Abstract
The connection between inflammation and cancer was initially recognized by Rudolf Virchow in the nineteenth century. During the last decades, a large body of evidence has provided support to his hypothesis, and now inflammation is recognized as one of the hallmarks of cancer, both in the etiopathogenesis and in ongoing tumor growth. Infection with the pathogen Helicobacter pylori is the primary causal factor in 90% of gastric cancer cases. As we increase our understanding of how chronic inflammation develops in the stomach and contributes to carcinogenesis, there is increasing interest in targeting cancer-promoting inflammation as a strategy to treat gastric cancer. Moreover, once cancer develops and anti-cancer immune responses are suppressed, there is evidence of a substantial shift in the microenvironment and new targets for immune therapy emerge. In this chapter, we provide insight into inflammation-related factors, including T lymphocytes, macrophages, pro-inflammatory chemokines and cytokines, which promote H. pylori-associated gastric cancer initiation and growth. While intervening with chronic inflammation is not a new practice in rheumatology or gastroenterology, this approach has not been fully explored for its potential to prevent carcinogenesis or to contribute to the treatment of gastric cancer. This review highlights current and possible strategies for therapeutic intervention including: i) targeting pro-inflammatory mediators, ii) targeting growth factors and pathways involved in angiogenesis in the gastric tumor microenvironment, and iii) enhancing anti-tumor immunity. In addition, we highlight a significant number of clinical trials and discuss the importance of individual tumor characterization towards offering personalized immune-related therapy.
Keywords: immunotherapy, gastric cancer, Helicobacter pylori, inflammation, immune modulation
1. Introduction
The connection between inflammation and cancer was initially recognized by Rudolf Virchow in the nineteenth century (Virchow 1863). During the last decades, a large body of evidence has provided support to his hypothesis, and now inflammation is recognized as one of the hallmarks of cancer (Colotta et al. 2009). Many types of cancer are preceded by a chronic inflammatory process, mostly initiated by infections or exposure to environmental factors. It is estimated that about 15% of new cancer cases worldwide in 2012 were attributable to carcinogenic infections, with H. pylori being the most important, accounting for about 770,000 cases of gastric cancer (GC) annually (Plummer et al. 2016).
GC is the fifth most common malignancy worldwide and the third leading cause of cancer-related mortality (Ferlay et al. 2015). Incidence rates of GC differ widely across geographic regions, with the highest rates observed in Asia, Eastern Europe, and some Latin American countries. Most GCs are adenocarcinomas but are highly heterogeneous with respect to histological architecture and molecular features (Gullo et al. 2018; Cancer Genome Atlas Research 2014; Lee et al. 2016). Histological classification systems (Lauren 1965; Lauwers et al. 2010) are clinically impractical to guide patient management. Due to differences in etiological and epidemiological factors, GCs are classified anatomically as cardia (proximal) and non-cardia (distal). It is estimated that ~90% of cases of non-cardia cancer worldwide are caused by H. pylori infection (Plummer et al. 2015).
GC is a lethal disease, mainly due to the high rates of diagnosis at advanced stages. With the exception of Japan and Korea, where screening programs for early detection have been implemented, overall five-year survival rates after diagnosis are below 35% (Cancer Stat Facts: Stomach Cancer, SEER Cancer Statistics Review, 1975–2015 2018; Zeng et al. 2018). Early GC is limited to the mucosa and submucosa, regardless of lymph node involvement, and surgical resection is the only curative treatment. Only a minority of patients with advanced disease responds to current modalities of treatment, which, according to the stage, include a combination of adjuvant or neoadjuvant therapies with surgery (Van Cutsem et al. 2016). Recent advances in targeted therapy such as trastuzumab, an antibody against human epidermal growth factor receptor 2 (HER2), and ramucirumab, an antibody against VEGFR2 (Bang et al. 2010; Fuchs et al. 2014; Wilke et al. 2014), and immunotherapy modalities (Fuchs et al. 2018a) have produced encouraging results in the treatment of patients with certain subtypes of advanced GC.
Due to the wide heterogeneity of GCs, new strategies to treat this disease are a priority. Although most cancer research has focused on the molecular changes of the neoplastic cells, it is now recognized that non-tumoral cells in the tumor microenvironment, especially immune cells, proliferate with the tumor and provide essential support for its growth and invasion. The recognized protective effect of non-steroidal anti-inflammatory drugs against GC and other gastrointestinal tumors (Abnet et al. 2009; Epplein et al. 2008; Kim et al. 2018) supports the role of chronic inflammation in carcinogenesis.
In this chapter, we provide insight into inflammation-related factors that promote H. pylori-associated GC initiation and growth, focusing on current and potential strategies for therapeutic intervention (Fig. 1). A section is dedicated to novel immunotherapy modalities, especially promising in certain subtypes of GC, such as Epstein-Barr virus (EBV)–associated and microsatellite instable (MSI) tumors. Of note, H. pylori is also the main etiologic factor of mucosa-associated lymphoid tissue lymphoma and will be discussed in chapter 4.
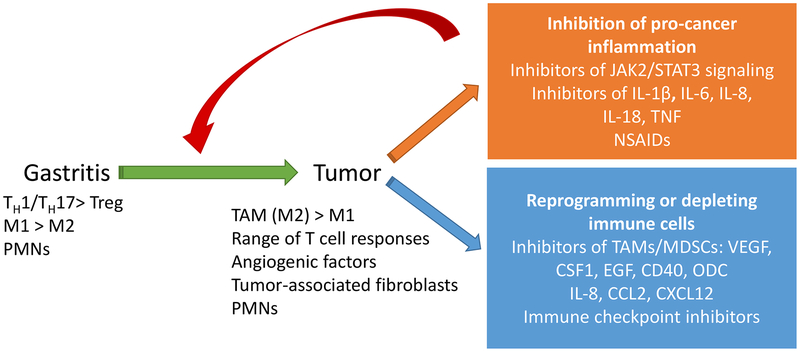
Figure 1. Resolution of gastric cancer-promoting inflammation: a novel strategy for anti-cancer therapy.
H. pylori infection leads to gastritis in infected persons, but only a subset will go on to develop GC. The microenvironment of the immune response during infection changes when a tumor develops. Immunotherapies could target several immune pathways. Some therapies could target the pro-inflammatory environment which drives the development of the tumor, while others would target the tumor microenvironment by reprogramming tumor infiltrating cells or inhibiting angiogenic factors.
2. Inflammatory mediators of gastritis and the TME
2.1. Chronic inflammation in H. pylori
Chronic inflammation of the gastric mucosa, termed gastritis, is a hallmark of H. pylori infection. H. pylori colonization leads to gastritis in all infected persons, but not all persons will develop symptoms. Both innate and adaptive immune cells are present and active during chronic inflammation as discussed in chapters 8 and 9 of this volume. Immune cell migration to the stomach and production of chemokines and cytokines culminates in ongoing activation of anti-microbial responses and the generation of reactive oxygen and nitrogen species (ROS and RNS). The chronic inflammatory response is believed to be required for the development of a sequence of epithelial transformations called the Correa cascade, which includes multifocal atrophic gastritis, intestinal metaplasia, dysplasia, and cancer (Correa et al. 1975; Mera et al. 2005; Mera et al. 2018; Correa et al. 2010).
Characteristic of mucosal surfaces like the intestines, gastric epithelial cells respond to microbes in the environment. Gastric epithelial cells respond to H. pylori and produce a number of pro-inflammatory cytokines and chemokines, including interleukin (IL)-1β, IL-6, IL-8 and IL-18. The local cytokine responses in human subjects indicate that there is increased tissue expression of IL-1β, IL-6, IL-8 and tumor necrosis factor (TNF, also referred to as TNF-α) (Lindholm et al. 1998). It has been suggested that elevated levels of many of these cytokines including IL-1β, IL-8 and TNF can also serve as biomarkers for GC (Macri et al. 2006). These cytokines and chemokines impact recruitment of immune cells – particularly polymorphonuclear cells (PMNs) and macrophages. As the infection persists, these cytokine responses also chronically persist. Many of these cytokines converge on signaling through Janus kinase/signal transducer and activator of transcription proteins (JAK/STATs) and activating nuclear factor-kappa B (NF-κB), which leads to upregulation of transcription of anti-apoptotic proteins, pro-inflammatory cytokines and chemokines, adhesion molecules and increased expression of inducible nitric oxide synthase (NOS2) or NADPH oxidase enzyme isoforms (Gobert and Wilson 2017). These changes in the microenvironment contribute to the development of carcinoma, because they can lead to increased DNA damage, dysfunctional DNA repair enzymes and genetic instability. Moreover, many of these inflammatory cytokines, such as IL-1β, IL-6, and IL-8, play a pivotal role in mediating the interaction between cancer stem cells and the microenvironment.
In addition to innate immune cell infiltration, cells of the adaptive arm of the immune system, including T lymphocytes and B lymphocytes, migrate into the gastric tissue in response to H. pylori infection. The T lymphocytes are predominantly CD4+ T helper cells (TH) and exhibit pro-inflammatory phenotypes (TH 1 and TH17) as they express interferon −gamma (IFNγ) and IL-17A, both pro-inflammatory cytokines associated with chronic inflammation. The differentiation of naïve T cells to activated TH1 or TH17 cells can be dictated by the innate cytokine environment. IL-1β, IL-6, IL-23 and transforming growth factor- beta (TGF-β) skew T cell response towards IL-17A producing cells while expression of IL-12 is likely to push naïve T cells towards IFNγ producing T cells. In humans (but not in mice), IL-23 also plays a role in TH1 cell differentiation. Increased gene expression of IFNγ, IL-12p40, IL-17A and IL-23 has been reported in stomach biopsies from H. pylori infected adults and children (Bhuiyan et al. 2014; Staples et al. 2013) and expression of IL-17A is associated with disease severity (Arachchi et al. 2017). Interestingly, not all studies have the same cytokine signature, in a study which evaluated H. pylori-positive gastritis patients versus H. pylori-negative gastritis patients, IL-12 expression was significantly elevated only in the H. pylori-positive patients, whereas many other cytokines were elevated in both groups, including TNF, IL-1β, IL-6 and IL-8 (Bauditz et al. 1999).
IL-17A and IFNγ subsequently further activate epithelial cells and macrophages in the tissue and can amplify the PMN response. Animal models have successfully defined roles for IFNγ and IL-17A in activating the proper signals required for the development of gastritis but also in activating anti-microbial responses against H. pylori (Dixon et al. 2016; Algood et al. 2009; Sjokvist Ottsjo et al. 2015). In H. pylori infected individuals, the frequencies of IFNγ and IL-17A+ cells were increased in the antrum (Luzza et al. 2000), particularly in patients with H. pylori induced gastric ulcers (Adamsson et al. 2017). Other T cell cytokines are also involved in the chronic inflammatory response including IL-21. Levels of IL-21 are increased in H. pylori infected samples from patients with gastritis (Caruso et al. 2007) and in H. pylori infected mice (Carbo et al. 2014). In human subjects, IL-21 expression correlates with activation of STAT3 and more severe gastritis (Bagheri et al. 2015). Mice deficient in IL-21 infected with H. pylori do not develop gastritis, but are colonized with a higher level of H. pylori than wild-type controls (Carbo et al. 2014). These data suggest again that IL-21 can drive inflammation but also that inflammation is necessary to bring anti-microbial responses to the stomach control H. pylori colonization.
Additional insight related to the role of macrophages in H. pylori immunopathogenesis and inflammation-associated cancer risk stems from studies related to polyamines. Polyamines are pleiotropic polycations that have many cellular functions, including regulation of gene transcription, protein translation, cell growth, proliferation, and differentiation (Hardbower et al. 2017; Pegg 2006, 2009). The production of the three major polyamines—putrescine, spermidine, and spermine—is tightly regulated and centers on the rate-limiting enzyme, ornithine decarboxylase (ODC1, hereafter referred to as ODC) (Asim et al. 2010). ODC uses the substrate, L-ornithine, to produce putrescine via a decarboxylation reaction (Asim et al. 2010). ODC has been implicated in several malignancies, including breast, colorectal, and gastric cancer. Most of the studies related to ODC have been focused on its role in epithelial cell function. However, ODC expression is upregulated in macrophages by H. pylori in vitro (Asim et al. 2010) and in infected gastritis tissues of mice and humans (Hardbower et al. 2017). Importantly, macrophage ODC is immunosuppressive, impairing M1-dependent host defense against H. pylori; mice with myeloid-specific deletion of Odc exhibited marked upregulation of M1 responses, including NOS2 expression/NO production; M1 gene signatures (Nos2, Il1b, Tnf, Il6, Il12a, Il12b) and M1 protein responses (IL-1β and TNF-α) as well as pro-inflammatory chemokines and IL-17A (Hardbower et al. 2017). In parallel, there was increased gastric inflammation (both acute and chronic) but a clear benefit of reduced H. pylori bacterial colonization levels. Another crucial observation was that the immunosuppressive effects of ODC activity were linked to the ability of putrescine to cause histone modifications (specific acetylation and methylation events) favoring suppression of gene transcription, thus blocking M1 response (Hardbower et al. 2017), suggesting that polyamines have a deleterious effect of restricting mucosal immune responses. These findings lead to the question of the potential role of ODC/polyamines in GC development induced by H. pylori; this is discussed in Section 4.2 below.
Many of these cytokines and signaling pathways likely contribute to the development of cancer. However, the microenvironment in the stomach of a patient with chronic gastritis likely differs from the tumor microenvironment.
2.2. The tumor microenvironment (TME) in gastric cancer
The TME is a complex network of tumor cells and numerous types of non-tumor cells, including lymphocytes, myeloid cells, endothelial cells, and fibroblasts. As a dynamic environment, the TME involves a large variety of molecules such as growth factors, cytokines, chemokines, antibodies, proteases, and metabolites as well as the extracellular matrix. Non-resolving inflammation derived from chronic infection with H. pylori is one of the characteristics of the TME in GC and is considered to play an essential role in tumor initiation and growth. In a long-term failed attempt to promote healing, this complex network of mediators in the gastric mucosa leads to the upregulation of pathways that increase cell survival, activate stem cells and promote epithelial proliferation.
Defined almost two decades ago, cancer immunoediting is the process by which the immune system can either restrain or promote cancer development, ultimately favoring the outgrowth of tumor cells with reduced immunogenicity (Shankaran et al. 2001; Schreiber et al. 2011). Mechanisms that lead to inhibition of anti-tumor immune responses involve multiple components within the TME. Tumor cells secrete cytokines and chemokines to recruit myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and tumor-associated macrophages (TAMs). These cells directly suppress the functions of natural killer (NK) cells and CD8+ T cells through the production and expression of various factors, ultimately favoring tumor growth and invasion (Kitamura et al. 2015).
The macrophage is a key player of the innate immune response that then modulates chronic inflammatory responses, therefore it plays an important role during H. pylori infection and carcinogenesis. Circulating monocytes are recruited across the vasculature into tumors by tumor-derived chemoattractants such as colony-stimulating factor 1 (CSF1), CC ligand 2 (CCL2), vascular endothelial growth factor A (VEGFA, commonly referred to as VEGF), or CXCL12 (Murdoch et al. 2008; Noy and Pollard 2014). In the tissues, macrophages adjust to the particular conditions of the environment, adopting either pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes (Mills 2012). M1 macrophages are activated by bacterial constituents and TH1 cytokines (e.g., IFNγ) and show antitumor activity through high antigen-presenting capacity, phagocytosis and upregulation of pro-inflammatory TH1 responses. In contrast, M2 macrophages are activated by TH2 cytokines (e.g., TGF-β, IL-4, IL-10, and IL-13), leading to suppression of adaptive immunity and promotion of tissue remodeling, angiogenesis and tumor growth (Fig. 2) (Tiemessen et al. 2007; Mills 2012). Multiple characteristics of tumors, including hypoxia and abundant cell death, help direct macrophage function towards attempting a “homeostatic” restoration (Ruffell et al. 2012). Thus, it has been argued that most TAMs exhibit a predominantly M2 phenotype (Mantovani et al. 2002). However, the large variety of functions in which TAMs are engaged suggests that extreme forms of M1/M2 polarization may not exist in the TME (Qian and Pollard 2010). In any case, high densities of TAMs or M2 macrophages have been associated with worse overall survival in several malignancies, including GC (Zhang et al. 2012; Jiang et al. 2017).
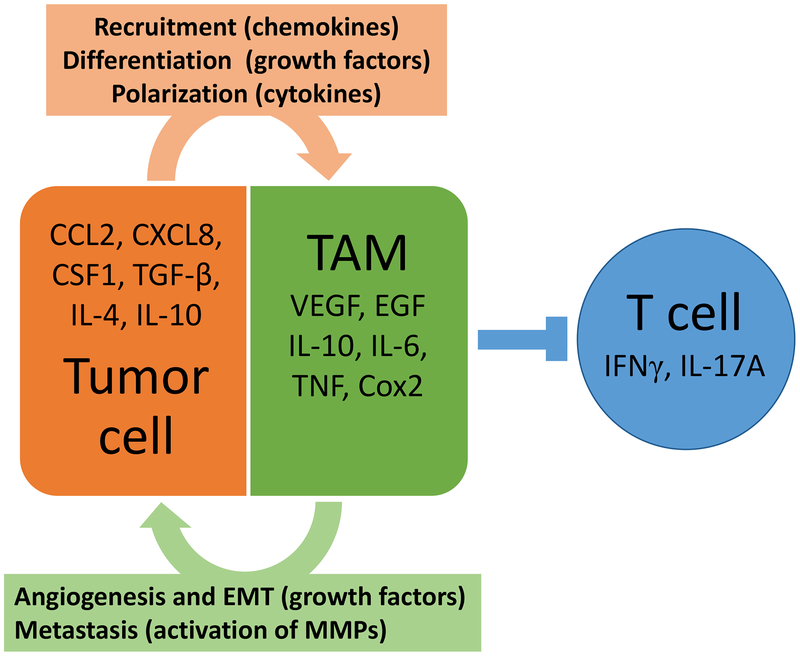
Figure 2. Tumor associated macrophages and their role in the tumor microenvironment.
Tumor cells can produce chemokines, cytokines and growth factors which drive recruitment, differentiation and polarization of TAMs. When the tumor associated macrophages enter the TME they can suppress anti-tumor T cell activity, contributes to angiogenesis of tumors cells through production of growth factors, and promote metastasis through activation of matrix metalloproteases.
TAMs promote the suppression of effective antitumor immunity via different pathways including production of anti-inflammatory cytokines (including IL-10 and TGF-β), prostaglandin E2 (PGE2), and expression of programmed death-ligand 1 (PD-L1) (Mantovani et al. 2014). In addition, TAMs regulate vascular programming of tumors by production of VEGF and other proangiogenic factors (Ruffell et al. 2012). Macrophage infiltration correlates significantly with tumor vascularity in human GC (Ohta et al. 2003). TAMs can also contribute to the invasiveness of tumor cells by remodeling the extracellular matrix and by opening the way to exit the tumor and colonize the surrounding tissues (Guiet et al. 2011). As the tumor progresses, hypoxic regions develop, caused by high metabolic and proliferative rates. Hypoxia is a potent inducer of VEGF, and this is mediated by the transcription factor hypoxia-inducible factor-1 (HIF-1) (Semenza 2003, 2012). In addition to the role in angiogenesis, VEGF that is secreted by tumor cells can function in an autocrine manner promoting proliferation, dedifferentiation and transition from an epithelial to mesenchymal phenotype, enhancing stromal invasion and tumor growth. This autocrine signaling, which is mediated by VEGFR2 and by neuropilins, could be necessary for the function of cancer stem cells because it seems to maintain the stem cell reserve and to sustain self-renewal (Goel and Mercurio 2013).
Recently, next-generation sequencing and large-scale genomics have led to new molecular classifications of GC (Cancer Genome Atlas Research 2014; Cristescu et al. 2015). The Cancer Genome Atlas (TCGA) project classified GC into four subtypes: EBV-positive, MSI), genomically stable, and chromosomally unstable tumors (Cancer Genome Atlas Research 2014). At the same time, advances in cancer immunotherapy have opened new frontiers for patient care across a variety of tumors. Among the GC subtypes, EBV and MSI are the most promising in this regard. Amplification of CD274 and PDCD1LG2, which encode PD-L1 and PDCD1LG2 respectively, is often found in EBV-positive tumors with high levels of PD-L1 protein expression detected on tumor cells (Derks et al. 2016). The interaction of PD-L1 with its receptor PD-1 (commonly found on T cells) inhibits T lymphocyte migration, proliferation and secretion of cytotoxic mediators, ultimately favoring tumor escape from the immune response.
Tumor-immune interactions are increasingly recognized as drivers of the clinical outcome and as potential targets for therapy. Recently, Thorsson and co-workers (Thorsson et al. 2018) characterized the immune TME of 33 cancer types (more than 10,000 tumors) into six “immune subtypes”: 1) wound healing, 2) IFNγ, 3) inflammatory, 4) lymphocyte depleted, 5) immunologically quiet, and 6) TGF-β dominant. About 80% of gastric adenocarcinomas were grouped within immune subtypes 1 (wound healing, characterized by elevated expression of angiogenic genes, a Th2 cell bias and high proliferating rate) and 2 (IFNγ dominant, with the highest M1/M2 polarization, a strong CD8 signal, but also a high proliferating rate). The best overall survival (combining all cancer sites) was observed in the immune subtype 3 (inflammatory, defined by elevated TH17 and TH1 genes and low/moderate tumor proliferation), while types 1 and 2 had less favorable outcomes despite having a substantial immune component (Thorsson et al. 2018). This study highlights the importance of the immune interactions within the TME on prognosis and the need for individual tumor characterization for effective personalized choice of immune-related therapies. Although the study by Thorsson and colleagues found that the immune subtype 3 TME (determined by immunogenetics) had the best overall survival of all cancer types, the situation may be different in the GC TME. A recent publication investigating the expression of several cytokines and their relationship with clinicopathological characteristics in GCs revealed that IL-17 expression (measured by immunohistochemistry) was associated with decreased survival (Kim et al. 2017). Taken together, these results indicate a need for a deeper understanding of T cell cytokines in the TME in GC.
3. Anti-cancer strategies targeting the pro-inflammatory mediators in the TME
3.1. Cell signaling inhibitors
One way to target several cytokines/inflammatory mediators is to inhibit the transcription factor NF-κB. Several drugs are available to modulate the NF-κB pathway and thereby reduce expression of IL-8 and other pro-inflammatory cytokines. Many of these drugs target NF-κB indirectly by reducing ROS production including resveratrol, anthocyanin, apigenin, and RK-1–123. Because resveratrol is a member of the polyphenol flavonoids class of antioxidants produced by a restricted number of plants it has received significant attention as a potential treatment/adjunct therapy for GC patients and several reviews have addressed it directly (Zulueta et al. 2015). Resveratrol has been shown to inhibit proliferation of a number of cancer cell lines, and it behaves as a chemo-preventive agent in assays that measure the three stages of carcinogenesis (Holian et al. 2002). While human pilot studies in patients with colorectal liver metastases have demonstrated that preoperative resveratrol reduced cancer cell proliferation (Patel et al. 2010) and increased apoptosis in resected tumor tissues (Howells et al. 2011), there have been no clinical trials with resveratrol in GC.
3.2. Cytokine antagonists (IL-1, IL-6, IL-18, TNF)
While many of these cytokines have been discussed in detail in other areas of this book (Chapters 8, 9, 12 and 13), here a review will be provided for cytokines that may be candidates for targeting during cancer therapy.
IL-1β is expressed by a number of different cell types in vitro in response to H. pylori and all of these cells may participate in the inflammatory response in vivo, including dendritic cells (DCs), monocytes/macrophages and gastric epithelial cells (Kim et al. 2013; Semper et al. 2014). IL-1β is a cytokine that has received significant attention because in several studies polymorphisms associated with increased expression of IL-1β are significantly associated with the development of GC (El-Omar et al. 2000; Camargo et al. 2006). Expression is notably correlated with clinical and pathological features of GC (Yin et al. 2016). Solid tumors in which IL-1β has been shown to be up-regulated include breast, colon, lung, head and neck cancers, and melanomas, and patients with IL-1β producing tumors have a generally poor prognosis. IL-1β inhibits acid secretion by downregulating H+/K+ATPase expression and gastrin expression (Smolka and Backert 2012). Moreover, transgenic expression of IL-1β in the stomach causes gastritis-associated GC with recruitment of MDSCs (Tu et al. 2008) and in human xenograph models, elevated levels of IL-1β are correlated with advanced metastatic disease (Lewis et al. 2006). There are several possibilities for targeting IL-1β including approved treatments already utilized to treat patients with rheumatoid arthritis (Nikfar et al. 2018). IL-1 receptor antagonist (IL-1RA, anakinra) is a naturally occurring protein has been shown to decrease tumor growth, angiogenesis, and metastases in murine xenograft models (Weinreich et al. 2003). This is the IL-1β blocking therapy that has received the most attention. Anakinra is well absorbed in humans, and its safety is well documented with few adverse reactions (Sota et al. 2018), making it a candidate to be tested in combination with standard chemotherapy in GC. Currently, there is only one clinical trial in the NCI database utilizing anakinra for treatment of cancer, and it is specifically focused on early stage multiple myeloma (clinicaltrials.gov ID# NCT02492750). In addition to the IL-1RA, there several other agents available to inhibit the inflammatory and tumor promoting effects of IL-1β including anti-interleukin-1 monoclonal antibodies, the soluble IL-1 receptor type II, IL-1β-converting enzyme inhibitors, and IL-1β cytokine traps.
IL-6 is another pleiotropic cytokine which impacts inflammatory T cell biology as well as tissue regeneration and carcinogenesis. While the findings on associations between IL-6 polymorphisms and risk of GC are controversial, there is increasing evidence that levels of IL-6 may be a prognostic marker for spread (Ashizawa et al. 2005). In inflammatory cells, IL-6 is well known for activating STAT3 signaling, which can induce a pro-carcinogenic, tumorigenic microenvironment. STAT3 signaling leads to activation of NF-κB in inflammatory cells and drives a positive feedback loop between immune cells and tumor cells that further stimulates the cancer stem cell components and may contribute to metastasis and resistance to cancer therapies. GC cell lines also express high levels of IL-6 receptor, and IL-6 activation of GC cells leads to STAT3 activation and VEGF production (Huang et al. 2004; Lee et al. 2010; Wang et al. 2013); moreover, in ex vivo assays this leads to human umbilical vein endothelial cell proliferation, tube formation and vascularization in a Matrigel plug assay (Huang et al. 2004). IL-6 also inhibits H2O2-induced apoptosis and blocks repair of oxidative DNA lesions in human GC cells through upregulation of anti-apoptotic gene, MCL-1 (Lin et al. 2001). Again, methods targeting IL-6 have been developed for the treatment of IL-6-associated diseases, such as rheumatoid arthritis and Castleman disease, but not for cancers. These therapeutics include anti-IL-6 antibodies (siltuximab and sirukumab), anti-IL-6 receptor antibodies (cilizumab and tocilizumab), soluble gp130 (also a receptor of IL-6, designed to inhibit IL-6 binding to IL-6R), and some selective small molecules which inhibit JAK/STAT signaling as described above. While the concept to inhibit IL-6 and/or IL-6 signaling is not new (Sansone and Bromberg 2012; Jones et al. 2011), few clinical trials have been performed with these therapeutics in solid tumors (Ruffell and Coussens 2015), and at the time of writing this chapter, no clinical trials were published utilizing these biological treatments in GC. Another way to block IL-6 indirectly is through the inhibition of STAT3. Napabucasin, an oral inhibitor of cancer stem cells through STAT3 signaling blocking, is being tested in many gastrointestinal tumors, either alone or in combination with standard chemotherapy. Currently there is one clinical trial evaluating the association between napabucasin and weekly paclitaxel (a first-line cytotoxic agent) as second-line therapy for patients with GC (NCT01278956). An interesting strategy in GC would be to investigate the efficacy and safety of napabucasin combined with an anti-IL-6 antagonist.
TNF is a pro-inflammatory cytokine, which was first recognized for its inhibitory effect in some tumors when present at high concentrations, but it is also key cytokine for orchestrating inflammation and the host immune response. TNF activated chemokine gradients recruit immune cells to the sites of infection/inflammation. TNF is induced during H. pylori infection (Lindholm et al. 1998). The level of TNF may dictate its functional consequences; for it is thought to be pro-angiogenic in tumors, but a potent anti-vascular cytokine at higher doses and can be used clinically to destroy tumor vasculature. Anti-TNF therapy is currently used for rheumatoid arthritis, Crohn’s disease, and other inflammatory diseases (reviewed in (Udalova et al. 2016)), but targeting TNF as an anti-cancer therapy has led to some scrutiny. One can appreciate why there are two very different hypothesizes as to the effect of anti-TNF therapy on cancer. On one hand, anti-TNF therapy could inhibit cancer development by reducing chronic inflammation, but on the other hand, if TNF induces apoptosis or has suppressive effects on gene expression, anti-TNF therapies may enhance the development of certain tumors. There have been many studies to assess the possibility that existing anti-TNF treatments increase risk for cancer, but because of heterogeneity within these studies there has been no consensus (reviewed in (Solomon et al. 2012)). Interestingly, Fan and colleagues (Chen et al. 2009) investigated the opposing approach of delivering the TNF protein (not anti-TNF) to gastric tumors. They fused TNF with a peptide (GX-1) known to target the human GC vasculature and injected the construct into the circulation of nude mice containing tumors of human GC cells. This targeted approach of delivering TNF delayed tumor growth and was less toxic than TNF alone. But, administering TNF has not been tested in clinical trials.
As mentioned earlier, some chronic inflammation is driven by T cell-derived cytokines including IL-17A and IFNγ. Targeting IFNγ to reduce inflammation or treat cancer has not been strongly considered because it has such an important role for control of infections- both viral and bacterial. The level of immunosuppression created by inhibiting this pro-inflammatory pathway would be unacceptable. On the other hand, targeting IL-17A has been considered both in chronic inflammatory disease and in cancer. There is evidence of increased IL-17A expression in GC tissue compared to normal gastric tissue (Yamada et al. 2012) and this may contribute to an imbalance of TH17/Treg cells (Li et al. 2013). On the other hand, there is conflicting evidence as to whether TH17 cells are increased in the peripheral blood of GC patients and good marker for tumor progression (Liu et al. 2012a; Zhang et al. 2008; Yamada et al. 2012). IL-17 inhibitors approved by the FDA include secukinumab and ixekizumab. These were designed to treat inflammatory disorders including psoriasis (Hueber et al. 2010; Sanford and McKeage 2015; Markham 2016), but they have not been used in a cancer setting and could be tested in patients with GC.
3.3. Non-steroidal anti-inflammatory drugs
Substantial evidence from epidemiological studies suggests that the use of aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) is protective against GC as well as other gastrointestinal tumors (Abnet et al. 2009; Epplein et al. 2009; Algra and Rothwell 2012; Huang et al. 2017; Kong et al. 2016; Rothwell et al. 2012; Zhang et al. 2014). Although the mechanisms by which NSAIDs protect GC are not completely understood, it is recognized that NSAIDs primarily reduce the production of prostaglandins (PGs) by inhibiting the activity of cyclooxygenase enzymes (Cox1 and/or Cox2). Cox1, encoded by PTGS1, is constitutively expressed and responsible for production of prostanoids during basal conditions in the gastrointestinal tract and other tissues. Cox2, encoded by PTGS2, is an inducible isoform upregulated at sites of inflammation and in some cancers, including GC (Ristimaki et al. 1997). Coxenzymes participate in the conversion of arachidonic acid into prostanoids, including PGs and thromboxane A2 (TxA2) (Wang and DuBois 2018). Besides promoting inflammation, prostanoids may facilitate tumor progression by several mechanisms, including stimulation of proliferation and inhibition of apoptosis of cancer cells, stimulation of tumor invasion and angiogenesis, and suppression of immune responses. A comprehensive review on the role of prostanoids in gastrointestinal cancer was recently published (Wang and DuBois 2018). Among prostanoids, PGE2 is the most abundant in human GC (Uefuji et al. 2000), and the measurement of its metabolite (PGE-M) levels in urine has shown PGE-M could be used as a biomarker for predicting GC risk and prognosis (Wang et al. 2017).
In the context of colorectal cancer, a −Cox2 selective inhibitor, celecoxib, was the first approved agent for patients with familial adenomatous polyposis. However, due to the cardiovascular side effects (Bresalier et al. 2005), long-term use of Cox2-selective inhibitors for cancer chemoprevention is no longer recommended. In contrast, long-term regular use of aspirin has proven beneficial for prevention of both cancer and cardiovascular diseases. Based on the evidence suggesting that aspirin therapy reduces the incidence of colorectal cancer after 5 to 10 years of use, the U. S. Preventive Services Task Force now recommends low dose aspirin use for the primary prevention of colorectal cancer in adults aged 50 to 59 years who meet certain criteria (Bibbins-Domingo and Force 2016; Chubak et al. 2016). Regarding GC, the evidence on the role of aspirin on prevention has been more limited and there is no current recommendation as a chemopreventive agent. Consistent with previous evidence, however, a recent meta-analysis (Huang et al. 2017) and a longitudinal study covering the whole population of South Korea concluded that long-term aspirin use was associated with reduction in GC risk (Kim et al. 2018). In this high GC risk population, the protective effect was significant in those individuals with cumulative aspirin daily dose use for at least three years. The evidence on the use of non-aspirin NSAIDs has shown less consistent results in protection against GC across studies (Kim et al. 2018; Abnet et al. 2009; Epplein et al. 2009; Huang et al. 2017). A recent study aimed to evaluate the protective effects of low-dose aspirin use after GC diagnosis found no association with GC-specific mortality after one year of follow-up (Spence et al. 2018). Currently, a phase III clinical trial assessing the long-term effects of regular aspirin use on recurrence and survival in various types of cancer is ongoing (Add-aspirin, NCT02804815). For the use of aspirin on GC prevention the well-known risks, including renal and platelet dysfunction, gastric ulceration and gastrointestinal bleeding (Lanas et al. 2011) should be considered. Nevertheless, the strength of the associations consistently seen in observational studies, along with the high GC mortality rate, supports the need for further research on the potential of NSAID chemoprevention trials, especially aspirin, among select high-risk populations.
Overall, blocking the inflammatory response in GC through the inhibition of pro-inflammatory cytokines comprises an attractive approach for clinical trials. A list of selected ongoing studies assessing therapeutic agents targeting cancer-promoting inflammation in patients with GC (excluding antiangiogenic agents) is presented in Table 1. However, it is unlikely that the blockade of a single cytokine would result in dramatic clinical effect. Rather, there is a good rationale to combine targeted agents directed to different cytokines in GC, including systemic chemotherapy. Yet, like most of the new clinical trials of advanced cancer, trials in GC should enroll molecularly-selected patients. In the context of cytokines, well-conducted studies should be undertaken to identify predictive biomarkers; with that information, patients would have their GC tissues tested for the biomarker in order to enroll into a specific “anti-inflammatory” trial.
Table 1.
Selected ongoing studies assessing therapeutic agents targeting cancer-promoting inflammation in patients with GC (excluding antiangiogenic agents)
| Strategy | Agent | Target | Mode of action | Combination or comparator | Tumor types | Phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| Blocking pro-inflammatory mediators | Napabucasin (BBI608) | STAT3 | Inhibitor of cancer cell stemness; indirectly inhibits IL-6 through STAT3 inhibition | Napabucasin + paclitaxel | Metastatic or locally recurrent GC or GEJC | III | NCT01278956 |
| Aspirin | COX1/COX2 | COX enzymes inhibition | Aspirin monotherapy | Non-metastatic solid tumors (adjuvant setting) | III | NCT02804815 | |
| Inhibiting recruitment of immune cells | Emactuzumab (RG7155) | CSFR1 | Monoclonal antibody | Emactuzumab + atezolizumab (anti-PD-L1 mAB) | Locally advanced or metastatic solid tumors | IB | NCT02323191 |
| Emactuzumab + selicrelumab (CD40 agonist)1 | Locally advanced or metastatic solid tumors | IA/IB | NCT02760797 | ||||
| Pexidartinib (PLX3397) | CSFR1 | Tyrosine kinase inhibitor | Pexidartinib + paclitaxel | Advanced, incurable solid tumors | IB | NCT01525602 | |
| HuMax-IL8 (BMS-986253) | IL-8 | Monoclonal antibody | HuMax-IL8 + nivolumab | Advanced, incurable solid tumors | I/II | NCT03400332 | |
| DKN-01 | DKK-1 | Monoclonal antibody | DKN-01 + paclitaxel or pembrolizumab | Metastatic or locally recurrent GC or GEJC | I | NCT02013154 | |
| Reprogramming TAMs from M2 to M1 | CDX-1140 | CD40 | Monoclonal antibody (CD40 agonist) | CDX-1140 + standard therapy | Advanced solid tumors | I | NCT03329950 |
| Selicrelumab (RO7009789) | CD40 | Monoclonal antibody (CD40 agonist) | Selicrelumab + atezolizumab (anti-PD-L1 mAB) | Locally advanced or metastatic solid tumors | IB | NCT02304393 | |
| Immuno-conjugates | CEA-TCB antibody (RO6958688) | CEA expressed in tumor tissues | Bispecific anti-CEA anti-CD3 monoclonal antibody | Monotherapy | Advanced CEA-expressing solid tumors | I | NCT02324257 |
This trial combines an inhibitor of immune cell recruitment and an agent to reprogram macrophages to M1 phenotype.
CD3: cluster of differentiation 3; CD40: cluster of differentiation 40; CEA: carcinoembrionic antigen; CEA-TCB: carcinoembryonic antigen T-cell bispecific; COX-1/COX-2: cyclooxygenases 1 and 2; CSF1R: colony-stimulating factor 1 receptor; DKK-1: Dickkopf-related protein 1; GC: gastric adenocarcinoma; GEJC: gastroesophageal adenocarcinoma; mAb: Monoclonal antibody; NCT: ClinicalTrials.gov identifier; PD-L1: programmed death-ligand 1; STAT3: Signal transducer and activator of transcription 3; TAMs: tumor-associated macrophages
4. Strategies targeting growth factors involved in angiogenesis
4.1. Growth Factors as target (VEGF/VEGFR2, CSF1/CSF1R, EGF/EGFR)
Vascular endothelial growth factor A (VEGFA, often referred to as VEGF), is a vascular permeability factor and the main regulator of tumor angiogenesis (Senger et al. 1983; Ferrara 2002). VEGF is a member of a family of growth factors and primarily binds to tyrosine kinase receptors VEGFR2 and VEGFR1 (Chung et al. 2010). VEGF is secreted by both tumor and non-tumor cells, such as macrophages, endothelial cells and fibroblasts (Goel and Mercurio 2013). VEGFR2 is mainly expressed by endothelial cells, but also by a variety of cells, including tumor cells. The binding of VEGF to VEGFR2 is considered critical for the regulation of tumor angiogenesis, by promoting the proliferation and migration of endothelial cells, as well as the degradation and remodeling of the extracellular matrix. Independent of the role of VEGF in tumor development, a large body of evidence supports a role for VEGF in the pathogenesis and maintenance of chronic inflammatory disorders. Notably, immune cells can express VEGF receptors, and the functions of these cells can be regulated by VEGF signaling. VEGF promotes the adherence of leukocytes to the vascular endothelium and the release of pro-inflammatory cytokines, such as IL-6 and TNF, which support tumor development in chronic inflammatory diseases (Waldner and Neurath 2012). The role of VEGF signaling in cancer-associated inflammation was demonstrated by Waldner and co-workers (Waldner et al. 2010). Patients with inflammatory bowel disease or with colitis-associated cancer showed increased expression of VEGFR2 on intestinal epithelial cells. Results from in vivo and in vitro experiments demonstrated that chronic inflammation induces VEGFR2 expression on intestinal epithelial cells and that VEGFR2 signaling is necessary for tumor growth (Waldner et al. 2010). There is also significant evidence that VEGF/VEGFR2 signaling has an important role in GC pathogenesis (Lieto et al. 2008; Murukesh et al. 2010; Suzuki et al. 2010) and the inhibition of this interaction is the main target of anti-VEGF therapeutics (Ferrara 2009).
The monoclonal anti-VEGF antibody bevacizumab was the first agent developed targeting the VEGF pathway. Bevacizumab is now approved for first- and/or second-line treatment of a variety of tumors including colorectal cancer, but clinical trials in GC have not obtained encouraging results (Ohtsu et al. 2011; Shen et al. 2015). Ramucirumab, a human monoclonal antibody that targets VEGFR2 (Spratlin et al. 2010), is the first drug targeting angiogenesis that showed to prolong survival in patients with previously treated advanced GC or gastroesophageal junction carcinomas (GEJC) in phase III clinical trials (Fuchs et al. 2014; Wilke et al. 2014). Currently, ramucirumab is indicated as a single agent or in combination with paclitaxel, for the treatment of patients with advanced or metastatic, GC or GEJC with disease progression on or after fluoropyrimidine- or platinum-containing chemotherapy (Ajani et al. 2016).
Apatinib, a selective VEGFR2 tyrosine kinase inhibitor, was tested in Asian patients with previously treated, advanced GC and showed prolonged overall and progression-free survival (Li et al. 2016). Apatinib was approved by the FDA in 2017 as a third-line therapy for refractory GC or GEJC. Experimental studies have suggested that apatinib not only has anti-angiogenesis effects but also possesses substantial angiogenesis-independent effects, inhibiting cell proliferation in vitro and delaying xenograph tumor growth in vivo (Lin et al. 2017). A large number of clinical trials including apatinib for patients with GC are currently underway, including phase III and IV trials ( NCT03042611 and NCT02426034). Regorafenib, a multikinase inhibitor that targets VEGFR2 and is used for refractory colorectal cancer (Wilhelm et al. 2011; Riechelmann and Grothey 2017), is currently being tested in clinical trials for GC and has provided promising results (Pavlakis et al. 2016).
Colony-stimulating factor 1 (CSF1 or also known as MCSF) is a hematopoietic growth factor constitutively expressed by many cell types (Hamilton et al. 2016). CSF1 is the major lineage regulator of most populations of macrophages, but it is also a chemotactic factor for macrophages. CSF1 exerts its effects through a tyrosine kinase receptor (CSF1R), which is expressed on monocytes and macrophages, but also on other myeloid cells within the TME (Cannarile et al. 2017). In human GC, elevated expression of CSF1 or CSF1R significantly correlated with disease progression and also with poor overall survival and disease-free survival (Okugawa et al. 2018). Targeting TAMs by inhibition of CSF1/CSF1R has shown encouraging results in preclinical cancer models in a variety of tumors, not only decreasing the number of TAMs, but also reprogramming remaining TAMs to support antigen presentation and bolster T-cell activation (Ries et al. 2014; Zhu et al. 2014; Pyonteck et al. 2013; Quail et al. 2016; DeNardo et al. 2011). Experimental evidence has shown that macrophages can mediate chemotherapy resistance by providing survival factors or activating anti-apoptotic pathways in cancer cells. In a mouse model of breast cancer, cytotoxic therapy showed to induce CSF1-dependent macrophage recruitment (DeNardo et al. 2011). In this model, blockade of macrophage recruitment with CSF1R-signaling antagonists, in combination with paclitaxel, showed promotion of TH1 responses and improved mouse survival by reduction in primary and metastatic tumors.
In human tumors, the most promising evidence targeting the CSF1/CSF1R axis has been documented in patients with the diffuse type of tenosynovial giant cell tumor (TGCT). TGCT is a rare neoplasm associated with inflammation and joint destruction, in part due to infiltration of CSFR1-bearing macrophages (Gelhorn et al. 2016). Because TGCT is associated with overexpression of CSF1, therapies targeting the CSF1/CSF1R axis have been tested in patients with locally advanced or relapsed TGCT (Brahmi et al. 2016). Studies have shown significant clinical improvement with emactuzumab (RG-7155), a recombinant humanized monoclonal antibody targeting CSF1R (Ries et al. 2014; Cassier et al. 2015), and with the small molecule CSF1R inhibitor pexidartinib (PLX3397) (Tap et al. 2015). In the context of GC, a large number of clinical trials assessing the potential of various CSFR1 inhibitors (emactuzumab, pexidartinib, DCC-3014, and others) either as monotherapies or in combination with other therapeutic modalities are underway (see Table 1).
Additional recent studies have implicated several other potential master regulators of macrophage function and polarization in the context of H. pylori infection and gastric carcinogenesis. We recently reported (Hardbower et al. 2016) that epidermal growth factor receptor (EGFR) signaling is a crucial component of the response of macrophages to bacterial infections, with H. pylori a prototypical example. While EGFR signaling in gastric epithelial cells has been documented and related to both ligand-dependent (EGF) and independent (TNF) responses (Yan et al. 2009), the response of mouse and human macrophage cell lines and mouse bone marrow-derived primary macrophages was ligand-independent and involved both tyrosine 1068 and serine 1046/7 phosphorylation sites (Hardbower et al. 2016). Importantly, human gastric tissues exhibited marked phosphorylation of EGFR in gastric macrophages along the entire cascade from gastritis to gastric adenocarcinoma, and mice with myeloid specific deletion of Egfr, exhibited attenuated gastric inflammation scores, increased H. pylori colonization, and reduction of M1 macrophage and TH1/TH17 responses.
It should be noted that these studies were in a model of chronic infection, not cancer. However, in the azoxymethane-dextran sulfate sodium model of colitis-associated colon carcinogenesis, mice with myeloid deletion of Egfr showed a marked reduction of tumor development (Hardbower et al. 2017). Moreover, these findings were associated with attenuation of M2 responses and angiogenesis and associated signaling. Surprisingly, mice with epithelial deletion of Egfr did not show protection from colon tumorigenesis. There are a series of studies from other groups similarly showing that deletion of Egfr in myeloid cells reduces liver cancer (Lanaya et al. 2014) and other colon cancer models (Srivatsa et al. 2017). There are also studies related to pancreatic cancer implicating macrophage EGFR signaling in M2 macrophage polarization (Ma et al. 2016).
The addition of the EGFR inhibitor, gefitinib, was very effective in rodent models of gastric cancer (Sierra et al. 2018). Specifically when added to the diet, this agent significantly reduced development of dysplasia and intramucosal carcinoma in H. pylori-infected INS-GAS mice and dysplasia and invasive gastric adenocarcinoma gastric cancer in infected gerbils. Gefitinib treatment reduced PMN infiltration and chemokine expression, as well as epithelial DNA damage in both rodent models. Gefitinib was effective if given as a pretreatment before infection or if administered after infection and inflammation was already established, and still had a benefit if given to animals after antibiotic eradication of the H. pylori (Sierra et al. 2018). It should be noted that the use of a pharmacologic approach does not distinguish the offending cellular source of EGFR signaling. To this end, the effect of gastric epithelial-specific deletion of Egfr was investigated; this resulted in less gastric inflammation, DNA damage and chemokine expression. Thus, the Wilson lab are generating mice with myeloid- and epithelial-specific deletion of Egfr on the cancer-prone FVB/N INS-GAS mouse and will be determining the effect on gastric carcinogenesis during H. pylori infection.
Despite the strong scientific rationale of antiangiogenic agents in GC and other solid tumors, the overall results of clinical trials with these agents have been quite modest, with survival improvements generally measured in weeks. Likewise, despite the promising preclinical data with anti-EGFR agents in GC, randomized trials with these agents, with the exception of trastuzumab for HER2-positive GC (Song et al. 2016), were not effective in patients with molecularly-unselected metastatic GC. Therefore, their future in the drug development process in GC is likely to be undermined by more innovative agents, such as immunotherapy.
4.2. Strategies inhibiting M1 to M2 transitions and promoting M1 phenotype
Besides the mentioned effects of targeting the CSF1/CSF1R axis on macrophage polarization, reprogramming TAMs into M1-phenotype macrophages can be achieved through a variety of other therapeutic modalities including chemotherapy, immunotherapy and radiotherapy (Ruffell et al. 2012; Genard et al. 2017). One of the modalities under investigation is based on the use of CD40 agonists. CD40 is a member of the TNF receptor superfamily that is present on a variety of immune cell types. CD40 activation plays a critical role in triggering T and B cell immunity, by activation of antigen-presenting cells, resulting in an enhanced anti-tumor immune response (Vonderheide 2018). By stimulating CD40, monoclonal antibodies against CD40 similarly have shown to reprogram TAMs from M2 phenotype to M1 macrophages. In a mouse model of pancreatic cancer, CD40-activated macrophages rapidly infiltrated tumors, showed anti-tumor properties, and facilitated the depletion of tumor stroma (Beatty et al. 2011). In addition, the activation of CD40 present on the surfaces of some solid tumor cells leads to direct tumor cell apoptosis and decreased tumor growth. In the context of GC, several clinical trials involving patients with a variety of advanced solid tumors are underway (Table 1). Some evidence has indicated that is also possible to re-educate TAMs by exposure to specific immunological mediators which may promote M1 macrophage development, such as IFNγ (Duluc et al. 2009; De Palma et al. 2008).
Having evidenced that loss of ODC enhances host defense against H. pylori (Hardbower et al. 2017), a related question is the potential to inhibit ODC in vivo. Efficacy of the pharmacologic agent α-difluoromethylornithine (DFMO), which blocks ODC activity, has been demonstrated in clinical trials related to prevention of colon polyps (Meyskens et al. 2008; Zell et al. 2010), and has also been used for the treatment of neuroblastoma (Bassiri et al. 2015; Evageliou et al. 2016; Saulnier Sholler et al. 2015). The mechanism of action is unproven, but has been conceptually related to reduction of epithelial cell growth and DNA replication. The downstream enzyme, spermine oxidase (SMOX), which generates oxidative stress during the back-conversion of spermine to spermidine, is upregulated by H. pylori in both macrophages (Chaturvedi et al. 2004) and gastric epithelial cells (Chaturvedi et al. 2014; Chaturvedi et al. 2011). Moreover this generation of ROS leads to oxidative DNA damage in the gastric epithelium (Chaturvedi et al. 2014; Chaturvedi et al. 2011), which is especially notable in the presence of gastric intestinal metaplasia (Chaturvedi et al. 2015). Inhibition of ODC with DFMO or inhibition of SMOX reduces DNA damage and gastric cancer development in gerbils infected with a gerbil-adapted H. pylori strain derived from a patient with gastric dysplasia from the Andean mountain region of Colombia, where gastric cancer risk is high (Chaturvedi et al. 2015).
Based on these studies we are currently conducting a phase II clinical trial using DFMO (eflornithine, 500 mg po per day) versus placebo in Latin American sites (Honduras and Puerto Rico, NCT02794428) in patients with precancerous gastric lesions (i.e. intestinal metaplasia). The primary endpoint is DNA damage at 6 months of treatment, with secondary endpoints of DNA damage at 18 months and histopathology at 18 months. The trial is ongoing, but final results will not be expected until 2020. An additional goal of the study is to determine effects of DFMO on immune cells versus epithelial cells.
4.3. Inhibition of MDSCs
Myeloid-derived suppressor cells (MDSCs), a heterogeneous group of immature myeloid cells that inhibit the antitumor activity of T lymphocytes and NK cells and which are absent in physiologic conditions, make an important component of TME. Studies in mice have demonstrated that the depletion of MDSCs is associated with delayed tumor growth (Schroder et al. 2017; Davis et al. 2017). Based on such findings, MDSCs have been investigated as therapeutic targets in GC. The Dickkopf-related protein 1 (DKK-1), a Wnt regulator, promotes immunosuppression in TME through the stimulation of MDSCs (Moehler et al. 2018). Two DKK-1 directed antibodies (BHQ880 and DKN-01) are currently being tested in several tumor types; an ongoing phase I trial is testing a DKK-1 monoclonal antibody in monotherapy or in combination with paclitaxel or pembrolizumab for patients with heavily pre-treated GC or GEJC ( NCT02013154). Another strategy to inhibit the functionality of MDSCs is through the blockade of the glucocorticoid-induced TNFR-related protein (GITR). This protein is expressed in normal monocytes, MDSCs and macrophages and its suppression has led to reduced tumor progression and increased T-cell infiltration in an animal model of pancreatic cancer (Moehler et al. 2018). Interestingly, the inhibition of GITR was associated with up regulation of cytotoxic T lymphocyte associated antigen 4 (CTLA4) on T cells and PD-L1 on tumor cells, proposing that a combination with checkpoint inhibitors may be required for this treatment to be effective.
4.4. Targeting chemokines and their receptors
The TME can also be targeted through the disruption of chemokines networks. Chemokines, small proteins normally involved in immune cell migration and lymphoid tissue expansion, are implicated in the TME immunosuppression through complex mechanisms, including the stimulation of MDSCs (Nagarsheth et al. 2017). Because chemokines and their receptors participate in major roles in inflammation and related-inflammatory diseases, research has been conducted to explore the modulation of certain chemokines as a form of cancer- (and TME-) directed therapy. Chemokine inhibitors, mostly in combination with immune checkpoint inhibitors, are undergoing clinical testing in different tumor types.
The overexpression of chemokine CCL2, the monocyte chemoattractant protein (MCP1), induces angiogenesis and tumorigenesis of GC in nude mice via macrophage recruitment (Kuroda et al. 2005). An elevated level of CCL2 has been reported in patients with GC, was correlated with lymph node metastasis, and was associated with lower overall survival rate (Tonouchi et al. 2002; Futagami et al. 2008; Tao et al. 2014). However, while the inhibition of the CCL2-CCR2 (CCL2 main receptor) signaling pathway represented an attractive approach in GC, clinical trials have shown disappointing results. Carlumab, also known as CNTO888, is a human IgG1k monoclonal antibody that binds CCL2 with high affinity and which has been tested in two different phase I trials in patients with solid tumors. In the first trial, carlumab was administered in monotherapy; it offered modest antitumor activity, with the best responses observed being tumor stabilization in ovarian cancer, ocular melanoma and neuroendocrine tumor (Sandhu et al. 2013). In a second phase I trial where carlumab was combined with different standard chemotherapeutic agents, only one out of 19 patients experienced a partial response (Brana et al. 2015). Another approach to CCL2/CCR2 interference is to inhibit the CCR2 receptor. A humanized IgG1 antibody, MLN1202, has been successful in several inflammation-related diseases such as multiple sclerosis and atherosclerosis. However, a phase II trial with this agent in patients with bone metastases did not show efficacy (Vela et al. 2015). Given the above results, further trials with CCL2/CCR2 inhibitors are on hold.
CXCR4 and CXCL12, its ligand, are immunohistochemically overexpressed in GC in comparison to normal gastric tissue and associated with survival, proliferation, angiogenesis, and migration of cancer cells. Studies have demonstrated that such chemokine expression patterns are prognostic factors for survival and metastases (Xue et al. 2017). Also, overexpression of CXCL12 in gastric mucosa contributes to carcinogenesis in the presence of inflammatory stimuli such as H. pylori (Shibata et al. 2013). CXCL12 also activates the PI3K/mTOR and MAPK/ERK signaling pathways (Rubie et al. 2016). The blockade of the CXCL12-CXCR4 axis is the target of drugs used to treat the human immunodeficiency virus (HIV) infection or to help with the mobilization and collection of CD34‐positive hematopoietic stem cells for transplantation in patients with certain hematological malignancies. Recently several pre-clinical studies in different tumor types, including GC, report encouraging antitumor effects from this biological class (Xue et al. 2017). However, clinical trials with CXCL12-CXCR4 inhibitors have just begun. A phase I trial of LY2510924, a peptide antagonist, which blocks stromal cell–derived factor-1 from CXCR4 binding, was conducted in patients with advanced and refractory solid tumors (although there were not any patients with GC in the study); while the safety profile was good, the best response observed was tumor stabilization in 20% of patients and the median duration of treatment was only 1.9 months (Galsky et al. 2014). Both randomized phase II trials in non-small cell lung cancer and renal cell carcinoma were negative for their primary endpoints of progression free survival (Salgia et al. 2017; Hainsworth et al. 2016). Despite these preliminary negative results, there are a number of ongoing clinical trials in multiple tumor types; but at the time of this publication, there are no trials in GC. An interesting approach in GC would be to combine CXCL12-CXCR4 inhibitors with other targeted agents that block the same pathways (e.g. mTOR inhibitors, MEK inhibitors) or with other chemokine inhibitors.
Interleukin-8 (IL-8) is a potent neutrophil chemotactic cytokine with potential impact on the tumor microenvironment. In chronic H. pylori-associated gastritis, gastric epithelial cells express increased levels of IL-8 within the lamina propria (Crabtree et al. 1994b). IL-8 is the single most up-regulated gene in whole genome profiling of H. pylori exposed gastric epithelial cells (Eftang et al. 2012). Moreover IL-8 expression is correlated with functional cagPAI status of H. pylori strains (Crabtree et al. 1994a; Crabtree et al. 1995), but IL-8 can also be strongly induced by IL-17 responses. The result of IL-8 expression is recruitment of PMNs to the tissue for higher degrees of PMN infiltration which is correlated with an increase in the secretion of TNF, IL-1β, IL-6 and IL-8 (Bauditz et al. 1999). In a study out of northern India, circulating levels of IL-8 were higher among patients with GC compared to healthy controls, but levels were comparable to patients with functional dyspepsia (Kumar et al. 2015). The expression of IL-8 directly correlates with a poor prognosis in GC (Yamada et al. 2013; Lee et al. 2004).
In addition to its potent neutrophilic chemotactic activity, IL-8 can induce proliferation and migration of cancer cells (Wilson et al. 1999; Brew et al. 2000). For this reason, there has been interest in targeting it as a cancer therapy. IL-8 increases the proliferation, migration and survival of endothelial cells, enhances epithelial-mesenchymal transition (EMT) and survival of cancer cells (Fernando et al. 2011). Moreover, there is evidence that IL-8 may enhance macrophage activity in tumors through activating VEGF expression, a potent angiogenic factor (Martin et al. 2009). IL-8 may modulate invasiveness and/or extracellular matrix remodeling through enhancement of matrix metalloprotease expression (MMP2/MMP9) (Li et al. 2005; Inoue et al. 2000; Kim et al. 2001). In addition to general mechanisms to block NF-κB signaling and IL-8 induction discussed above, small molecule inhibitors targeting IL-8 receptors (CXCR1 and CXCR2) have been developed. G31P and SCH-527123 were initially developed for use in prostate and colon cancers, respectively, with the intention of reducing cell migration and increasing apoptosis of cancer cells (Liu et al. 2012b; Ning et al. 2012). Repertaxin, another inhibitor of CXCR1 and CXCR2, has shown to decrease tumor proliferation in the GC cell lineMKN45 (Wang et al. 2016). These inhibitors have only been tested in vitro on GC cells and no clinical trials have been performed with them in GC.
Meanwhile there is also interest in directly blocking IL-8, especially since the findings that high serum IL-8 levels correlate with poor prognosis in various tumors (Sanmamed et al. 2014). Moreover, IL-8 was found to stimulate recruitment of MDSCs and promote EMT in tumors conferring resistance to immune-mediated killing (reviewed in (David et al. 2016)). An IL-8-specific monoclonal antibody, known as HuMax-IL8, has been shown to reduce mesenchymal features in cancer cells leading to enhanced susceptibility to NK and T cell-mediated lysis and to decrease the frequency of granulocytic MDSCs in xenograft models (Collins et al. 2018). The HuMax-IL8 drug (BMS-986253) is also designed to inhibit IL-8 and thus far the only clinical trial utilizing this drug is to test it in combination with nivolumab in patients with advanced solid tumors, but no results have been published ( NCT03400332).
5. Enhancing Anti-tumor Immunity (Therapy-induced inflammation)
Another form of targeting inflammation as an anti-cancer strategy is to modulate the immunosuppressive TME through therapies that stimulate the antitumor immune response. The immune checkpoint inhibitors, currently the monoclonal antibodies against PD-1/PD-L1 and CTLA4, represent a successful class and have been approved to treat numerous solid tumors and hematological malignancies. The use of immunotherapy in GC has just started and will be discussed below.
5.1. Checkpoint Inhibitors (anti-PD-1/L1, anti-CTLA4)
Currently, two immune checkpoint inhibitors (ICPi), pembrolizumab and nivolumab, have been approved in many countries for advanced/metastatic GC and both are anti-PD-1 monoclonal antibodies. The landmark phase II trial by Le D et al (Le et al. 2017) demonstrated dramatic tumor shrinkage and significantly prolonged progression free survival with pembrolizumab for patients with heavily pre-treated MSI solid tumors, mostly Lynch syndrome-associated colorectal cancer. These results led to the FDA’s first ever site-agnostic approval. Given that tumor lymphocytic infiltration is one of the hallmarks of MSI tumors, it was conceived that ICPi would work mostly in “inflamed” tumors. This has proven to be true; larger magnitudes of benefit have been observed in inflammation-associated tumors with higher mutation burden, such as lung cancer, melanoma, and Merkel cell carcinoma. In GC, the overall benefit of immunotherapy has been modest. However, in specific subgroups where cancer-associated inflammation is present, i.e. MSI, higher response rates and prolonged disease control have been reported. While EBV-positive nasopharyngeal carcinoma patients are more likely to benefit from ICPi (Kao et al. 2015), clinical data in GC have not been published, but are eagerly awaited. Currently it is unknown whether concurrent or prior H. pylori infection predicts response and survival in GC.
In the KEYNOTE-059 multi-cohort trial, pembrolizumab was administered in different settings of patients with advanced GC or GEJC (Wainberg et al. 2017). Cohort 1 enrolled 259 patients with at least two prior lines of therapy and 57% of the patients had PD-L1 positive tumors (defined as positive immunohistochemistry expression in at least 1% in tumor cells); in this cohort, pembrolizumab monotherapy offered an overall objective response rate of 12% (16% in PD-L1 positive and 6% in PD-L1 negative, respectively), with 3% of patients achieving complete response, regardless of PD-L1 status. As expected, among the 4% of patients with MSI tumors, the objective response rate was 57.1% (Fuchs et al. 2017). This uncontrolled phase II trial led to the approval of this drug in the third line setting of PD-L1-positive GC. Recently a phase III trial of pembrolizumab versus paclitaxel in second-line was negative for its primary endpoint, overall survival, for patients with combined positive score ≥ 1 tumors – the number of PD-L1 positive staining tumor, lymphocytes or macrophages divided by the total number of viable tumor cells and multiplied by 100 (Fuchs et al. 2018b). Interestingly, the study showed that the higher the combined positive score, the higher the benefit of pembrolizumab when compared to paclitaxel, reinforcing that the more intense the tumor inflammation, the higher the likelihood of anti-tumor activity from treatment with an ICPi.
In the phase III trial named ATTRACTION-2, 493 Asian patients with advanced GC or GEJC previously treated with chemotherapy were randomized to receive nivolumab or placebo until disease progression (Kang et al. 2017). Similar to the overall clinically irrelevant, albeit statistically significant, results with pembrolizumab, this trial demonstrated a modest gain (measured in days) in overall survival. In contrast to the pembrolizumab trial, PD-L1 expression was not predictive of a survival benefit with nivolumab. Based on these results, nivolumab was approved in Japan for GC.
Overall clinical trials in GC with anti-CTLA4 monoclonal antibodies have showed discouraging results, with limited efficacy signals (Bang et al. 2017; Ralph et al. 2010). Similarly, the results with the combination of anti-PD-L1 and anti-CTLA4 agents were disappointing. In two cohorts of the CheckMate 032 phase I/II trial where one-quarter to one-third of patients had PD-L1 positive tumors, nivolumab in combination with ipilimumab was delivered on two different dose schedules (Janjigian et al. 2017). These treatments led to overall objective response in 8% and 24% of patients, respectively, but with median overall survival times of only 6.9 and 4.8 months. These results clearly demonstrate that although immunotherapy is one of greatest advances in modern oncology, it does not work in molecularly unselected GC cancer patients. The molecularly distinct subtypes of GC (Cancer Genome Atlas Research 2014; Cristescu et al. 2015) should be explored for predictive biomarkers to ICPi responsiveness.
5.2. Other immunotherapies–immunoconjugates
A new class of immunotherapeutic agents, immunoconjugates, is being tested and demonstrating promising results in certain tumor types. A bispecific anti-carcinoembryonic antigen (CEA) anti-CD3 antibody carcinoembryonic antigen-T-cell bispecific antibody (CEA-TCB) has shown impressive results in phase I trials in colorectal cancer (Parkhurst et al. 2011). The CEA-TCB RO6958688 was given as monotherapy or in combination with atezolizumab, an anti-PD-L1 monoclonal antibody, to patients with advanced solid tumors, the majority with colorectal cancer, with positive immunohistochemistry expression of CEA (Tabernero et al. 2017); tumor inflammation was evidenced in computerized tomographies (CT) scans within a few days of administration in the higher dose levels cohorts, and after 4 weeks, metabolic response by 18-FDG PET scan was observed in 28% of patients in the monotherapy group and in 60% of those in the combination arm. Interestingly, the most common adverse events were inflammation-related, such as pyrexia (56.3%), and dose-limiting toxicities were associated with inflammation in metastatic lesions. Although preliminary, the CEA-TCB RO6958688 could be tested in a great variety of CEA+ tumors ( NCT02324257, NCT02650713). Indeed, phase III trials are planned in advanced colorectal and GC.
Conclusions
In this chapter, we have provided insights into inflammation-related factors that promote H. pylori-associated GC initiation and growth. Strategies for immune therapy could fall into 3 major classifications including: targeting pro-cancer inflammation and reprogramming or depleting immune cells in the TAM to increase anti-cancer immune responses. Some of these strategies will likely be most successful when therapy is provided as an adjunct to current chemotherapy, whereas, others may prove to be more effective as monotherapy. For example, anti-VEGF agents provide some benefit when combined with chemotherapy, but offer limited efficacy as monotherapy. On the other hand, immunoconjugates and immune checkpoint inhibitors seem to offer antitumor activity alone or combined among them, while the addition of chemotherapy does not appear to improve outcomes in GC. Moreover, because of the diversity of the microenvironments (gastritis to tumor) which have been reported and the array of potential targets, it is clear that immune subtyping and tumor characterization are crucial for the field to move to more successful immune-therapies to treat persons at risk of GC and/or to treat existing cancer. And lastly, the drug development process, including pharma and academia, should concentrate efforts to identify predictive biomarkers in GC and to design clinical trials with enriched populations so we can offer GC patients treatments that make a significant difference in their lives.
Acknowledgments
This work was supported by U. S. National Institutes of Health (NIH) grants R01AT004821, R01CA190612, P01CA116087, P01CA028842; Office of Medical Research, Veterans Affairs Merit Review grants IBX000915A(to H.M.S.A.) and I01BX001453(to K.T.W.); and Vanderbilt University Digestive Disease Research Center supported by NIH grant P30DK058404.
References
- Abnet CC, Freedman ND, Kamangar F, Leitzmann MF, Hollenbeck AR, Schatzkin A (2009) Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. British journal of cancer 100 (3):551–557. doi: 10.1038/sj.bjc.6604880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamsson J, Ottsjo LS, Lundin SB, Svennerholm AM, Raghavan S (2017) Gastric expression of IL-17A and IFNgamma in Helicobacter pylori infected individuals is related to symptoms. Cytokine 99:30–34. doi: 10.1016/j.cyto.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H (2016) Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN 14 (10):1286–1312 [DOI] [PubMed] [Google Scholar]
- Algood HM, Allen SS, Washington MK, Peek RM Jr., Miller GG, Cover TL (2009) Regulation of gastric B cell recruitment is dependent on IL-17 receptor A signaling in a model of chronic bacterial infection. Journal of immunology 183 (9):5837–5846. doi: 10.4049/jimmunol.0901206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algra AM, Rothwell PM (2012) Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. The Lancet Oncology 13 (5):518–527. doi: 10.1016/S1470-2045(12)70112-2 [DOI] [PubMed] [Google Scholar]
- Arachchi PS, Fernando N, Weerasekera MM, Senevirathna B, Weerasekera DD, Gunasekara CP (2017) Proinflammatory Cytokine IL-17 Shows a Significant Association with Helicobacter pylori Infection and Disease Severity. Gastroenterology research and practice 2017:6265150. doi: 10.1155/2017/6265150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, Aoki T, Ohnuma S, Aoki T (2005) Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 8 (2):124–131. doi: 10.1007/s10120-005-0315-x [DOI] [PubMed] [Google Scholar]
- Asim M, Chaturvedi R, Hoge S, Lewis ND, Singh K, Barry DP, Algood HS, de Sablet T, Gobert AP, Wilson KT (2010) Helicobacter pylori induces ERK-dependent formation of a phospho-c-Fos c-Jun activator protein-1 complex that causes apoptosis in macrophages. The Journal of biological chemistry 285 (26):20343–20357. doi: 10.1074/jbc.M110.116988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri N, Azadegan-Dehkordi F, Shirzad M, Zamanzad B, Rahimian G, Taghikhani A, Rafieian-Kopaei M, Shirzad H (2015) Mucosal interleukin-21 mRNA expression level is high in patients with Helicobacter pylori and is associated with the severity of gastritis. Central-European journal of immunology 40 (1):61–67. doi: 10.5114/ceji.2015.50835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang YJ, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, Yamaguchi K, Balogh A, Sanchez T, Moehler M (2017) Efficacy of Sequential Ipilimumab Monotherapy versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 23 (19):5671–5678. doi: 10.1158/1078-0432.CCR-17-0025 [DOI] [PubMed] [Google Scholar]
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK, To GATI (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376 (9742):687–697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- Bassiri H, Benavides A, Haber M, Gilmour SK, Norris MD, Hogarty MD (2015) Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Translational pediatrics 4 (3):226–238. doi: 10.3978/j.issn.2224-4336.2015.04.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauditz J, Ortner M, Bierbaum M, Niedobitek G, Lochs H, Schreiber S (1999) Production of IL-12 in gastritis relates to infection with Helicobacter pylori. Clinical and experimental immunology 117 (2):316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331 (6024):1612–1616. doi: 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan TR, Islam MM, Uddin T, Chowdhury MI, Janzon A, Adamsson J, Lundin SB, Qadri F, Lundgren A (2014) Th1 and Th17 responses to Helicobacter pylori in Bangladeshi infants, children and adults. PloS one 9 (4):e93943. doi: 10.1371/journal.pone.0093943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Force USPST (2016) Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine 164 (12):836–845. doi: 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- Brahmi M, Vinceneux A, Cassier PA (2016) Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis. Current treatment options in oncology 17 (2):10. doi: 10.1007/s11864-015-0385-x [DOI] [PubMed] [Google Scholar]
- Brana I, Calles A, LoRusso PM, Yee LK, Puchalski TA, Seetharam S, Zhong B, de Boer CJ, Tabernero J, Calvo E (2015) Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Targeted oncology 10 (1):111–123. doi: 10.1007/s11523-014-0320-2 [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA, Adenomatous Polyp Prevention on Vioxx Trial I (2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. The New England journal of medicine 352 (11):1092–1102. doi: 10.1056/NEJMoa050493 [DOI] [PubMed] [Google Scholar]
- Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE (2000) Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine 12 (1):78–85. doi: 10.1006/cyto.1999.0518 [DOI] [PubMed] [Google Scholar]
- Camargo MC, Mera R, Correa P, Peek RM Jr., Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG (2006) Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 15 (9):1674–1687. doi: 10.1158/1055-9965.EPI-06-0189 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513 (7517):202–209. doi: 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Stat Facts: Stomach Cancer, SEER Cancer Statistics Review, 1975–2015. (2018) National Cancer Institute; https://seer.cancer.gov/csr/1975_2015/. 2018 [Google Scholar]
- Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D (2017) Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. Journal for immunotherapy of cancer 5 (1):53. doi: 10.1186/s40425-017-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbo A, Olivares-Villagomez D, Hontecillas R, Bassaganya-Riera J, Chaturvedi R, Piazuelo MB, Delgado A, Washington MK, Wilson KT, Algood HM (2014) Systems modeling of the role of interleukin-21 in the maintenance of effector CD4+ T cell responses during chronic Helicobacter pylori infection. mBio 5 (4):e01243–01214. doi: 10.1128/mBio.01243-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R, Fina D, Peluso I, Fantini MC, Tosti C, Del Vecchio Blanco G, Paoluzi OA, Caprioli F, Andrei F, Stolfi C, Romano M, Ricci V, MacDonald TT, Pallone F, Monteleone G (2007) IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. Journal of immunology 178 (9):5957–5965 [DOI] [PubMed] [Google Scholar]
- Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, Ries C, Brillouet A, Muller C, Jegg AM, Broske AM, Dembowski M, Bray-French K, Freilinger C, Meneses-Lorente G, Baehner M, Harding R, Ratnayake J, Abiraj K, Gass N, Noh K, Christen RD, Ukarma L, Bompas E, Delord JP, Blay JY, Ruttinger D (2015) CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. The Lancet Oncology 16 (8):949–956. doi: 10.1016/S1470-2045(15)00132-1 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Piazuelo MB, Yan F, Barry DP, Sierra JC, Delgado AG, Hill S, Casero RA Jr., Bravo LE, Dominguez RL, Correa P, Polk DB, Washington MK, Rose KL, Schey KL, Morgan DR, Peek RM Jr., Wilson KT (2014) Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology 146 (7):1739–1751 e1714. doi: 10.1053/j.gastro.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, Israel DA, Casero RA Jr., Correa P, Gobert AP, Polk DB, Peek RM Jr., Wilson KT (2011) Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141 (5):1696–1708 e1691–1692. doi: 10.1053/j.gastro.2011.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA Jr., Wilson KT (2004) Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. The Journal of biological chemistry 279 (38):40161–40173. doi: 10.1074/jbc.M401370200 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, Sierra JC, Hardbower DM, Delgado AG, Schneider BG, Israel DA, Romero-Gallo J, Nagy TA, Morgan DR, Murray-Stewart T, Bravo LE, Peek RM Jr., Fox JG, Woster PM, Casero RA Jr., Correa P, Wilson KT (2015) Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 34 (26):3429–3440. doi: 10.1038/onc.2014.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Cao S, Zhang Y, Wang X, Liu J, Hui X, Wan Y, Du W, Wang L, Wu K, Fan D (2009) A novel peptide (GX1) homing to gastric cancer vasculature inhibits angiogenesis and cooperates with TNF alpha in anti-tumor therapy. BMC cell biology 10:63. doi: 10.1186/1471-2121-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubak J, Whitlock EP, Williams SB, Kamineni A, Burda BU, Buist DS, Anderson ML (2016) Aspirin for the Prevention of Cancer Incidence and Mortality: Systematic Evidence Reviews for the U.S. Preventive Services Task Force. Annals of internal medicine 164 (12):814–825. doi: 10.7326/M15-2117 [DOI] [PubMed] [Google Scholar]
- Chung AS, Lee J, Ferrara N (2010) Targeting the tumour vasculature: insights from physiological angiogenesis. Nature reviews Cancer 10 (7):505–514. doi: 10.1038/nrc2868 [DOI] [PubMed] [Google Scholar]
- Collins JM, Heery CR, Donahue RN, Palena C, Mada RA, Strauss J, Gatti-Mays ME, Schlom J, Gulley JL, Bilusic M (2018) Phase I trial of BMS-986253, an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. Paper presented at the 2018 ACOS Annual Meeting, Chicago, Illinois, June 4, 2018 [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30 (7):1073–1081. doi: 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M (1975) A model for gastric cancer epidemiology. Lancet 2 (7924):58–60 [DOI] [PubMed] [Google Scholar]
- Correa P, Piazuelo MB, Wilson KT (2010) Pathology of gastric intestinal metaplasia: clinical implications. The American journal of gastroenterology 105 (3):493–498. doi: 10.1038/ajg.2009.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, Perry S, Lindley IJ, Rappuoli R (1995) Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. Journal of clinical pathology 48 (1):41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JE, Farmery SM, Lindley IJ, Figura N, Peichl P, Tompkins DS (1994a) CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. Journal of clinical pathology 47 (10):945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, Primrose JN, Lindley IJ (1994b) Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. Journal of clinical pathology 47 (1):61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nature medicine 21 (5):449–456. doi: 10.1038/nm.3850 [DOI] [PubMed] [Google Scholar]
- David JM, Dominguez C, Hamilton DH, Palena C (2016) The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 4 (3). doi: 10.3390/vaccines4030022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, Silvin C, Van Waes C, Allen C (2017) Anti-PD-L1 Efficacy Can Be Enhanced by Inhibition of Myeloid-Derived Suppressor Cells with a Selective Inhibitor of PI3Kdelta/gamma. Cancer research 77 (10):2607–2619. doi: 10.1158/0008-5472.CAN-16-2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, Falini A, Guidotti LG, Galli R, Naldini L (2008) Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer cell 14 (4):299–311. doi: 10.1016/j.ccr.2008.09.004 [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer discovery 1 (1):54–67. doi: 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, Rabkin CS, Bass AJ (2016) Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget 7 (22):32925–32932. doi: 10.18632/oncotarget.9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon BR, Radin JN, Piazuelo MB, Contreras DC, Algood HM (2016) IL-17a and IL-22 Induce Expression of Antimicrobials in Gastrointestinal Epithelial Cells and May Contribute to Epithelial Cell Defense against Helicobacter pylori. PloS one 11 (2):e0148514. doi: 10.1371/journal.pone.0148514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P (2009) Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. International journal of cancer 125 (2):367–373. doi: 10.1002/ijc.24401 [DOI] [PubMed] [Google Scholar]
- Eftang LL, Esbensen Y, Tannaes TM, Bukholm IR, Bukholm G (2012) Interleukin-8 is the single most up-regulated gene in whole genome profiling of H. pylori exposed gastric epithelial cells. BMC microbiology 12:9. doi: 10.1186/1471-2180-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr., Rabkin CS (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404 (6776):398–402. doi: 10.1038/35006081 [DOI] [PubMed] [Google Scholar]
- Epplein M, Nomura AM, Hankin JH, Blaser MJ, Perez-Perez G, Stemmermann GN, Wilkens LR, Kolonel LN (2008) Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer causes & control : CCC 19 (8):869–877. doi: 10.1007/s10552-008-9149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epplein M, Nomura AM, Wilkens LR, Henderson BE, Kolonel LN (2009) Nonsteroidal antiinflammatory drugs and risk of gastric adenocarcinoma: the multiethnic cohort study. American journal of epidemiology 170 (4):507–514. doi: 10.1093/aje/kwp162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evageliou NF, Haber M, Vu A, Laetsch TW, Murray J, Gamble LD, Cheng NC, Liu K, Reese M, Corrigan KA, Ziegler DS, Webber H, Hayes CS, Pawel B, Marshall GM, Zhao H, Gilmour SK, Norris MD, Hogarty MD (2016) Polyamine Antagonist Therapies Inhibit Neuroblastoma Initiation and Progression. Clinical cancer research : an official journal of the American Association for Cancer Research 22 (17):4391–4404. doi: 10.1158/1078-0432.CCR-15-2539 [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 136 (5):E359–386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C (2011) IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer research 71 (15):5296–5306. doi: 10.1158/0008-5472.CAN-11-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N (2002) VEGF and the quest for tumour angiogenesis factors. Nature reviews Cancer 2 (10):795–803. doi: 10.1038/nrc909 [DOI] [PubMed] [Google Scholar]
- Ferrara N (2009) VEGF-A: a critical regulator of blood vessel growth. European cytokine network 20 (4):158–163. doi: 10.1684/ecn.2009.0170 [DOI] [PubMed] [Google Scholar]
- Fuchs C, Doi T, Jang RW-J, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DVT, Bang Y, Wang J, Koshiji M, Dalal R, Yoon HH (2017) KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. Paper presented at the American Society of Clinical Oncology, Chicago, Illinois, 2017 [Google Scholar]
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH (2018a) Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA oncology 4 (5):e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Ozguoglu M, Bang Y, Bartolomeo MD, Mandala M, Ryu M-H, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Goekkurt E, WMansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Kang SP, Mayo CA, Ohtsu A, Shitara K Pembrolizumab (pembro) vs paclitaxel (PTX) for previously treated advanced gastric or gastroesophageal junction (G/GEJ) cancer: Phase 3 KEYNOTE-061 trial. In: ASCO Annual Meeting, Chicago, Illinois, June 4, 2018 2018b. Journal of Clinical Oncology, [Google Scholar]
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J, Investigators RT (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383 (9911):31–39. doi: 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- Futagami S, Tatsuguchi A, Hiratsuka T, Shindo T, Horie A, Hamamoto T, Ueki N, Kusunoki M, Miyake K, Gudis K, Tsukui T, Sakamoto C (2008) Monocyte chemoattractant protein 1 and CD40 ligation have a synergistic effect on vascular endothelial growth factor production through cyclooxygenase 2 upregulation in gastric cancer. Journal of gastroenterology 43 (3):216–224. doi: 10.1007/s00535-007-2151-8 [DOI] [PubMed] [Google Scholar]
- Galsky MD, Vogelzang NJ, Conkling P, Raddad E, Polzer J, Roberson S, Stille JR, Saleh M, Thornton D (2014) A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 20 (13):3581–3588. doi: 10.1158/1078-0432.CCR-13-2686 [DOI] [PubMed] [Google Scholar]
- Gelhorn HL, Tong S, McQuarrie K, Vernon C, Hanlon J, Maclaine G, Lenderking W, Ye X, Speck RM, Lackman RD, Bukata SV, Healey JH, Keedy VL, Anthony SP, Wagner AJ, Von Hoff DD, Singh AS, Becerra CR, Hsu HH, Lin PS, Tap WD (2016) Patient-reported Symptoms of Tenosynovial Giant Cell Tumors. Clinical therapeutics 38 (4):778–793. doi: 10.1016/j.clinthera.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genard G, Lucas S, Michiels C (2017) Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Frontiers in immunology 8:828. doi: 10.3389/fimmu.2017.00828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert AP, Wilson KT (2017) Human and Helicobacter pylori Interactions Determine the Outcome of Gastric Diseases. Current topics in microbiology and immunology 400:27–52. doi: 10.1007/978-3-319-50520-6_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Mercurio AM (2013) VEGF targets the tumour cell. Nature reviews Cancer 13 (12):871–882. doi: 10.1038/nrc3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiet R, Van Goethem E, Cougoule C, Balor S, Valette A, Al Saati T, Lowell CA, Le Cabec V, Maridonneau-Parini I (2011) The process of macrophage migration promotes matrix metalloproteinase-independent invasion by tumor cells. Journal of immunology 187 (7):3806–3814. doi: 10.4049/jimmunol.1101245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo I, Carneiro F, Oliveira C, Almeida GM (2018) Heterogeneity in Gastric Cancer: From Pure Morphology to Molecular Classifications. Pathobiology : journal of immunopathology, molecular and cellular biology 85 (1–2):50–63. doi: 10.1159/000473881 [DOI] [PubMed] [Google Scholar]
- Hainsworth JD, Reeves JA, Mace JR, Crane EJ, Hamid O, Stille JR, Flynt A, Roberson S, Polzer J, Arrowsmith ER (2016) A Randomized, Open-Label Phase 2 Study of the CXCR4 Inhibitor LY2510924 in Combination with Sunitinib Versus Sunitinib Alone in Patients with Metastatic Renal Cell Carcinoma (RCC). Targeted oncology 11 (5):643–653. doi: 10.1007/s11523-016-0434-9 [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Cook AD, Tak PP (2016) Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nature reviews Drug discovery 16 (1):53–70. doi: 10.1038/nrd.2016.231 [DOI] [PubMed] [Google Scholar]
- Hardbower DM, Coburn LA, Asim M, Singh K, Sierra JC, Barry DP, Gobert AP, Piazuelo MB, Washington MK, Wilson KT (2017) EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis. Oncogene 36 (27):3807–3819. doi: 10.1038/onc.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardbower DM, Singh K, Asim M, Verriere TG, Olivares-Villagomez D, Barry DP, Allaman MM, Washington MK, Peek RM Jr., Piazuelo MB, Wilson KT (2016) EGFR regulates macrophage activation and function in bacterial infection. The Journal of clinical investigation 126 (9):3296–3312. doi: 10.1172/JCI83585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holian O, Wahid S, Atten MJ, Attar BM (2002) Inhibition of gastric cancer cell proliferation by resveratrol: role of nitric oxide. American journal of physiology Gastrointestinal and liver physiology 282 (5):G809–816. doi: 10.1152/ajpgi.00193.2001 [DOI] [PubMed] [Google Scholar]
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ (2011) Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer prevention research 4 (9):1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT (2004) Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. Journal of biomedical science 11 (4):517–527. doi: 10.1159/000077902 [DOI] [PubMed] [Google Scholar]
- Huang XZ, Chen Y, Wu J, Zhang X, Wu CC, Zhang CY, Sun SS, Chen WJ (2017) Aspirin and non-steroidal anti-inflammatory drugs use reduce gastric cancer risk: A dose-response meta-analysis. Oncotarget 8 (3):4781–4795. doi: 10.18632/oncotarget.13591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Psoriasis Study G, Durez P, Tak PP, Gomez-Reino JJ, Rheumatoid Arthritis Study G, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Uveitis Study G, Rose K, Haider A, Di Padova F (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Science translational medicine 2 (52):52ra72. doi: 10.1126/scitranslmed.3001107 [DOI] [PubMed] [Google Scholar]
- Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP (2000) Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 6 (5):2104–2119 [PubMed] [Google Scholar]
- Janjigian YY, Ott PA, Calvo E, Kim JW, Ascierto PA, Sharma P (2017) Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy-refractory (CTx-R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study. Journal of Clinical Oncology 35 (15):4014–4014. doi: 10.1200/JCO.2017.35.15_suppl.4014 [DOI] [Google Scholar]
- Jiang W, Liu K, Guo Q, Cheng J, Shen L, Cao Y, Wu J, Shi J, Cao H, Liu B, Tao K, Wang G, Cai K (2017) Tumor-infiltrating immune cells and prognosis in gastric cancer: a systematic review and meta-analysis. Oncotarget 8 (37):62312–62329. doi: 10.18632/oncotarget.17602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Scheller J, Rose-John S (2011) Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. The Journal of clinical investigation 121 (9):3375–3383. doi: 10.1172/JCI57158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390 (10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- Kao HF, Hsu C, Huang HC, Wang CW, Cheng JD, Hong RL (2015) Correlation between plasma Epstein-Barr virus DNA and clinical response to pembrolizumab in patients with advanced or metastatic nasopharyngeal carcinoma. Paper presented at the European Cancer Congress, Vienna, Austria, [Google Scholar]
- Kim DJ, Park JH, Franchi L, Backert S, Nunez G (2013) The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori infected dendritic cells. European journal of immunology 43 (10):2650–2658. doi: 10.1002/eji.201243281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Bae BN, Kang G, Kim HJ, Park K (2017) Cytokine expression associated with Helicobacter pylori and Epstein-Barr virus infection in gastric carcinogenesis. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 125 (9):808–815. doi: 10.1111/apm.12725 [DOI] [PubMed] [Google Scholar]
- Kim MH, Chang J, Kim WJ, Banerjee S, Park SM (2018) Cumulative Dose Threshold for the Chemopreventive Effect of Aspirin Against Gastric Cancer. The American journal of gastroenterology 113 (6):845–854. doi: 10.1038/s41395-018-0097-5 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ (2001) Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia 3 (1):33–42. doi: 10.1038/sj/neo/7900124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Pollard JW (2015) Immune cell promotion of metastasis. Nature reviews Immunology 15 (2):73–86. doi: 10.1038/nri3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong P, Wu R, Liu X, Liu J, Chen S, Ye M, Yang C, Song Z, He W, Yin C, Yang Q, Jiang C, Liao F, Peng R, Zhou Z, Xu D, Xia L (2016) The Effects of Anti-inflammatory Drug Treatment in Gastric Cancer Prevention: an Update of a Meta-analysis. Journal of Cancer 7 (15):2247–2257. doi: 10.7150/jca.16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kumari N, Mittal RD, Mohindra S, Ghoshal UC (2015) Association between pro-(IL-8) and anti-inflammatory (IL-10) cytokine variants and their serum levels and H. pylori-related gastric carcinogenesis in northern India. Meta gene 6:9–16. doi: 10.1016/j.mgene.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T, Kitadai Y, Tanaka S, Yang X, Mukaida N, Yoshihara M, Chayama K (2005) Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clinical cancer research : an official journal of the American Association for Cancer Research 11 (21):7629–7636. doi: 10.1158/1078-0432.CCR-05-0798 [DOI] [PubMed] [Google Scholar]
- Lanas A, Wu P, Medin J, Mills EJ (2011) Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 9 (9):762–768 e766. doi: 10.1016/j.cgh.2011.05.020 [DOI] [PubMed] [Google Scholar]
- Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic M, Sieghart W, Trauner M, Wang H, Sibilia M (2014) EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nature cell biology 16 (10):972–977. doi: 10.1038/ncb3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren P (1965) The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta pathologica et microbiologica Scandinavica 64:31–49 [DOI] [PubMed] [Google Scholar]
- Lauwers G, Carneiro F, Graham D (2010) Gastric carcinoma. WHO classification of tumours of the digestive system, 4th edn International Agency for Research on Cancer, Lyon, France [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357 (6349):409–413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Bass AJ, Ajani JA (2016) Gastric Adenocarcinoma: An Update on Genomics, Immune System Modulations, and Targeted Therapy. American Society of Clinical Oncology educational book American Society of Clinical Oncology Meeting 35:104–111. doi: 10.14694/EDBK_159091 [DOI] [PubMed] [Google Scholar]
- Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH, Song SK, Kim HS (2004) Relationship between urokinase-type plasminogen receptor, interleukin-8 gene expression and clinicopathological features in gastric cancer. Oncology 66 (3):210–217. doi: 10.1159/000077997 [DOI] [PubMed] [Google Scholar]
- Lee SA, Choi SR, Jang JS, Lee JH, Roh MH, Kim SO, Kim MC, Kim SJ, Jeong JS (2010) Expression of VEGF, EGFR, and IL-6 in gastric adenomas and adenocarcinomas by endoscopic submucosal dissection. Digestive diseases and sciences 55 (7):1955–1963. doi: 10.1007/s10620-009-0967-1 [DOI] [PubMed] [Google Scholar]
- Lewis AM, Varghese S, Xu H, Alexander HR (2006) Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. Journal of translational medicine 4:48. doi: 10.1186/1479-5876-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK (2005) Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis 8 (1):63–71. doi: 10.1007/s10456-005-5208-4 [DOI] [PubMed] [Google Scholar]
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H (2016) Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34 (13):1448–1454. doi: 10.1200/JCO.2015.63.5995 [DOI] [PubMed] [Google Scholar]
- Li Q, Li Q, Chen J, Liu Y, Zhao X, Tan B, Ai J, Zhang Z, Song J, Shan B (2013) Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncology reports 30 (3):1215–1222. doi: 10.3892/or.2013.2570 [DOI] [PubMed] [Google Scholar]
- Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F, Galizia G (2008) Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Annals of surgical oncology 15 (1):69–79. doi: 10.1245/s10434-007-9596-0 [DOI] [PubMed] [Google Scholar]
- Lin MT, Juan CY, Chang KJ, Chen WJ, Kuo ML (2001) IL-6 inhibits apoptosis and retains oxidative DNA lesions in human gastric cancer AGS cells through up-regulation of anti-apoptotic gene mcl-1. Carcinogenesis 22 (12):1947–1953 [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhai E, Liao B, Xu L, Zhang X, Peng S, He Y, Cai S, Zeng Z, Chen M (2017) Autocrine VEGF signaling promotes cell proliferation through a PLC-dependent pathway and modulates Apatinib treatment efficacy in gastric cancer. Oncotarget 8 (7):11990–12002. doi: 10.18632/oncotarget.14467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm C, Quiding-Jarbrink M, Lonroth H, Hamlet A, Svennerholm AM (1998) Local cytokine response in Helicobacter pylori-infected subjects. Infection and immunity 66 (12):5964–5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N, Zhang J, Liu X, Li N, Guo G, Tong W, Zhuang Y, Zou Q (2012a) Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. Journal of clinical immunology 32 (6):1332–1339. doi: 10.1007/s10875-012-9718-8 [DOI] [PubMed] [Google Scholar]
- Liu X, Peng J, Sun W, Yang S, Deng G, Li F, Cheng JW, Gordon JR (2012b) G31P, an antagonist against CXC chemokine receptors 1 and 2, inhibits growth of human prostate cancer cells in nude mice. The Tohoku journal of experimental medicine 228 (2):147–156 [DOI] [PubMed] [Google Scholar]
- Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F (2000) Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. Journal of immunology 165 (9):5332–5337 [DOI] [PubMed] [Google Scholar]
- Ma X, Wu D, Zhou S, Wan F, Liu H, Xu X, Xu X, Zhao Y, Tang M (2016) The pancreatic cancer secreted REG4 promotes macrophage polarization to M2 through EGFR/AKT/CREB pathway. Oncology reports 35 (1):189–196. doi: 10.3892/or.2015.4357 [DOI] [PubMed] [Google Scholar]
- Macri A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, Teti D, Famulari C (2006) Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 11 (2):184–193. doi: 10.1080/13547500600565677 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454 (7203):436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology 23 (11):549–555 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Vecchi A, Allavena P (2014) Pharmacological modulation of monocytes and macrophages. Current opinion in pharmacology 17:38–44. doi: 10.1016/j.coph.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Markham A (2016) Ixekizumab: First Global Approval. Drugs 76 (8):901–905. doi: 10.1007/s40265-016-0579-y [DOI] [PubMed] [Google Scholar]
- Martin D, Galisteo R, Gutkind JS (2009) CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. The Journal of biological chemistry 284 (10):6038–6042. doi: 10.1074/jbc.C800207200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P (2005) Long term follow up of patients treated for Helicobacter pylori infection. Gut 54 (11):1536–1540. doi: 10.1136/gut.2005.072009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Schneider BG, Morgan DR, Peek RM Jr., Correa P, Wilson KT, Piazuelo MB (2018) Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 67 (7):1239–1246. doi: 10.1136/gutjnl-2016-311685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL Jr., McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW (2008) Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer prevention research 1 (1):32–38. doi: 10.1158/1940-6207.CAPR-08-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD (2012) M1 and M2 Macrophages: Oracles of Health and Disease. Critical reviews in immunology 32 (6):463–488 [DOI] [PubMed] [Google Scholar]
- Moehler M, Gopfert K, Lenz HJ (2018) Outlook: Immunotherapy in Gastrointestinal Carcinoma - Innovative Strategies. Oncology research and treatment 41 (5):313–315. doi: 10.1159/000489047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nature reviews Cancer 8 (8):618–631. doi: 10.1038/nrc2444 [DOI] [PubMed] [Google Scholar]
- Murukesh N, Dive C, Jayson GC (2010) Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. British journal of cancer 102 (1):8–18. doi: 10.1038/sj.bjc.6605483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarsheth N, Wicha MS, Zou W (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nature reviews Immunology 17 (9):559–572. doi: 10.1038/nri.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikfar S, Saiyarsarai P, Tigabu BM, Abdollahi M (2018) Efficacy and safety of interleukin-1 antagonists in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology international. doi: 10.1007/s00296-018-4041-1 [DOI] [PubMed] [Google Scholar]
- Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Nagulapalli Venkata KC, Louie SG, Petasis NA, Ladner RD, Lenz HJ (2012) The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Molecular cancer therapeutics 11 (6):1353–1364. doi: 10.1158/1535-7163.MCT-11-0915 [DOI] [PubMed] [Google Scholar]
- Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41 (1):49–61. doi: 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K (2003) Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. International journal of oncology 22 (4):773–778 [PubMed] [Google Scholar]
- Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29 (30):3968–3976. doi: 10.1200/JCO.2011.36.2236 [DOI] [PubMed] [Google Scholar]
- Okugawa Y, Toiyama Y, Ichikawa T, Kawamura M, Yasuda H, Fujikawa H, Saigusa S, Ohi M, Araki T, Tanaka K, Inoue Y, Tanaka M, Miki C, Kusunoki M (2018) Colony-stimulating factor-1 and colony-stimulating factor-1 receptor co-expression is associated with disease progression in gastric cancer. International journal of oncology 53 (2):737–749. doi: 10.3892/ijo.2018.4406 [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA (2011) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Molecular therapy : the journal of the American Society of Gene Therapy 19 (3):620–626. doi: 10.1038/mt.2010.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K (2010) Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer research 70 (19):7392–7399. doi: 10.1158/0008-5472.CAN-10-2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, Bang YJ, Alcindor T, O’Callaghan CJ, Burnell MJ, Tebbutt NC, Rha SY, Lee J, Cho JY, Lipton LR, Wong M, Strickland A, Kim JW, Zalcberg JR, Simes J, Goldstein D (2016) Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34 (23):2728–2735. doi: 10.1200/JCO.2015.65.1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE (2006) Regulation of ornithine decarboxylase. The Journal of biological chemistry 281 (21):14529–14532. doi: 10.1074/jbc.R500031200 [DOI] [PubMed] [Google Scholar]
- Pegg AE (2009) Mammalian polyamine metabolism and function. IUBMB life 61 (9):880–894. doi: 10.1002/iub.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S (2016) Global burden of cancers attributable to infections in 2012: a synthetic analysis. The Lancet Global health 4 (9):e609–616. doi: 10.1016/S2214-109X(16)30143-7 [DOI] [PubMed] [Google Scholar]
- Plummer M, Franceschi S, Vignat J, Forman D, de Martel C (2015) Global burden of gastric cancer attributable to Helicobacter pylori. International journal of cancer 136 (2):487–490. doi: 10.1002/ijc.28999 [DOI] [PubMed] [Google Scholar]
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature medicine 19 (10):1264–1272. doi: 10.1038/nm.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141 (1):39–51. doi: 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, Holland EC, Sutton JC, Joyce JA (2016) The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352 (6288):aad3018. doi: 10.1126/science.aad3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph C, Elkord E, Burt DJ, O’Dwyer JF, Austin EB, Stern PL, Hawkins RE, Thistlethwaite FC (2010) Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 16 (5):1662–1672. doi: 10.1158/1078-0432.CCR-09-2870 [DOI] [PubMed] [Google Scholar]
- Riechelmann R, Grothey A (2017) Antiangiogenic therapy for refractory colorectal cancer: current options and future strategies. Therapeutic advances in medical oncology 9 (2):106–126. doi: 10.1177/1758834016676703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord JP, Levitsky H, Blay JY, Ruttinger D (2014) Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer cell 25 (6):846–859. doi: 10.1016/j.ccr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M (1997) Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer research 57 (7):1276–1280 [PubMed] [Google Scholar]
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW (2012) Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 379 (9826):1602–1612. doi: 10.1016/S0140-6736(11)61720-0 [DOI] [PubMed] [Google Scholar]
- Rubie C, Kauffels A, Kolsch K, Glanemann M, Justinger C (2016) CXCL12/CXCR4 display an inverse mRNA expression profile in gastric carcinoma that correlates with tumor progression. Oncology letters 11 (1):360–364. doi: 10.3892/ol.2015.3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Affara NI, Coussens LM (2012) Differential macrophage programming in the tumor microenvironment. Trends in immunology 33 (3):119–126. doi: 10.1016/j.it.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Coussens LM (2015) Macrophages and therapeutic resistance in cancer. Cancer cell 27 (4):462–472. doi: 10.1016/j.ccell.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R, Stille JR, Weaver RW, McCleod M, Hamid O, Polzer J, Roberson S, Flynt A, Spigel DR (2017) A randomized phase II study of LY2510924 and carboplatin/etoposide versus carboplatin/etoposide in extensive-disease small cell lung cancer. Lung cancer 105:7–13. doi: 10.1016/j.lungcan.2016.12.020 [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, Wang G, Tromp BJ, Puchalski TA, Balkwill F, Berns B, Seetharam S, de Bono JS, Tolcher AW (2013) A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer chemotherapy and pharmacology 71 (4):1041–1050. doi: 10.1007/s00280-013-2099-8 [DOI] [PubMed] [Google Scholar]
- Sanford M, McKeage K (2015) Secukinumab: first global approval. Drugs 75 (3):329–338. doi: 10.1007/s40265-015-0359-0 [DOI] [PubMed] [Google Scholar]
- Sanmamed MF, Carranza-Rua O, Alfaro C, Onate C, Martin-Algarra S, Perez G, Landazuri SF, Gonzalez A, Gross S, Rodriguez I, Munoz-Calleja C, Rodriguez-Ruiz M, Sangro B, Lopez-Picazo JM, Rizzo M, Mazzolini G, Pascual JI, Andueza MP, Perez-Gracia JL, Melero I (2014) Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clinical cancer research : an official journal of the American Association for Cancer Research 20 (22):5697–5707. doi: 10.1158/1078-0432.CCR-13-3203 [DOI] [PubMed] [Google Scholar]
- Sansone P, Bromberg J (2012) Targeting the interleukin-6/Jak/stat pathway in human malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30 (9):1005–1014. doi: 10.1200/JCO.2010.31.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier Sholler GL, Gerner EW, Bergendahl G, MacArthur RB, VanderWerff A, Ashikaga T, Bond JP, Ferguson W, Roberts W, Wada RK, Eslin D, Kraveka JM, Kaplan J, Mitchell D, Parikh NS, Neville K, Sender L, Higgins T, Kawakita M, Hiramatsu K, Moriya SS, Bachmann AS (2015) A Phase I Trial of DFMO Targeting Polyamine Addiction in Patients with Relapsed/Refractory Neuroblastoma. PloS one 10 (5):e0127246. doi: 10.1371/journal.pone.0127246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331 (6024):1565–1570. doi: 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- Schroder M, Loos S, Naumann SK, Bachran C, Krotschel M, Umansky V, Helming L, Swee LK (2017) Identification of inhibitors of myeloid-derived suppressor cells activity through phenotypic chemical screening. Oncoimmunology 6 (1):e1258503. doi: 10.1080/2162402X.2016.1258503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nature reviews Cancer 3 (10):721–732. doi: 10.1038/nrc1187 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148 (3):399–408. doi: 10.1016/j.cell.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semper RP, Mejias-Luque R, Gross C, Anderl F, Muller A, Vieth M, Busch DH, Prazeres da Costa C, Ruland J, Gross O, Gerhard M (2014) Helicobacter pylori-induced IL-1beta secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. Journal of immunology 193 (7):3566–3576. doi: 10.4049/jimmunol.1400362 [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219 (4587):983–985 [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410 (6832):1107–1111. doi: 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y, Xu R, Chen L, Liu Y, Yu S, Bu L, Piao Y (2015) Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 18 (1):168–176. doi: 10.1007/s10120-014-0351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata W, Ariyama H, Westphalen CB, Worthley DL, Muthupalani S, Asfaha S, Dubeykovskaya Z, Quante M, Fox JG, Wang TC (2013) Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut 62 (2):192–200. doi: 10.1136/gutjnl-2011-301824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra JC, Asim M, Verriere TG, Piazuelo MB, Suarez G, Romero-Gallo J, Delgado AG, Wroblewski LE, Barry DP, Peek RM Jr., Gobert AP, Wilson KT (2018) Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut 67 (7):1247–1260. doi: 10.1136/gutjnl-2016-312888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjokvist Ottsjo L, Flach CF, Nilsson S, Malefyt Rde W, Walduck AK, Raghavan S (2015) Defining the Roles of IFN-gamma and IL-17A in Inflammation and Protection against Helicobacter pylori Infection. PloS one 10 (7):e0131444. doi: 10.1371/journal.pone.0131444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka AJ, Backert S (2012) How Helicobacter pylori infection controls gastric acid secretion. Journal of gastroenterology 47 (6):609–618. doi: 10.1007/s00535-012-0592-1 [DOI] [PubMed] [Google Scholar]
- Solomon DH, Mercer E, Kavanaugh A (2012) Observational studies on the risk of cancer associated with tumor necrosis factor inhibitors in rheumatoid arthritis: a review of their methodologies and results. Arthritis and rheumatism 64 (1):21–32. doi: 10.1002/art.30653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Zhu J, Lu D (2016) Molecular-targeted first-line therapy for advanced gastric cancer. The Cochrane database of systematic reviews 7:CD011461. doi: 10.1002/14651858.CD011461.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sota J, Vitale A, Insalaco A, Sfriso P, Lopalco G, Emmi G, Cattalini M, Manna R, Cimaz R, Priori R, Talarico R, de Marchi G, Frassi M, Gallizzi R, Soriano A, Alessio M, Cammelli D, Maggio MC, Gentileschi S, Marcolongo R, La Torre F, Fabiani C, Colafrancesco S, Ricci F, Galozzi P, Viapiana O, Verrecchia E, Pardeo M, Cerrito L, Cavallaro E, Olivieri AN, Paolazzi G, Vitiello G, Maier A, Silvestri E, Stagnaro C, Valesini G, Mosca M, de Vita S, Tincani A, Lapadula G, Frediani B, De Benedetti F, Iannone F, Punzi L, Salvarani C, Galeazzi M, Angotti R, Messina M, Tosi GM, Rigante D, Cantarini L, Working Group” of Systemic Autoinflammatory Diseases of SIR (2018) Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clinical rheumatology. doi: 10.1007/s10067-018-4119-x [DOI] [PubMed] [Google Scholar]
- Spence AD, Busby J, Johnston BT, Baron JA, Hughes CM, Coleman HG, Cardwell CR (2018) Low-Dose Aspirin Use Does Not Increase Survival in 2 Independent Population-Based Cohorts of Patients With Esophageal or Gastric Cancer. Gastroenterology 154 (4):849–860 e841. doi: 10.1053/j.gastro.2017.10.044 [DOI] [PubMed] [Google Scholar]
- Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O’Bryant C, Chow LQ, Serkova NJ, Meropol NJ, Lewis NL, Chiorean EG, Fox F, Youssoufian H, Rowinsky EK, Eckhardt SG (2010) Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28 (5):780–787. doi: 10.1200/JCO.2009.23.7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsa S, Paul MC, Cardone C, Holcmann M, Amberg N, Pathria P, Diamanti MA, Linder M, Timelthaler G, Dienes HP, Kenner L, Wrba F, Prager GW, Rose-John S, Eferl R, Liguori G, Botti G, Martinelli E, Greten FR, Ciardiello F, Sibilia M (2017) EGFR in Tumor-Associated Myeloid Cells Promotes Development of Colorectal Cancer in Mice and Associates With Outcomes of Patients. Gastroenterology 153 (1):178–190 e110. doi: 10.1053/j.gastro.2017.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples E, Ingram RJ, Atherton JC, Robinson K (2013) Optimising the quantification of cytokines present at low concentrations in small human mucosal tissue samples using Luminex assays. Journal of immunological methods 394 (1–2):1–9. doi: 10.1016/j.jim.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri R, Fujimura T, Heldin CH, Ooi A (2010) Clinicopathological significance of platelet-derived growth factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-beta phosphorylation, and microvessel density in gastric cancer. BMC cancer 10:659. doi: 10.1186/1471-2407-10-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero J, Melero I, Ros W, Argiles G, Marabelle A, Rodriguez-Ruiz M (2017) Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). Journal of Clinical Oncology, vol 35 10.1200/JCO.2017.35.15_suppl.3002 [DOI] [Google Scholar]
- Tao LL, Shi SJ, Chen LB, Huang GC (2014) Expression of monocyte chemotactic protein-1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World journal of gastroenterology 20 (15):4421–4427. doi: 10.3748/wjg.v20.i15.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, Staddon AP, Cohn AL, Shapiro GI, Keedy VL, Singh AS, Puzanov I, Kwak EL, Wagner AJ, Von Hoff DD, Weiss GJ, Ramanathan RK, Zhang J, Habets G, Zhang Y, Burton EA, Visor G, Sanftner L, Severson P, Nguyen H, Kim MJ, Marimuthu A, Tsang G, Shellooe R, Gee C, West BL, Hirth P, Nolop K, van de Rijn M, Hsu HH, Peterfy C, Lin PS, Tong-Starksen S, Bollag G (2015) Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. The New England journal of medicine 373 (5):428–437. doi: 10.1056/NEJMoa1411366 [DOI] [PubMed] [Google Scholar]
- Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CE, Cancer Genome Atlas Research N, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich L (2018) The Immune Landscape of Cancer. Immunity 48 (4):812–830 e814. doi: 10.1016/j.immuni.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS (2007) CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proceedings of the National Academy of Sciences of the United States of America 104 (49):19446–19451. doi: 10.1073/pnas.0706832104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonouchi H, Miki C, Tanaka K, Kusunoki M (2002) Profile of monocyte chemoattractant protein-1 circulating levels in gastric cancer patients. Scandinavian journal of gastroenterology 37 (7):830–833 [PubMed] [Google Scholar]
- Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC (2008) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer cell 14 (5):408–419. doi: 10.1016/j.ccr.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udalova I, Monaco C, Nanchahal J, Feldmann M (2016) Anti-TNF Therapy. Microbiology spectrum 4 (4). doi: 10.1128/microbiolspec.MCHD-0022-2015 [DOI] [PubMed] [Google Scholar]
- Uefuji K, Ichikura T, Mochizuki H (2000) Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 6 (1):135–138 [PubMed] [Google Scholar]
- Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. Lancet 388 (10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- Vela M, Aris M, Llorente M, Garcia-Sanz JA, Kremer L (2015) Chemokine receptor-specific antibodies in cancer immunotherapy: achievements and challenges. Frontiers in immunology 6:12. doi: 10.3389/fimmu.2015.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R (1863) Die Krankhaften Geschwülste (translation, Diseased Tumors). Hirschwald, Berlin, Germany [Google Scholar]
- Vonderheide RH (2018) The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer cell 33 (4):563–569. doi: 10.1016/j.ccell.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg ZA, Jalal S, Muro K, Yoon HH, Garrido M, Golan T, Doi T, DCatenacci DV, Geva R, Ku G, Bleeker J, Bang Y, Hara H, Chung H, Savage M, Want J, Koshiji M, Dalal R, Fuchs CS (2017) KEYNOTE-059 Update: Efficacy and safety of pembrolizumab alone or in combination with chemotherapy in patients with advanced gastric or gastroesophageal (G/GEJ) cancer. Annuals of Oncology 28 (suppl 5):v616–v617 [Google Scholar]
- Waldner MJ, Neurath MF (2012) Targeting the VEGF signaling pathway in cancer therapy. Expert opinion on therapeutic targets 16 (1):5–13. doi: 10.1517/14728222.2011.641951 [DOI] [PubMed] [Google Scholar]
- Waldner MJ, Wirtz S, Jefremow A, Warntjen M, Neufert C, Atreya R, Becker C, Weigmann B, Vieth M, Rose-John S, Neurath MF (2010) VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. The Journal of experimental medicine 207 (13):2855–2868. doi: 10.1084/jem.20100438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, DuBois RN (2018) Role of prostanoids in gastrointestinal cancer. The Journal of clinical investigation. doi: 10.1172/JCI97953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hu W, Wang K, Yu J, Luo B, Luo G, Wang W, Wang H, Li J, Wen J (2016) Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. International journal of oncology 48 (4):1341–1352. doi: 10.3892/ijo.2016.3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Cai H, Zheng W, Michel A, Pawlita M, Milne G, Xiang YB, Gao YT, Li HL, Rothman N, Lan Q, Shu XO, Epplein M (2017) A Prospective Study of Urinary Prostaglandin E2 Metabolite, Helicobacter pylori Antibodies, and Gastric Cancer Risk. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 64 (10):1380–1386. doi: 10.1093/cid/cix106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Si X, Xu A, Meng X, Gao S, Qi Y, Zhu L, Li T, Li W, Dong L (2013) Activation of STAT3 in human gastric cancer cells via interleukin (IL)-6-type cytokine signaling correlates with clinical implications. PloS one 8 (10):e75788. doi: 10.1371/journal.pone.0075788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich DM, Elaraj DM, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Spiess PJ, Alexander HR (2003) Effect of interleukin 1 receptor antagonist gene transduction on human melanoma xenografts in nude mice. Cancer research 63 (18):5957–5961 [PubMed] [Google Scholar]
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D (2011) Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International journal of cancer 129 (1):245–255. doi: 10.1002/ijc.25864 [DOI] [PubMed] [Google Scholar]
- Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A, Group RS (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. The Lancet Oncology 15 (11):1224–1235. doi: 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Byron K, Gibson PR (1999) Interleukin-8 stimulates the migration of human colonic epithelial cells in vitro. Clinical science 97 (3):385–390 [PubMed] [Google Scholar]
- Xue LJ, Mao XB, Ren LL, Chu XY (2017) Inhibition of CXCL12/CXCR4 axis as a potential targeted therapy of advanced gastric carcinoma. Cancer medicine 6 (6):1424–1436. doi: 10.1002/cam4.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Kato S, Matsuhisa T, Makonkawkeyoon L, Yoshida M, Chakrabandhu T, Lertprasertsuk N, Suttharat P, Chakrabandhu B, Nishiumi S, Chongraksut W, Azuma T (2013) Predominant mucosal IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer. World journal of gastroenterology 19 (19):2941–2949. doi: 10.3748/wjg.v19.i19.2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Saito H, Ikeguchi M (2012) Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. The Journal of surgical research 178 (2):685–691. doi: 10.1016/j.jss.2012.07.055 [DOI] [PubMed] [Google Scholar]
- Yan F, Cao H, Chaturvedi R, Krishna U, Hobbs SS, Dempsey PJ, Peek RM Jr., Cover TL, Washington MK, Wilson KT, Polk DB (2009) Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology 136 (4):1297–1307, e1291–1293. doi: 10.1053/j.gastro.2008.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Lan C, Pei H, Zhu Z (2016) Expression of interleukin 1beta in gastric cancer tissue and its effects on gastric cancer. OncoTargets and therapy 9:31–35. doi: 10.2147/OTT.S94277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell JA, McLaren CE, Chen WP, Thompson PA, Gerner EW, Meyskens FL (2010) Ornithine decarboxylase-1 polymorphism, chemoprevention with eflornithine and sulindac, and outcomes among colorectal adenoma patients. Journal of the National Cancer Institute 102 (19):1513–1516. doi: 10.1093/jnci/djq325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J (2018) Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. The Lancet Global health 6 (5):e555–e567. doi: 10.1016/S2214-109X(18)30127-X [DOI] [PubMed] [Google Scholar]
- Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G (2008) The prevalence of Th17 cells in patients with gastric cancer. Biochemical and biophysical research communications 374 (3):533–537. doi: 10.1016/j.bbrc.2008.07.060 [DOI] [PubMed] [Google Scholar]
- Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS one 7 (12):e50946. doi: 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang XQ, Ding XW, Yang RK, Huang SL, Kastelein F, Bruno M, Yu XJ, Zhou D, Zou XP (2014) Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: a meta-analysis. British journal of cancer 110 (9):2378–2388. doi: 10.1038/bjc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer research 74 (18):5057–5069. doi: 10.1158/0008-5472.CAN-13-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulueta A, Caretti A, Signorelli P, Ghidoni R (2015) Resveratrol: A potential challenger against gastric cancer. World journal of gastroenterology 21 (37):10636–10643. doi: 10.3748/wjg.v21.i37.10636 [DOI] [PMC free article] [PubMed] [Google Scholar]