Abstract
Objective
Hypercholesterolemia is associated with the development of a pro-inflammatory state and is a documented risk factor for progression to insulin resistance, nonalcoholic fatty liver and cardiovascular diseases. Sitagliptin is an incretin enhancer that improves glucose tolerance by inhibiting dipeptidyl peptidase-4, but it also has reported anti-inflammatory effects. The current study was thus undertaken to examine the interactions of dietary Cholesterol (Cho) and sitagliptin on markers of inflammation.
Methods
Male Sprague–Dawley rats were provided diets high in Cho and gavaged with vehicle or an aqueous suspension of sitagliptin (100 mg/kg/day) from day 10 through day 35. Molecular methods were used to analyze the lipid profile and inflammatory markers in liver and serum samples. H&E-stained liver sections were used for histopathological evaluation. Hepatic influx of mononuclear cells and necrosis were assessed by immunohistochemistry.
Results
Sitagliptin reduced triglyceride and Cho levels in serum of rats on the control diet but these effects were abrogated in rats on the high-Cho diet. Sitagliptin produced a significant increase in the expression of hepatic inflammatory markers (Tnfa, Il1b, and Mcp1) and a corresponding increase in serum TNFα and IL-1β in rats on the high-Cho diet, but it had no effect on rats on the control diet. Additionally, sitagliptin had no effect on liver morphology in rats on the control diet, but it produced hepatic histopathological changes indicative of necrosis and mononuclear cell infiltration in rats on the high-Cho diet. These mononuclear cells were identified as macrophages and T cells.
Conclusion
When provided in the context of a high-Cho diet, these findings reveal previously unrecognized hepato-inflammatory effects of sitagliptin that are accompanied by evidence of hepatic necrosis and mononuclear cell infiltration.
Keywords: DPP-4 inhibitor, Hepatic necrosis, Hypercholesterolemia, Inflammation
Introduction
Inflammation is a relevant phenomenon in almost all acute and chronic liver diseases. Non-alcoholic fatty liver (NAFL) is one of the most common chronic liver diseases which is characterized by hepatic lipid accumulation (steatosis) in the absence of significant alcohol consumption [1]. A significant proportion of patients with NAFL may progress to a more severe stage which is referred to as non-alcoholic steatohepatitis (NASH) [2]. In addition to steatosis, histological characteristics of NASH include inflammation, necrosis and varying degrees of fibrosis. Under physiological conditions, cytokine production in the liver is either minimal or absent. However, under pathological stimuli such as steatosis, hepatic immune cells become activated and secrete copious amounts of inflammatory cytokines. Pro-inflammatory cytokines such as TNF-α, IL-1β and chemokines such as MCP-1 have been shown to play an important role in hepatic inflammation, a crucial aspect in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) [3–8].
Diet is one of the key modifiable risk factors and its importance in lifestyle management and reduction of NAFLD incidence cannot be ignored. Western diets are comprised of meat, poultry and dairy products that are rich in cholesterol (Cho). Epidemiological studies have provided a link between increased dietary intake of Cho and risk or severity of NAFLD [9, 10]. Although the precise mechanisms of Cho lipotoxicity are not known, studies suggest that increased Cho content can trigger ER stress and mitochondrial dysfunction leading to cell death [11–13]. Furthermore, free Cho can activate liver-resident macrophages (Kupffer cells) and hepatic stellate cells resulting in inflammation and fibrogenesis [14–17].
As NAFLD is frequently associated with obesity, weight loss is an important first approach in the treatment of the disease. Novel incretin-based therapies that include glucagon-like peptide-1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors have also been investigated for the treatment of NAFLD. Sitagliptin is a DPP-4 inhibitor that enhances the action of incretin hormones (GLP-1 and glucose dependent insulinotropic peptide) and lowers blood glucose levels through its insulinotropic effects. It is frequently prescribed in the context of metabolic disease by virtue of its hypoglycemic efficacy and its additional effects to improve endothelial function, cardio-renal function, and lipid profile [18–22]. Additionally, sitagliptin was shown to be beneficial in alleviating both NAFL [23] and NASH [24, 25]. Sitagliptin has also been shown to impact lipid levels, and in type 2 diabetic patients with hyperlipidemia, treatment with this drug improved lipid profile by lowering serum levels of triglycerides and total Cho [26]. However, a search of the literature found that adverse effects of sitagliptin had been reported in both animal and human studies. In a study conducted in mice, sitagliptin caused pancreatic injury which was exacerbated upon feeding a high-fat diet [27]. A small number of cases of hepatic concerns have also been reported in type 2 diabetics who did not have a history of alcohol abuse or chronic liver disease [28]. In a patient with 10-year history of type 2 diabetes and alcohol abuse, sitagliptin was associated with acute hepatic damage [29]. Discontinuation of sitagliptin normalized the elevated hepatic enzymes to baseline levels. Additionally, worsening of heart failure during the use of DPP-4 inhibitors has been reported in patients with type 2 diabetes [30].
Given that hepatic accumulation of Cho can be a critical factor in the progression of NAFLD, and experimental evidence showing that hypercholesterolemia can be associated with inflammatory responses involved in the progression of NAFLD [31], the present work was undertaken to assess the impact of sitagliptin on inflammation in the context of dietary hypercholesterolemia. Using Sprague–Dawley (SD) rats gavaged with vehicle or an aqueous suspension of sitagliptin and fed high-Cho diet, we report that sitagliptin accentuated the effects of high dietary Cho on hepatic and peripheral markers of inflammation [32].
Research design and methods
All animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals, and were approved by the Institutional Animal Care and Use Committee of the Pennington Biomedical Research Center, Baton Rouge, LA.
Animals and diets
Adult male SD rats (6 weeks old) weighing 225–250 g were obtained from Envigo RMS, Inc. (Indianapolis, IN) and used in all experiments. Purina #5001 Chow containing 25.05% carbohydrate, 24.1% protein and 11.4% fat was supplemented with 0.5% cholic acid and 2.0% maltose dextrin and used as the control (Con) diet. The experimental diets were formulated by Dyets (Bethlehem, PA) by enriching the Con diet with 2.0% Cho to produce the high-Cho diet. The energy content of Con and Cho diets were 12.71 kJ/g and12.77 kJ/g, respectively. Food and water were provided ad libitum. Rats were housed individually in cages with standard bedding in a temperature and humidity-controlled room with a 12-h day/night cycle regulation.
Animal experiment
Adult male rats were weight-matched and randomly assigned to Con and Cho groups (n = 16 per dietary group). After 10 days on their respective diets, half of the rats in each dietary group were orally gavaged with an aqueous suspension of sitagliptin (100 mg/kg/day) [33, 34] while the remaining half were gavaged with vehicle (water). The diet and drug regimen were continued for an additional 25 days. Food intake, body weight, body composition and fasting blood glucose were measured at weekly intervals. On day 36, after a 4-h fast, CO2 inhalation was used to produce respiratory arrest, followed by cardiac puncture to obtain blood samples and rapid harvest of livers.
Measurement of body composition
Body composition (lean mass and fat mass) was measured at the designated intervals in each experiment using NMR spectroscopy (Bruker Minispec, Billerica, MA) and calibration standards provided by the manufacturer.
Sample collection
A fasting blood sample (initial) was collected by retro-orbital puncture under anesthesia (isoflurane–oxygen inhalation) at the beginning of each experiment. The final blood sample was collected at the end of the study by cardiac puncture (after CO2 inhalation just before euthanasia). Serum was separated and stored at − 80 °C for the lipid and inflammatory marker(s) analysis. Immediately after harvest, a small segment from the largest lobe of the liver was processed for fixation, paraffin embedding, and sectioning for histological analysis. The remaining tissue was snap frozen in liquid nitrogen and stored at − 80 °C for further analysis.
Histology
The liver samples were fixed in 10% neutral buffer formalin and processed on a TissueTek VIP 6 Vacuum Infiltration Processor. Liver tissue was embedded in paraffin and sectioned into 5 μm and stained with hematoxylin and eosin (H&E) for microscopy and histopathological examination. The H&E staining was performed using a Leica St 5020 Autostainer. Slides were also scanned at 20X using a Hamamatsu Nanozoomer Digital Pathology system (Hamamatsu City, Japan). After blinding the identity of the specimens, the liver slides were evaluated by the pathologist. The specimens were evaluated for necrosis, fat infiltration, fibrosis and mononuclear cell infiltration, and were assigned a score between 1 and 4 where 1 had the lowest lesion and 4 had the highest.
RNA isolation and quantitative real-time PCR
Approximately 50–100 mg of each liver sample was mixed with 300 µL of TRIzol (MRC, Inc., Cincinnati, OH, USA) and homogenized using a hand-held homogenizer. After incubation for 5 min at room temperature, 30 µL of 1-bromo-3-cholopropane (Sigma-Aldrich, St. Louis, MO, USA) was added and vortexed. After centrifugation at 12,000 rpm for 15 min at 4 °C, the supernatant was transferred to a fresh tube for the addition of 70% ethanol (1:1). Total RNA was isolated using RNeasy mini kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol and RNA samples were quantified on a NanoDrop spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). 2.0 μg of total RNA was reverse-transcribed using oligo-(dT)20 primers and M-MLV reverse transcriptase using the kit from Promega (Madison, WI) and 10 ng of cDNA was used to conduct quantitative real-time PCR on a Step One Plus System (Applied Biosystems, Foster City, CA, USA). The sequences of primers are provided in Table 1. Target gene expression in each sample was normalized to the endogenous control gene cyclophilin in respective samples.
Table 1.
List of primers used for QRT-PCR analysis
| Gene | Forward primer sequence (5′–3′) | Forward primer sequence (5′–3′) |
|---|---|---|
| CypA | TATCTGCACTGCCAAGACTGAGTG | CTTCTTGCTGGTCTTGCCATTCC |
| Tnfa | AGACCCTCACACTCAGATCA | GTCTTTGAGATCCATGCCATTG |
| Mcp1 | ATGATCCCAATGAGTCGGC | AGTTTTCTAATGTACTTCTGGACCC |
| Il1b | CAAGCAACGACAAAATCCCTG | GACAAACCGCTTTTCCATCTTC |
| Cd11b | TCCCATCTTTCCCGCTAATTC | ACTCAGTTTTGTCGGTCCTG |
| Cd68 | CTCATCATTGGCCTGGTCC | GTTGATTGTCGTCTGCGGG |
| Iba1 | GCCTCATCGTCATCTCCCCA | AGGAAGTGCTTGTTGATCCCA |
| Cd3 | GAAGAACGAGCAGCTGTATCA | GAGAAACCTCCATCTCAAGACC |
| Cd4 | GCTTTCTGGTTTTCACGGG | GATTGTGGCTTTTCTGCATCC |
| Cd8 | GCGATATTTACATCTGGGCACC | AATTTCTCTGAAGGTCTGGGC |
| Ccl3 | CATTGCTGACTATTTTGAGACCAG | TCAGGCATTTAGTTCCAGCTC |
| Cxcl2 | CATGAAGTTTGTCTCAACCCTG | CTTTTCTCTTTGATTCTGCCCG |
| Vcam1 | GAAAGGATCGTACAGTCTGGTG | ATAGCTTGGTTTGTGGAGGG |
| Icam1 | AAGTCTGTCAAACGGGAGATG | CGCAATGATCAGTACCAACAC |
Serum metabolite analyses
Serum IL-1β and TNF-α were measured using Quantikine enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN, USA) following the manufacturer’s protocol.
Measurement of triglycerides and cholesterol
Triglycerides in serum were assayed using colorimetric assay kits from Cayman (Ann Arbor, MI, USA) following the manufacturer’s instructions. Total cholesterol in serum was assayed with a colorimetric assay kit from Cell Biolabs (San Diego, CA, USA).
Immunohistochemistry
For immunohistochemistry, formalin-fixed paraffin-embedded liver sections were used. Slides were briefly deparaffinized with xylene, dehydrated with ethanol and then pressure heated for 20 min at 100 °C in Na citrate buffer. Endogenous peroxidase activities were inactivated in 3% H2O2 in TBS for 12 min at room temperature. Samples were then incubated with protein block for 30 min to block non-specific antibody-binding sites. Overnight incubation at 4 °C was performed using anti-IBA-1 (Waco), anti-CD-3 (Abcam) and anti-HMGB1 (Abcam) primary antibodies. A negative antibody control was included in each case by replacing the primary antibody with antibody diluent. Secondary detection was performed via Leica Bond Polymer Refine kit and slides were counterstained with hematoxylin. Slides were scanned using a Hamamatsu NDP system (Hamamatsu City, Japan). Using 4–6 random fields per slide, positive staining was quantified and analyzed with the help of ImageJ software.
Statistical analyses
The change in body weight, lean mass, and fat mass over time were analyzed using a repeated measure two-way analysis of variance (ANOVA) with diet and sitagliptin treatment as classification variables. The variables measured at the end of the experiment were analyzed using two-way ANOVA with diet and sitagliptin treatment as main effects. Least squares means for each diet and drug treatment combination were compared using the Tukey correction for multiple comparisons (GraphPad software, La Jolla, CA, USA).
Results
The effects of high-Cho diet and sitagliptin on triglyceride and cholesterol levels: model validation
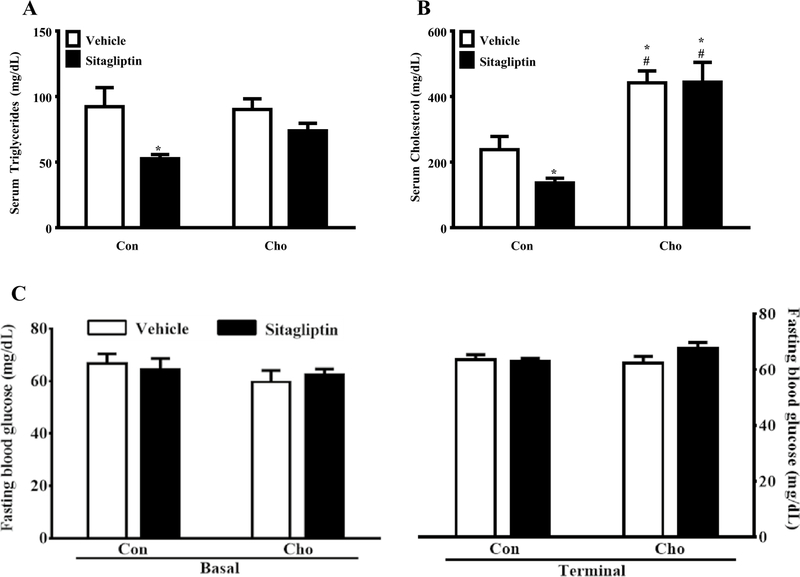
To validate the model used in our study, we examined some of the well-established and beneficial effects of sitagliptin treatment. Since sitagliptin has been documented to improve lipid profiles, we tested the effects of sitagliptin on serum triglycerides and cholesterol. Sitagliptin lowered serum triglyceride levels in rats fed the Con diet, but it was without effect on rats fed the high-Cho diet (Fig. 1a). Diet alone had no effect on serum triglyceride in rats gavaged with vehicle (Fig. 1a). A similar pattern emerged with respect to the effects of diet and sitagliptin on serum cholesterol with two notable differences. First, diet increased serum cholesterol in the high-Cho group compared to Con and sitagliptin had no effect on serum cholesterol in this group (Fig. 1b). Second, the significantly lower serum cholesterol levels in the Con group were further lowered by sitagliptin (Fig. 1b). Therefore, in line with the results obtained by other investigators, we also found that sitagliptin reduced lipid levels. However, these beneficial effects of sitagliptin were abrogated in case of hypercholesterolemic rats. Additionally, fasting blood glucose levels were measured which remained unaffected by sitagliptin and high-Cho diet in the given experimental duration (Fig. 1c).
Fig. 1.
Effects of sitagliptin and high-Cho diet on serum triglyceride, serum cholesterol and fasting blood glucose levels. SD rats were fed Con (control) or high Cho (cholesterol) diets ad libitum. From day 10 through day 35 as described in “Materials and methods”, half the animals of each group were orally gavaged with vehicle and the remaining half with an aqueous suspension of sitagliptin (100 mg/kg/day) for the duration of the study. a Sitagliptin reduced serum triglycerides in rats fed the Con diet. *p < 0.05 vs. Con + Vehicle, Cho + Vehicle and Cho + Sitagliptin groups. b Sitagliptin reduced total Cho levels in the serum of rats fed the Con diet. *p < 0.05 vs. Con + Vehicle and #p < 0.001 vs. Con + Sitagliptin group. c Fasting basal and terminal blood samples were collected to measure blood glucose levels. Sitagliptin did not affect the fasting blood glucose levels irrespective of the diets. All data are presented as the mean ± SEM (n = 7–8 per group)
The effects of high-Cho diet and sitagliptin on hepatic and peripheral markers of inflammation
The primary objective of the current study was to assess the impact of dietary Cho and sitagliptin on hepatic and peripheral markers of inflammation. Using the expression of Tnfa, Il1b and Mcp1 as hepatic markers of inflammation, we found that sitagliptin was without effect on hepatic inflammatory markers in rats on the Con diet (Fig. 2a–c). In contrast, expression of these markers was modestly increased in rats on the high-Cho diet gavaged with vehicle, but in this case, the increase did not reach the threshold for statistical significance (Fig. 2a–c). However, in rats on the high-Cho diet gavaged with sitagliptin, Tnfa mRNA was increased 15-fold compared to Con rats (Fig. 2a), Il1b mRNA was increased sevenfold compared to Con rats (Fig. 2b), and Mcp1 mRNA was increased eightfold relative to Con rats (Fig. 2c).
Fig. 2.
Effects of sitagliptin and high-Cho diet on expression of hepatic and peripheral inflammatory markers. SD rats were fed Con and Cho diets ad libitum. From day 10 through day 35 as described in “Materials and methods”, half the animals of each group were orally gavaged with vehicle and the remaining half with an aqueous suspension of sitagliptin (100 mg/kg/day) for the duration of the experiment. a–c Hepatic gene expression of inflammatory markers were measured. In rats fed the high-Cho diet, sitagliptin exacerbated the expression of a Tnfa (*,#,$p < 0.01 vs. Con + Vehicle, Con + Sitagliptin and Cho + Vehicle groups respectively), b Il1b (*p < 0.01 vs. Con + Vehicle, #p < 0.001 vs. Con + sitagliptin and $p < 0.01 vs. Cho + Vehicle groups), and cMcp1 (*,#p < 0.0001 vs. Con + Vehicle, Con + Sitagliptin groups, respectively, and $p < 0.01 vs. Cho + Vehicle group). d, e Serum levels of inflammatory markers were measured by ELISA. In rats on the high-Cho diet, sitagliptin exacerbated the circulating protein levels of d IL-1β (*,#p < 0.01 vs. Con + Vehicle, Con + Sitagliptin groups, respectively, and $p < 0.05 vs. Cho + Vehicle group) and e TNF-α (*p < 0.05 vs. Cho + Vehicle; t test analysis show differences within the Cho group). All data are presented as the mean ± SEM (n = 7–8 per group)
Serum IL-1β levels were unchanged by sitagliptin in rats on the Con diet, and the high-Cho diet alone did not produce a significant increase in IL-1β (Fig. 2d). However, when rats on the high-Cho diet were gavaged with sitagliptin, a significant increase in serum IL-1β was detected (Fig. 2d). In addition, serum TNF-α was undetectable in rats on the Con diet, irrespective of whether they were administered sitagliptin or not (Fig. 2e). However, serum TNF-α was detectable in rats on the high-Cho diet gavaged with vehicle (Fig. 2e). More importantly, rats on the high-Cho diet gavaged with sitagliptin showed a further 14-fold increase in serum TNF-α (Fig. 2e). Collectively, these findings establish that sitagliptin acts synergistically with the high-Cho diet to increase expression of hepatic and circulating markers of inflammation.
The effects of high-Cho diet and sitagliptin on structural changes in liver
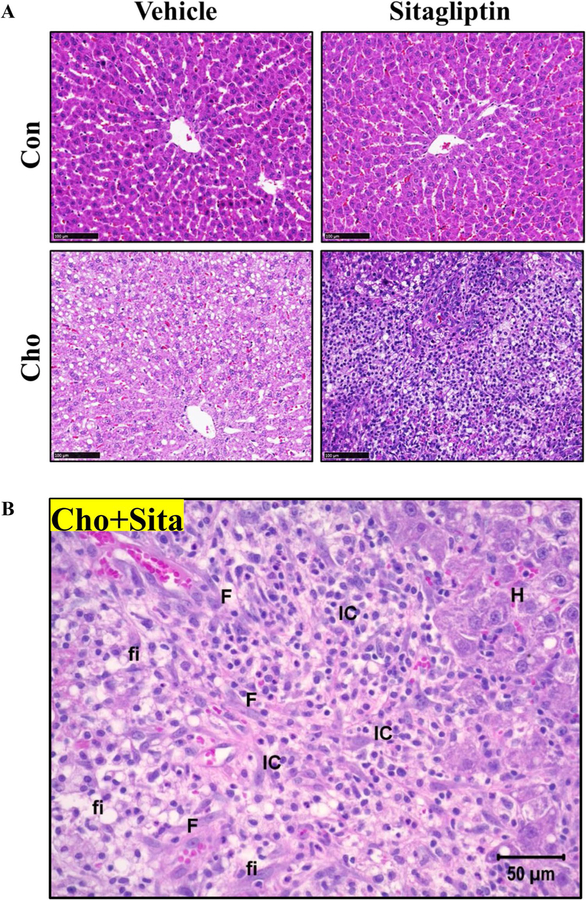
To test for corresponding histopathological changes in liver, H&E-stained liver sections were examined and scored (1–4) to quantify the severity of hepatic lesions. The scores of 1–4 corresponded to minimal, mild, moderate and severe hepatic lesions, respectively. The lesions monitored were necrosis, fat infiltration, fibrosis and mononuclear cell infiltration. As anticipated, no hepatic lesions were detected in rats on the Con diet regardless of whether they were gavaged with vehicle or sitagliptin (Fig. 3, Table 2). However, fat infiltration and necrosis were observed in livers from rats on high-Cho diet. For example, in vehicle-administered rats on high-Cho diet, 75% had minimal necrosis (Score 1) and moderate to severe fat infiltration (scores 3–4) (Fig. 3, Table 2). However, when sitagliptin was administered to rats on the high-Cho diet, fat infiltration was not reduced but necrosis became severe and further confounded by fibrosis and infiltration of mononuclear cells. Fat infiltration was observed in 75% of these rats, while necrosis was present in 50% of the rats, with scores ranging from 3 to 4 and 2 to 3 for the respective pathologies. Additionally, moderate fibrosis and mononuclear cell infiltration were observed in 50% of these rats, corresponding to a score of 2 (Fig. 3, Table 2). In summary, the histopathological evaluation of liver tissues reveals a significant interaction between sitagliptin and the high-Cho diet that exacerbates the severity of hepatic lesions.
Fig. 3.
Effects of sitagliptin and high-Cho diet on liver histology. SD rats were fed Con or Cho diets ad libitum. From day 10 through day 35 as described in “Materials and methods”, half the animals in each group were orally gavaged with vehicle and the remaining half with an aqueous suspension of sitagliptin (100 mg/kg/day) for the duration of the experiment. Sections from the largest lobe of the liver from each animal were used for histopathological evaluation. a, b Representative sections of H&E-stained liver tissue from the Con and Cho groups gavaged with vehicle or sitagliptin. a Sitagliptin exacerbated hepatic damage in rats fed the high-Cho diet. Scale bars = 100 µm. b The hepatic lesions assessed are represented in a magnified liver image of Cho + Sitagliptin group. Scale bar = 50 µm. F fibrosis, IC inflammatory cells (macrophages), fi fat infiltration, H swollen hepatocytes
Table 2.
Histological scoring of liver samples of SD rats fed Con or Cho diets and gavaged with vehicle or sitagliptin
| Specimen ID | Con vehicle |
Cho vehicle |
||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | 7 | 1 | 2 | 5 | 8 | |
| Histopathology findings | N | N | N | |||||
| Necrosis | <1> | <1> | – | <1> | <1> | |||
| Fat infiltration | − | [4] | [3–4] | [3] | [2] | |||
| Fibrosis | − | − | − | − | − | |||
| Mononuclear cell infiltration | − | − | − | − | − | |||
| Specimen ID | Con sitagliptin |
Con sitagliptin |
||||||
| 6 | 7 | 8 | 9 | 1 | 2 | 4 | 8 | |
| Histopathology findings | N | N | N | |||||
| Necrosis | <1> | <2> | <3> | <1> | <1> | |||
| Fat infiltration | − | [2] | [4] | [3] | [3] | |||
| Fibrosis | − | [2] | [2] | − | − | |||
| Mononuclear cell infiltration | − | [2] | [2] | − | − | |||
| Severity score | Lesion distribution | |||||||
| 1 = Minimal | ( ) = Focal | |||||||
| 2 = Mild | < > = Multifocal | |||||||
| 3 = Moderate | [ ] = Diffuse | |||||||
| 4 = Marked | − = Lesion absent | |||||||
Specimen ID denotes individual animal IDs in each group
N no histopathology findings
The effects of high-Cho diet and sitagliptin on hepatic necrosis
Necrosis is one form of cell deaths that has been recognized to have role in various acute and chronic liver pathologies. To corroborate our histopathological findings and to explore whether necrosis was increased in livers of rats fed high Cho and gavaged with sitagliptin, we assessed the expression of high mobility group box 1 (HMGB1) protein by staining the liver sections with HMGB1 antibody (Fig. 4). HMGB1 is a ubiquitous nuclear protein and is passively released by necrotic cells. Liver sections of rats fed Con diet revealed nuclear staining of HMGB1 (Fig. 4). A slight increase in cytoplasmic translocation of HMGB1 in addition to nuclear staining was detectable in rats on Cho diet (Fig. 4). Interestingly, when sitagliptin was gavaged to high-Cho group, the cytoplasmic staining of HMGB1 (indicative of necrosis) was markedly increased (Fig. 4).
Fig. 4.
Effect of sitagliptin and high-Cho diet on hepatic necrosis. SD rats were fed Con or Cho diets ad libitum. From day 10 through day 35 as described in “Materials and methods”, half the animals in each group were orally gavaged with vehicle and the remaining half with an aqueous suspension of sitagliptin (100 mg/kg/day) for the duration of the experiment. Representative IHC images of liver sections using anti-HMGB1 antibody are shown. Nuclear staining of HMGB1 is evident in rats fed the Con diet irrespective of gavage with vehicle or sitagliptin. An increase in the cytoplasmic staining is visible in rats fed the high-Cho diet which is further exacerbated by sitagliptin. Arrows represent cytoplasmic translocation of HMGB1 which is indicative of necrosis
The effects of high-Cho diet and sitagliptin on mononuclear cell infiltration in the liver
Histopathological data from our studies demonstrated increased hepatic influx of mononuclear cells in rats on high-Cho diet and gavaged with sitagliptin (Fig. 5a). Next, we aimed to identify the cellular nature of hepatic inflammation resulting from the high-Cho diet and sitagliptin. Among the mononuclear cells, macrophages and T cells have been demonstrated to play crucial roles in the pathogenesis of NAFLD. Therefore, using real-time PCR and IHC, markers specific to macrophages and T cells were analyzed in the liver tissues. Using mRNA expression of Cd11b, Cd68 and Iba1 as markers of macrophage infiltration, we found that sitagliptin increased hepatic expression of each of these genes in rats fed the high-Cho diet (Fig. 5b–d). Some of these markers were directly influenced by diet alone. For example, the high-Cho diet had no effect on Cd11b expression (Fig. 5b). However, the mRNA levels of Cd68 and Iba1 were notably increased by high-Cho diet alone (Fig. 5c, d). Unlike the high-Cho group, sitagliptin was without effect on expression of these markers in rats on the Con diet (Fig. 5b–d). Additionally, like macrophage markers, a synergistic interaction between sitagliptin and the high-Cho diet was observed with respect to T-cell marker expression (Fig. 5e–g). Cd3, which is a pan-T-cell-specific marker, was shown to be upregulated in the high-Cho group gavaged with sitagliptin (Fig. 5e). Expression of Cd3 was not increased by the high-Cho diet alone (Fig. 5e). In addition, the Con diet did not affect the expression of Cd3 with or without sitagliptin (Fig. 5e). To further characterize the specific T cell population as T helper or T cytotoxic, mRNA expression of Cd4 and Cd8, respectively, were evaluated. Compared to controls, Cd4 expression was increased only in the livers of rats fed high-Cho diet (Fig. 5f). Although sitagliptin did not produce a significant increase in Cd4 expression in Cho group, it was still significantly greater than in rats on Con diet (Fig. 5f). Further, the high-Cho diet alone did not increase Cd8 expression, but gavage of this group with sitagliptin produced a significant increase in Cd8 expression (Fig. 5g).
Fig. 5.
Effect of sitagliptin and diets on hepatic mononuclear cell ► infiltration. SD rats were fed Con or Cho diets ad libitum. From day 10 through day 35 as described in “Materials and methods”, half the animals in each group were orally gavaged with vehicle and the remaining half with an aqueous suspension of sitagliptin (100 mg/kg/day) for the duration of the experiment. a Representative sections of H&E-stained liver tissue from the Con and Cho groups gavaged with vehicle or sitagliptin. Arrows indicate diffuse mononuclear cell infiltration in rats fed high Cho and gavaged with sitagliptin. b–g Hepatic gene expression of macrophage and T-cell-specific markers were measured. In rats fed the high-Cho diet, sitagliptin increased the mRNA levels of b Cd11b (*,#p < 0.01 vs. Con + Vehicle, Con + Sitagliptin groups, respectively, and $p < 0.05 vs. Cho + Vehicle group), c Cd68 (*p < 0.05, **p < 0.0001 vs. Con + Vehicle group; #p < 0.05, ##p < 0.0001 vs. Con + Sitagliptin group; $p < 0.01 vs. Cho + Vehicle group), d Iba1 (*p < 0.05, **p < 0.0001 vs. Con + Vehicle group; #p < 0.05, ##p < 0.0001 vs. Con + Sitagliptin group; $p < 0.01 vs. Cho + Vehicle group), e Cd3 (*p < 0.001 vs. Con + Vehicle group, #p < 0.01 vs. Con + Sitagliptin group and $p < 0.05 vs. Cho + Vehicle group), f Cd4 (*p < 0.05, **p < 0.01 vs. Con + Vehicle group; #p < 0.05, ##p < 0.01 vs. Con + Sitagliptin group) and g Cd8 (*,#p < 0.01 vs. Con + Vehicle, Con + Sitagliptin groups, respectively, and $p < 0.05 vs. Cho + Vehicle group). h–i Representative IHC images of liver sections using anti-IBA-1 and anti-CD3 antibodies where brown positive staining is indicative of h macrophages and i T cells, respectively. j, k These images were analyzed using ImageJ. Sitagliptin enhanced infiltration of j macrophages (*,$p < 0.001 vs. Con + Vehicle, Cho + Vehicle groups, respectively, and #p < 0.0001 vs. Con + Sitagliptin group) and k T cells (*,$p < 0.05 vs. Con + Vehicle, Cho + Vehicle groups, respectively, and #p < 0.01 vs. Con + Sitagliptin group) in the liver of rats fed the high-Cho diet. l Corresponding H&E and IHC images demonstrating macrophages as the predominant cells infiltrating the livers of rats fed high Cho and gavaged with sitagliptin. m–p Hepatic mRNA levels of markers of cell recruitment were measured. In rats fed the high-Cho diet, sitagliptin increased the gene expression of m Ccl3 (*,#p < 0.01 vs. Con + Vehicle, Con + Sitagliptin groups, respectively, and $p < 0.05 vs. Cho + Vehicle group), n Cxcl2 (*,#p < 0.01 vs. Con + Vehicle, Con + Sitagliptin groups, respectively, and $p < 0.05 vs. Cho + Vehicle group), o Vcam1 (*p < 0.05, **p < 0.001 vs. Con + Vehicle group; #p < 0.05, ##p < 0.001 vs. Con + Sitagliptin group; $p < 0.05 vs. Cho + Vehicle group) and p Icam1 (*,#p < 0.01 vs. Con + Vehicle, Con + Sitagliptin groups, respectively, and $p < 0.05 vs. Cho + Vehicle group). All data are presented as the mean ± SEM (n = 7–8 per group)
To further support our findings, immunohistochemistry using anti-IBA-1 and anti-CD-3 antibodies was performed to assess the hepatic influx of macrophages and T cells, respectively (Fig. 5h, i). Consistent with our gene expression data, liver histology showed that rats fed high Cho and gavaged with sitagliptin had severe hepatic inflammation with inflammatory cell clusters composed of macrophages (Fig. 5h) and T cells (Fig. 5i), with macrophages being the predominant cells infiltrating the liver (Fig. 5l). Quantitative analysis using ImageJ software revealed a significant influx of macrophages (Fig. 5j) and T cells (Fig. 5k) in the livers of rats fed high-Cho diet and gavaged with sitagliptin. Recruitment of these inflammatory cells in livers of this group was mediated by a marked increase in chemoattractant such as Ccl3 (Fig. 5m), Cxcl2 (Fig. 5n) and endothelialexpressed cell adhesion molecules such as Vcam1 (Fig. 5o) and Icam1 (Fig. 5p).
The effects of high-Cho diet and sitagliptin on body composition and energy balance
To assess whether the experimental diet and sitagliptin were affecting inflammatory markers independently through effects on body weight or body composition, the effects of each combination on energy balance were examined. Analysis of final body weight, lean mass and adiposity did not show significant differences across the groups (Table 3). Also, the food and energy intake per unit body weight did not differ among the groups (Table 3). Thus, feeding diets enriched with Cho and administration of sitagliptin did not have major effects on body weight, composition, and energy balance (Table 3). Although sitagliptin increased the expression of inflammatory markers in rats fed the high-Cho diet, it seems unlikely that this response is related to changes in body weight or body composition among the groups.
Table 3.
The effect of sitagliptin and diets on body composition and energy balance in SD rats
| Response variable | Con |
Cho |
||
|---|---|---|---|---|
| Vehicle | Sitagliptin | Vehicle | Sitagliptin | |
| Body weight (g) | 348.4 ± 13.23 | 340.1 ± 11.48 | 335.9 ± 5.91 | 340.1 ± 6.71 |
| Lean Mass (%) | 79 ± 0.37 | 78.6 ± 0.43 | 79.4 ± 0.77 | 78.5 ± 0.66 |
| Adiposity (%) | 4.8 ± 0.39 | 5.2 ± 0.45 | 5.2 ± 0.68 | 5.3 ± 0.83 |
| Food intake (g/g BW/day) | 0.08 ± 0.001 | 0.08 ± 0.001 | 0.08 ± 0.001 | 0.08 ± 0.002 |
| Energy intake (Kcal/gBW/day) | 0.23 ± 0.004 | 0.24 ± 0.004 | 0.23 ± 0.004 | 0.24 ± 0.006 |
SD rats were fed Con and Cho diets ad libitum from day 10 through day 35 as described in the Materials and Methods. Half the animals of each group were orally gavaged with vehicle and the remaining half with an aqueous suspension of sitagliptin (100 mg/kg/day) for the duration of the study
Body weight and composition were determined prior to being euthanized. The lean mass and adiposity were calculated as a percentage of body weight. Group means for food and energy intake per unit body weight were calculated for the 35-day experimental period. Sitagliptin and high Cho diet did not affect body weight, composition and energy balance significantly. All data are presented as the mean ± SEM (n = 8 per group)
Discussion
A large number of genetic and environmental factors such as lifestyle and diet have been associated with obesity, insulin resistance and diabetes which are recognized as important risk factors for the development of NAFLD [35]. Several studies have reported a positive association between Western dietary pattern and the risk of NAFLD [36]. Western diets are primarily comprised of meat, poultry and dairy products which contain high amounts of Cho. Emerging evidence suggests that Cho is an important risk factor in NAFL/NASH pathogenesis, in addition to triglycerides and free fatty acids [13, 37–41]. Therefore, increased consumption of meat, poultry and dairy products directly would translate to higher intake of Cho, and thus contribute to the disease burden of NAFL and its progression to more severe inflammatory stage, NASH.
The inflammatory response is particularly relevant, as it is a key factor in the development and progression of not only NAFLD but also CVD, which can be life threatening. Therefore, we investigated the effects of dietary enrichment with Cho in relation to inflammation in adult male SD rats. Rodents generally do not experience elevated serum Cho when fed high-Cho diet unless it is a transgenic strain, such as ApoE–/–, or they consume a high-Cho diet supplemented with cholic acid or other bile salts [42]. Therefore, to induce hypercholesterolemia in the experiment presented here, rats were fed custom-made diets enriched with 2% Cho containing 0.5% cholic acid. As anticipated, the serum Cho levels of rats fed high-Cho diet were significantly higher compared to rats on Con diet, thus proving the diets were effective in inducing hypercholesterolemia in these animals. We carried out intervention studies with sitagliptin, due to its known antiinflammatory and other health benefits that are independent of its hypoglycemic properties [18–24]. Sitagliptin belongs to the class of DPP-4 inhibitors and is an oral medication in clinical practice for the management of type 2 diabetes.
Sitagliptin has also been shown to impact lipid levels, and in type 2 diabetic patients with hyperlipidemia, treatment with this drug improved lipid profile by lowering serum levels of triglycerides and total Cho [26]. In the current study, sitagliptin mediated a reduction in circulating triglyceride as well as Cho levels in the serum of the rodents consuming the Con diet. Feeding a high-Cho diet, however, masked the beneficial effect of sitagliptin on lipid profile, providing a strong case that Cho is a significant risk factor and high-Cho diets have the potential to undermine the beneficial effects of sitagliptin.
Importantly, although it was anticipated that sitagliptin would reduce the inflammatory profiles of hypercholesterolemic animals, these rats, in fact, experienced an increase in hepatic inflammation. These results were highly reproducible, and we were able to unequivocally demonstrate the hepato-inflammatory effects of sitagliptin in a high-Cho background in three independent animal studies conducted by our group. A search of the literature indicates a relationship between hypercholesterolemia and pathological alterations in the liver [43]. Consistent with these studies, we also found minimal necrosis and mild fat infiltration in the liver of hypercholesterolemic rats. Upon dosing with sitagliptin, there was an additional increase in the severity of hepatic lesions. Rats consuming the high-Cho diet that were treated with sitagliptin had higher injury scores and other lesions such as fibrosis and infiltration of mononuclear cells. Further, macrophages and T cells were identified as the major cells contributing to hepatic inflammation evident in rats fed high Cho and gavaged with sitagliptin.
Sitagliptin has been shown to reduce food intake and also has weight-neutral effects [44]. There were no differences between food intake or body weight and composition between the dietary groups, nor did the parameters change with sitagliptin treatment for the duration of our study (35 days). Additionally, fasting blood glucose levels were not significantly different among the groups at any point during the study, irrespective of the intervention with the drug. Although sitagliptin is known to attenuate hyperglycemia, the animals in this study were euglycemic and did not experience hyperglycemia and were, therefore, unable to respond to the drug in this manner.
The phenomenal increase in hepatotoxic markers by sitagliptin in rats fed high Cho is very interesting and merits additional investigations. This unanticipated finding is contrary to the anti-inflammatory properties of this drug as reported by previous workers [45, 46]. However, based on some recent reports and our own findings, it is possible that previously reported anti-inflammatory effects of DPP-4 inhibitors may vary based on the extent and duration of the dietary intake of Cho. Since there are reports of increased cardiovascular, and hepatic and pancreatic pathologies due to sitagliptin/DPP-4 inhibitor therapies, more studies are needed to better understand potential diet/drug interactions that may compromise the beneficial effects of the drug. Also, whether the observed increase in inflammatory responses due to sitagliptin is specific, or a generalized effect of all DPP-4 inhibitors, merits further investigations. Multiple mechanisms may culminate in sitagliptin-mediated exacerbation of hepatotoxicity of rats on high-Cho diet. The studies on the mechanisms of hepatotoxic effects of sitagliptin in hypercholesterolemia, however, are beyond the scope of the present manuscript. The current work would pave way for mechanistic studies by other investigators in addition to our own efforts.
In summary, data from our studies explicitly demonstrate that sitagliptin exacerbates hepatic inflammation in rats on the high-Cho diet. Future studies in diabetic animal models fed a high-Cho diet and administered sitagliptin would shed light on the safety of this drug in hyperglycemic conditions combined with hypercholesterolemia. This would be relevant in furthering our understanding of the management of hyperglycemia in diabetic patients who are hypercholesterolemic and, therefore, we believe our findings may have important translational implications.
Acknowledgements
The funding support from the Louisiana Biomedical Research Network is gratefully acknowledged. We thank the services provided by the Cell Biology and Bioimaging Core at Pennington Biomedical Research Center.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
References
- 1.Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. [DOI] [PubMed] [Google Scholar]
- 2.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- 3.Kanazawa I, Tanaka K-I, Sugimoto T. DPP-4 inhibitors improve liver dysfunction in type 2 diabetes mellitus. Med Sci Monit 2014;20:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology 2010;139:323 e7–34 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesmana CRA, Hasan I, Budihusodo U, Gani RA, Krisnuhoni E, Akbar N, et al. Diagnostic value of a group of biochemical markers of liver fibrosis in patients with non-alcoholic steatohepatitis. J Digest Dis 2009;10:201–6. [DOI] [PubMed] [Google Scholar]
- 6.Valenti L, Fracanzani AL, Dongiovanni P, Santorelli G, Branchi A, Taioli E, et al. Tumor necrosis factor α promoter polymorphisms and insulin resistance in nonalcoholic fatty liver disease. Gastroenterology 2002;122:274–80. [DOI] [PubMed] [Google Scholar]
- 7.Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res 2007;37:50–7. [DOI] [PubMed] [Google Scholar]
- 8.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006;44:1167–74. [DOI] [PubMed] [Google Scholar]
- 9.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003;37:909–16. [DOI] [PubMed] [Google Scholar]
- 10.Yasutake K, Nakamuta M, Shima Y, Ohyama A, Masuda K, Haruta N, et al. Nutritional investigation of non-obese patients with non-alcoholic fatty liver disease: the significance of dietary cholesterol. Scand J Gastroenterol 2009;44:471–7. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Hossain MA, Sadat S, Hager L, Liu L, Tam L, et al. Lecithin cholesterol acyltransferase (LCAT) null mice are protected from diet-induced obesity and insulin resistance in a gender specific manner through multiple pathways. J Biol Chem 2011;286(20):17809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hager L, Li L, Pun H, Liu L, Hossain MA, Maguire GF, et al. Lecithin: cholesterol acyltransferase deficiency protects against cholesterol-induced hepatic endoplasmic reticulum stress in mice. J Biol Chem 2012;287:20755–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, et al. Mitochondrial free cholesterol loading sensitizes to TNF-and Fas-mediated steatohepatitis. Cell Metab 2006;4:185–98. [DOI] [PubMed] [Google Scholar]
- 14.Bieghs V, Verheyen F, van Gorp PJ, Hendrikx T, Wouters K, Lütjohann D, et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PLoS ONE 2012;7:e34378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leroux A, Ferrere G, Godie V, Cailleux F, Renoud M-L, Gaudin F, et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol 2012;57:141–9. [DOI] [PubMed] [Google Scholar]
- 16.Schwabe RF, Maher JJ. Lipids in liver disease: looking beyond steatosis. Gastroenterology 2012;142:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, et al. Free cholesterol accumulation in hepatic stellate cells: Mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 2014;59:154–69. [DOI] [PubMed] [Google Scholar]
- 18.Panchapakesan U, Pollock C. The role of dipeptidyl peptidase–4 inhibitors in diabetic kidney disease. Front Immunol 2015;6:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its antiinflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J 2013;77:1337–444. [DOI] [PubMed] [Google Scholar]
- 20.Kubota Y, Miyamoto M, Takagi G, Ikeda T, Kirinoki-Ichikawa S, Tanaka K, et al. The dipeptidyl peptidase-4 inhibitor sitagliptin improves vascular endothelial function in type 2 diabetes. J Korean Med Sci 2012;27:1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez N, Touihri K, Matheeussen V, Costa AM, Mahmoudabady M, Mathieu M, et al. Dipeptidyl peptidase IV inhibition improves cardiorenal function in overpacing-induced heart failure. Eur J Heart Fail 2012;14:14–211. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto Y, Oyama J-I, Ikeda H, Kuroki S, Gondo S, Iwamoto T, et al. Effects of sitagliptin beyond glycemic control: focus on quality of life. Cardiovasc Diabetol 2013;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akaslan SB, Degertekin CK, Yilmaz G, Cakir N, Arslan M, Toruner FB. Effects of sitagliptin on nonalcoholic fatty liver disease in diet-induced obese rats. Metab Syndr Relat Disord 2013;11:243–50. [DOI] [PubMed] [Google Scholar]
- 24.Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes 2011;60(4):1246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmanová I, Škrha P, Šedo A, Anděl M. Dynamic change of focal fatty sparing in non-alcoholic fatty liver disease after treatment with sitagliptin. Ultrasound Int Open 2016;2:E98–E9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shigematsu E, Yamakawa T, Kadonosono K, Terauchi Y. Effect of sitagliptin on lipid profile in patients with type 2 diabetes mellitus. J Clin Med Res 2014;6:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouse R, Xu L, Stewart S, Zhang J. High fat diet and GLP-1 drugs induce pancreatic injury in mice. Toxicol Appl Pharmacol 2014;276:104–14. [DOI] [PubMed] [Google Scholar]
- 28.Shahbaz A, Aziz K, Umair M, Sharifzadeh M, Sachmechi I (2018) Acute liver injury induced by sitagliptin: report of two cases and review of literature. Cureus 2018;10(6):e2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyoda-Akui M, Yokomori H, Kaneko F, Shimizu Y, Takeuchi H, Tahara K, et al. A case of drug-induced hepatic injury associated with sitagliptin. Intern Med 2011;50:1015–20. [DOI] [PubMed] [Google Scholar]
- 30.Packer M. Worsening heart failure during the use of DPP-4 inhibitors: pathophysiological mechanisms, clinical risks, and potential influence of concomitant antidiabetic medications. JACC Heart Fail 2018;6:445–51. [DOI] [PubMed] [Google Scholar]
- 31.Kim EJ, Kim B-H, Seo HS, Lee YJ, Kim HH, Son H-H, et al. Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS ONE 2014;9:e97841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak R, Kumar A, Palfrey HA, Gettys TW, Murthy SN. Sitagliptin exacerbates expression of inflammatory markers in sprague Dawley rats fed a high cholesterol diet. Toxicol Suppl Toxicol Sci 2018;150(1):Abstract #1327. [Google Scholar]
- 33.Hausenloy DJ, Whittington HJ, Wynne AM, Begum SS, Theodorou L, Riksen N, et al. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol 2013;12:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim S, Choi SH, Shin H, Cho BJ, Park HS, Ahn BY, et al. Effect of a dipeptidyl peptidase-IV inhibitor, des-fluoro-sitagliptin, on neointimal formation after balloon injury in rats. PLoS ONE 2012;7:e35007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 2006;40:S5–S10. [DOI] [PubMed] [Google Scholar]
- 36.Mirmiran P, Amirhamidi Z, Ejtahed H-S, Bahadoran Z, Azizi F. Relationship between diet and non-alcoholic fatty liver disease: a review article. Iran J Public Health 2017;46:1007. [PMC free article] [PubMed] [Google Scholar]
- 37.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007;46:1081–90. [DOI] [PubMed] [Google Scholar]
- 38.Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, et al. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 2013;57:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerr TA, Davidson NO. Cholesterol and nonalcoholic fatty liver disease: renewed focus on an old villain. Hepatology 2012;56:1995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wouters K, van Bilsen M, van Gorp PJ, Bieghs V, Lütjohann D, Kerksiek A, et al. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS Lett 2010;584:1001–5. [DOI] [PubMed] [Google Scholar]
- 41.Van Rooyen DM, Farrell GC. SREBP-2: A link between insulin resistance, hepatic cholesterol, and inflammation in NASH. J Gastroenterol Hepatol 2011;26:789–92. [DOI] [PubMed] [Google Scholar]
- 42.Chiang M, Chen Y, Huang A. Plasma lipoprotein cholesterol levels in rats fed a diet enriched in cholesterol and cholic acid. Int J Vitam Nutr Res Internationale Zeitschrift fur Vitamin-und Ernahrungsforschung Journal international de vitaminologie et de nutrition 1998;68:328–34. [PubMed] [Google Scholar]
- 43.Csonka C, Baranyai T, Tiszlavicz L, Fébel H, Szűcs G, Varga ZV, et al. Isolated hypercholesterolemia leads to steatosis in the liver without affecting the pancreas. Lipids Health Dis 2017;16:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta V. Pleiotropic effects of incretins. Indian J Endocrinol Metab 2012;16:S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung Y-A, Choi Y-K, Jung G-S, Seo H-Y, Kim H-S, Jang BK, et al. Sitagliptin attenuates methionine/choline-deficient diet-induced steatohepatitis. Diabetes Res Clin Pract 2014;105:47–57. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Hausding M, Weng S-Y, Kim YO, Steven S, Klein T, et al. Gliptins suppress inflammatory macrophage activation to mitigate inflammation, fibrosis, oxidative stress, and vascular dysfunction in models of nonalcoholic steatohepatitis and liver fibrosis. Antioxid Redox Signal 2017;28:87–109. [DOI] [PubMed] [Google Scholar]