Abstract
Background
Transforming growth factor beta 1 (TGFβ) is known to be a regulator of autoimmunity. Loss of TGFβ leads to severe multi-organ autoimmunity in mice. In skin, role of TGFβ in suppressing autoimmunity is unclear.
Objective
Determine whether Keratinocyte (KC)-derived TGFβ is required for skin immune homeostasis.
Methods
We generated K14-CreERT2 TGFβ1fl/fl (TGFβΔKC) mice allowing for tamoxifen-induced deletion of TGFβ1 in KC. The phenotype of skin was analyzed and compared to mice in which epidermal activation of TGFβ is impaired.
Results
KC was the major source of TGFβ in epidermis. Topical tamoxifen application led to efficient TGFβ1 deletion. The expected acanthosis was observed but no inflammatory infiltrate or altered numbers of resident immune cells were evident. Similarly, Itgb6−/− x K14Cre Itgb8f/f (Itgb6−/−Itgb8ΔKC) mice lacking both epidermal TGFβ-activating integrins showed no evidence of cutaneous inflammation.
Conclusions
KC-derived TGFβ and epidermal TGFβ activation are not required to suppress skin autoimmunity in steady state.
1. Introduction
Transforming growth factor beta 1 (TGFβ) is a pleiotropic cytokine belonging to the transforming growth factor superfamily [1]. TGFβ is secreted as a biologically inactive dimer. It becomes active when mature TGFβ disassociates from its latency associated peptide (LAP) [2]. Dissociation of LAP from TGFβ is primarily mediated by the RGD binding integrins αvβ6 and αvβ8 [3–5]. Active TGFβ binds to a heterodimeric TGFβ receptor complex and plays an essential role in cell cycle [6], tissue repair [7], and immune homeostasis and tolerance [8].
As a potent regulator of the immune and inflammatory systems, TGFβ generally exerts an immunosuppressive effect [8]. TGFβ−/− mice exhibit no gross developmental abnormalities, but develop an acute wasting syndrome accompanied by severe mucosal inflammation due to the requirement of TGFβ acting on CD4+ T cells to promote tolerance [9–13] Mice with ablation of TGFβ in CD4+ T cells develop global spontaneous autoimmunity indicating that TGFβ acts in a paracrine manner on CD4+ T cells [14]. Similarly, Itgb6−/− mice which have defective TGFβ-activation at barrier surfaces develop inflammatory infiltrates in lung and intestine with age [15]. A similar phenotype has been reported in skin, though it is much more subtle possibly due to the expression of both αvβ6 and αvβ8 in skin but only αvβ6 in lung and intestine [16]. TGFβ also has a direct effect on KC and inhibits proliferation in vitro through regulation of growth-related gene expression [6,17]. Transgenic overexpression in KC of both active and latent TGFβ results in a severe psoriasis-like skin phenotype in mice [18,19]. Thus, TGFβ can participate in growth regulation, maintenance of tolerance and induction of inflammation in the skin.
TGFβ is also required for retention of immune cell in epidermis during steady-state conditions. Autocrine TGFβ expressed by Langerhans cells (LC) is required for their maintenance in the epidermis as LC-specific ablation of TGFβ or TGFβRII results in spontaneous migration of LC from the epidermis to regional lymph nodes [20–22]. Similarly, Itgb6−/−Itgb8ΔKC mice that lack both TGFβ-activating integrins αvβ6 and αvβ8 in the epidermis have a complete absence of epidermal LC. In addition, epidermal CD8+ tissue-resident memory T cells (TRM) require active TGFβ for their persistence. These cells are absent from Itgb6−/−Itgb8ΔKC mice and in mice treated with neutralizing mAbs to αvβ6 [5,23,24]. Despite the clear importance of TGFβ for the maintenance of skin homeostasis, the cellular source of TGFβ in steady-state skin remains unclear. Herein, we established that KC represent the major cell type expressing TGFβ in the skin and developed mice with a KC-specific ablation of TGFβ (TGFβΔKC mice). We observed increased KC growth but found that skin immune homeostasis was largely unaffected. From these data, we conclude that KC-derived TGFβ is not required for skin immune homeostasis.
2. Materials and methods
2.1. Mice
TGF-βloxP, K14Cre, K14-CreERT2 and huLangerin-DTA mice have been previously described [14,21,25]. Itgb6−/− and Itgb8loxP mice were provided by D. Sheppard (University of California, San Francisco). We crossed K14-Cre mice with Itgb8loxP and Itgb6−/− mice to obtain Itgb6−/−Itgb8ΔKC mice[5]. K14CreERT2 mice were bred with TGFβloxP mice and ROSA26.LSL.YFP (Jackson Laboratories) reporter mice resulting TGFβΔKC mice. All experiments were performed on age- (6–12 week) and sex-matched mice. All mice were maintained under specific-pathogen-free conditions and all animal experiments were approved by University of Pittsburgh Institutional Animal Care and Use Committee.
2.2. Tamoxifen treatment
Tamoxifen (T5648; Sigma-Aldrich) was dissolved in 1/10th volume of 200 proof ethanol following incubation at 37 °C for 15–30 min with occasional vortexing. Corn oil (Sigma-Aldrich) was added for a final concentration of Tamoxifen at 10 mg/ml and was administered to mice for 5 consecutive days by intraperitoneal injection at 0.05 mg per g body weight. 5 mg of 4-Hydroxytamoxifen (H7904–5MG; Sigma-Aldrich) was dissolved in 1.25ml ≥99.5% acetone following incubation at 37 °C for 5–10 min with occasional vortexing. Corn oil (Sigma-Aldrich) was added for a final concentration of 2 mg/ml and was administered to mice for 2 consecutive days by topical application (40 ul/shaved flank and 10 ul/ear).
2.3. Cell Sorting by Flow Cytometry and Quantitative PCR
Single-cell suspension of epidermal cells was obtained as below. LC, KC, and DETC were sorted using a FACSAria cell sorter based as CD45+MHCII+CD207+, CD45− MHCII−, CD45+MHCII−CD3+TCR γδ+, respectively. RNA was isolated using an RNeasy Mini Kit (Qiagen) and quantified from NanoDrop readings. cDNA was generated using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). TaqMan Gene Expression Master Mix, TaqMan Gene Expression Assays for TGF-β1 and ABI Prism 7900HT (Applied Biosystems) were used for quantitative PCR. All kits were performed according the manufacturer’s instructions. All Ct values were normalized to hypoxanthine phosphoribosyltransferase (Hprt) or glyceraldehyde phosphate dehydrogenase (Gapdh) expression.
2.4. Flow cytometry
Preparation of epidermal and dermal single cells suspension from tissues were performed as previously described [25]. For preparing single cells suspension from the skin, shaved skin was harvested and fat tissues was removed mechanically by forceps. Float their dermal side down in the Petri dish containing pre-warmed 37°C 0.3% trypsin (Sigma-Aldrich) solution in 150 mM NaCl, 0.5 mM KCl and 0.5 mM glucose. Incubate in CO2 incubator at 37° for 45–60 minutes. The epidermis was physically separated from the dermis and disrupted by mincing and vigorous pipetting. Wash dermis with PBS. The dermis was minced finely with scissors and resuspended in RPMI1640 (Gibco, Grand Island, NY) with 2.5 mg/ml collagenase XI (Sigma-Aldrich), 0.25 mg/ml hyaluronidase (Sigma-Aldrich), 0.1 mg/ml DNase (Sigma-Aldrich), 0.01 M HEPES (Sigma-Aldrich), and 10% FBS followed by incubation in a shaking incubator for 1 h at 37°C at 250 rpm. The resulting cells were filtered through a 40 um cell strainer (BD Biosciences). Single-cell suspensions were blocked with 2.4G2 culture supernatant (American Type Culture Collection) and were stained with antibodies to extracellular markers, in PBS with 2% calf serum (Hyclone) and 0.05% Azide for 30 min at 4°C or 37°C. Fluorochrome-conjugated antibodies CD8 (53–6.7), CD45.2 (104), CD3 (17A2), TCR β (H57–597), Thy1.2 (30-H12), CD69 (H1.2F3), CD103 (2E7), MHCII (M5/114.15.2), and CD11b (M1/70), TCR γ/δ(GL3), CD 64 (X54–5/7.1), CD34 (HM34), CD24 (M1/69) were purchased from BioLegend. Foxp3(R16–715) and CD4 (GK1.5) were purchased from BD Biosciences. Anti-Langerin (929F3.01) was purchased from Novus Biologicals (Littleton, CO). Sca-1 (D7) was purchased from ebioscience. The fixable viability dye eFluor 780 (eBioscience) was used for live-dead discrimination. Intracellular staining of langerin, foxp3, and green fluorescent protein was performed with a BD Bioscience Cytofix/Cytoperm kit (BD Biosciences) in accordance with the manufacturer’s instructions. The intracellular cytokine staining was performed with Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. A BD LSRFORTESSA (BD Biosciences) and Flowjo software (TreeStar, Ashland, OR) were used for analysis.
2.5. Immunofluorescence of epidermis
Epidermal sheets were prepared as previously described [25]. Shaved defatted flank skin was affixed to slides with double-sided adhesive (3M, St. Paul, MN). Slides were incubated in 10 mM EDTA in PBS for 90 min at 37°C, followed by physical removal of the dermis. The epidermal sheets were fixed in 4% PFA at room temperature (RT) for 30 min. The epidermal sheets were blocked with PBS containing 0.1% tween-20, 2% BSA and 2% rat serum for 1 hours at RT before staining overnight with Alexa Fluor 488–conjugated anti–GFP, Alexa Fluor 594–conjugated anti–mouse I-A/I-E and Alexa Fluor 647– conjugated anti-TCR γ/δ antibody in PBS containing 0.1% tween-20 and 0.5% BSA. Images were captured on a IX83 fluorescent microscope (Olympus Tokyo, Japan) using a x10 objective; image analysis was performed using cell Sens Dimension software (Olympus). Both LC and DETC in epidermis were counted either by ImageJ software or manually in a blinded fashion.
2.6. H&E staining
Mouse skin was embedded in OCT compound and frozen in dry ice. Transverse mouse skin sections that were 8-μm thick were prepared from frozen blocks using a cryostat and were fixed for 30 min in 4% PFA. Hematoxylin and eosin staining was performed as follows: 0.1% Mayer’s hematoxylin staining for 15 min, two-three quick rinses in distillated water, section under 10 min running tap water and rinses in distillated water. Apply 95% ethanol for 30s, 0.5% eosin staining for 30 s and rinse in 80% ethanol. Then mounting media was used and the section was observed and photographed with a Nikon Eclipse E400 microscope. Acanthosis was quantified by measuring epidermal thickness on the section every 200 um for 5 points and the average was used for the value of the acanthosis.
2.7. Statistical analysis
Two groups were compared with Prism software (GraphPad) using the two-tailed unpaired Student’s t test. For three groups, Turkey’s multiple comparisons was used. Data are presented as mean only or mean ± standard error of the mean (s.e.m.). p < 0.05 was considered significant.
3. Results
3.1. KC is the major source of TGFβ in the epidermis
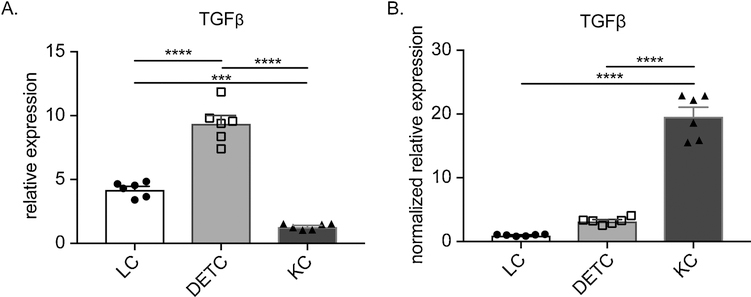
Epidermal cells including KC, dendritic epidermal T cells (DETC) and Langerhans Cells (LC) have all been reported to produce TGFβ [26,27]. First, we sought to determine which cell type is the dominant source of TGFβ in the epidermis. Single cell suspensions of epidermis were FACSsorted to obtain pure populations of KC (CD45−MHCII−), DETC (CD45+MHCII−CD3+TCRγδ+) and LC (CD45+MHCII+). mRNA was generated and analyzed by qPCR for Tgfb1 expression levels which were normalized to Hprt. On a per cell basis, DETC expressed more TGFβ than LC and KC (Fig. 1a). However, these cell types account for different proportions of the epidermis. DETC, LC and KC represent approximately 2%, 2% and 95% of total epidermal cells, respectively (unpublished observations). After normalizing for the number of each cell type, TGFβ contributed by KC was 6 times higher than DETC and 22 times higher than LC (Fig. 1b). Thus, we concluded that KC represented the major cellular source of TGF-β in epidermis during steady state conditions.
Fig. 1. Keratinocytes are the major source of TGFβ in the epidermis.
(A) TGFβ1 mRNA expression levels from FACSsorted LC, DETC and KC as analyzed by qRT-PCR normalized to Hprt. (B) TGFβ1 mRNA expression as in (A) normalized to relative percentage of each cell type in epidermis. Each symbol represent data from an individual animal. Data are presentative of at least 2 independent experiments. Data are means ± SEM. *** p<0.001; **** p<0.0001.
3.2. Generation and validation of TGFβΔKC mice
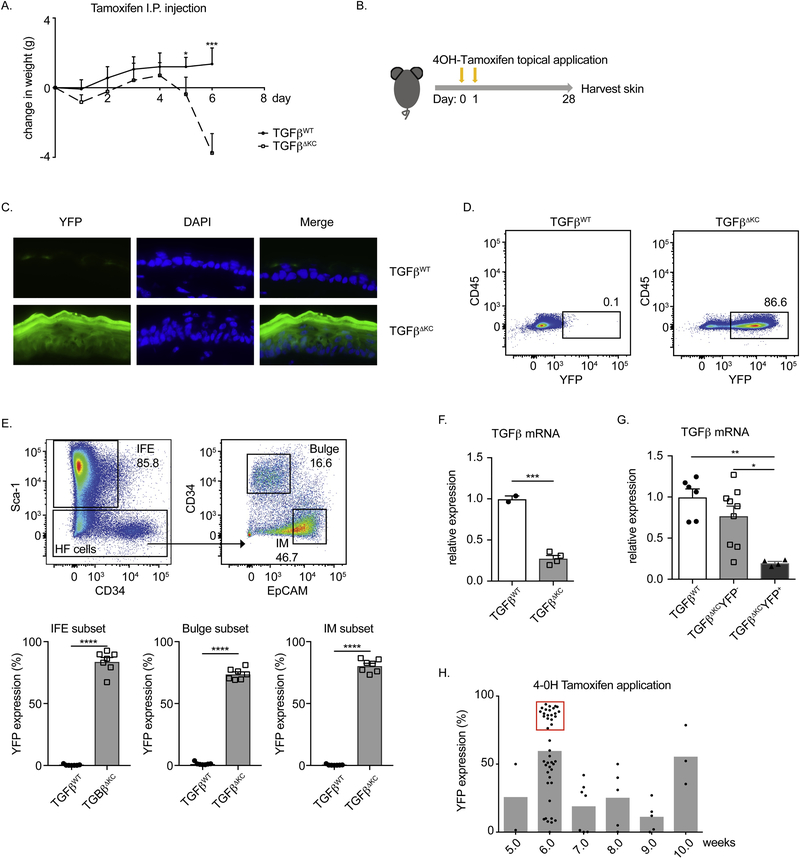
To determine the requirement of KC-derived TGFβ, we generated tamoxifen (TAM)-inducible keratinocyte-specific TGFβ-deficient mice. K14CreERT2 mice were bred with TGFβf/f mice and ROSA26.YFP reporter mice resulting in K14-CreERT2 TGFβ1fl/fl (TGFβΔKC). We first administered TAM i.p. over five consecutive days to allow for the inducible deletion of TGFβ in KC. Unexpectedly, the majority of TGFβΔKC but not TAM treated control mice (TGFβWT) developed severe weight loss necessitating euthanasia first observable on day 5 after the first treatment of TAM (Fig. 2a). Gross and histologic observations of the skin of these mice showed no obvious phenotype (unpublished observations). K14 is reported to be expressed in mucosal epithelia [28]. Thus, the lethal phenotype may result from the inducible deletion of TGFβ at these sites.
Fig. 2. Generation and validation of TGFβΔKC mice.
(A) 10mg/ml tamoxifen was injected i.p. at 5ul per g animal weight for 5 consecutive days (day 0–4). Time course of weight change during/after i.p. tamoxifen is shown for control TGFβWT (solid line) and TGFβΔKC (dashed line) mice. (B) 4OH-Tamoxifen topical application protocol: 2mg/ml 4OH-Tamoxifen was applied to shaved skin on two consecutive days and the skin was analyzed 28 days after the first 4OH-tamoxifen. (C) Immunofluorescent microscopic visualization of TGFβWT and TGFβΔKC flank skin illustrates epidermal expression of YFP (green) and DAPI nuclear label (blue). (D) Flow cytometry staining of YFP in KC (live single CD45−). (E) Gating strategy of KC population: interfollicular: IFE-, Bulge- and isthmus: IM-KC subsets and frequency of YFP expression in each KC subsets. Cells were gated on live single CD45− cells. (F) Tgfb1 mRNA relative expression level normalized to Gapdh in epidermis from TGFβWT and TGFβΔKC mice. The average gene expression level in control mice was taken as 1. (G) Relative Tgfb1 mRNA expression normalized to Gapdh in KC sorted from TGFβWT mice and YFP+ and YFP− KC from TGFβΔKC mice. The average gene expression level in KC from TGFβWT mice was taken as 1. (H) An analysis of YFP induction efficiency in KC isolated in 4OH-tamoxifen treated mice of the indicated age. The red box indicated mice used in subsequent experiments. n=4–5 mice (A) and each symbol represents data from an individual animal (E-H). Data are representative of at least 2 independent experiments. Data are means ± SEM. * p<0.05; ** p<0.01; ***p<0.001, **** p<0.0001.
To specifically test the requirement of KC-derived TGFβ in skin, we next applied 2mg/ml 4OH-Tamxifen (4OH-TAM) topically on TGFβΔKC and control mice for two consecutive days (Fig. 2b). After 28 days, the skin was harvested and analyzed by immunofluorescent microscopy for expression of YFP to determine the efficiency of Cre activation. We observed high levels of YFP expression in 4OH-TAM treated TGFβΔKC but not control mice (Fig. 2c). Flow cytometric analysis revealed a similar high degree of efficiency with >80% of KC expressing YFP (Fig. 2d). Sub-setting of keratinocyte population into interfollicular KC (IFE:CD45−MHCII−CD34−Sca-1+), bulge KC (CD45− MHCII−Sca-1−CD34+), and isthmus KC (IM:CD45−MHCII−Sca-1−EpCAM+) revealed a similar high degree of YFP expression in all populations (Fig. 2e).
To ensure that expression of YFP accurately reflected the absence of TGFβ, we obtained mRNA from epidermis 28 days following topical tamoxifen application. As expected, levels of TGFβ1 were greatly reduced in 4OH-TAM treated TGFβΔKC but not control mice (Fig. 2f). In addition, we isolated YFP+ and YFP− KC from 4OH-TAM treated TGFβΔKC mice and found greatly reduced levels of TGFβ1 in YFP+ but not YFP− KC (Fig. 2g). From these data, we conclude that topical 4OH-tamoxifen application to TGFβΔKC mice allows for the efficient deletion of TGFβ and that expression of YFP accurately reports the loss of TGFβ expression.
During these experiments, we observed that often some cohorts of TGFβΔKC would display greatly reduced efficiency of YFP expression. An analysis of mouse age at the time of 4OH-TAM treatment revealed that topical tamoxifen applied only at 6 weeks of age resulted in efficient expression of YFP (Fig. 2h). Even when TAM was applied at 6 weeks of age, some cohorts expressed low YFP expression. In all subsequent experiments, all mice were analyzed for efficiency of YFP expression and only those showing >80% YFP+ KC were used for analysis (Fig. 2h, red box).
3.3. KC-derived TGFβ is not necessary to maintain skin immune homeostasis
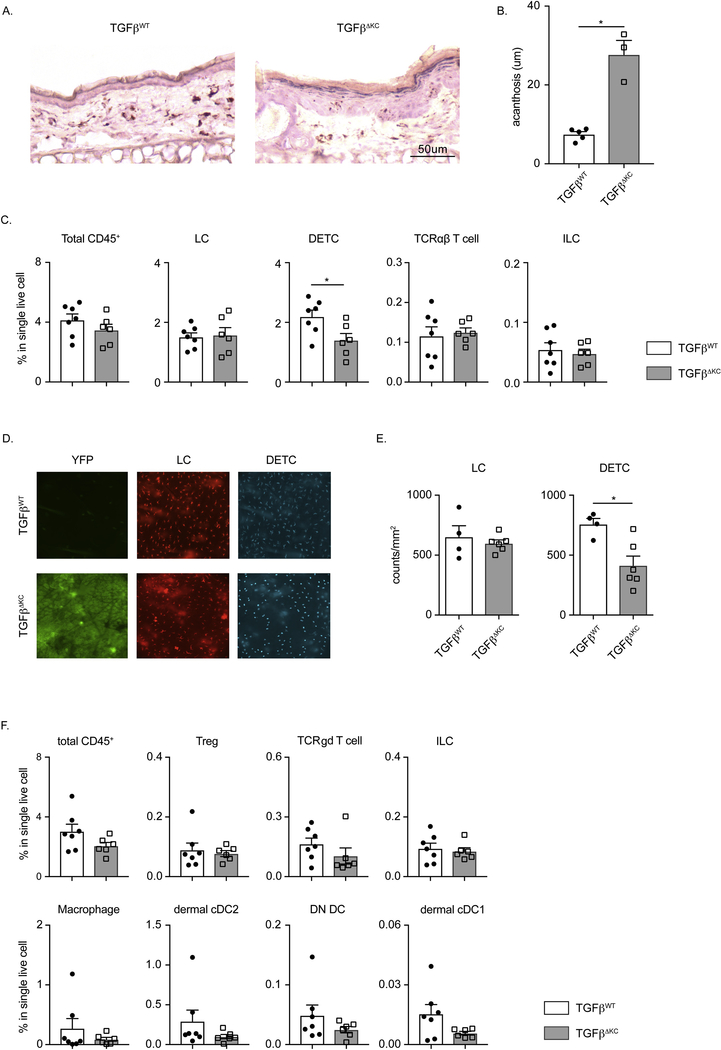
Using the same experimental strategy as above, we first histologically examined back skin 28 days following 4OH-TAM application. Acanthosis (thickened epidermis) was clearly evident on transverse H&E sections from TAM treated TGFβΔKC but not control mice (Fig. 3a). Quantification determined that the increased acanthosis was significant (Fig. 3b). TGFβ has been reported to suppress KC proliferation directly in vitro [6]. The increased epidermal thickness is consistent with this report and functionally validated the absence of TGFβ in 4OH-TAM treated TGFβΔKC mice. Notably, we did not observe evidence of an inflammatory infiltrate on H&E staining.
Fig. 3. KC-derived TGFβ is not necessary to maintain skin immune homeostasis.
(A) Representative H&E staining of transverse ear section of TGFβWT and TGFβΔKC mice. (B) Epidermal thickness in TGFβWT and TGFβΔKC mice is shown. (C) Flow cytometry analysis of epidermal single cell suspension from TGFβWT and TGFβΔKC mice. Frequency of the indicated population in total live single cells are shown. (D) Immunofluorescent microscopic visualization whole mounted epidermis from TGFβΔWT and TGFβΔKC for YFP (green), LC (red, MHCII) and DETC (blue, TCRγδ) is shown. (E) Quantification of cell numbers in (D) is shown. (F) Flow cytometry analysis of dermal single cell suspension from TGFβWT and TGFβΔKC mice. The percentages of the indicated population in single live cells are shown. Each symbol represents data from an individual animal. Data are representative of at least 2 independent experiments. Data are means ± SEM. * p<0.05.
To determine whether more subtle changes occurred in the cellular composition of skin occurred in 4OH-TAM treated TGFβΔKC mice, we analyzed single cell epidermal and dermal skin suspensions by flow cytometry. In the epidermis, we did not observe any alterations in the frequency of total CD45+ cells, LC, TCRαβ T cells or innate lymphoid cells (ILC) (Fig. 3c). To confirm these results, we examined epidermal whole mounts by immunofluorescent microscopy. The epidermis of TGFβΔKC mice showed a modest decrease of DETC numbers which was also seen with flow cytometry but no alteration in the number of LC (Fig. 3d, e).
In dermis, the frequency of CD45+ cells was not altered in 4OH-TAM treated TGFβΔKC mice (Fig. 3f) compared with control mice. In addition, no changes in the frequency of Treg (CD45+Thy1.2+TCRb-CD3+CD4+Foxp3+), γδ T cells (CD45+Thy1.2+TCRb−CD3mid), ILC (CD45+Thy1.2+TCRb−CD3−), Macrophages (CD45−MHCII+CD64+CD11b+) and dendritic cells subsets (dermal cDC2, CD45−MHCII+CD64−CD11b+; DN DC, CD45−MHCII+CD64−CD11b−; dermal cDC1, CD45−MHCII+CD64−CD207+CD103+). Taken together, we conclude that the absence of KC-derived TGFβ has no effect on immune homeostasis in steady state.
3.4. KC-mediated activation of TGFβ is not necessary to maintain skin immune homeostasis
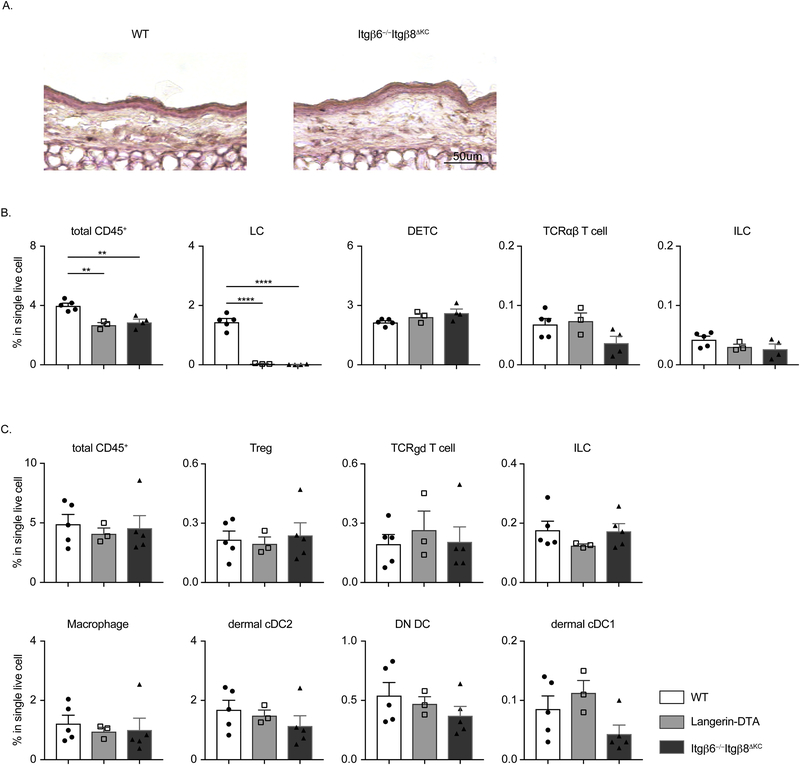
We next sought to determine whether activation of TGFβ by integrins αvβ6 and αvβ8 expressed by keratinocytes is required to maintain skin immune homeostasis. We used Itgb6−/− x K14Cre Itgb8f/f (Itgb6−/−Itgb8ΔKC) mice that lack both TGFβ-activating integrins in the epidermis. A prior report has suggested that Itgb6−/− develop mild spontaneous skin inflammation at the site of occasional hair loss [15]. We did not observe increased incidence of the hair loss in our mice colony compared to B6 wild type mice (unpublished observations). Histological examination did not reveal an obvious alteration in the cellular infiltrate in Itgb6−/−Itgb8ΔKC mice and no evidence of acanthosis (Fig. 4a). As above, we analyzed single cell suspensions of epidermis and dermis for more subtle alterations in the frequency of various immune subsets. We have previously demonstrated, Itgb6−/− Itgb8ΔKC mice, have a dramatic absence of LC. To ensure that any observe phenotype derived from the absence of αvβ6 and αvβ8 and not LC, we also examined the cellular composition of skin from huLangerin-DTA that have a constitutive absence of LC. In the epidermis, as expected, we noted the absence of LC in Itgb6−/−Itgb8ΔKC and huLangerin-DTA mice (Fig. 4b). We did not observe a significant alteration in the frequency of total CD45+ cells, TCR αβ T cells, DETC or ILC in any group. Notably, unlike TGFβΔKC mice, the frequency of DETC were unaffected. In dermis, the frequency of Treg, TCR γδ T cells, ILC, Macrophages and DC was unaltered in Itgb6−/−Itgb8ΔKC mice (Fig. 4c). From these data, we conclude that KC-mediated activation of TGFβ is required for epidermal maintenance of LC and to suppress acanthosis but has no other nonredundant role in skin immune homeostasis during steady state.
Fig. 4. KC-mediated activation of TGFβ is not necessary to maintain skin immune homeostasis.
(A) Representative H&E staining of transverse ear section of WT and Itgb6−/−Itgb8ΔKC mice. (B and C) Flow cytometry analysis of epidermal (B) and dermal (C) single cell suspension from WT, huLangerin-DTA and Itgb6−/−Itgb8ΔKC mice. Frequency of the indicated population is shown. Data are representative of at least 2 independent experiments. Data are means ± SEM. ** p<0.01; **** p<0.0001.
4. Discussion
Herein, we have demonstrated that ablation of TGFβ in keratinocytes does not result in the development of an inflammatory infiltrate or altered numbers of resident immune cells. Similarly, integrins αvβ6 and αvβ8 in the epidermis were not required to maintain skin homeostasis beyond residency of epidermal leukocytes. Taken together, these data support the conclusion that KC-derived TGFβ and epidermal activation of TGFβ are not required for maintenance of skin immune homeostasis. The role of TGFβ from other epidermal sources and dermis remains important to be explored.
Given the known importance of TGFβ to suppress effector CD4+ T cells and Tregs which can be found in the skin [29–31], we anticipate that inducible ablation of KC-derived TGFβ or ablation αvβ6 and αvβ8-mediated TGFβ-activation would result in loss of T cell tolerance to skin antigens. The absence of spontaneous inflammation indicates that epidermal TGFβ is either not required or redundant. It is likely that autocrine/paracrine TGFβ and αvβ8 expressed by subsets of dendritic cells found in the dermis is sufficient [32,33]. The clear absence of an inflammatory infiltrate in the skin double αvβ6- and αvβ8- deficient mice is surprising given on prior observations of mild occasional spontaneous skin inflammation [15]. We speculate that either further back crossing onto the C57BL/6 background or an altered cutaneous microbiome may explain this discrepancy. We previously reported that autocrine/paracrine TGFβ was required for the maintenance of LC [21,22]. Our data showing that loss of KC-derived TGFβ did not affect LC number in epidermis further supports this finding and demonstrated that KC-derived TGFβ is not required.
A caveat for all Cre-mediated loss-of-function studies is whether the achieved reduction of a given gene is sufficiently robust to yield a given phenotype. Topical tamoxifen treatment of TGFβΔKC mice yield >80% efficiency of TGFβ deletion as measured by direct measure of TGFβ mRNA and expression of YFP which we demonstrated efficiently phenocopied levels of TGFβ mRNA. Importantly, we did observe the expected phenotype of increased acanthosis indicating a functional absence of TGFβ. We also noted a modest decrease in DETC numbers in tamoxifen treated TGFβΔKC mice. We do not think that epidermal retention of DETC is dependent on TGFβ given the normal numbers of DETC observed in Itgb6−/−Itgb8ΔKC mice and TGFβ−/− mice [20]. Rather, we suspect that reduced DETC numbers is an indirect effect in TGFβΔKC mice.
There were two unexpected and unusual observations in the TGFβΔKC model. First, the majority of TGFβΔKC but not control mice developed severe weight loss shortly after systematic application of tamoxifen. We suspect this phenotype is related to expression of K14 in other tissue such as the oropharynx and esophagus since TGFβ−/− do develop severe esophagal and pharyngeal inflammation [34]. We also noted that highly efficient ablation of TGFβ occurs following topical application to 6 week old mice with another peak at 10 weeks. This timing is similar to the mouse hair anagen cycle[35]. It is possible that topical tamoxifen targeting genes in KC may have heightened efficiency during anagen. Alternatively, given the importance of TGFβ and αvβ6 during hair development, this phenomena may only occur when the targeting of TGFβ [36,37].
In this report, we have focused exclusively on analysis of immune homeostasis under steady-state conditions. An examination of the requirement for KC-derived TGFβ in the context of autoimmunity, fibrosis and infection remain as exciting future directions.
Highlights.
K14-CreERT2 TGFβ1fl/fl mice develop acanthosis
K14-CreERT2 TGFβ1fl/fl mice do not develop spontaneous skin inflammation
Itgb6−/− x K14Cre Itgb8f/f mice do not develop spontaneous skin inflammation
Acknowledgments
We thank the members of the Kaplan laboratory and members throughout the departments of Dermatology and Immunology for helpful discussions. We also thank the Division of Laboratory Animal Resources of the University of Pittsburgh for excellent animal care.
Funding
This work benefitted from SPECIAL BD LSRFORTESSATM funded by NIH 1S10OD011925-01. YY was financially supported by China Scholarship Council (CSC) No 201706370257., T.H. by JSPS Overseas Reasearch Fellowships and DHK by R01AR060744.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Massagué J, The transforming growth factor-beta family, Annu. Rev. Cell Biol 6 (1990) 597–641. [DOI] [PubMed] [Google Scholar]
- [2].Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. , Latent TGF-β structure and activation, Nature. 474 (2011) 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, et al. , Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice, J. Cell Biol 176 (2007) 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, et al. , Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice, J. Cell. Sci 122 (2009) 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mohammed J, Beura LK, Bobr A, Astry B, Chicoine B, Kashem SW, et al. , Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β, Nat. Immunol 17 (2016) 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pietenpol JA, Holt JT, Stein RW, Moses HL, Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation, Proc. Natl. Acad. Sci. U.S.a 87 (1990) 3758–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Penn JW, Grobbelaar AO, Rolfe KJ, The role of the TGF-β family in wound healing, burns and scarring: a review, Int J Burns Trauma. 2 (2012) 18–28. [PMC free article] [PubMed] [Google Scholar]
- [8].Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA, Transforming growth factor-beta regulation of immune responses, Annu. Rev. Immunol 24 (2006) 99–146. [DOI] [PubMed] [Google Scholar]
- [9].Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. , Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease, Nature. 359 (1992) 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, et al. , Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death, Proc. Natl. Acad. Sci. U.S.a 90.2 (1993): 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oh SA, Li MO, TGF-β: guardian of T cell function, J Immunol. 191 (2013) 3973–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li MO, Sanjabi S, and Flavell RA, Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and-independent mechanisms, Immunity. 25.3 (2006): 455–471. [DOI] [PubMed] [Google Scholar]
- [13].Marie JC, Liggitt D, Immunity AR, 2006, Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor, Immunity 2006. September 1;25(3):455–71. [DOI] [PubMed] [Google Scholar]
- [14].Marie JC, Liggitt D, Rudensky AY, Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor, Immunity. 25 (2006) 441–454. [DOI] [PubMed] [Google Scholar]
- [15].Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, et al. , Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin, J. Cell Biol 133 (1996) 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lowell CA, Mayadas TN, Overview: studying integrins in vivo, Methods Mol. Biol 757 (2012) 369–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Coffey RJ, Bascom CC, Sipes NJ, Graves-Deal R, Weissman BE, Moses HL, Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta, Mol. Cell. Biol 8 (1988) 3088–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sellheyer K, Bickenbach JR, Rothnagel JA, Bundman D, Longley MA, Krieg T, et al. , Inhibition of skin development by overexpression of transforming growth factor beta 1 in the epidermis of transgenic mice, Proc. Natl. Acad. Sci. U.S.a 90 (1993) 5237–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li AG, Wang D, Feng X-H, Wang X-J, Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder, Embo J. 23 (2004) 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Borkowski TA, Letterio JJ, Farr AG, Udey MC, A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells, J. Exp. Med 184 (1996) 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ, Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells, J. Exp. Med 204 (2007) 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bobr A, Igyarto BZ, Haley KM, Li MO, Flavell RA, Kaplan DH, Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration, Proc. Natl. Acad. Sci. U.S.a 109 (2012) 10492–10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, et al. , The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP–dependent activation of TGF-β1, J Cell Biol. 157.3 (2002): 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. , A mechanism for regulating pulmonary inflammation and fibrosis: the integrin αvβ6 binds and activates latent TGF β1, Cell. 96.3 (1999): 319–328. [DOI] [PubMed] [Google Scholar]
- [25].Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ, Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity, Immunity. 23 (2005) 611–620. [DOI] [PubMed] [Google Scholar]
- [26].Gruschwitz MS, Hornstein OP, Expression of transforming growth factor type beta on human epidermal dendritic cells, J. Invest. Dermatol 99 (1992) 114–116. [DOI] [PubMed] [Google Scholar]
- [27].Granucci F, Girolomoni G, Lutz MB, Foti M, Marconi G, Gnocchi P, et al. , Modulation of cytokine expression in mouse dendritic cell clones, Eur. J. Immunol 24 (1994) 2522–2526. [DOI] [PubMed] [Google Scholar]
- [28].Smith CM, Hayamizu TF, Finger JH, Bello SM, McCright IJ, Xu J, et al. , The mouse Gene Expression Database (GXD): 2019 update, Nucleic Acids Res. 47 (2019) D774–D779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marie JC, Letterio JJ, Gavin M, Rudensky AY, TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells, J. Exp. Med 201 (2005) 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA, Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis, J. Exp. Med 199 (2004) 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ali N, Rosenblum MD, Regulatory T cells in skin, Immunology. 152 (2017) 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, et al. , Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice, Nature. 449 (2007) 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D, Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice, J. Clin. Invest 120 (2010) 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, et al. , Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse, Blood. 87 (1996) 1439–1445. [PubMed] [Google Scholar]
- [35].Hou C, Miao Y, Wang X, Chen C, Lin B, Hu Z, Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in the hair cycle, Exp Ther Med. 12 (2016) 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, et al. , Control of murine hair follicle regression (catagen) by TGF-β1 in vivo, The FASEB Journal. 14.5 (2000): 752–760. [DOI] [PubMed] [Google Scholar]
- [37].Xie Y, McElwee KJ, Owen GR, Häkkinen L, and Larjava HS, Integrin β6-deficient mice show enhanced keratinocyte proliferation and retarded hair follicle regression after depilation, J Invest Dermatol. 132.3 (2012): 547–555. [DOI] [PubMed] [Google Scholar]