Abstract
Citrus greening or huanglongbing (HLB) is the main threat to the European citrus industry since one of its vectors, the African citrus psyllid, Trioza erytreae, has recently become established in mainland Europe. In this context, classical biological control programmes should be implemented to reduce the spread of the psyllid. The aims of this study were to: i) disentangle the parasitoid complex of T. erytreae combining morphological and molecular characterization; and ii) to study the biology of its main parasitoids in its area of origin in South Africa for their future importation into Europe. The main citrus producing areas of South Africa were surveyed during 2017. In contrast to previous studies, the parasitoid complex of T. erytreae included three species of primary parasitoids: Tamarixia dryi, Psyllaephagus pulvinatus and another parasitoid of the genus Tamarixia. Molecular analysis showed that it is a new species closely related to T. dryi. Tamarixia dryi was the most abundant parasitoid but its relative abundance varied among sampling sites. The sex ratio (males/females) of T. dryi and Tamarixia sp. decreased with T. erytreae size and became female biased when psyllid nymphs were larger than 0.6 and 1.2 mm2, respectively. These parasitoids were attacked by three species of hyperparasitoids, Aphidencyrtus cassatus, Marietta javensis and a species of the genus Aphanogmus. Aphidencyrtus cassatus, the most abundant hyperparasitoid, tended to emerge from large nymphs, and adult females lived as long as those of T. dryi. The implications of these results are discussed within the framework of the introduction of T. dryi into Europe.
Subject terms: Agroecology, Entomology, Invasive species
Introduction
Citrus greening or huanglongbing (HLB) is one of the most devastating citrus diseases in the world1–4. It is associated with the three phloem α-proteobacterias “Candidatus Liberibacter asiaticus” (CLas), “Ca. Liberibacter americanus” (CLam) and “Ca. Liberibacter africanus” (CLaf). The three bacteria are transmitted by two insect vectors: the Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Liviidae) and the African citrus psyllid Trioza erytreae (Del Guercio) (Hemiptera: Triozidae)2,5. Since its first record in Taiwan in 19086, D. citri has spread and has been reported transmitting CLas in Asia, parts of North and South America, Africa and numerous islands in the Atlantic and Pacific oceans2,7–10. In contrast, T. erytreae is associated with CLaf and, since its first record in 1929 in South Africa11,12, has been recorded along all the African continent, Yemen and a few Atlantic Ocean islands2. It has recently been reported from Portugal and Spain13 even though HLB has not been detected yet in European countries14,15.
HLB disease manifests as asymmetrical yellow mottles or severe chlorosis of the foliage, fruit drop and dieback2,16, leading to significant economic losses17,18. As an example of the destructive potential of HLB, citrus production in Florida has dropped by more than 70% since HLB was detected in 200519. In the Mediterranean Basin, HLB detection could be a critical turning point not only because it is the main producing area of citrus for the fresh market in the World20, but also because Mediterranean citriculture is based on small farming, where managing HLB and its vectors is more complex than in larger commercial orchards21.
In Europe, T. erytreae was first detected in 1994 in Madeira (Portugal)22 and later on in the Canary Islands (Spain) in 200223,24. Until then, it seemed to be restricted to non-continental areas, but in 2014 it first appeared in the north-western Iberian Peninsula13. Despite the initial insecticide treatments to eradicate it, T. erytreae is now spreading from the north-west to the south-west of the Iberian Peninsula15. Parasitoids of the genus Tamarixia are amongst the most effective natural enemies of HLB vectors5,25–27. However, no native parasitoids have been recorded from T. erytreae in the Atlantic islands or in the Iberian Peninsula15,28. In this context, classical biological control seems to be the most feasible measure for preventing T. erytreae to spread further in the Mediterranean citrus growing areas.
The complex of parasitoids in South Africa and Swaziland was analyzed in detail during the 1960’s and 70 s29,30 and in Cameroon twenty years ago31. According to these studies, the two main primary parasitoids of T. erytreae in Southern Africa are Tamarixia dryi (synonym Tetrastichus dryi) (Waterston) (Hymenoptera: Eulophidae) and Psyllaephagus pulvinatus (Waterston) (Hymenoptera: Encyrtidae). Both are solitary koinobiont parasitoids. The former is an ectoparasitoid, whereas the encyrtid is an endoparasitoid. These primary parasitoids are frequently attacked by a complex of hyperparasitoids26,32 that, accordingly to Van Der Berg and Greenland26, severely decrease the impact of the primary parasitoids. Tamarixia dryi was used in a classical biological control programme in Reunion Island when T. erytreae was detected in 197425,33. In less than five years, T. dryi became established and controlled T. erytreae25,26. Similarly, in 1982, T. dryi was imported into Mauritius34. In these islands both T. erytreae and D. citri coexisted as well as the African and Asian forms of HLB. However, only the classical biological control of T. erytreae with T. dryi was successful34,35. The lack of alternative hosts for T. erytreae, the presence of alternative hosts for T. dryi and the absence of hyperparasitoids were considered key aspects for the establishment of T. dryi and the successful control of T. erytreae populations36–39. Whether T. dryi would find these conditions in mainland Europe its unknown. However, T. dryi is highly specific. The parasitoid did not parasitize and develop in any of the eleven alternative host species that were offered in host-specificity tests40.
In this study, we propose a classical biological control programme to introduce the main parasitoids of T. erytreae from its area of origin into Europe. We first identified the parasitoid complex of T. erytreae in several areas of South Africa using morphological and molecular characterisation. We then determined several aspects of the biology of the main parasitoids: sex ratio, longevity and hyperparasitism. Implications of using T. dryi in classical biological control programmes of T. erytreae are discussed.
Results
Insect survey
The Western Cape was the only province in South Africa where neither T. erytreae nor its symptoms were recorded (see supplementary material Table S1). Leaves of trees from 17 out of the 65 sampled sites in the other provinces had the characteristic open gall-like structures, indicating the presence of T. erytreae. Live nymphs were collected from five out of the 17 sites and parasitoids were recorded from four sites.
Parasitoid emergence and species abundance
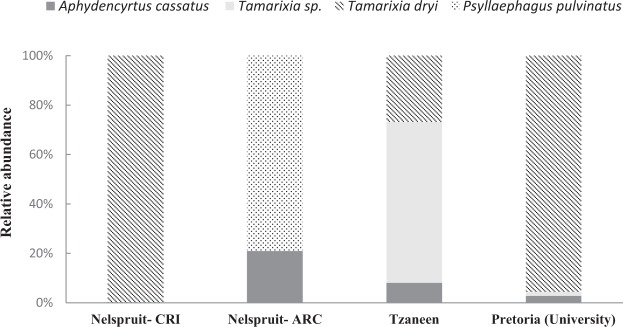
A total of 580 parasitized T. erytreae individuals were collected during the survey. From these samples 334 parasitoids belonging to five species emerged. The parasitoids in the remaining 246 psyllids failed to develop. The parasitoid complex was composed of at least five species whose relative abundance varied with sampling site (Fig. 1). Tamarixia dryi was the most abundant primary parasitoid in Pretoria and Nelspruit (>95% of the emerged parasitoids). This parasitoid species was also present in Tzaneen. On the other hand, the primary parasitoids P. pulvinatus (79%) and Tamarixia sp. (65%) were the most abundant species in Nelspruit and Tzaneen, respectively. The most abundant hyperparasitoid was Aphidencyrtus cassatus Annecke (Hymenoptera: Encyrtidae), which was recovered in Nelspruit, Tzaneen and Pretoria. One specimen of an Aphanogmus sp. (Hymenoptera: Ceraphronidae) was recorded in Tzaneen.
Figure 1.
Relative abundance of Trioza erytreae parasitoids collected from individual parasitized nymphs at four sites in South Africa in 2017.
Parasitism rates ranged between 0.72 ± 0.12 in Pretoria and 0.41 ± 0.21 in Nelspruit (Table 1). Parasitoid emergence ranged between 0.57 ± 0.04 and 0.66 ± 0.19. Hyperparasitism rates ranged between 0 and 0.09.
Table 1.
Parasitism and hyperparasitism rates of Trioza erytreae in four locations from South Africa.
| Date | Location | Parasitism assessment | |||
|---|---|---|---|---|---|
| No. of nymphs examined | Parasitism rate [Mean ± EE (n)] | Parasitoid emergence [Mean ± EE (n)] | Hyperparasitism rate [Mean ± EE (n)] | ||
| 9/28/2017 | Nelspruit- CRI* | 72 | 0.45 ± 0.19 (5) | 0.64 ± 0.09 (5) | 0 (5) |
| 09/29/2017 | Nelspruit- ARC* | 266 | 0.41 ± 0.21 (4) | 0.66 ± 0.19 (4) | 0.09 ± 0.09 (4) |
| 10/05/2017 | Tzaneen | 742 | 0.42 ± 0.07 (3) | 0.57 ± 0.004 (3) | 0.08 ± 0.04 (3) |
| 10/05/2017 | Pretoria (University) | 142 | 0.72 ± 0.12 (5) | 0.65 ± 0.12 (5) | 0.02 ± 0.003(5) |
*CRI: Citrus Research International (Nelspruit).
*ARC: Agricultural Research Council (Nelspruit).
DNA barcoding of Tamarixia and Trioza specimens
The sequences were submitted to the GenBank public sequence repository, with the following accession numbers for T. dryi (MK293946-MK293954), Tamarixia sp. (MK302489-MK302491) and T. erytreae (MK285548-MK285560). In a BLAST search, the COI barcode sequence obtained using the specific primers for T. erytreae showed 100% homology with the South African accessions KY754590 (TeSA7) and KY754594 (TeSA1) identified by Khamis et al.41 as T. erytreae, confirming that all specimens collected at Pretoria, Nelspruit and Tzaneen corresponded indeed to these species (see Supplementary Material Fig. S1). The COI barcode fragment sequenced from T. dryi and a new species of Tamarixia collected at Pretoria, Nelspruit and Tzaneen, shared 86–91% of identity to COI barcode fragment accessions from other Tamarixia species available in GenBank [Tamarixia radiata (Waterston)(GQ912272), Tamarixia drukyulensis (Yefremova and Yegorenkova) (KY986293) and Tamarixia triozae (Burks) (GQ912288)] (Fig. 2). The new species of Tamarixia collected at Tzaneen showed a higher identity (90%) to T. dryi sequences than to T. radiata, T. drukyulensis and T. triozae (87, 88 and 86%, respectively).
Figure 2.
Nucleotide sequence of COI barcode fragment for Tamarixia dryi and T. sp generated in the present work. Deduced amino acid (aa) sequence of the corresponding polypeptide is shown under each triplet. Nucleotide changes and non-conserved aa in the sequence of of T. sp COI fragment are represented in boldface and in black boxes, respectively. The coding region of Tamarixia spp COI gene and standard primers position used for the amplification of the barcode fragment −652 bp without including the sequence of the standard primers– are shown for schematic purposes.
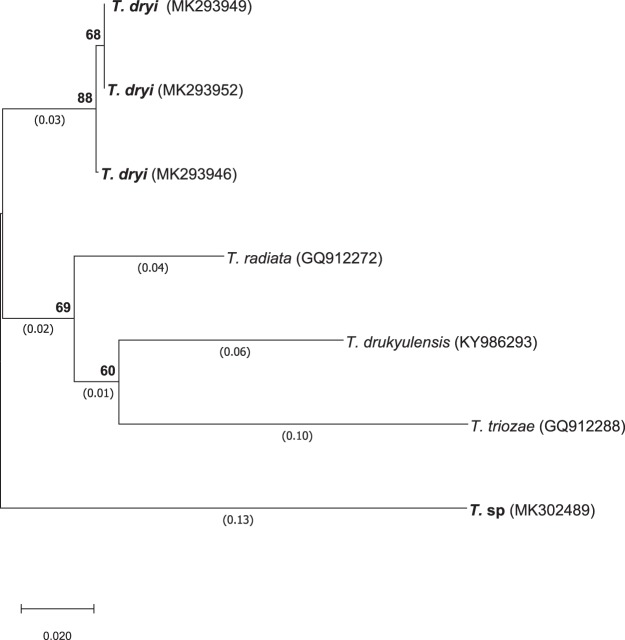
The phylogenetic tree of these Tamarixia species was paraphyletic with two distinct branches (Fig. 3). The first branch separated into two clusters. The first cluster grouped together the COI barcode sequences obtained in this work from the T. dryi specimens, while the second cluster hosted the T. radiata, T. drukyulensis and T. triozae GenBank accessions included in the analysis. The COI barcode sequence from the new Tamarixia sp. branched separately from the rest of the Tamarixia species included in this analysis.
Figure 3.
Rooted phylogenetic analysis showing the evolutionary relationships between Tamarixia species, based on DNA sequences of COI barcode fragment. The analysis involved seven nucleotide sequences including those of Tamarixia dryi and one T. sp generated in this study, and three closest sequences retrieved from GenBank [T. radiata (GQ912272), T. drukyulensis (KY986293) and T. triozae (GQ812288)].
Seasonal trend of the parasitoid complex of T. erytreae
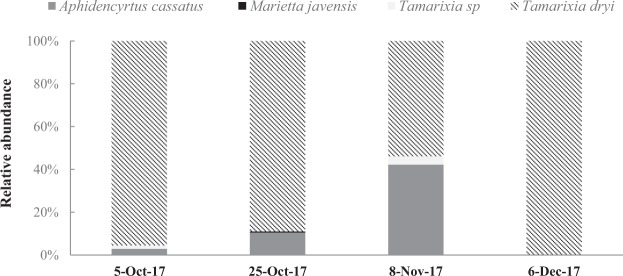
The most abundant parasitoid was T. dryi, followed by its hyperparasitoid A. cassatus, but their relative abundance showed different trends (Fig. 4). While the relative abundance of T. dryi decreased over the five weeks, the number of hyperparasitoids increased during the same period. The primary parasitoid Tamarixia sp. was recorded during the first and third sampling periods, and the hyperparasitoid Marietta javensis (Howard) (Hymenoptera: Aphelinidae) was found only at the second sampling date.
Figure 4.
Relative abundance of Trioza erytreae parasitoids in a citrus orchard from the University of Pretoria (Pretoria, South Africa) throughout the spring of 2017.
Effect of host size on the secondary sex ratio of T. dryi and Tamarixia sp
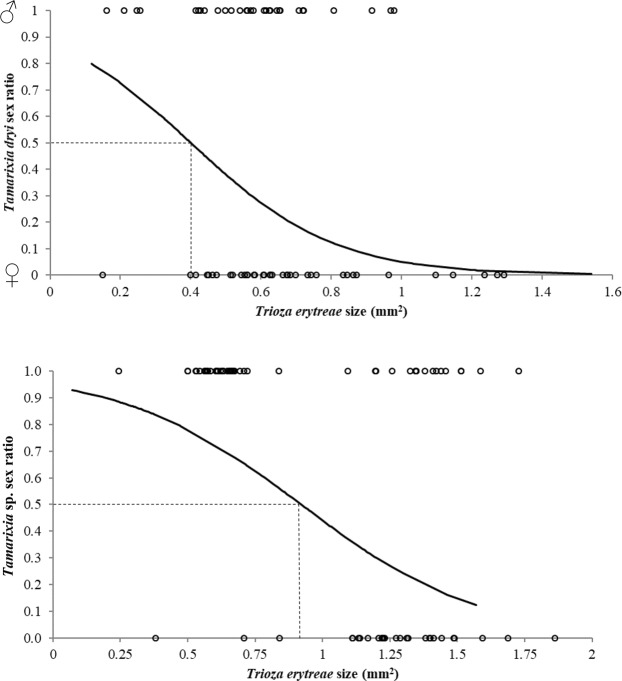
The secondary sex ratio of the primary parasitoids T. dryi and Tamarixia sp. depended on T. erytreae size. In both species, females emerged from larger-sized hosts than males (T. dryi: F = −3.34; df = 1, 86; P < 0.001; Tamarixia sp.: F = −3.99; df = 1, 78; P < 0.001) (Fig. 5A,B). Sex ratio in T. dryi turned female-biased around 0.40 mm2 and in Tamarixia sp. at around 0.90 mm2.
Figure 5.
Effect of Trioza erytreae size on the secondary sex ratio of Tamarixia dryi (A) and Tamarixia sp (B). Sex ratio turns female-biased around 0.4 mm2 in T. dryi and 0.9 mm2 in Tamarixia sp.
Effect of host size on A. cassatus emergence
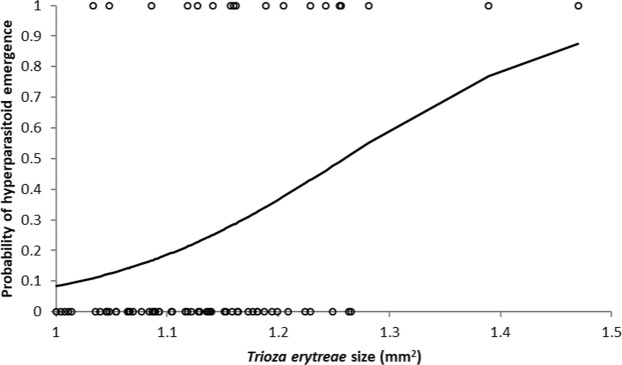
Hyperparasitism also depended on the size of T. erytreae individuals. The hyperparasitoid A. cassatus tended to emerge from large hosts (F = 3.144; df = 1, 80; P = 0.002) (Fig. 6). Hyperparasitism rates became higher than 50% when hosts were larger than 1.65 mm2.
Figure 6.
Effect of Trioza erytreae size on the probability that an individual of Syrphophagus cassatus emerged from the nymph.
Longevity of T. dryi and its hyperparasitoid A. cassatus
Survival of T. dryi differed between sexes (χ21 = 4.29; P = 0.038) (Fig. 7). Females lived 19.6 ± 1.86 days on average and males 14.75 ± 1.47 days. Survival of A. cassatus was also higher for females than for males (25.5 ± 1.14 and 17.71 ± 2.89 days, respectively) (χ21 = 5.16; P = 0.023). When females of both species were compared, no significant differences were found with respect to their longevity (χ21 = 4.10; P = 0.21).
Figure 7.
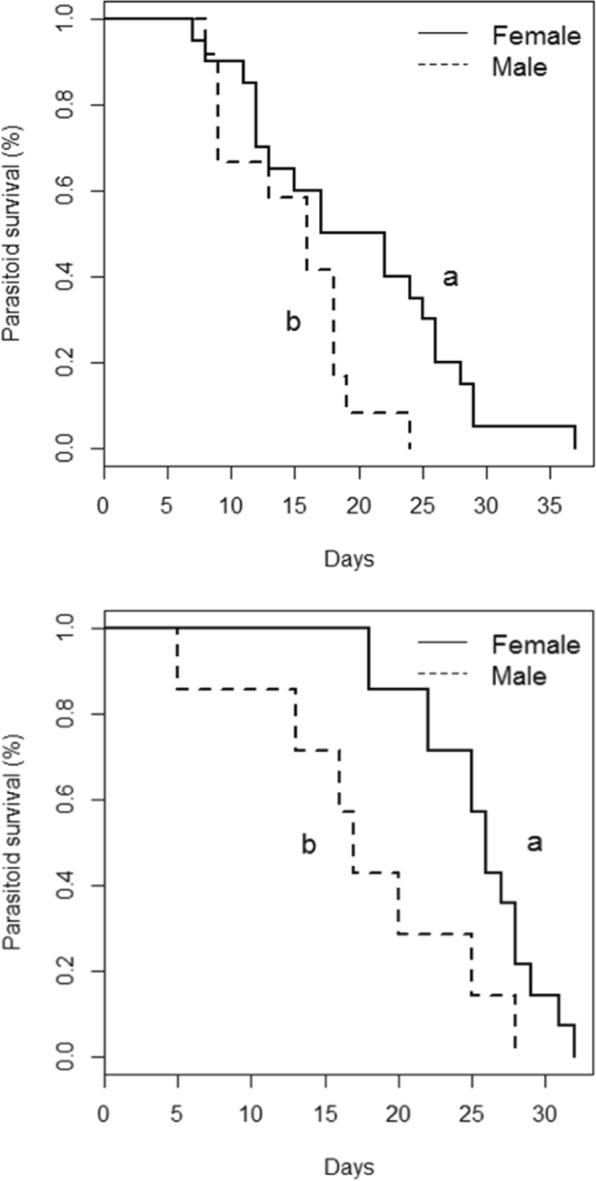
Cumulative survival function of Tamarixia dryi (A) and its hyperparasitoid Syrphophagus cassatus (B) for both sexes.
Discussion
Trioza erytreae was highly parasitized by several species of hymenopteran parasitoids. Parasitism rates were high in all the sampled areas in spring and ranged between 0.40 and 0.70. These rates are similar to those reported by van der Merwe42 (1923) and Catling43 in South Africa and Swaziland, respectively, and Tamesse et al.44 in Cameroon. Therefore, as demonstrated by Catling in the 1960s, parasitoids are important biotic regulators of T. erytreae in those areas where insecticides are not sprayed in South Africa, e.g. abandoned and experimental orchards and public gardens29,45. This result reinforces the suggestion of introducing exotic parasitoids to those areas where T. erytreae has arrived and where effective native parasitoids are absent. This is the case of Maderia (Portugal), the Canary Islands (Spain) and, more recently, mainland Europe15.
Among the three species of primary parasitoids of T. erytreae, T. dryi was the most effective and abundant, as has been previously demonstrated by other studies in South Africa and Swaziland30,43. Its relative abundance was higher than 90% in two sites. Similar values were obtained by Catling43 in South Africa. Another primary parasitoid of T. erytreae, P. pulvinatus, was only present in Nelspruit-ARC, where it was the most abundant species (80%). The high abundance of P. pulvinatus at this site may be due to the absence of T. dryi. Although P. pulvinatus parasitizes younger nymphs than T. dryi, its developmental time is longer than that of T. dryi30. This reason might partially explain why T. dryi tends to be more abundant where T. erytreae is present at low population densities.
A new parasitoid species from the genus Tamarixia was recorded in Tzaneen and Pretoria. It was the most abundant species in Tzaneen (70%), coexisting with T. dryi and the hyperparasitoid A. cassatus. In Pretoria, it was recorded sporadically. This new species could be the same species named as “Tetrastichus sp. n” in Western and Eastern Africa and classified as a primary parasitoid (Aubert 1986). In other studies in Cameroon, Zimbabwe and Malawi, an unknown “Tetrastichus sp.” was also found, but it was recorded as a hyperparasitoid30,34,44. The high abundance of Tamarixia sp. and the fact that we never observed the pupae or larvae of any Tamarixia species attacked by other larvae suggest that it is a primary parasitoid. The molecular analysis confirmed that this new species has not been reported yet in the database Genbank and it is closely related to T. dryi.
The sex ratio of T. dryi and Tamarixia sp. turned female biased when the size of T. erytreae was greater than 0.40 and 0.90 mm2, respectively. Many species of solitary parasitoids lay male eggs on small hosts and female eggs on large hosts46,47. This host-size-dependent sex ratio is presumed to be advantageous because females gain a greater benefit from the resulting increase in body size than do males46,48. According to Waage49, this occurs mostly in idiobionts, which paralyze the host, because in koinobiont parasitoids, hosts continue growing and its size at oviposition is a less reliable predictor of the resources that offspring will have available for development50,51. In our case, although T. dryi is a koinobiont, host size increases only slightly after parasitism (personal observations). Therefore, host size could be a reliable predictor of the resources when T. dryi recognizes and assesses the size of T. erytreae individuals. These results are important for mass-rearing the parasitoids and maximizing the production of females52.
Hyperparasitism rates were low and ranged between zero and 0.1 at the four sites. In Pretoria, where the seasonal trend of the parasitoids was determined in spring, hyperparasitism also reached a maximum of 0.1. These values were higher than the ones obtained by Catling and Anneke in the Letaba District [Limpopo (then Transvaal province) South Africa] from 1965 to 1967, although hyperparasitism was thought to be of little apparent significance, later on Mc Daniel and Moran30 and Anneke and Moran53 determined that it was the main factor in the increase of T. erytreae population levels in citrus. Among the hyperparasitoids, A. cassatus was the most abundant and widely distributed. Aphidencyrtus cassatus is considered the most abundant hyperparasitoid of T. erytreae and it attacks, at least, the two primary parasitoids T. dryi and P. pulvinatus43,44,54. Moreover, A. cassatus has been observed host feeding in the thoracic region of the primary parasitoid host, which sometimes killed the host30. This mortality and the high temperatures reached at the end of spring could have caused the high mortality rates of the primary parasitoids observed in Pretoria.
Two traits of the biology of A. cassatus could explain the negative impact that this hyperparasitoid has on T. dryi. Firstly, the probability that the hyperparasitoid A. cassatus emerged from T. erytreae nymphs increased with the size of the nymph. Since T. dryi females develop in larger nymphs than males, A. cassatus may affect the secondary sex ratio of T. dryi and more males will emerge. Secondly, females of the hyperparasitoid A. cassatus lived for more than 30 days when they had access to carbohydrates, and their longevity was similar to that of T. dryi. Therefore, the hyperparasitoid is capable of surviving long periods of host scarcity or, at least, as long as the primary parasitoid. Although hyperaparasitoids can regulate herbivore populations by stabilising host-parasitoid interactions55–57, from the point of view of classical biological control, the accidental introduction of A. cassatus could impair the establishment of T. dryi58. Therefore, great care should be taken to exclude this parasitoid when importing T. dryi to Europe, for example introducing only adult parasitoids, establishing isolines and following quarantine procedures before the release59,60.
Materials and Methods
Insect survey
Sampling took place in citrus producing areas in four provinces, Gauteng, Limpopo, Mpumalanga and Western Cape, of South Africa. A total of 65 citrus orchards, 5 public parks and 60 private properties were examined from September 21st to December 9th of 2017. At all sites, the sampling date, the number of trees sampled, the variety of the trees and the presence of T. erytreae or its visual symptoms and parasitoids were recorded (see supplementary material Table S1). Visual symptoms of T. erytreae on citrus, in contrast to those of Diaphorina citri, consist of pit galls protruding from the upper surface of the leaves, chlorosis and leave twisting15,61.
Parasitoid identification, relative abundance and parasitism rates
From those areas and trees where T. erytreae was collected, the psyllids were transported to the laboratory to identify potential parasitoids, determine their relative abundance and parasitism rates in each location. From each tree, 3 to 20 leaves infested by T. erytreae were collected and transported in enclosed individualized plastic bags to the laboratory. Due to the scarcity of T. erytreae in some of the orchards, the number of sampled trees and leaves was variable (see supplementary material Table S1). Once in the laboratory, the number of live psyllids and psyllids suitable for parasitism (2nd-to the 5th-instar nymph) and parasitized psyllids was recorded using a stereomicroscope. In order to identify all the parasitoids and calculate the rate of emerging parasitoids, psyllid nymphs were placed individually in 1 ml microtubes closed with cotton wool. Afterwards, the microtubes tubes were kept in an incubator (LabconTM 2 LTGC 20, Laboratory Marketing Services cc, South Africa) under controlled conditions (12 L: 12 Dh, 25 °C, 60–70% RH) and checked daily until parasitoids emerged. Once emerged, their sex was determined and the identification at species level was carried out using the key of Tamesse62. The morphological identifications of T. erytreae and Tamarixia parasitoids were confirmed by David Ouvrard (Natural History Museum, London) and Roger Burks (University of California, Riverside), respectively. Moreover, voucher specimens of all the parasitoid species collected during this study were deposited at the University of California, Riverside and labeled with the database number UCRC_ENT from 00517324 to 00517336.
In order to calculate the parasitism rate, each tree was used as a sampling unit because T. erytreae, as other psyllids, has an aggregative distribution pattern63–65.
DNA extraction, PCR and sequencing of barcode fragment
The morphological identifications were verified with molecular identifications using cytochrome c oxidase subunit 1 (COI) sequences of T. erytreae and of the parasitoids T. dryi and Tamarixia sp. The insects were collected at three different locations, Nelspruit, Pretoria, and Tzaneen, in South Africa. DNA was extracted from individual insects using a salting out method66 adapted from Monzó et al.67. Polymerase chain reaction (PCR) was performed to amplify the mitochondrial COI gene. Standard primers LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCA GGGTGACCAAAAAATCA-3′)68 were used for the amplification of a 708-bp fragment of the COI gene of Tamarixia species, while Te-6U30 (5′-ATTTTTAAGCACTAATCATAAAATTATTGG-3′) and Te-720L26 (5′-TATACTTCAGGATGTCCAAAAAATCA-3′) specific primers were used to amplify a COI barcode fragment of a 714-bp fragment of the COI of T. erytreae. PCR was carried out in a total reaction volume of 20 µl containing 1X Reaction Buffer (Mg free), 1625 µM MgCl2, 250 µM of all four dNTPs, 0.25 µM of each primer, 1 U of DNA polymerase (1 U/µl, Biotools), and 1 µl of DNA in a thermal cycler (Eppendorf Mastercycler). Reactions were cycled as follow: initial denaturation at 95 °C for 120 seconds (s); 40 cycles of 94 °C for 60 s, 45 °C for Tamarixia or 54 °C for T. erytreae for 60 s and 72 °C for 90 s; and a final extension at 72 °C for 600 s. Amplified PCR products were resolved in a 1.2% agarose gel and successfully amplified barcode fragments were cleaned up using the UltraClean PCR Clean-up DNA Purification Kit (MO BIO Laboratories Inc., Carlsbad - California, USA). Amplified barcode fragments were bidirectional sequenced using both the forward and reverse PCR primers. Sanger-sequencing was performed by capillary electrophoresis using a 3130XL Genetic Analyzer (Applied Biosystems, Carlsbad - California, USA), at the Sequencing Service of the IBMCP (Valencia, Spain). Sequences were analysed and trimmed to remove primer sequences, using Sequencer DNA Sequence Analysis Software (Gene Codes Corporation, Ann Arbor – Michigan, USA). Forward and reverse high-quality reads obtained for each individual were assembled into consensus sequences and submitted to the GeneBank public sequence repository.
Sequence data and phylogenetic analysis
The phylogenetic analysis of the two species of Tamarixia was carried out using the COI barcode sequences obtained in this work together with those already included in the GenBank: T. radiata (Waterston), T. drukyulensis Yefremova and Yegorenkov and T. trioza (Burks). Consensus sequences corresponding to the amplified COI barcode fragment of the Tamarixia parasitoids were first used as query to BLASTN against the non-redundant nucleotide collection of the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi), optimising for highly similar sequences. The barcode based taxonomic assignment at the genus level was set at 94% identity over a 90% sequence overlap. Multiple alignments of consensus sequences and closest sequence identities were done using Clustal Omega software69. The phylogenetic analysis was carried out in MEGA X70, using the Neighbor-Joining method71. The reliability of the tree pattern was evaluated using a bootstrap test with 10000 replicates72, and the evolution distances, given as units of the number of base substitution per sites, were computed using the Maximum Composite Likelihood method73. The rate variation among sites was modelled with a gamma distribution (shape parameter = 1).
Seasonal trend of the parasitoid complex of T. erytreae
The seasonal trend of T. erytreae and its parasitoids was studied in an infested lemon orchard located at the University of Pretoria Experimental Farm (25°44′51.1″S 28°15′31.2″E). The orchard was ~10 years old, and the trees sampled were not treated with pesticidesduring the sampling period. From October to December, five infested leaves from five trees were sampled every two weeks. Samples were processed following the same methodology described above until parasitoids emerged and were identified.
Effect of host size on secondary sex ratio and hyperparasitism of parasitoids
The effects of host size on the secondary sex ratio of T. dryi and Tamarixia sp. and on hyperparasitism were analyzed. After parasitoids emerged they were identified and their sex was determined. The psyllid nymphs have an oval shape and host size was determined by calculating the area of an ellipse by multiplying r1 × r2 × π (r1: major radius, r2: minor radius). Both sex and hyperparasitism ratios were analyzed using generalized linear models assuming binomial errors. The assumed error structure was assessed by a heterogeneity factor equal to the residual deviance divided by the residual degrees of freedom. If an over- or an under dispersion was detected, we re-evaluated the significance of the explanatory variables using an F-test after rescaling the statistical model by a Pearson’s χ2 divided by the residual degrees of freedom. All data analyses were performed with the R freeware statistical package (Version 1.0.143)74.
Longevity of Tamarixia dryi and its hyperparasitoid Aphidencyrtus cassatus
Tamarixia dryi and A. cassatus longevity was recorded. From the individual nymph sampled at the University of Pretoria Experimental Farm, a total of 20 females and 12 males of T. dryi as well as 14 females and 7 males of A. cassatus were selected. Individual parasitoids were placed singly in 1 ml microtubes tubes closed with cotton wool and 1 M sucrose drop renewed every two days. The microtubes were kept under controlled conditions in an incubator (Labcon 2 LTGC 20, Laboratory Marketing Services CC, Roodepoort, South Africa) at 12L: 12D, 25 ± 2 °C and 60–70% RH, and the survival of the insects was checked daily. The Cox regression model was used to determine differences between sexes within the same species and between species. Analyses were carried out using the R freeware statistical package (Version 1.0.143)74.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Supplementary information
Acknowledgements
We thank Dr Johan Burger (Stellenbosch University) and Dr Tim Grout (Citrus Research International) and their staff for their assistance during the sampling. We also thank Dr David Ouvrard (Natural History Museum, London) and Dr Roger Burks (University of California, Riverside) for the morphological identifications of T. erytreae and Tamarixia parasitoids respectively and Dr Serguei Triapitsyn for the official deposition of voucher specimens at the University of California, Riverside. We also thank Dr. Susan Halbert and an anonymous reviewer for their comments. This research was partly funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 727459 “Insect-borne prokaryote-associated diseases in tropical and subtropical perennial crops” - TROPICSAFE, the Spanish Ministry of Economy and Competitiveness under grant agreement INIA E-RTA RTA2015-00005-C06 “Control and confinement strategies for Trioza erytreae, vector of citrus huanglongbing” and the Conselleria d’Agricultura, Pesca i Alimentació de la Generalitat Valenciana. This research was also supported by a pre-doctoral grant to J. Pérez-Rodríguez from Generalitat Valenciana (Spain), and M. Pérez-Hedo was the recipient of a research fellowship from the INIA Spain (Subprogramme DOC-INIA-CCAA).
Author Contributions
Field and laboratory assays were designed and performed by J.P., K.K. and A.T. Data were subsequently analysed by J.P. and A.T. Molecular analyses were performed by O.R. and M.P. All authors wrote, read and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45294-w.
References
- 1.do Carmo Teixeira D, et al. ‘Candidatus Liberibacter americanus’, associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Int. J. Syst. Evol. Microbiol. 2005;55:1857–1862. doi: 10.1099/ijs.0.63677-0. [DOI] [PubMed] [Google Scholar]
- 2.Bové JM. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. Journal of Plant Pathology. 2006;88:7–37. doi: 10.4454/jpp.v88i1.828. [DOI] [Google Scholar]
- 3.Gottwald T, Graça JVD, Bassanezi RB. Citrus Huanglongbing: The Pathogen and its impact. Plant Heal. Prog. 2007;8:1–36. doi: 10.1094/PHP-2007-0906-01-RV. [DOI] [Google Scholar]
- 4.Lee JA, et al. Asymptomatic spread of huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. 2015;112:7605–7610. doi: 10.1073/pnas.1508253112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grafton-Cardwell EE, Stelinski LL, Stansly PA. Biology and Management of Asian Citrus Psyllid, Vector of the Huanglongbing Pathogens. Annu. Rev. Entomol. 2013;58:413–432. doi: 10.1146/annurev-ento-120811-153542. [DOI] [PubMed] [Google Scholar]
- 6.Kuwayama. Psylliden Japans. I. Trans. Sapporo Nat. Hist. Soc. 2, 149–189 (1908).
- 7.Knapp JL, et al. The Asian citrus psyllid and citrus greening disease. Citrus Ind. 1998;79:28–29. [Google Scholar]
- 8.French JV, Kahlke CJ, Da Graça JV. First record of the Asian citrus psylla, Diaphorina citri Kuwayama (Homoptera: Psyllidae) in Texas. Subtrop. Plant Sci. 2001;53:14–15. [Google Scholar]
- 9.Lopes SA, et al. Liberibacters Associated with Citrus Huanglongbing in Brazil: ‘Candidatus Liberibacter asiaticus’ Is Heat Tolerant, ‘Ca. L. americanus’ Is Heat Sensitive. Plant Dis. 2009;93:257–262. doi: 10.1094/PDIS-93-3-0257. [DOI] [PubMed] [Google Scholar]
- 10.Shimwela Mpoki M., Narouei-Khandan Hossein A., Halbert Susan E., Keremane Manjunath L., Minsavage Gerald V., Timilsina Sujan, Massawe Deogracious Protas, Jones Jeffrey B., van Bruggen Ariena H. C. First occurrence of Diaphorina citri in East Africa, characterization of the Ca. Liberibacter species causing huanglongbing (HLB) in Tanzania, and potential further spread of D. citri and HLB in Africa and Europe. European Journal of Plant Pathology. 2016;146(2):349–368. doi: 10.1007/s10658-016-0921-y. [DOI] [Google Scholar]
- 11.Jagoueix S, Bove JM, Garnier M. The phloem-limited bacterium of greening disease of citrus is a member of the α Subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994;44:379–386. doi: 10.1099/00207713-44-3-379. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Hartung JS, Levy L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods. 2006;66:104–115. doi: 10.1016/j.mimet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Otero RP, Vázquez JPM, Del Estal P. Detección de la psila africana de los cítricos, Trioza erytreae (Del Guercio, 1918)(Hemiptera: Psylloidea: Triozidae), en la Península Ibérica. Arq. Entomolóxicos. 2015;13:119–122. [Google Scholar]
- 14.Otero RP, Lorenzo SP, Vázquez JPM. La psila africana de los cítricos (Trioza erytreae Del Guercio) y su situación actual en Galicia. Rev. Frutic. 2016;48:48–59. [Google Scholar]
- 15.Cocuzza GEM, et al. A review on Trioza erytreae (African citrus psyllid), now in mainland Europe, and its potential risk as vector of huanglongbing (HLB) in citrus. J. Pest Sci. 2017;90:1–17. doi: 10.1007/s10340-016-0804-. [DOI] [Google Scholar]
- 16.Zhang Y, et al. Lifetime gains and patterns of accumulation and mobilization of nutrients in females of the synovigenic parasitoid, Diglyphus isaea Walker (Hymenoptera: Eulophidae), as a function of diet. J. Insect Physiol. 2011;57:1045–1052. doi: 10.1016/j.jinsphys.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Manjunath KL, et al. Detection of ‘Candidatus Liberibacter asiaticus’ in Diaphorina citri and its importance in the management of citrus huanglongbing in Florida. Phytopathology. 2008;98:387–396. doi: 10.1094/PHYTO-98-4-0387. [DOI] [PubMed] [Google Scholar]
- 18.Paula BMD, et al. Active taste compounds in juice from oranges symptomatic for Huanglongbing (HLB) citrus greening disease. LWT. 2018;91:518–525. doi: 10.1016/j.lwt.2018.01.083. [DOI] [Google Scholar]
- 19.Hodges, A. W. & Spreen, T. H. Economic impacts of citrus greening (HLB) in Florida, 2006/07-2010/11. Univ. Florida IFAS Ext. doi:FE903 (2015).
- 20.Jacas Josep Anton, Karamaouna Filitsa, Vercher Rosa, Zappalà Lucia. Integrated Management of Arthropod Pests and Insect Borne Diseases. Dordrecht: Springer Netherlands; 2010. Citrus Pest Management in the Northern Mediterranean Basin (Spain, Italy and Greece) pp. 3–27. [Google Scholar]
- 21.Reig-Martínez E, Picazo-Tadeo AJ. Analysing farming systems with data envelopment analysis: citrus farming in Spain. Agric. Syst. 2004;82:17–30. doi: 10.1016/j.agsy.2003.12.002. [DOI] [Google Scholar]
- 22.Fernandes, E. & Franquinho-Aguiar, A. M. Evolution of two quarantine pests of Toxoptera citricida (Kirkaldy) and Trioza erytreae (Del Guercio) in Madeira Island [Portugal].Boletín de Sanidad Vegetal. Plagas (España) (2001).
- 23.Pérez Padrón F, Carnero Hernández A. Presence of Trioza erytreae the African citrus psyllid on the island of Tenerife. Rev. Granja. 2002;9:54–57. [Google Scholar]
- 24.Hernández AG. Trioza erytreae (Del Guercio 1918): nueva plaga de los citricos en Canarias. Phytoma España La Rev. Prof. Sanid. Veg. 2003;153:112–118. [Google Scholar]
- 25.Etienne, J. & Aubert, B. Biological control of psyllid vectors of greening disease on Reunion Island. In International Organization of Citrus Virologists Conference Proceedings 8, 118–121 (1980).
- 26.Van den Berg MA, Greenland J. Tamarixia dryi, parasitoid of the citrus psylla, Trioza erytreae: a review. African Plant Prot. 2000;6:25–28. [Google Scholar]
- 27.Hoddle MS, Pandey R. Host range testing of Tamarixia radiata (Hymenoptera: Eulophidae) sourced from the Punjab of Pakistan for classical biological control of Diaphorina citri (Hemiptera: Liviidae: Euphyllurinae: Diaphorinini) in California. J. Econ. Entomol. 2014;107:125–136. doi: 10.1603/EC13318. [DOI] [PubMed] [Google Scholar]
- 28.Aguiar AF, Martin JH. Psyllids (Homoptera: Psylloidea) from Madeira Island: an updated checklist including new records. Sociedade Portuguesa de entomologia. 1999;6:335–342. [Google Scholar]
- 29.Catling HD. The bionomics of the South African citrus psylla, Trioza erytreae (Del Guercio)(Homoptera: Psyllidae) I. The influence of the flushing rhythm of citrus and factors which regulate flushing. J. Entomol. Soc. South. Afr. 1969;32:191–208. [Google Scholar]
- 30.Mc Daniel JR, Moran VC. The parasitoid complex of the citrus psylla Trioza erytreae (Del Guercio)[Homoptera: Psyllidae] Entomophaga. 1972;17:297–317. doi: 10.1007/BF02371184. [DOI] [Google Scholar]
- 31.Tamesse JL, Messi J. Receptivity to Trioza erytreae (Del Guercio) of citrus varieties in Cameroon. Fruits (Paris) 2000;55:389–400. [Google Scholar]
- 32.Tamesse JL, Messi J. Incidence de Trioza erytreae (del Guercio)(Homoptera: Triozidae), Psylle Vecteur du Greening sur la Sensibilité des Plantules d’Agrumes dans une Pépinière au Cameroun. Int. J. Trop. Insect Sci. 2002;22:97–103. doi: 10.1017/S1742758400015174. [DOI] [Google Scholar]
- 33.Aubert B, Bové JM, Etienne J. La lutte contre la maladie du «greening» des agrumes à l’île de la Réunion. Résultats et perspectives. Fruits. 1980;35:605–624. [Google Scholar]
- 34.Aubert, B. & Quilici, S. Monitoring adult psyllas on yellow traps in Reunion Island. In International Organization of Citrus Virologists Conference Proceedings (1957–2010) 10 (1988).
- 35.Bové J, Heat-tolerant Asian HLB. meets heat-sensitive African HLB in the Arabian Peninsula! Why? J. Citrus Pathol. 2014;1:1–78. [Google Scholar]
- 36.Husain, M. & Nath, D. The citrus psylla (Diaphorina citri, Kuw.) [Psyllidae: Homoptera]. Mem. Dep. Agric. India, Entomol. Ser. 10, 1–27 (1927).
- 37.Toorawa, P. La maladie du Huanglongbing (Greening) des agrumes à l’île Maurice. Détection de Candidatus Liberobacter Asiaticum et Candidatus Liberobacter Africanum dans les agrumes et les insectes vecteurs. Doctoral dissertation, Bordeaux 2 (1998).
- 38.Chen X, Stansley P. Biology of Tamarixia radiata (Hymenoptera: Eulophidae), parasitoid of the citrus greening disease vector Diaphorina citri (Hemiptera: Psylloidea): a mini review. Florida Entomol. 2014;97:1404–1413. doi: 10.1653/024.097.0415. [DOI] [Google Scholar]
- 39.Parra JRP, Alves GR, Diniz AJF, Vieira JM. Tamarixia radiata (Hymenoptera: Eulophidae) × Diaphorina citri (Hemiptera: Liviidae): mass rearing and potential use of the parasitoid in Brazil. J. Integr. Pest Manag. 2016;7:1–5. doi: 10.1093/jipm/pmw003. [DOI] [Google Scholar]
- 40.Urbaneja-Bernat, P. et al. Host range testing of Tamarixia dryi (Hymenoptera: Eulophidae) sourced from South Africa for classical biological control of Trioza erytreae (Hemiptera: Psyllidae) in Europe. Biol. Control In Press (2019).
- 41.Khamis FM, et al. DNA Barcode Reference Library for the African Citrus Triozid, Trioza erytreae (Hemiptera: Triozidae): Vector of African Citrus Greening. J. Econ. Entomol. 2017;110:2637–2646. doi: 10.1093/jee/tox283. [DOI] [PubMed] [Google Scholar]
- 42.Van der Merwe, C. P. The citrus psylla (Trioza merwei, Pettey). J. Dept. Agric., Union S. Afr., Pretoria. Gov. Print. Station. Off. Repr. (1923).
- 43.Catling HD. The bionomics of the South African citrus psylla, Trioza erytreae (Del Guercio)(Homoptera: Psyllidae) 2. The influence of parasites and notes on the species involved. J. Entomol. Soc. South. Afr. 1969;32:209–223. [Google Scholar]
- 44.Tamesse JL, et al. The parasitoid complex of Trioza erytreae (Del Guercio)(Homoptera: Triozidae), citrus psylla, in Cameroon. Fruits-Paris. 2002;57:19–28. doi: 10.1051/fruits:2002003. [DOI] [Google Scholar]
- 45.Catling HD. Distribution of the psyllid vectors of citrus greening disease, with notes on the biology and bionomics of Diaphorina citri. FAO Plant Prot. Bull. 1970;18:8–15. [Google Scholar]
- 46.Charnov EL, Los-Den Hartogh RL, Jones WT, Van Den Assem J. Sex ratio evolution in a variable environment. Nature. 1981;289:27. doi: 10.1038/289027a0. [DOI] [PubMed] [Google Scholar]
- 47.Beltrà A, Pekas A, Soto A, Tena A. Employing evolutionary theory to improve biological pest control: causes of non-adaptive sex allocation behavior in parasitoid wasps and implications. Basic Appl. Ecol. 2014;15:625–632. doi: 10.1016/j.baae.2014.09.002. [DOI] [Google Scholar]
- 48.Godfray, H. C. J. Parasitoids: behavioral and evolutionary ecology. (Princeton University Press 1994).
- 49.Waage JK. Sib‐mating and sex ratio strategies in scelionid wasps. Ecol. Entomol. 1982;7:103–112. doi: 10.1111/j.1365-2311.1982.tb00648.x. [DOI] [Google Scholar]
- 50.Askew, R. R. & Shaw, M. R. Parasitoid communities: their size, structure and development. In Waage, J. and Greathead, D. (eds), Insect Parasitoids, 13th Symposium of Royal Entomological Society of London 225–264 (Academic Press, London: now Elsevier 1986).
- 51.West SA, Sheldon BC. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- 52.Bernal JS, Luck RF, Morse JG. Host influences on sex ratio, longevity, and egg load of two Metaphycus species parasitic on soft scales: implications for insectary rearing. Entomol. Exp. Appl. 1999;92:191–204. doi: 10.1046/j.1570-7458.1999.00538.x. [DOI] [Google Scholar]
- 53.Annecke, D. P. & Moran, V. C. Insects and mites of cultivated plants in South Africa. (Butterworth 1982).
- 54.Aubert B. Trioza erytreae Del Guercio and Diaphorina citri Kuwayama(Homoptera: Psylloidea), the two vectors of citrus greening disease: Biological aspects and possible control strategies. Fruits. 1987;42:149–162. [Google Scholar]
- 55.Hassell MP, Waage JK. Host-parasitoid population interactions. Annu. Rev. Entomol. 1984;29:89–114. doi: 10.1146/annurev.en.29.010184.000513. [DOI] [Google Scholar]
- 56.Briggs CJ. Competition among parasitoid species on a stage-structured host and its effect on host suppression. Am. Nat. 1993;141:372–397. doi: 10.1086/285479. [DOI] [Google Scholar]
- 57.Sullivan DJ, Völkl W. Hyperparasitism: multitrophic ecology and behavior. Annu. Rev. Entomol. 1999;44:291–315. doi: 10.1146/annurev.ento.44.1.291. [DOI] [PubMed] [Google Scholar]
- 58.Tougeron K, Tena A. Hyperparasitoids as new targets in biological control in a global change context. Biol. Control. 2018;130:164–171. doi: 10.1016/j.biocontrol.2018.09.003. [DOI] [Google Scholar]
- 59.Heimpel, G. E. & Mills, N. J. Biological control: ecology and applications, 10.1017/9781139029117 (Cambridge University Press, Cambridge, UK 2010).
- 60.van Lenteren JC, et al. Environmental risk assessment of exotic natural enemies used in inundative biological control. Biol. Control. 2003;48:3–38. [Google Scholar]
- 61.Catling HD. Notes on the biology of the South African citrus psylla, Trioza erytreae (Del Guercio)(Homoptera: Psyllidae) J. Entomol. Soc. South. Afr. 1973;36:299–306. [Google Scholar]
- 62.Tamesse JL. Key for identification of the Hymenopteran parasitoids of the African citrus psylla Trioza erytreae Del Guercio (Hemiptera: Triozidae) in Cameroon. African. J. Agric. Res. 2009;4:85–91. [Google Scholar]
- 63.Samways MJ, Manicom BQ. Immigration, frequency distributions and dispersion patterns of the psyllid Trioza erytreae (del Guercio) in a citrus orchard. J. Appl. Ecol. 1983;20:463–472. doi: 10.2307/2403520. [DOI] [Google Scholar]
- 64.Chi-kun Y, Fasheng L. Nine new species and a new genus of psyllids from Yunnan (Homoptera: Psyllidae) Entomotaxonomia. 1984;4:1. [Google Scholar]
- 65.Butler CD, Trumble JT. Spatial dispersion and binomial sequential sampling for the potato psyllid (Hemiptera: Triozidae) on potato. Pest Manag. Sci. 2012;68:865–869. doi: 10.1002/ps.3242. [DOI] [PubMed] [Google Scholar]
- 66.Sunnucks P, Hales DF. Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae) Mol. Biol. Evol. 1996;13:510–524. doi: 10.1093/oxfordjournals.molbev.a025612. [DOI] [PubMed] [Google Scholar]
- 67.Monzó C, Sabater-Muñoz B, Urbaneja A, Castañera P. The ground beetle Pseudophonus rufipes revealed as predator of Ceratitis capitata in citrus orchards. Biol. Control. 2011;56:17–21. doi: 10.1016/j.biocontrol.2010.09.004. [DOI] [Google Scholar]
- 68.Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 69.Sievers F, et al. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 72.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution (N. Y). 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 73.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Team, Rs RStudio: integrated development for R. RStudio, Inc., Boston, MA42, http//www.rstudio.com (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.