention of HZ for persons 60 years of age or older [1]. In 2008, it was recommended by the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) for this indication [2]. Notably, the efficacy of LAV to prevent HZ decreases with age, from ~70% efficacy for persons 50–59 years of age to 64% for persons 60 to 69 years of age, and down to approximately 37% for persons 70 years of age or older [2, 4, 5]. Injection site reactions (ISRs) were generally mild [2, 5], and LAV efficacy was found to wane in the Long-Term Prevention Study (LTPS) [6]. More recently, LAV was FDA approved in persons 50 years of age or older [2]; however, in 2014, the ACIP elected to leave its age recommendation unchanged, in part based upon an unpublished cost-effectiveness analysis (CEA) [7].
In October 2017, the FDA approved an HZ subunit vaccine (SUV) containing recombinant varicella-zoster virus glycoprotein E and the AS01B adjuvant system for the prevention of HZ in people aged 50 years and older (Shingrix, GlaxoSmithKline Biologicals, Research Triangle Park, NC [GSK]) [8]. SUV was studied as a 2-dose series administered 2 months apart in 2 randomized, placebo-controlled trials, ZOE-50 (adults 50 years of age and older) and ZOE-70 (adults 70 years of age and older), demonstrating 96.2% and 87.7% efficacy at reducing the risk of HZ in the total vaccinated cohorts, respectively [9, 10]. Pooled analysis for persons 70 years of age and older in ZOE-50 and ZOE-70 demonstrated efficacy of 89.9% against HZ and 78.9% against PHN in the total vaccinated cohort [10]. There were more ISRs and systemic reactions compared with placebo, but serious adverse events occurred with similar frequencies, and 95.5% returned for their second immunization [9, 10].
There have been numerous studies that have analyzed cost-effectiveness for LAV in the form of cost per quality-adjusted life-year (QALY) and incremental cost-effectiveness ratio (ICER) compared with no vaccine (NoV), with variable results based on age at vaccination, epidemiology, utility estimates, duration of vaccine protection, and cost of the vaccine [11–18]. In general, LAV has been found to be effective in preventing HZ and PHN in persons aged 50 years and older; however, it has been most consistently found to be cost-effective in persons aged 60 and above [11–18].
On January 26, 2018, the ACIP recommended the use of SUV for the prevention of HZ and related complications for immunocompetent adults aged ≥50 years, including those who have previously received LAV [8]. The ACIP stated a preference for SUV over LAV for the prevention of HZ and related complications [8]. These recommendations were in part driven by CEAs by the CDC [19], Le and Rothberg [20], GSK [21], and Merck. Additional independent CEAs are prudent. The following analysis compares the cost-effectiveness of SUV with LAV and a strategy of no vaccine.
METHODS
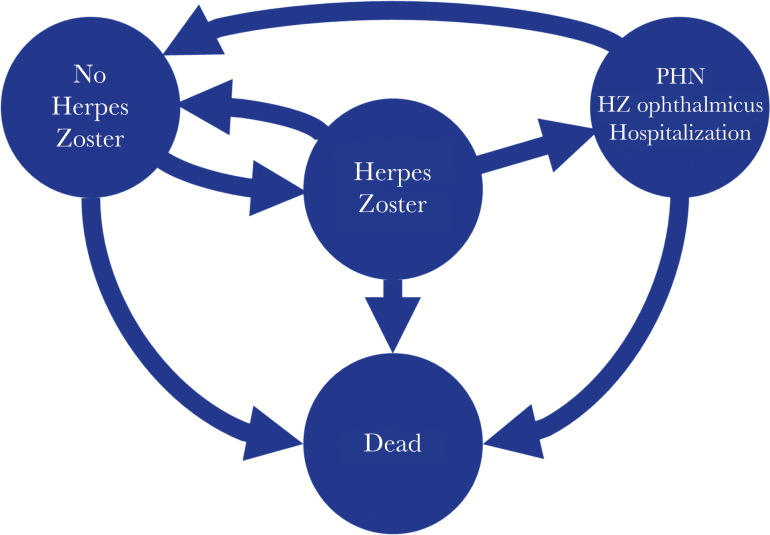
To estimate the cost utility of SUV vs the LAV and no vaccination strategies, a Markov decision model was developed (Figure 1) based on published randomized controlled trials and data on vaccine efficacy, durability of protection, quality of life, resource utilization, costs, and disease epidemiology, utilizing Microsoft Excel 2016 (Microsoft Corporation). The target population was stratified by age, allowing entry into the model at age 50 years or older, and data were modeled until the last cohort member was assumed to have died at age 100 years. The cycle length was 1 year, with a lifelong time horizon, and data for men and women were modeled together. The CEA took a societal perspective in its cost and utility assessments, with both costs (in 2018 US dollars) and QALYs discounted at 3% per year, and it was structured to allow comparisons based on 3 vaccination strategies: SUV, LAV, and NoV.
Figure 1.
Four state Markov model structure. No Vaccination, Subunit Vaccination, and Live Attenuated Vaccine differ in the rates at which individuals transition from the “No Herpes Zoster” state to the “Herpes Zoster” state. The reduction in herpes zoster incidence with vaccination depends on the initial vaccine efficacy (based on age of vaccination) and the number of years since vaccination (where the linear vaccine waning reduces efficacy each year). Abbreviations: HZ, herpes zoster; PHN, post-herpetic neuralgia.
Individuals entering the model were assumed to be immunocompetent adults at risk for HZ and PHN based upon 2011 US age group risk estimates [22], decreased as appropriate based on the efficacy associated with each vaccine strategy. Modeled complications included PHN, acute ocular involvement, hospitalization, and death. These complications were assumed to be independent, and individuals were returned to the health state “at risk for HZ” unless they died from HZ or other causes. Utility was assumed to be lost for acute ocular complications and PHN for that episode cycle year, and for death utility was lost as a permanent decrement in QALYs. The outcomes included costs (vaccine-associated costs, direct medical costs, and indirect productivity costs) and effectiveness (reductions in HZ cases and PHN cases, increases in QALYs) for each strategy. The ICER was estimated as the incremental cost divided by the incremental change in QALYs of each strategy vs the other strategies.
Model inputs and assumptions for the base case analysis are presented in Table 1. Data were derived primarily from studies of the general US population without immune-compromising conditions and include epidemiologic parameters, vaccination parameters, costs, and QALYs [2, 4, 5, 9, 10, 14]. Age-specific mortality rates were based on 2011 US life tables [23]. Measures of the risk of PHN, degree of severity, and associated loss of utility were derived from Le and Rothberg [14] and from Harvey [24].
Table 1.
Model Parameters: Epidemiologic Data, Vaccination Efficacy and Adverse Events, Utilities and Costs With Base Case Values, and Ranges Used for Sensitivity Analyses
| Variable | Base Case Value | Range | Source |
|---|---|---|---|
| HZ incidence per 1000 person-years | Johnson et al. [22], 2015 | ||
| 50–59 y | 6.74 | 6.66 to 6.82 | |
| 60–69 y | 9.32 | 9.20 to 9.44 | |
| 70–79 y | 12.02 | 11.79 to 12.25 | |
| 80–100 y | 12.78 | 12.49 to 13.07 | |
| Complications given HZ, % | |||
| Probability of ocular complications | Harvey [24], 2016 | ||
| 50–59 y | 0.03 | 0.00 to 0.05 | |
| 60–69 y | 0.04 | 0.02 to 0.06 | |
| 70–79 y | 0.05 | 0.03 to 0.07 | |
| 80–100 y | 0.07 | 0.05 to 0.09 | |
| PHN by age | Le et al. [14], 2015 | ||
| 50–59 y | 0.038 | 0.023 to 0.053 | |
| 60–69 y | 0.069 | 0.042 to 0.096 | |
| 70–100 y | 0.185 | 0.142 to 0.228 | |
| Probability of moderate or severe PHN | Harvey [24], 2016 | ||
| 50–59 y | 0.46 | 0.36 to 0.56 | |
| 60–69 y | 0.56 | 0.46 to 0.66 | |
| 70–79 y | 0.61 | 0.51 to 0.71 | |
| 80–84 y | 0.65 | 0.55 to 0.75 | |
| 85–100 y | 0.68 | 0.58 to 0.78 | |
| Probability of moderate PHN in individuals with mod–severe PHN | 0.5 | 0.03 to 0.08 | Harvey [24], 2016 |
| Probability of pain given HZ | 0.05 | 0.00 to 0.27 | Harvey [24], 2016 |
| No pain given HZ | 0.11 | 0.04 to 0.43 | |
| Mild pain given HZ | 0.37 | 0.19 to 0.57 | |
| Moderate pain given HZ | 0.47 | 0.26 to 0.63 | |
| Severe pain given HZ | |||
| Death due to HZ per 100 000 cases of HZ | CDC WONDER [31], 2017 | ||
| 50–59 y | 1.26 | 0.86 to 1.67 | |
| 60–69 y | 2.22 | 1.72 to 2.72 | Le et al. [14], 2015 |
| 70–79 y | 6.18 | 5.32 to 7.03 | |
| 80–89 y | 23.96 | 21.88 to 26.03 | |
| 90–100 y | 152.13 | 143.76 to 160.5 | |
| Vaccination efficacy and adverse events | |||
| LAV efficacy | 0.698 | 0.489 to 0.907 | Oxman et al. [5], 2005, and Harvey [24], 2016 |
| 50–59 y | |||
| 60–69 y | 0.657 | 0.460 to 0.854 | |
| 70–79 y | 0.407 | 0.285 to 0.529 | |
| 80–100 | 0.157 | 0.110 to 0.204 | |
| LAV waning rate (and assumed SUV waning rate) | –0.0544 | –0.072 to –0.037 | Morrison et al. [6], 2015, and Le et al. [14], 2015 |
| SUV efficacy | Lal et al. [9], 2015, and Cunningham et al. [10], 2016 | ||
| Aged 50–59 y | 0.969 | 0.906 to 0.994 | |
| Aged 60–69 y | 0.941 | 0.856 to 0.981 | |
| Aged 70–79 y | 0.899 | 0.846 to 0.937 | |
| Aged 80 y and above | 0.897 | 0.786 to 0.958 | |
| SUV waning rate (2 doses) | –0.0544 | –0.072 to –0.037 | Assumption |
| SUV waning rate (1 dose) | –0.0800 | Assumption | |
| Adverse events above placebo | |||
| LAV injection site reaction | 0.317 | 0.283 to 0.326 | Morrison et al. [6], 2015 |
| LAV serious adverse event | 0.001 | 0 to 0.002 | Morrison et al. [6], 2015 |
| SUV injection site reaction | 0.669 | 0.6021 to 0.7359 | Lal et al. [9], 2015, and Cunningham et al. [20], 2016 |
| SUV serious adverse event | 0.00058 | 0 to 0.003 | Lal et al. [9], 2015, and Cunningham et al. [10], 2016 |
| Utilities | |||
| Acute HZ QALYs lost | Lieu et al. [29], 2008 | ||
| No pain HZ | 0.020 | 0.014 to 0.026 | |
| Mild HZ | 0.041 | 0.029 to 0.053 | |
| Moderate HZ | 0.047 | 0.033 to 0.061 | |
| Severe HZ | 0.058 | 0.040 to 0.075 | |
| Average Acute HZ QALYs lost | 0.050 | ||
| PHN QALYs lost (1 y) | Harvey [24], 2016 | ||
| Mild pain PHN | 0.31 | 0.211 to 0.433 | |
| Moderate pain PHN | 0.55 | 0.389 to 0.731 | |
| Severe pain PHN | 0.77 | 0.498 to 0.992 | |
| Ocular complications QALYs lost (1 y) | 0.24 | 0.178 to 0.311 | Harvey [24], 2016 |
| Common adverse reaction QALYs lost per dose | 0.001 | 0.0005 to 0.002 | Harvey [24], 2016 |
| Serious adverse event QALYs lost per dose | 2.13E-05 | 6.41E-06 to 4.57E-05 | Harvey [24], 2016 |
| Costs | |||
| LAV | $134.16 | $100.62 to $167.70 | CDC (CMS Cost/Dose) [27], 2018 |
| SUV | $204.38 | $153.29 to $255.48 | CDC (CMS Cost/Dose) [27], 2018 |
| Vaccine administration cost (HCPCS 90471) | $20.88 | $15.66 to $26.10 | CMS Fee Schedule [28], 2018 |
| Direct medical costs, $/case, adjusted for inflation | Harvey [24], 2016 | ||
| Acute HZ | $957 | $867 to $1051 | |
| PHN | $5831 | $4055 to $7936 | |
| Ocular complications | $4163 | $2986 to $5543 | |
| Serious adverse event | $9778.32 | $5975.64 to $13 581.00 | Dunn et al. [32], 2014 |
| Productivity costs HZ, h | Harvey [24], 2016 | ||
| No pain HZ | 5 | 3 to 6 | |
| Mild | 6 | 4 to 8 | |
| Moderate HZ | 22 | 15 to 30 | |
| Severe HZ | 61 | 39 to 82 | |
| Mild pain, PHN | 4 | 3 to 5 | |
| Moderate pain, PHN | 30 | 20 to 41 | |
| Severe pain, PHN | 81 | 53 to 110 | |
| Average hourly wages | $26.61 | $19.96 to $33.26 |
Abbreviations: CDC, Centers for Disease Control and Prevention; CMS, Centers for Medicare and Medicaid Services; HCPCS, Healthcare Common Procedure Coding System; HZ, herpes zoster; ICER, incremental cost-effectiveness ratio; LAV, live attenuated herpes zoster vaccine; NoV, no vaccine; PHN, post-herpetic neuralgia; QALY, quality-adjusted life-year, SUV, subunit vaccine.
Vaccine efficacy rates were obtained from published randomized controlled trials [4, 8, 9]. Adherence to the second dose of SUV was 95.5% in the combined data for ZOE-50 and ZOE-70; as such, the efficacy data utilized were from the total vaccinated cohorts to account for reduced efficacy due to the reduced adherence to the second dose [9, 10]. The efficacy of LAV over time has been studied in the LTPS for persons aged 60 years and older, with limited long-term efficacy data for persons aged 50 to 59 years [6]. Le and Rothberg, in their cost-effectiveness analysis of HZ for persons aged 50 years, calculated a linear slope waning rate of –0.0544 per year with the assumption that LAV waned at the same rate regardless of age at vaccination, and they utilized this waning rate estimate for SUV [6, 14]. We similarly utilized –0.0544 per year as the waning rate for the slope in our model (ie, vaccine efficacy decreased linearly at 5.44% per year) for LAV and after 2 doses of SUV, and we assumed a more rapid waning rate of –0.08 per year when only 1 dose of SUV was received. Prosser, in her economic evaluation of LAV and SUV for the prevention of HZ and related complications, estimated similar waning rates for SUV (–0.0515 per year for both doses and –0.0909 per year for 1 dose) based on data from Cunningham et al. and other assumptions [10, 19].
Costs, including those associated with acute HZ, PHN, and other complications, were derived from prior estimates with adjustment to 2018 US dollars [24–26]. As of June 1, 2018, the CDC listed its vaccine contract price for LAV at $134.16, and the initial CDC vaccine contract price for SUV was $102.19 per dose or $204.38 total for both doses [27]. Immunization administration cost was $20.88 per dose based on the 2018 Centers for Medicare and Medicaid Services payment schedule [28]. Injection site reactions were assumed to incur no financial costs, and serious adverse events due to the vaccine had costs estimated per dose, with productivity costs due to HZ stratified by severity [24]. Utility estimates were obtained from multiple sources [24, 29]. QALYs were adjusted for age based on estimates of US QALYs for noninstitutionalized adults utilizing 7 health-related quality-of-life scores [30]. Mortality rates due to HZ were obtained from the CDC WONDER online database [31].
Sensitivity analyses were performed to assess assumptions and methodological uncertainties by varying the age at vaccination, the waning rate, efficacy, adherence to the second SUV dose, and other variables. Both 1-way and 2-way sensitivity analyses were performed.
RESULTS
For the base case analysis (Table 2), a strategy of NoV with a cohort entering the model at age 50, 60, and 70 years of age resulted in 31.3%, 26.2%, and 19.7% of individuals developing HZ, respectively. Administration of LAV at these ages resulted in absolute HZ lifetime cumulative incidence reductions of 3.3%, 3.9%, and 1.9%, respectively, whereas SUV had greater reductions at 6.5%, 7.9%, and 8.1%, respectively. Similarly, PHN developed in 3.8%, 3.8%, and 3.6% of people entering the model at age 50, 60, and 70 years, with absolute PHN lifetime cumulative incidence reduced by 0.1%, 0.3%, and 0.4% for LAV, and 0.3%, 0.8%, and 1.5% for SUV, respectively. As a validation of the model, the cumulative lifetime incidence of HZ and PHN are similar to those found in the literature (Supplementary Table 1).
Table 2.
Lifetime Costs and Effectiveness of NoV vs LAV vs SUV Among Persons Aged 50, 60, and 70 Years With LAV Priced at $134 and SUV Priced at $204
| Age at Vaccination, y | Strategy | HZ Cases, % | HZ Cases Prevented vs NoV, % | PHN Cases, % | PHN Cases Prevented vs NoV, % | Total Cost, $ | Incremental Cost vs NoV, $ | QALYs | Incremental QALYs vs NoV | ICER vs NoV, $/QALY |
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | NoV | 31.28 | 3.85 | 519.06 | 16.047 | |||||
| LAV | 27.99 | 3.29 | 3.71 | 0.14 | 602.64 | 83.58 | 16.047 | 0.000705 | 118 535 | |
| SUV | 24.82 | 6.47 | 3.54 | 0.31 | 630.29 | 111.24 | 16.048 | 0.001220 | 91 156 | |
| 60 | NoV | 26.24 | 3.82 | 540.16 | 12.801 | |||||
| LAV | 22.35 | 3.89 | 3.51 | 0.31 | 602.09 | 61.93 | 12.802 | 0.001450 | 42 712 | |
| SUV | 18.34 | 7.90 | 3.03 | 0.79 | 599.05 | 58.90 | 12.804 | 0.003052 | 19 300 | |
| 70 | NoV | 19.70 | 3.65 | 509.19 | 9.267 | |||||
| LAV | 17.76 | 1.94 | 3.29 | 0.36 | 600.40 | 91.21 | 9.268 | 0.001034 | 88 251 | |
| SUV | 11.63 | 8.07 | 2.15 | 1.49 | 516.78 | 7.59 | 9.273 | 0.005392 | 1407 |
Abbreviations: ICER, incremental cost-effectiveness ratio; LAV, live attenuated herpes zoster vaccine; NoV, no vaccine; PHN, post-herpetic neuralgia; QALY, quality-adjusted life-year, SUV, subunit vaccine.
The utility benefits for 1 million adults vaccinated with LAV at ages 50, 60, and 70 years were 705, 1450, and 1034 QALYs saved, and for SUV, the QALYs saved were 1220, 3052, and 5392, respectively. At a price of $134.16 for LAV and total SUV price for both doses of $204.38, incremental costs for LAV and SUV vs NoV were estimated at $84 and $111 for an adult aged 50 years, $62 and $59 for an adult aged 60 years, and $91 and $8 for an adult aged 70 years at vaccination, respectively.
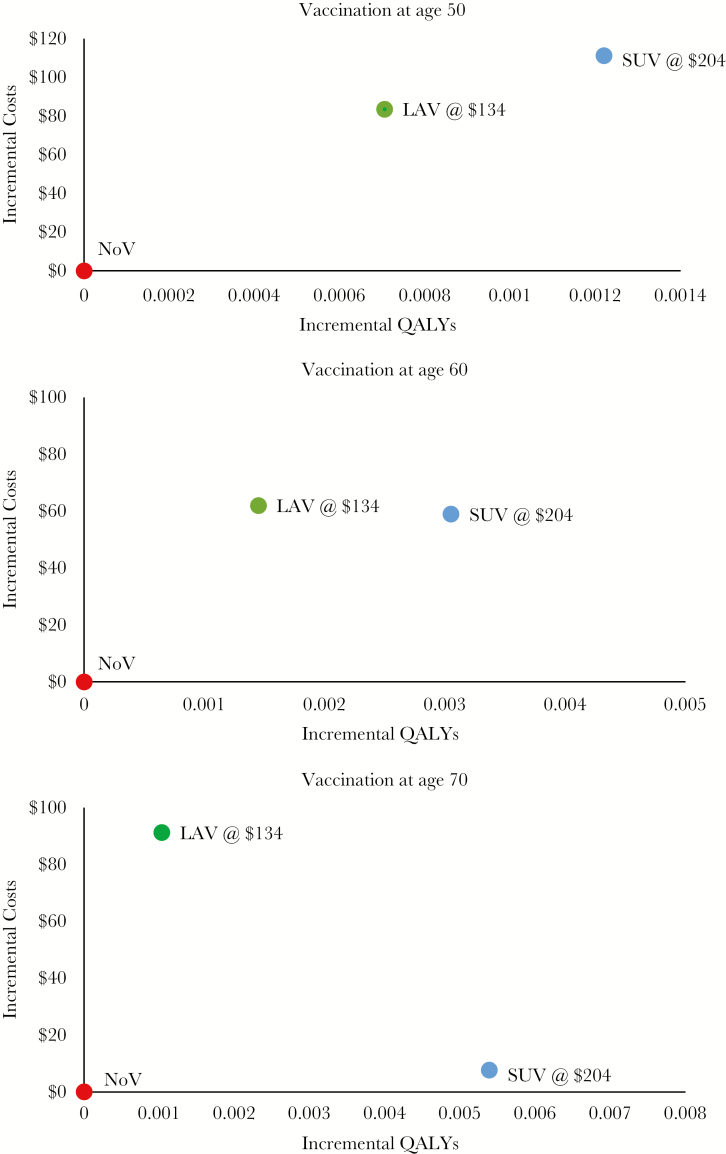
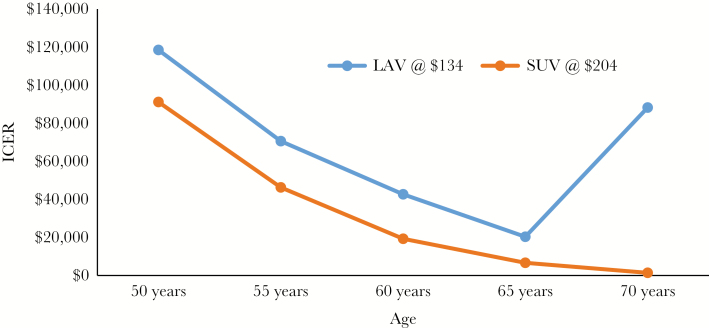
The ICERs for LAV vs NoV at age 50, 60, and 70 years are $118 535, $42 712, and $88 251 per QALY, respectively (Table 2). This nonlinear behavior stems from the low risk of HZ at age 50 and the short duration of protection with the vaccine, which leads to a high cost-effectiveness ratio at age 50, but the lack of initial efficacy at older ages leads to a high cost-effectiveness ratio at age 70. For SUV, the corresponding ICER for each age group compared with NoV is $91 156, $19 300, and $1407 per QALY, respectively. Figure 2 consists of cost-effectiveness planes for individuals aged 50, 60, and 70 years. The plane for the first age group (Figure 2A) is notable for SUV demonstrating extended dominance over LAV; for the older age groups (Figure 2B and C), LAV has fewer QALYs saved at a higher incremental cost than SUV, indicating that LAV is dominated by SUV. Figure 3 demonstrates higher ICERs for LAV vs SUV compared with no vaccination at all age groups. We used the model to calculate the price of SUV that would result in achieving willingness-to-pay thresholds from $50 000 to $100 000 per QALY for vaccination at age 50, 60, and 70 years vs the NoV strategy (Table 3). At the FDA-approved and ACIP-recommended age of vaccination of 50 years, SUV would be cost-effective at a total price for both doses of $153 for a willingness-to-pay threshold of $50 000 per QALY and at $216 for a willingness-to-pay threshold of $100 000 per QALY. The price for these ICER thresholds is substantially higher the older the age at vaccination.
Figure 2.
Cost-effectiveness planes for SUV at different costs, LAV and no vaccination. Abbreviations: LAV, live attenuated herpes zoster vaccine; QALY, quality-adjusted life-year, SUV, subunit vaccine.
Figure 3.
ICERs vs no vaccination varied by age at vaccination. Abbreviations: ICER, incremental cost-effectiveness ratio; LAV, live attenuated herpes zoster vaccine; QALY, quality-adjusted life-year, SUV, subunit vaccine.
Table 3.
SUV Price to Achieve ICER Target Relative to NoV
| Age | Willingness-to-Pay Threshold, $ | SUV Price to Achieve Willingness-to-Pay Threshold, $/QALY |
|---|---|---|
| 50 | 50 000 | 152.65 |
| 75 000 | 184.07 | |
| 100 000 | 215.50 | |
| 60 | 50 000 | 300.88 |
| 75 000 | 379.46 | |
| 100 000 | 458.04 | |
| 70 | 50 000 | 474.25 |
| 75 000 | 613.09 | |
| 100 000 | 751.93 |
Abbreviations: ICER, incremental cost-effectiveness ratio; NoV, no vaccine; QALY, quality-adjusted life-year, SUV, subunit vaccine.
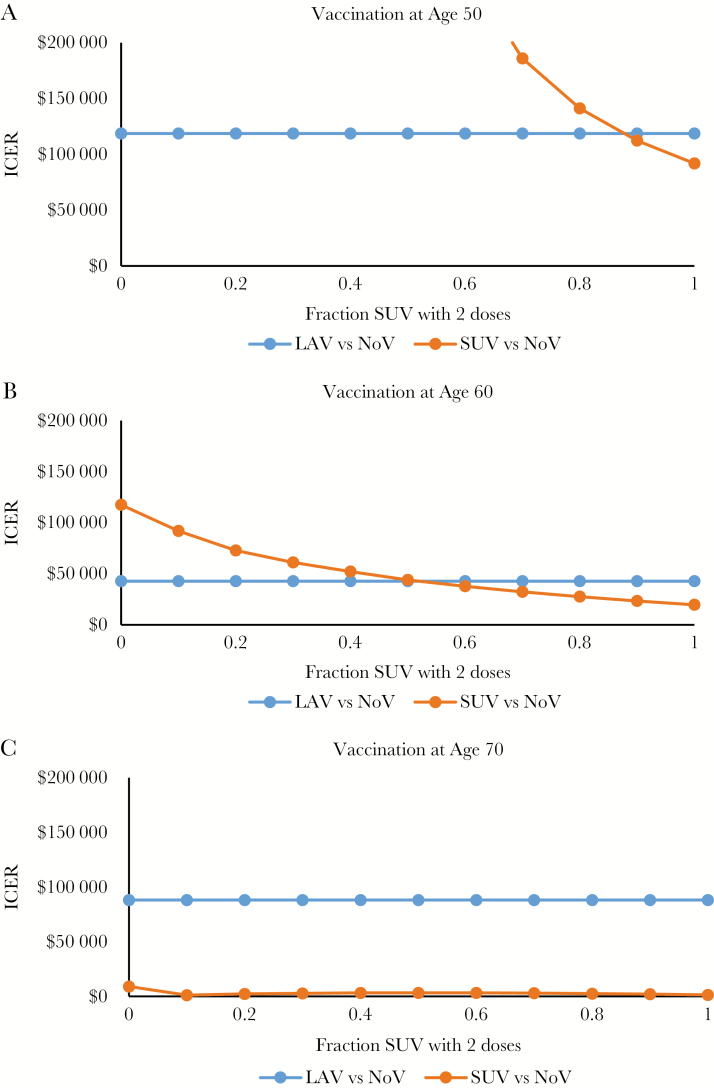
One-way sensitivity analysis of multiple factors is found in Table 4 for age of vaccination of 50 years, 60 years, and 70 years. Notably, the long-term effectiveness of the vaccine (how quickly effectiveness wanes) is a strong driver of cost-effectiveness. If the SUV efficacy waning rate is –0.072 per year, vaccinating 60-year-olds would have an ICER of $55 082 per QALY, but if SUV wanes more slowly at –0.037 per year, vaccinating 60-year-olds would actually result in cost savings. An additional 1-way analysis of the percentage receiving the second SUV dose by age is found in Figure 4, with assumptions including that a single SUV dose is only 80% as effective as 2 doses initially and that 1 dose had an efficacy waning rate of –0.08 per year (resulting in lost efficacy by 10 years). For those vaccinated at age 50 years (Figure 4A), ~90% must adhere to the second dose for LAV to maintain its cost-effectiveness advantage over SUV; however, only ~50% of 60-year-old vaccine recipients (Figure 4B) need to adhere to maintain the advantage, and by age 70 years (Figure 4C), the cost-effectiveness advantage is maintained regardless of adherence rate.
Table 4A.
One-way Sensitivity Analysis (Impact on ICER for Those Vaccinated at Age 50)
| Variable | Base Case Value | Range (Low–High) | ICER of LAV vs NoV (Low) | ICER of LAV vs NoV (High) | ICER of UV vs NoV (Low) | ICER of UV vs NoV (High) |
|---|---|---|---|---|---|---|
| HZ incidence per 1000 person-years | ||||||
| 50–59 y | 6.74 | 6.66 to 6.82 | $122 293 | $114 944 | $93 947 | $88 476 |
| 60–69 y | 9.32 | 9.20 to 9.44 | $119 128 | $117 947 | $92 398 | $89 937 |
| 70–79 y | 12.02 | 11.79 to 12.25 | $118 535 | $118 535 | $91 156 | $91 156 |
| 80–100 y | 12.78 | 12.49 to 13.07 | $118 535 | $118 535 | $91 156 | $91 156 |
| Complications given HZ, % | ||||||
| Probability of ocular complications | ||||||
| 50–59 y | 0.03 | 0.00 to 0.05 | $118 535 | $118 535 | $91 156 | $91 156 |
| 60–69 y | 0.04 | 0.02 to 0.06 | $118 535 | $118 535 | $91 156 | $91 156 |
| 70–79 y | 0.05 | 0.03 to 0.07 | $118 535 | $118 535 | $91 156 | $91 156 |
| 80–100 y | 0.07 | 0.05 to 0.09 | $118 535 | $118 535 | $91 156 | $91 156 |
| PHN by age | ||||||
| 50–59 y | 0.038 | 0.023 to 0.053 | $147 097 | $98 262 | $111 284 | $76 338 |
| 60–69 y | 0.069 | 0.042 to 0.096 | $124 608 | $112 953 | $104 309 | $80 523 |
| 70–100 y | 0.185 | 0.142 to 0.228 | $118 535 | $118 535 | $91 156 | $91 156 |
| Probability of moderate or severe PHN | ||||||
| 50–59 y | 0.46 | 0.36 to 0.56 | $125 132 | $112 582 | $95 711 | $87 001 |
| 60–69 y | 0.56 | 0.46 to 0.66 | $119 950 | $117 153 | $93 971 | $88 499 |
| 70–79 y | 0.61 | 0.51 to 0.71 | $118 535 | $118 535 | $91 156 | $91 156 |
| 80–84 y | 0.65 | 0.55 to 0.75 | $118 535 | $118 535 | $91 156 | $91 156 |
| 85–100 y | 0.68 | 0.58 to 0.78 | $118 535 | $118 535 | $91 156 | $91 156 |
| Probability of moderate PHN in individuals with moderate or severe PHN | 0.5 | 0.03 to 0.08 | $113 217 | $127 477 | $86 068 | $99 955 |
| Probability of pain given HZ | ||||||
| No pain given HZ | 0.05 | 0.00 to 0.27 | $12 545 | $103 649 | $94 460 | $79 034 |
| Mild pain given HZ | 0.11 | 0.04 to 0.43 | $131 068 | $82 868 | $101 579 | $62 354 |
| Moderate pain given HZ | 0.37 | 0.19 to 0.57 | $134 138 | $103 522 | $104 586 | $78 449 |
| Severe pain given HZ | 0.47 | 0.26 to 0.63 | $141 224 | $98 034 | $111 358 | $73 277 |
| Death due to HZ per 100 000 | ||||||
| 50–59 y | 1.26 | 0.86 to 1.67 | $118 774 | $118 292 | $91 320 | $90 989 |
| 60–69 y | 2.22 | 1.72 to 2.72 | $118 567 | $118 504 | $91 215 | $91 097 |
| 70–79 y | 6.18 | 5.32 to 7.03 | $118 535 | $118 535 | $91 156 | $91 156 |
| 80–89 y | 23.96 | 21.88 to 26.03 | $118 535 | $118 535 | $91 156 | $91 156 |
| 90–100 y | 152.13 | 143.76 to 160.5 | $118 535 | $118 535 | $91 156 | $91 156 |
| Vaccination efficacy and adverse events | ||||||
| LAV efficacy (take) | ||||||
| 50–59 y | 0.698 | 0.489 to 0.907 | Dominated | $21 980 | $91 156 | $91 156 |
| LAV waning rate (reduction in efficacy per year) | –0.0544 | –0.072 to –0.037 | $289 148 | $38 318 | $91 156 | $91 156 |
| SUV efficacy (take) | ||||||
| Aged 50–59 y | 0.969 | 0.906 to 0.994 | $118 535 | $118 535 | $148 649 | $76 381 |
| SUV waning rate (reduction in efficacy per year) | –0.0544 | –0.072 to –0.037 | $118 535 | $118 535 | $279 166 | $19 330 |
| Adverse events above placebo | ||||||
| LAV injection site reaction | 0.317 | 0.283 to 0.326 | $118 535 | $118 535 | $91 156 | $91 156 |
| LAV serious adverse event | 0.001 | 0 to 0.002 | $118 535 | $118 535 | $91 156 | $91 156 |
| SUV injection site reaction | 0.669 | 0.6021 to 0.7359 | $118 535 | $118 535 | $79 783 | $106 311 |
| SUV serious adverse event | 0.00058 | 0 to 0.003 | $118 535 | $118 535 | $90 321 | $94 831 |
| Utilities | ||||||
| Acute HZ QALYs lost | ||||||
| No pain HZ | 0.02 | 0.014 to 0.026 | $119 726 | $117 368 | $92 139 | $90 194 |
| Mild HZ | 0.041 | 0.029 to 0.053 | $124 327 | $113 259 | $95 953 | $86 816 |
| Moderate HZ | 0.047 | 0.033 to 0.061 | $142 874 | $101 282 | $111 548 | $77 067 |
| Severe HZ | 0.058 | 0.040 to 0.075 | $161 894 | $93 495 | $127 923 | $70 806 |
| PHN QALYs lost | ||||||
| Mild pain PHN | 0.31 | 0.211 to 0.433 | $123 419 | $112 981 | $95 483 | $86 297 |
| Moderate pain PHN | 0.55 | 0.389 to 0.731 | $123 993 | $112 946 | $96 430 | $85 876 |
| Severe pain PHN | 0.77 | 0.498 to 0.992 | $130 683 | $110 177 | $103 058 | $83 304 |
| Ocular complications QALYs lost | 0.24 | 0.178 to 0.311 | $118 535 | $118 535 | $91 156 | $91 156 |
| Adverse reactions and adverse events | ||||||
| Common AR QALYs lost per dose | 0.001 | 0.0005 to 0.002 | $74 809 | Dominated | $53 222 | Dominated |
| Serious AE QALYs lost per dose | 0.0000213 | 6.4E-06 to 4.6E-05 | $116 507 | $122 016 | $90 631 | $92 029 |
| Costs | ||||||
| LAV | $134.16 | $100.62 to $167.70 | $72 353 | $164 718 | $91 156 | $91 156 |
| SUV | $204.38 | $153.29 to $255.48 | $118 535 | $118 535 | $50 509 | $131 812 |
| Vaccine administration cost (HCPCS 90471) | $20.88 | $15.66 to $26.10 | $111 348 | $125 723 | $83 037 | $99 275 |
| Direct medical costs, $/case, adjusted for inflation | ||||||
| Acute HZ | $957 | $867 to $1151 | $122 257 | $114 648 | $95 167 | $86 967 |
| PHN | $5831 | $4055 to $7936 | $121 582 | $114 925 | $94 808 | $86 828 |
| Ocular complications | $4163 | $2986 to $5543 | $118 535 | $118 535 | $91 156 | $91 156 |
| Serious adverse event, $/vaccine dose | $0.18 | $0.11 to $0.25 | $118 439 | $118 632 | $91 124 | $91 188 |
| Productivity costs HZ, h | ||||||
| No pain HZ | 5 | 3 to 5 | $118 640 | $118 483 | $91 268 | $91 100 |
| Mild | 6 | 4 to 8 | $120 515 | $116 555 | $93 290 | $89 022 |
| Moderate HZ | 22 | 15 to 30 | $121 219 | $115 469 | $94 048 | $87 851 |
| Severe HZ | 61 | 39 to 82 | $129 268 | $108 290 | $102 722 | $80 116 |
| Mild pain, PHN | 4 | 3 to 5 | $118 558 | $118 513 | $91 182 | $91 130 |
| Moderate pain, PHN | 30 | 20 to 41 | $118 639 | $118 422 | $91 285 | $91 014 |
| Severe pain, PHN | 81 | 53 to 110 | $118 825 | $118 235 | $91 518 | $90 781 |
| Average hourly wages, $ | $26.61 | $19.96 to $33.26 | $129 939 | $107 132 | $103 496 | $78 816 |
Abbreviations: AE, adverse event; AR, adverse reaction; HCPCS, Healthcare Common Procedure Coding System; HZ, herpes zoster; ICER, incremental cost-effectiveness ratio; LAV, live attenuated HZ vaccine; NoV, no vaccine; PHN, post-herpetic neuralgia; QALY, quality-adjusted life-year, SUV, subunit vaccine.
Figure 4.
One-way analysis of adherence to second SUV dose. Abbreviations: ICER, incremental cost-effectiveness ratio; LAV, live attenuated herpes zoster vaccine; NoV, no vaccine; QALY, quality-adjusted life-year, SUV, subunit vaccine.
Table 4B.
One-Way Sensitivity Analysis (Impact on ICER for Those Vaccinated at Age 60)
| Variable | Base Case Value | Range (Low–High) | ICER of AV vs NoV (Low) | ICER of AV vs NoV (High) | ICER of UV vs NoV (Low) | ICER of UV vs NoV (High) |
|---|---|---|---|---|---|---|
| HZ incidence per 1000 person-years | ||||||
| 60–69 y | 9.32 | 9.20 to 9.44 | $44 209 | $41 269 | $20 125 | $18 498 |
| 70–79 y | 12.02 | 11.79 to 12.25 | $42 980 | $42 447 | $19 795 | $18 814 |
| 80–100 y | 12.78 | 12.49 to 13.07 | $42 712 | $42 712 | $19 300 | $19 300 |
| Complications given HZ, % | ||||||
| Probability of ocular complications | ||||||
| 60–69 y | 0.04 | 0.02 to 0.06 | $42 712 | $42 712 | $19 300 | $19 300 |
| 70–79 y | 0.05 | 0.03 to 0.07 | $42 712 | $42 712 | $19 300 | $19 300 |
| 80–100 y | 0.07 | 0.05 to 0.09 | $42 712 | $42 712 | $19 300 | $19 300 |
| PHN by age | ||||||
| 60–69 y | 0.069 | 0.042 to 0.096 | $58 423 | $32 254 | $26 478 | $14 048 |
| 70–100 y | 0.185 | 0.142 to 0.228 | $44 435 | $41 080 | $22 318 | $16 684 |
| Probability of moderate or severe PHN | ||||||
| 60–69 y | 0.56 | 0.46 to 0.66 | $45 391 | $40 311 | $20 310 | $18 372 |
| 70–79 y | 0.61 | 0.51 to 0.71 | $43 260 | $42 177 | $20 041 | $18 604 |
| 80–84 y | 0.65 | 0.55 to 0.75 | $42 712 | $42 712 | $19 300 | $19 300 |
| 85–100 y | 0.68 | 0.58 to 0.78 | $42 712 | $42 712 | $19 300 | $19 300 |
| Probability of moderate PHN in individuals with moderate or severe PHN | 0.5 | 0.03 to 0.08 | $40 162 | $47 132 | $17 845 | $21 870 |
| Probability of pain given HZ | ||||||
| No pain given HZ | 0.05 | 0.00 to 0.27 | $43 496 | $39 588 | $19 606 | $18 067 |
| Mild pain given HZ | 0.11 | 0.04 to 0.43 | $45 148 | $34 366 | $20 298 | $15 802 |
| Moderate pain given HZ | 0.37 | 0.19 to 0.57 | $46 355 | $38 839 | $21 275 | $17 176 |
| Severe pain given HZ | 0.47 | 0.26 to 0.63 | $48 808 | $36 480 | $23 157 | $15 299 |
| Death due to HZ per 100 000 | ||||||
| 60–69 y | 2.22 | 1.72 to 2.72 | $42 712 | $42 712 | $19 300 | $19 300 |
| 70–79 y | 6.18 | 5.32 to 7.03 | $42 762 | $42 663 | $19 317 | $19 283 |
| 80–89 y | 23.96 | 21.88 to 26.03 | $42 718 | $42 707 | $19 306 | $19 294 |
| 90–100 y | 152.13 | 143.76 to 160.5 | $42 712 | $42 712 | $19 300 | $19 300 |
| Vaccination efficacy and adverse events | ||||||
| LAV | ||||||
| 60–69 y | 0.657 | 0.460 to 0.854 | $334 733 | $682 | $19 300 | $19 300 |
| LAV waning rate | –0.0544 | –0.072 to –0.037 | $90 956 | $8690 | $19 300 | $19 300 |
| SUV | ||||||
| Aged 60–69 y | 0.941 | 0.856 to 0.981 | $42 712 | $42 712 | $40 084 | $12 720 |
| SUV waning rate | –0.0544 | –0.072 to –0.037 | $42 712 | $42 712 | $55 082 | Cost-saving |
| Adverse events above placebo | ||||||
| LAV injection site reaction | 0.317 | 0.283 to 0.326 | $42 712 | $42 712 | $19 300 | $19 300 |
| LAV serious adverse event | 0.001 | 0 to 0.002 | $42 712 | $42 712 | $19 300 | $19 300 |
| SUV injection site reaction | 0.669 | 0.6021 to 0.7359 | $42 712 | $42 712 | $18 286 | $20 432 |
| SUV serious adverse event | 0.00058 | 0 to 0.003 | $42 712 | $42 712 | $19 203 | $19 714 |
| Utilities | ||||||
| Acute HZ QALYs lost | ||||||
| No pain HZ | 0.02 | 0.014 to 0.026 | $42 953 | $42 474 | $19 399 | $19 202 |
| Mild HZ | 0.041 | 0.029 to 0.053 | $43 865 | $41 619 | $19 774 | $18 848 |
| Moderate HZ | 0.047 | 0.033 to 0.061 | $47 255 | $38 967 | $21 153 | $17 745 |
| Severe HZ | 0.058 | 0.040 to 0.075 | $50 316 | $37 105 | $22 383 | $16 963 |
| PHN QALYs lost | ||||||
| Mild pain PHN | 0.31 | 0.211 to 0.433 | $44 242 | $40 954 | $20 043 | $18 450 |
| Moderate pain PHN | 0.55 | 0.389 to 0.731 | $45 214 | $40 211 | $20 586 | $18 033 |
| Severe pain PHN | 0.77 | 0.498 to 0.992 | $48 363 | $38 994 | $22 233 | $17 424 |
| Ocular complications QALYs lost | 0.24 | 0.178 to 0.311 | $42 712 | $42 712 | $19 300 | $19 300 |
| Adverse reactions and adverse events | ||||||
| Common AR QALYs lost per dose | 0.001 | 0.0005 to 0.002 | $33 464 | $95 504 | $15 112 | $43 299 |
| Serious AE QALYs lost per dose | 0.0000213 | 6.4E-06 to 4.6E-05 | $42 364 | $43 296 | $19 257 | $19 371 |
| Costs | ||||||
| LAV | $134.16 | $100.62 to $167.70 | $20 253 | $65 171 | $19 300 | $19 300 |
| SUV | $204.38 | $153.29 to $255.48 | $42 712 | $42 712 | $3046 | $35 557 |
| Vaccine administration cost (HCPCS 90471) | $20.88 | $15.66 to $26.10 | $39 217 | $46 208 | $16 053 | $22 547 |
| Direct medical costs, $/case, adjusted for inflation | ||||||
| Acute HZ | $957 | $867 to $1151 | $44 873 | $40 455 | $21 283 | $17 228 |
| PHN | $5831 | $4055 to $7936 | $46 035 | $38 774 | $23 017 | $14 895 |
| Ocular complications | $4163 | $2986 to $5543 | $42 712 | $42 712 | $19 300 | $19 300 |
| Serious adverse event, $/vaccine dose | $0.18 | $0.11 to $0.25 | $42 666 | $42 759 | $19 287 | $19 313 |
| Productivity costs HZ, h | ||||||
| No pain HZ | 5 | 3 to 5 | $42 773 | $42 682 | $19 356 | $19 272 |
| Mild | 6 | 4 to 8 | $43 862 | $41 563 | $20 355 | $18 245 |
| Moderate HZ | 22 | 15 to 30 | $44 270 | $40 932 | $20 730 | $17 666 |
| Severe HZ | 61 | 39 to 82 | $48 944 | $36 764 | $25 020 | $13 840 |
| Mild pain, PHN | 4 | 3 to 5 | $42 733 | $42 692 | $19 322 | $19 278 |
| Moderate pain, PHN | 30 | 20 to 41 | $42 847 | $42 565 | $19 453 | $19 131 |
| Severe pain, PHN | 81 | 53 to 110 | $43 088 | $42 323 | $19 730 | $18 855 |
| Average hourly wages | $26.61 | $19.96 to $33.26 | $49 546 | $35 879 | $25 660 | $12 940 |
Abbreviations: AE, adverse event; AR, adverse reaction; HCPCS, Healthcare Common Procedure Coding System; HZ, herpes zoster; ICER, incremental cost-effectiveness ratio; LAV, live attenuated HZ vaccine; NoV, no vaccine; PHN, post-herpetic neuralgia; QALY, quality-adjusted life-year, SUV, subunit vaccine.
Table 4C.
One-Way Sensitivity Analysis (Impact on ICER for Those Vaccinated at Age 70)
| Variable | Base Case Value | Range (Low–High) | ICER of AV vs NoV (Low) | ICER of LAV vs NoV (High) | ICER of SUV vs NoV (Low) | ICER of SUV vs NoV (High) |
|---|---|---|---|---|---|---|
| HZ incidence per 1000 person-years | ||||||
| 70–79 y | 12.02 | 11.79 to 12.25 | $92 457 | $84 319 | $2149 | $697 |
| 80–100 y | 12.78 | 12.49 to 13.07 | $88 251 | $88 251 | $1559 | $1257 |
| Complications given HZ, % | ||||||
| Probability of ocular complications | ||||||
| 70–79 y | 0.05 | 0.03 to 0.07 | $88 251 | $88 251 | $1407 | $1407 |
| 80–100 y | 0.07 | 0.05 to 0.09 | $88 251 | $88 251 | $1407 | $1407 |
| PHN by age | ||||||
| 70–100 y | 0.185 | 0.142 to 0.228 | $125 194 | $66 297 | $6394 | Cost-saving |
| Probability of moderate or severe PHN | ||||||
| 70–79 y | 0.61 | 0.51 to 0.71 | $99 717 | $79 072 | $1800 | $1066 |
| 80–84 y | 0.65 | 0.55 to 0.75 | $88 251 | $88 251 | $1465 | $1351 |
| 85–100 y | 0.68 | 0.58 to 0.78 | $88 251 | $88 251 | $1414 | $1401 |
| Probability of moderate PHN in individuals with moderate or severe PHN | 0.5 | 0.03 to 0.08 | $79 649 | $105 022 | $953 | $2235 |
| Probability of pain given HZ | ||||||
| No pain given HZ | 0.05 | 0.00 to 0.27 | $89 400 | $83 536 | $1405 | $1420 |
| Mild pain given HZ | 0.11 | 0.04 to 0.43 | $91 716 | $75 425 | $1448 | $1251 |
| Moderate pain given HZ | 0.37 | 0.19 to 0.57 | $92 750 | $83 277 | $1958 | $784 |
| Severe pain given HZ | 0.47 | 0.26 to 0.63 | $94 971 | $81 042 | $2885 | Cost-saving |
| Death due to HZ per 100 000 | ||||||
| 70–79 y | 6.18 | 5.32 to 7.03 | $88 352 | $88 153 | $1408 | $1407 |
| 80–89 y | 23.96 | 21.88 to 26.03 | $88 251 | $88 251 | $1408 | $1407 |
| 90–100 y | 152.13 | 143.76 to 160.5 | $88 251 | $88 251 | $1407 | $1407 |
| Vaccination efficacy and adverse events | ||||||
| LAV | ||||||
| 70–79 y | 0.407 | 0.285 to 0.529 | $616 280 | $27 345 | $1407 | $1407 |
| LAV waning rate | –0.0544 | –0.072 to –0.037 | $147 990 | $44 236 | $1407 | $1407 |
| SUV | ||||||
| Aged 70–79 y | 0.899 | 0.846 to 0.937 | $88 251 | $88 251 | $6236 | Cost-saving |
| SUV waning rate | –0.0544 | –0.072 to –0.037 | $88 251 | $88 251 | $11 242 | Cost-saving |
| Adverse events above placebo | ||||||
| LAV injection site reaction | 0.317 | 0.283 to 0.326 | $88 251 | $88 251 | $1407 | $1407 |
| LAV serious adverse event | 0.001 | 0 to 0.002 | $88 251 | $88 251 | $1407 | $1407 |
| SUV injection site reaction | 0.669 | 0.6021 to 0.7359 | $88 251 | $88 251 | $1367 | $1451 |
| SUV serious adverse event | 0.00058 | 0 to 0.003 | $88 251 | $88 251 | $1385 | $1501 |
| Utilities | ||||||
| Acute HZ QALYs lost | ||||||
| No pain HZ | 0.02 | 0.014 to 0.026 | $88 599 | $87 906 | $1412 | $1403 |
| Mild HZ | 0.041 | 0.029 to 0.053 | $89 906 | $86 656 | $1427 | $1389 |
| Moderate HZ | 0.047 | 0.033 to 0.061 | $94 620 | $82 686 | $1481 | $1341 |
| Severe HZ | 0.058 | 0.040 to 0.075 | $98 695 | $79 806 | $1527 | $1306 |
| PHN QALYs lost | ||||||
| Mild pain PHN | 0.31 | 0.211 to 0.433 | $93 114 | $82 874 | $1463 | $1344 |
| Moderate pain PHN | 0.55 | 0.389 to 0.731 | $97 963 | $79 402 | $1520 | $1300 |
| Severe pain PHN | 0.77 | 0.498 to 0.992 | $111 620 | $75 372 | $1668 | $1249 |
| Ocular complications QALYs lost | 0.24 | 0.178 to 0.311 | $88 251 | $88 251 | $1407 | $1407 |
| Adverse reactions and adverse events | ||||||
| Common AR QALYs lost per dose | 0.001 | 0.0005 to 0.002 | $64 445 | $337 906 | $1225 | $2007 |
| Serious AE QALYs lost per dose | 0.0000213 | 6.4E-06 to 4.6E-05 | $87 291 | $89 871 | $1406 | $1410 |
| Costs | ||||||
| LAV | $134.16 | $100.62 to $167.70 | $56 744 | $119 758 | $1407 | $1407 |
| SUV | $204.38 | $153.29 to $255.48 | $88 251 | $88 251 | Cost-saving | $10 609 |
| Vaccine administration cost (HCPCS 90471) | $20.88 | $15.66 to $26.10 | $83 348 | $93 155 | Cost-saving | $3245 |
| Direct medical costs, $/case, adjusted for inflation | ||||||
| Acute HZ | $957 | $867 to $1151 | $89 834 | $86 599 | $2582 | $180 |
| PHN | $5831 | $4055 to $7936 | $94 028 | $81 404 | $5696 | -$3676 |
| Ocular complications | $4163 | $2986 to $5543 | $88 251 | $88 251 | $1407 | $1407 |
| Serious adverse event, $/vaccine dose | $0.18 | $0.11 to $0.25 | $88 185 | $88 317 | $1400 | $1415 |
| Productivity costs HZ, h | ||||||
| No pain HZ | 5 | 3 to 5 | $88 296 | $88 229 | $1440 | $1391 |
| Mild | 6 | 4 to 8 | $89 093 | $87 410 | $2033 | $782 |
| Moderate HZ | 22 | 15 to 30 | $89 392 | $86 948 | $2255 | $439 |
| Severe HZ | 61 | 39 to 82 | $92 815 | $83 895 | $4796 | Cost-saving |
| Mild pain, PHN | 4 | 3 to 5 | $88 283 | $88 219 | $1431 | $1384 |
| Moderate pain, PHN | 30 | 20 to 41 | $88 501 | $87 976 | $1595 | $1201 |
| Severe pain, PHN | 81 | 53 to 110 | $88 951 | $87 526 | $1933 | $864 |
| Average hourly wages | $26.61 | $19.96 to $33.26 | $93 694 | $82 809 | $5453 | Cost-saving |
Abbreviations: AE, adverse event; AR, adverse reaction; HCPCS, Healthcare Common Procedure Coding System; HZ, herpes zoster; ICER, incremental cost-effectiveness ratio; LAV, live attenuated HZ vaccine; NoV, no vaccine; PHN, post-herpetic neuralgia; QALY, quality-adjusted life-year, SUV, subunit vaccine.
Two-way sensitivity analysis shows that both waning and age at vaccination can be important to the cost-effectiveness of the SUV (Table 5). If the vaccine waning rate is high (7.2%) and 50-year-olds are vaccinated, the ICER is $279 166 per QALY. But if the vaccine waning rate is low (3.7% per year) and 60-year-olds are vaccinated, the vaccine is cost-saving. Supplementary Table 2 has an additional threshold analysis on the waning efficacy of SUV.
Table 5.
Two-Way Sensitivity Analysis on Age and SUV Waning
| Age at Immunization, y | |||||||
|---|---|---|---|---|---|---|---|
| ICER of SUV vs NoV | 50 | 55 | 60 | 65 | 70 | 75 | |
| SUV waning rate | –0.072 | $279 166 | $128 762 | $55 082 | $25 350 | $11 242 | $14 271 |
| –0.065 | $176 504 | $90 444 | $38 576 | $17 130 | $7180 | $10 266 | |
| –0.058 | $113 844 | $59 093 | $25 230 | $10 026 | $3304 | $6505 | |
| –0.051 | $73 511 | $35 805 | $14 233 | $3804 | Cost-saving | $3043 | |
| –0.044 | $42 451 | $18 259 | $5204 | Cost-saving | Cost-saving | Cost-saving | |
| –0.037 | $19 381 | $5239 | Cost-saving | Cost-saving | Cost-saving | Cost-saving | |
Abbreviations: ICER, incremental cost-effectiveness ratio; NoV, no vaccine; SUV, subunit vaccine.
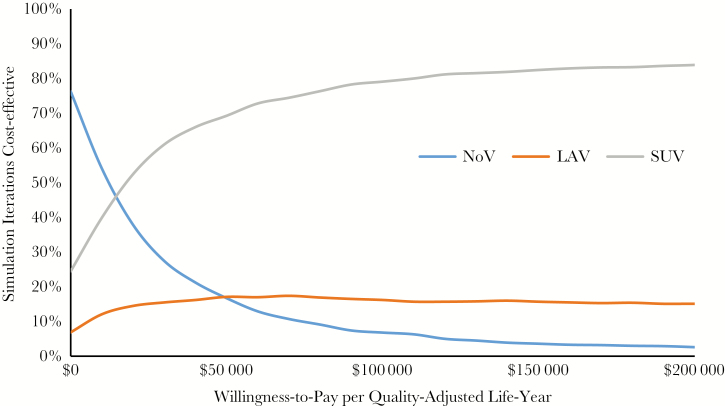
The probabilistic sensitivity analysis (Figure 5) also suggests that SUV is highly likely to be cost-effective for a weighted average of ages from 50 to 70 years. It is 69% likely to be cost-effective at an ICER of $50 000 per QALY and 82% likely to be cost-effective at an ICER of $150 000 per QALY.
Figure 5.
Probabilistic sensitivity analysis: cost-effectiveness acceptability curves. Abbreviations: LAV, live attenuated herpes zoster vaccine; NoV, no vaccine; SUV, subunit vaccine.
DISCUSSION
This CEA demonstrates that for many cost and age at vaccination scenarios, SUV is likely to offer very good value in comparison to an NoV strategy. In most of these scenarios, SUV demonstrates dominance over the currently available LAV. Although LAV has an indication for persons aged 50 years and above, it is only recommended by the ACIP for persons aged 60 years and above due to diminished cost-effectiveness. In contrast, SUV received both an FDA indication and an ACIP recommendation for persons aged 50 and above, with our study and others demonstrating its value for individuals aged 50 to 60 years [9, 20–22]. Targeting persons aged 60 years and above (similar to LAV), SUV appears much more cost-effective, and remains so even with unfavorable variation in its estimated waning rate.
The results of our analysis are very consistent with the analyses by Le et al. [14] and Prosser [19], as well as the GSK analysis subsequently published by Curran et al. [21], despite differences in model structure, assumptions, and inputs. Our model had slightly different functional forms and parameterization for vaccine effectiveness and how PHN affects patients. All 4 analyses found SUV to be cost-effective compared with LAV for the age groups studied. The cost per QALY was in general highest in the youngest age groups, where the incidence of HZ and PHN was the lowest. All models were sensitive to a number of inputs, including vaccine efficacy, age at vaccination, vaccine waning rate, and completion rates for the second dose of SUV. Unlike the studies by Le et al. [14] and Curran et al. [21], our analysis examines the cost-effectiveness of SUV for a younger population aged 50 and finds that SUV could be cost-effective for that population.
There are several limitations to our analysis. Perhaps most significant is the assumption that the waning rate of SUV is comparable to LAV. As noted, few data on this assumption are available; however, the waning rate for SUV is unlikely to be significantly faster than that of LAV based on results extending to 4 years for the ZOE-70 and ZOE-50 trials [9, 10], and SUV might end up having a significantly slower waning rate than LAV. This assumption is thus more likely to result in an underestimate of the value of SUV.
A second important limitation is the assumption that 95.5% of individuals would return for their second SUV dose, as was observed in the phase 3 trials [9, 10]. ISR and the cost of the second dose of the vaccine could result in a lower real-world return rate, resulting in both lower efficacy and lower costs and consequentially mixed ICER variations depending on age at vaccination. As demonstrated in our analysis, when vaccinated at younger ages, adherence to the second dose must be high to maintain cost-effectiveness. At older ages, adherence appears less important from the CEA perspective; however, this older adult population, having more often witnessed the morbidity of HZ and PHN, might be more motivated to adhere to the second dose, regardless of cost or the risk of ISRs. Strategies to optimize adherence will be essential to achieve adherence values at or near those observed in clinical trials [9, 10].
Another limitation in the Markov model is that it does not account for the possibility that patients who develop HZ might develop enhanced immunity against redeveloping HZ and PHN; however, this consideration is controversial [2], and if present, the limitation would affect all 3 strategies comparably, and thus it would be less likely to have significant impact in the final comparison. Furthermore, our analysis does not take into account the potential for a reduction of PHN beyond that afforded by the reduction in HZ; without this additional reduction in PHN incidence, we might be overestimating the associated ICERs for each vaccine strategy. Finally, another important limitation is that the model could not account for individuals vaccinated with SUV who had previously received LAV (ACIP recommends SUV regardless of prior LAV vaccination status) [8].
Additional information will be forthcoming on long-term efficacy, as a subgroup of ZOE-50 and ZOE-70 patients are being monitored for continued efficacy of the vaccine beyond the 4-year study periods. In addition, given the information available on the persistence of LAV efficacy, the role of a booster vaccination for LAV should be considered (and depending on the outcome of the persistence studies for SUV, a booster vaccination for SUV might also need to be considered). Furthermore, the role of combining these vaccines (ie, providing both LAV and SUV in an undetermined sequence separated by an undetermined interval) might also deserve study. Unlike LAV, SUV is not a live vaccine [5, 9, 10], and as such its role in immunocompromised patients is important to study, as this population is generally at even higher risk for HZ and PHN than members of the general population.
In conclusion, immunization of adults aged 50 years and above with SUV appears to be of good value, given the current pricing of SUV and assuming a waning rate comparable to that of LAV. Even at higher prices (especially in older age groups), with faster waning rates, and with poorer adherence to the second dose, our model suggests that SUV remains cost-effective at a willingness-to-pay threshold of $100 000 per QALY. Forthcoming data on SUV waning rate will allow for a more robust comparison between SUV, LAV, and NoV, and additional studies are required to determine the role of booster doses for these vaccines.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. C.F.C. has served on an advisory board for GlaxoSmithKline. A.A., G.P, J.S., and D.H. have no conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cohen JI. Clinical practice: herpes zoster. N Engl J Med 2013; 369:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC) Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30; quiz CE2–4. [PubMed] [Google Scholar]
- 3. White RR, Lenhart G, Singhal PK, et al. Incremental 1-year medical resource utilization and costs for patients with herpes zoster from a set of US health plans. Pharmacoeconomics 2009; 27:781–92. [DOI] [PubMed] [Google Scholar]
- 4. Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 6. Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 55:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR; Centers for Disease Control and Prevention (CDC) Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014; 63:729–31. [PMC free article] [PubMed] [Google Scholar]
- 8. Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018; 67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lal H, Cunningham AL, Godeaux O, et al. ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 10. Cunningham AL, Lal H, Kovac M, et al. ZOE-70 Study Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 11. Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25:8326–37. [DOI] [PubMed] [Google Scholar]
- 12. Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis 2007; 44:1280–8. [DOI] [PubMed] [Google Scholar]
- 13. Brisson M, Pellissier JM, Levin MJ. Cost-effectiveness of herpes zoster vaccine: flawed assumptions regarding efficacy against postherpetic neuralgia. Clin Infect Dis 2007; 45:1527–9. [DOI] [PubMed] [Google Scholar]
- 14. Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med 2015; 163:489–97. [DOI] [PubMed] [Google Scholar]
- 15. Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31:125–36. [DOI] [PubMed] [Google Scholar]
- 16. Kawai K, Preaud E, Baron-Papillon F, et al. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32:1645–53. [DOI] [PubMed] [Google Scholar]
- 17. de Boer PT, Wilschut JC, Postma MJ. Cost-effectiveness of vaccination against herpes zoster. Hum Vaccin Immunother 2014; 10:2048–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med 2006; 145:317–25. [DOI] [PubMed] [Google Scholar]
- 19. Prosser LA. Economic evaluation of vaccination for prevention of herpes zoster and related complications. Paper presented at: Advisory Committee on Immunization Practices; 25 October 2017; Atlanta, GA: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-03-prosser.pdf. Accessed April 2018 [Google Scholar]
- 20. Le P, Rothberg MB. Cost-effectiveness of the adjuvanted herpes zoster subunit vaccine in older adults. JAMA Intern Med 2018; 178:248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine 2018; 36:5037–45. [DOI] [PubMed] [Google Scholar]
- 22. Johnson BH, Palmer L, Gatwood J, et al. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis 2015; 15:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Center for Health Statistics, Centers for Disease Control and Prevention, US Department of Health and Human Services. National vital statistics reports. United States life tables, 2011. 2015. http://www.bls.gov/cps/cpsaat03.htm. Accessed December 2016.
- 24. Harvey MH. Making Good Decisions: Examining the Cost-effectiveness and Optimal Timing of the Herpes Zoster Vaccine [doctoral dissertation]. Ann Arbor, MI: University of Michigan; 2016. https://deepblue.lib.umich.edu/handle/2027.42/135809. Accessed April 2017 [Google Scholar]
- 25. Drolet M, Levin MJ, Schmader KE, et al. Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine 2012; 30:2047–50. [DOI] [PubMed] [Google Scholar]
- 26. US Bureau of Economic Analysis. Gross Domestic Product: Implicit Price Deflator (GDPDEF), retrieved from FRED, Federal Reserve Bank of St. Louis. https://fred.stlouisfed.org/series/GDPDEF. Accessed December 2018. [Google Scholar]
- 27. CDC Vaccine Price List. Vaccines for Children Program (VFC) https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html. Accessed January 2019. [Google Scholar]
- 28. Centers for Medicare & Medicaid Services. Physician fee schedule search https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed April 2018.
- 29. Lieu TA, Ortega-Sanchez I, Ray GT, et al. Community and patient values for preventing herpes zoster. Pharmacoeconomics 2008; 26:235–49. [DOI] [PubMed] [Google Scholar]
- 30. Hanmer J, Lawrence WF, Anderson JP, et al. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making 2006; 26:391–400. [DOI] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention, National Center for Health Statistics. Compressed mortality file 1999–2015 on CDC WONDER online database, released December 2016. Data are from the Compressed Mortality File 1999–2015 Series 20 no. 2U, 2016, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. http://wonder.cdc.gove/cmf-icd10.html. Accessed April 2017. [Google Scholar]
- 32. Dunn JD, Sclar DA. Anaphylaxis: a payor’s perspective on epinephrine autoinjectors. Am J Med 2014; 127:S45–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.