Abstract
Rationale: “Noninfectious” pulmonary complications are significant causes of morbidity and mortality after allogeneic hematopoietic cell transplant. Early-onset viral reactivations or infections are common after transplant. Whether the first-onset viral infection causes noninfectious pulmonary complications is unknown.
Objectives: To determine whether the first-onset viral infection within 100 days after transplant predisposes to development of noninfectious pulmonary complications.
Methods: We performed a retrospective review of 738 allogeneic hematopoietic cell transplant patients enrolled from 2005 to 2011. We also established a novel bone marrow transplantation mouse model to test whether herpesviral reactivation after transplant causes organ injury.
Measurements and Main Results: First-onset viral infections with human herpesvirus 6 or Epstein-Barr virus within 100 days after transplant increase the risk of developing idiopathic pneumonia syndrome (adjusted hazard ratio [aHR], 5.52; 95% confidence interval [CI], 1.61–18.96; P = 0.007; and aHR, 9.21; 95% CI, 2.63–32.18; P = 0.001, respectively). First infection with human cytomegalovirus increases risk of bronchiolitis obliterans syndrome (aHR, 2.88; 95% CI, 1.50–5.55; P = 0.002) and grade II–IV acute graft-versus-host disease (aHR, 1.59; 95% CI, 1.06–2.39; P = 0.02). Murine roseolovirus, a homolog of human herpesvirus 6, can also be reactivated in the lung and other organs after bone marrow transplantation. Reactivation of murine roseolovirus induced an idiopathic pneumonia syndrome–like phenotype and aggravated acute graft-versus-host disease.
Conclusions: First-onset herpesviral infection within 100 days after allogeneic hematopoietic cell transplant increases risk of pulmonary complications. Experimentally reactivating murine roseolovirus causes organ injury similar to phenotypes seen in human transplant recipients.
Keywords: herpesvirus reactivation, idiopathic pneumonia syndrome, bronchiolitis obliterans syndrome, graft-versus-host disease, murine roseolovirus
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary complications are a leading cause of morbidity and nonrelapse mortality after allogeneic hematopoietic cell transplantation (HCT). Early-onset viral infections are common after HCT. Recent studies reported occult viral infections in HCT recipients with noninfectious pulmonary complications. Whether first-onset viral infections predispose allogeneic HCT recipients to noninfectious pulmonary complications remains undetermined.
What This Study Adds to the Field
This is the first study to reveal that first-onset herpesviral infection after allogeneic HCT is associated with noninfectious pulmonary complications. This study also establishes the first murine model to causally link herpesvirus reactivation with the development of lung pathology similar to idiopathic pneumonia syndrome.
Pulmonary complications are a leading cause of morbidity and nonrelapse mortality (NRM) after allogeneic hematopoietic cell transplantation (allo-HCT). Pulmonary complications can be infectious or noninfectious in etiology, and are common, occurring in 30–60% of allo-HCT recipients (1, 2). Noninfectious pulmonary complications, including idiopathic pneumonia syndrome (IPS) and bronchiolitis obliterans syndrome (BOS), are associated with significant morbidity and mortality after HCT (3–5).
During periods of immune suppression, such as after HCT, latent human herpesviruses (HHVs) can reactivate to the lytic cycle (6). HHVs typically infect individuals in the first two decades of life and establish life-long latency to escape immune surveillance and detection (6). In addition, primary HHV infections can occur in seronegative patients receiving grafts from seropositive donors (7). Recent human studies have highlighted a role for HHVs in the pathogenesis of pulmonary complications after HCT (6, 8, 9).
Viral infections after HCT cause morbidity and mortality by altering host immune responses in the lungs and other host target organs (10–12). Using a syngeneic murine transplant model, we previously reported that infections with murine gammaherpesvirus 68 (MHV-68), a mouse homolog of human Epstein-Barr virus (EBV) and Kaposi sarcoma–associated herpesvirus, given after HCT engraftment caused severe pneumonitis and lung fibrosis 3 weeks after infection, a time period when lytic viral infection was cleared (10, 13). In those experiments, we identified aberrant lung pathology (pneumonitis and fibrosis) driven by an abnormal CD4+ T-helper cell response skewed toward pathogenic type 17 polarization in infected mice (10). Clinical data and several mouse models suggest the expansion of type 17 CD4+ T cells contributes to IPS (14–16).
We hypothesized that first-onset viral infection (FOVI; i.e., the first viral infection or reactivation within 100 d after HCT) will predispose patients to the development of noninfectious pulmonary complications. We focused on FOVI because accumulating evidence indicates that the first episode of viral infection or reactivation may be the critical determinant in setting the immunologic tone, such as through specific cytokine production, T-cell polarization, or suppression (10, 17–19). First viral infection and subsequent therapy are also likely to impact secondary infections (20, 21). We conducted a retrospective review of FOVIs and the subsequent development of IPS and BOS in a large cohort of allo-HCT recipients. In addition, we also examined the association with graft-versus-host disease (GVHD). Here we report a significant association between FOVIs and the development of pulmonary complications and NRM in HCT recipients. To demonstrate biologic plausibility for these causal associations between reactivated viral infection, lung injury, and GVHD, we developed a new animal model of neonatal infection with a murine herpesvirus that demonstrates reactivation from latency following allogeneic bone marrow transplant (BMT). Notably, mice that received BMT after neonatal viral infection showed evidence of lung injury and acute GVHD (aGVHD) in other organs. Results of some of these studies have been previously reported in the form of an abstract (22).
Methods
Ethics Statement
All human studies were approved by the institutional review board at the University of Michigan (HUM00117533), with all patients or their surrogates signing informed consent permitting data collection. All murine experiments were approved by the Institutional Animal Care and Use Committee at the University of Michigan (protocol 6821).
Human Study Population and Study Design
Allogeneic HCT recipients transplanted at the University of Michigan Medical Center between 2005 and 2011 were included in this retrospective analysis. Quantitative PCR (qPCR) for cytomegalovirus (CMV) was performed weekly on plasma through 100 days after HCT. Viral positivity of HHV-6 (both HHV-6A and HHV-6B), EBV, herpes simplex virus (HSV), or community-acquired respiratory viruses (CARV) was analyzed as clinically indicated. IPS, BOS, and GVHD were diagnosed according to NIH consensus guidelines (3, 4). Additional detail on the methods for detecting viral positivity is provided in the online supplement.
Murine Roseolovirus and Infection
Murine roseolovirus (MRV) was previously termed murine thymic lymphotropic virus, or murine thymic virus and has been sequenced by Dr. W.M.Y.’s laboratory recently (23). Litters of newborn BALB/c mice within 24 hours after birth were infected with MRV as previously described (23). See the online supplement for further details.
Mouse Minor Histocompatibility Antigen–mismatched BMT
B10.D2 (H2d) and BALB/c (H2d) mice were purchased from Jackson Laboratory. BALB/c mice were infected with MRV within 24 hours after birth and allowed to age for 8–10 weeks for the virus to complete lytic infection and establish latency. BMT was performed as previously reported (24). Mice were then assessed at various time points for evidence of MRV reactivation, clinical signs of GVHD, and tissue pathology. See the online supplement for further details.
Tumor Necrosis Factor-α and Total Protein Measurement
Mice were killed and BAL was performed with 1 ml of phosphate-buffered saline containing 5 mM ethylenediaminetetraacetic acid. The levels of tumor necrosis factor-α and total protein in BAL fluid were measured by ELISA and BCA assay, respectively. See the online supplement for further details.
Quantitative PCR for MRV Genes
Total RNA was extracted from mouse tissues using Ambion TRIzol reagent (ThermoFisher) and genomic DNA was prepared from 100 μl mouse BAL fluid using a DNA blood kit (Qiagen). Information about PCR analyses for viral lytic genes and genomic abundance is provided in the online supplement.
Murine Histology
Whole lungs were inflated and fixed with 10% formalin followed by sectioning for hematoxylin and eosin staining as previously described (10). Images were taken on an Olympus BX-51 microscope by a DP-70 camera.
Statistical Analysis
We used a Cox proportional hazards regression model with a time-dependent covariate for FOVIs to estimate overall mortalities. We used stabilized inverse probability censored weighting methodology adjustments to Cox proportional hazards regression to analyze other time-to-event outcomes (25, 26). In multivariate modeling of time-to-events, forward selection was initially used to identify independently predictive demographic and clinical risk factors at the 0.05 significance level with exceptions in some of the analyses. Additional detail on statistical analysis of human data is provided in the online supplement. All analyses were done using R version 3.3.2 (R Foundation for Statistical Computing) (27).
For preclinical mouse studies, Student’s t tests were used to determine significance between two groups. When three groups or more were compared, one-way ANOVA was used with a Tukey multiple comparisons test to determine significance.
Results
Patient Clinical Characteristics and Demographics
Seven hundred thirty-eight patients undergoing allo-HCT at the University of Michigan Medical Center were analyzed. Clinical characteristics and demographics are reported in Table 1. The median follow-up was 121 weeks (95% confidence interval [CI], 93–157 wk), with a median patient age of 51 years (range, 7 mo to 73 yr).
Table 1.
Patient Clinical Characteristics and Demographics
| Characteristics | Total Allogeneic HCT Patients (n = 738) |
|---|---|
| Age at HCT, median (range), yr | 51 (0–73) |
| Follow-up time, median (95% CI), wk* | 121 (93–157) |
| Sex, n (%) | |
| M | 438 (59) |
| F | 300 (41) |
| Indication, n (%) | |
| Malignant | 623 (84) |
| Nonmalignant | 115 (16) |
| HLA disparity, n (%) | |
| Matched | 583 (79) |
| Mismatched | 155 (21) |
| Donor relationship, n (%) | |
| Related | 355 (48) |
| Unrelated | 383 (52) |
| Number of HCT, n (%) | |
| First | 652 (88) |
| Second/third | 86 (12) |
| Conditioning by irradiation, n (%) | |
| TBI-based | 185 (25) |
| Chemotherapy-based | 553 (75) |
| Conditioning by intensity, n (%) | |
| Myeloablative | 518 (70) |
| Nonmyeloablative | 220 (30) |
| Graft source, n (%) | |
| PB | 600 (81) |
| BM | 87 (12) |
| CB | 51 (7) |
Definition of abbreviations: BM = bone marrow; CB = umbilical cord blood; CI = confidence interval; HCT = hematopoietic cell transplantation; PB = peripheral blood; TBI = total body irradiation.
Values are indicated as the number (percentage), except age at HCT and follow-up time as specified.
Median follow-up time and the corresponding 95% CI was estimated by a Kaplan-Meier method.
Detection of Viral Infections
Viral infections were defined as isolation of viral proteins or nucleic acid from any body fluid or tissue specimen using viral cultures, qPCR, immunohistochemical analysis, or in situ hybridization. FOVI was defined by the presence of the first viral pathogen within 100 days after HCT.
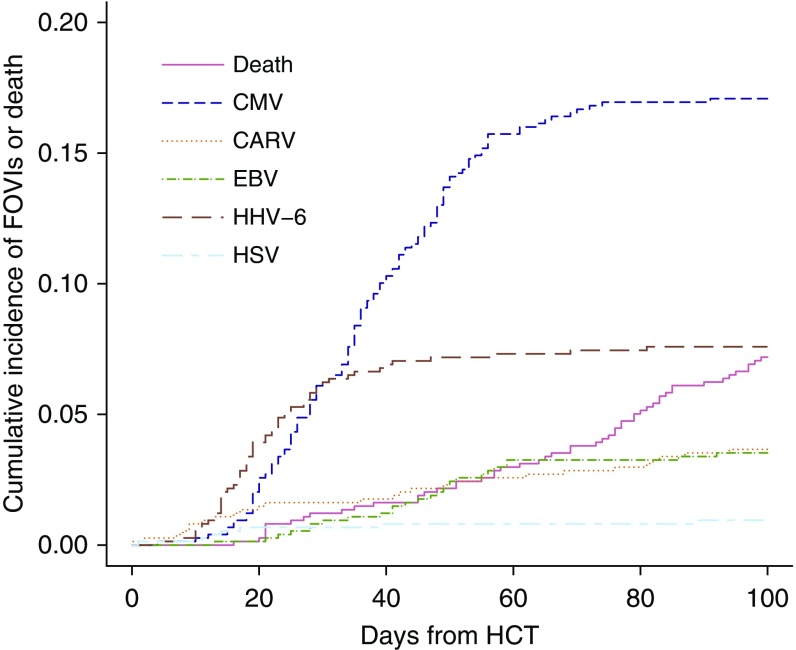
FOVIs were detected in 242 (32.8%) patients (Table 2), with CMV (126 patients) and HHV-6 (56 patients) (Table 2) being the most frequently observed viral pathogens. HHV-6 was detected earlier after HCT than CMV (Figure 1). The median time to first onset of HHV-6, CMV, or EBV among the patients with infection was 21 days (interquartile range [IQR], 14–29), 39 days (IQR, 28–54), or 55 days (IQR, 41–162). The risk factors for FOVIs with CMV, HHV-6, EBV, and CARV after HCT are reported in the online supplement (see Tables E1 and E2 in the online supplement).
Table 2.
First Viral Infection Detection during 100 Days after HCT (n = 738)
| Viral Positivity | Patients [n (% of Total)] | Sample Sources (n) |
|---|---|---|
| Any viral | 242 (32.8) | |
| CMV | 126 (17.1) | Plasma (126) |
| HHV-6 | 56 (7.6) | Plasma (55) |
| BAL fluid (1) | ||
| CARV | 27 (3.7) | Nasopharynx (12) |
| Feces (8) | ||
| Plasma (6) | ||
| BAL fluid (1) | ||
| EBV | 26 (3.5) | Plasma (15) |
| Whole blood (9) | ||
| Serum (1) | ||
| CSF (1) | ||
| HSV | 7 (0.9) | Oral mucosa (4) |
| Serum (3) |
Definition of abbreviations: CARV = community-acquired respiratory viruses; CMV = cytomegalovirus; CSF = cerebrospinal fluid; EBV = Epstein-Barr virus; HCT = hematopoietic cell transplantation; HHV-6 = human herpesvirus 6; HSV = herpes simplex virus.
Figure 1.
The cumulative incidence of first-onset viral infection or death after allogeneic hematopoietic cell transplantation at the University of Michigan Medical Center. The study cohort consisted of 738 hematopoietic cell transplantation recipients. Cytomegalovirus DNA testing was performed routinely. Epstein-Barr virus, community-acquired respiratory viruses, human herpesvirus 6, and herpes simplex virus were undertaken when clinically indicated. CARV = community-acquired respiratory viruses; CMV = cytomegalovirus; EBV = Epstein-Barr virus; FOVI = first-onset viral infection; HCT = hematopoietic cell transplantation; HHV-6 = human herpesvirus 6; HSV = herpes simplex virus.
Overall Survival, Relapse, Nonrelapse Mortality, and GVHD after FOVIs
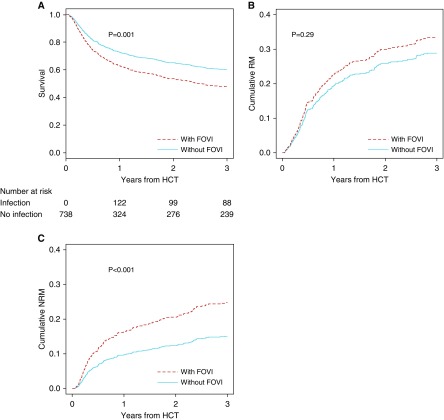
Overall survival was significantly lower in patients with FOVIs versus those without after adjustment for age, sex, time-dependent aGVHD, underlying diagnosis, HLA disparity, source of hematopoietic cells, donor relationship, and conditioning regimen (adjusted hazard ratio [aHR], 1.45; 95% CI, 1.16–1.82; P = 0.001) (Figure 2A). The cumulative incidence of relapse mortality in the FOVI group was not significantly higher than those patients without FOVI (aHR, 1.19; 95% CI, 0.86–1.65; P = 0.29) (Figure 2B). In contrast, a significant increase in NRM was seen for those with FOVIs (aHR, 1.74; 95% CI, 1.27–2.38; P < 0.001) (Figure 2C). Among the types of FOVIs, first infection with HHV-6 was significantly associated with increased NRM, and the association between first infection with EBV or CMV and NRM showed borderline significance (Figure E1). Thus, the association between FOVIs and mortality seems primarily driven by NRM rather than relapse of underlying malignancy.
Figure 2.
Overall survival, relapse, and nonrelapse mortality after hematopoietic cell transplantation (HCT) by first-onset viral infections (FOVIs). Viral infections within 100 days after HCT were modeled as time-dependent covariates. An individual is counted as infection free during at-risk periods before their first infection and only changes status to infected for their first infection type. The curves are adjusted for age, sex, time-dependent acute graft-versus-host disease, underlying diagnosis, HLA disparity, source of hematopoietic cells, donor relationship, and conditioning regimen. (A) Estimation of overall survival stratified by FOVIs. The number of patients at risk of death at the beginning of HCT, 1, 2, or 3 years after HCT for FOVI group were 0, 122, 99, and 88, respectively, and for non-FOVI group were 738, 324, and 276, respectively. (B) Inverse weighted cumulative incidence of relapse mortality stratified by FOVIs. (C) Inverse weighted cumulative incidence of nonrelapse mortality stratified by FOVIs. NRM = nonrelapse mortality; RM = relapse mortality.
Patients with FOVIs had a significantly higher cumulative incidence of subsequent grade II–IV aGVHD, but not chronic GVHD, than the group without prior FOVIs after adjustment for confounders (Figure E2 and Tables E3 and E4). Specifically, FOVI with CMV is significantly associated with the development of subsequent grade II–IV aGVHD after adjustment for confounders (Table E5). When we further adjust the year of HCT in our multivariable model, FOVI with HHV-6 was significantly associated with subsequent grade II–IV aGVHD (Table E6).
FOVIs Are Associated with Development of IPS in HCT Recipients
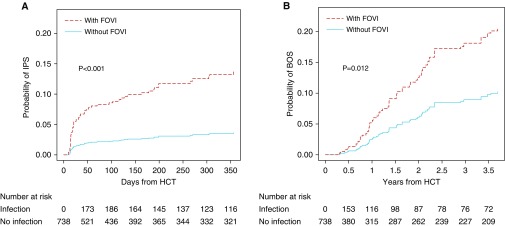
We next examined the relationship between FOVIs and the development of noninfectious pulmonary complications after HCT in our study cohort, in which 41 patients (5.5%) developed IPS. The clinical characteristics of patients with IPS are summarized in Table E7. For patients who had FOVIs and subsequently developed IPS, the median time from FOVI to onset of IPS was 15 days (IQR, 5–107 d) (Figure E3A).
In a univariate analysis, FOVIs with HHV6, EBV, HSV, HLA mismatched transplant status, younger age at transplant, and cord blood transplant were associated with the development of IPS (Table E8). After adjustment for age, HLA disparity, and source of hematopoietic cells, patients with an FOVI had a significantly higher cumulative incidence of subsequent IPS compared with patients without a FOVI (aHR, 3.97; 95% CI, 1.91–8.25; P < 0.001) (Figure 3A; Table E9). To determine the association between specific FOVI types and the development of IPS, we used multivariable Cox regression analyses to adjust for confounding effects. Only HHV-6 (aHR, 5.52; 95% CI, 1.61–18.96; P = 0.007), EBV (aHR, 9.21; 95% CI, 2.63–32.18; P = 0.001), and HSV (aHR, 11.0; 95% CI, 2.56–47.19; P = 0.001) remained as independent risk factors for the development of IPS (Table 3). The significance of these associations is not changed after adjustment for the year of HCT performed or the development of acute or chronic GVHD (Tables E10 and E11).
Figure 3.
Cumulative incidence of idiopathic pneumonia syndrome (IPS) and bronchiolitis obliterans syndrome (BOS) by first-onset viral infections (FOVIs). (A) Inverse-weighted cumulative incidence of IPS in days after hematopoietic cell transplantation (HCT) stratified by FOVIs (patients without FOVI, n = 496; patients with FOVIs, n = 242). Number of patients at risk of developing IPS at each time point is presented under the plot. (B) Inverse weighted cumulative incidence of BOS in years after HCT stratified by FOVIs. Number of patients at risk of developing BOS at each time point is presented under the plot.
Table 3.
Multivariable Inverse Weighted Analysis of Risk Factors for Time from HCT to IPS and BOS, Respectively
| Multivariable Analysis | IPS C-Index = 0.742 |
BOS C-Index = 0.661 |
||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | P Value | aHR | 95% CI | P Value | |
| First CMV vs. none* | 1.68 | 0.53–5.34 | 0.38 | 2.88 | 1.50–5.55 | 0.002 |
| First HHV-6 vs. none* | 5.52 | 1.61–18.96 | 0.007 | 2.20 | 0.81–5.98 | 0.12 |
| First EBV vs. none* | 9.21 | 2.63–32.18 | 0.001 | 1.60 | 0.35–7.39 | 0.55 |
| First HSV vs. none* | 11.00 | 2.56–47.19 | 0.001 | 4.98 | 0.78–31.82 | 0.09 |
| First CARV vs. none* | 3.17 | 0.73–13.66 | 0.12 | —† | —† | —† |
| Age, 10 yr | 0.90 | 0.77–1.06 | 0.20 | 1.01 | 0.86–1.18 | 0.91 |
| HLA mismatched | 1.41 | 0.60–3.33 | 0.44 | 1.69 | 0.85–3.33 | 0.13 |
| CBT | 0.92 | 0.23–3.75 | 0.91 | 0.51 | 0.12–2.10 | 0.35 |
Definition of abbreviations: aHR = adjusted hazard ratio; BOS = bronchiolitis obliterans syndrome; CARV = community-acquired respiratory viruses; CBT = umbilical cord blood transplant; CI = confidence interval; C-index = concordance index; CMV = cyclomegalovirus; EBV = Epstein-Barr virus; HCT = hematopoietic cell transplantation; HHV-6 = human herpesvirus 6; HSV = herpes simplex virus; IPS = idiopathic pneumonia syndrome.
First-onset viral infection status is a time-dependent categorical predictor. The additional three variables were forward selected in an inverse probability weighted Cox regression model for at least one of pulmonary complications.
Infections modeled with time-dependent covariates through the first 100 days.
No CARV infection up to the first 100 days after HCT in patients who developed BOS.
FOVIs Are Associated with Development of BOS in HCT Recipients
Among our allo-HCT cohort, 49 patients (6.6%) developed BOS over follow-up. The clinical characteristics of patients with BOS are summarized in Table E7. The duration from viral infection to BOS was evenly distributed over the first 2 years of follow-up (Figure E3B). For patients who had FOVIs followed by BOS, the median time from FOVI to onset of BOS was 412 days (IQR, 149–699 d). Patients with a FOVI had significantly higher cumulative incidence of BOS than patients without a FOVI (aHR, 2.13; 95% CI, 1.19–3.85; P = 0.012) (Figure 3B and Table E9) after adjustment for age, HLA disparity, and source of hematopoietic cells. Both univariate and multivariable analyses identified CMV as the only significant risk factor for the development of BOS after HCT (aHR, 2.88; 95% CI, 1.50–5.55; P = 0.002) (Tables 3 and E3). The significance of the association is not changed after adjustment for the year of HCT performed or the development of acute or chronic GVHD (Tables E10 and E11).
Mouse Model of Post-BMT MRV Reactivation
We next sought to establish an allogeneic BMT mouse model using a murine herpesvirus to test whether reactivation of herpesvirus can cause subsequent organ complications after HCT. A recent study has reported that MRV is a mouse homolog of HHV-6/HHV-7 based on their similarities in viral genomic sequence and viral tropism (23). MRV infection in BALB/c mice within 24 hours after birth via intraperitoneal injection causes thymic necrosis and depletion of CD4+ and CD4+CD8+ T lymphocytes at 10 days after infection (Figure E4), but the infected thymi are recovered at 6 weeks after infection (28).
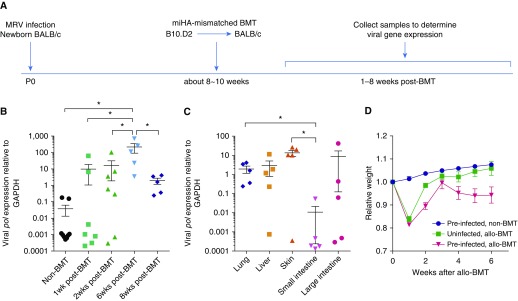
To closely mimic HHV-6 infections in humans and minor histocompatibility antigen (miHA)-mismatched allo-HCT clinical scenarios, we infected BALB/c (H2d) newborn mice with MRV and allowed them to recover and establish latent infection before performing a miHA-mismatched BMT at 8–10 weeks of age. We conditioned the mice with total body irradiation before transplanting bone marrow cells and splenocytes isolated from miHA-mismatched B10.D2 (H2d) mice (Figure 4A). We found that some preinfected BMT mice expressed the lytic viral DNA polymerase gene, pol, in the lungs within 1–2 weeks after miHA-mismatched transplant, and by 6 weeks, all tested BMT mice had lytic infections in the lungs (Figure 4B). By 8 weeks after BMT, the expression levels of pol were significantly reduced in those preinfected mice, presumably because of the virus reentering the latent phase (Figure 4B). The expression levels and frequencies of pol seem to be different among various organs, with higher levels and frequencies noted in the lung and skin, but lower levels in small intestine at 8 weeks after miHA-mismatched BMT (Figure 4C). Preinfected bone marrow recipients lost more body weight at 2 weeks after miHA-mismatched BMT and again 4–6 weeks after BMT (Figure 4D, red line). No mice died during the course of study.
Figure 4.
Murine roseolovirus (MRV) can be reactivated by minor histocompatibility antigen (miHA)-mismatched bone marrow transplant (BMT). (A) Schematic of experimental timeline. Newborn BALB/c (H2d) mice were infected with MRV via intraperitoneal injection within 24 hours after birth. Mice were allowed to grow for 8–10 weeks to establish latent infection before receiving miHA-mismatched BMT by transplanting 5 × 106 BM cells and 5 × 106 splenocytes isolated from B10.D2 (H2d) mice. Lung tissues were collected at 8–10 weeks of age (non-BMT control animals) or 1–8 weeks after BMT. (B) Expression levels of viral lytic gene pol in lung tissue after miHA-mismatched BMT. Each symbol represents a unique mouse. Dot plots are presented with mean ± SEM. *P < 0.05. (C) Expression levels of pol in various organs at 8 weeks after BMT (n = 5). The expression levels of pol are normalized to the expression of host GAPDH. Each symbol represents a unique mouse. Dot plots are presented with mean ± SEM. *P < 0.05. (D) Percentage of weight change in mice after miHA-mismatched BMT. Data are representative of three replicate experiments with five mice per group. Error bars indicate SEM.
MRV after miHA-mismatched BMT Reactivation Causes IPS-like Pathology
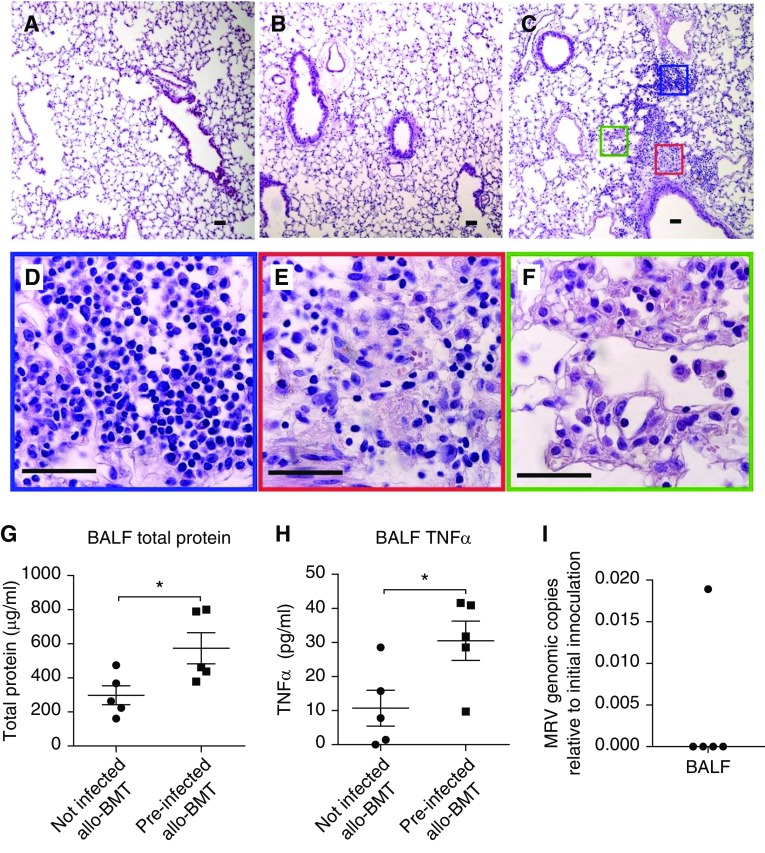
We assessed whether reactivation of MRV after miHA-mismatched BMT could cause IPS-like pathology. Late-onset IPS usually develops 3–6 weeks after HCT in humans (3). Interestingly, we noted a second wave of losing weight in preinfected miHA-mismatched BMT mice 4–6 weeks after transplant (Figure 4D); thus, we inspected lung histology at 6 weeks after BMT. Infection with MRV in neonates did not cause lung inflammation in adult mice, and miHA-mismatched BMT alone caused only mild airway edema (Figures 5A and 5B). However, lungs from preinfected miHA-mismatched BMT mice exhibited increased infiltration of inflammatory cells around both pulmonary vessels and bronchioles (Figure 5C). An infiltrate predominantly composed of monocytes and lymphocytes was seen in the artery adventitia (Figure 5D), whereas a more complex mixture of neutrophils, macrophages, monocytes, eosinophils, and lymphocytes within a fibrin matrix was found in airway submucosa (Figure 5E). Infiltration of neutrophils and macrophages in the interstitium and in alveolar spaces was associated with interstitial thickening (Figure 5F).
Figure 5.
Murine roseolovirus (MRV) reactivation by minor histocompatibility antigen–mismatched bone marrow transplant (BMT) causes idiopathic pneumonia syndrome–like pulmonary pathology. BALB/c (H2d) mice at 8–10 weeks old with or without preinfection with MRV as newborns underwent minor histocompatibility antigen–mismatched BMT procedure receiving 5 × 106 BM cells and 5 × 106 splenocytes isolated from B10.D2 (H2d) mice as described in Figure 4. (A–C) Lung histology (hematoxylin and eosin staining) in age-matched preinfected non-BMT mice (A), uninfected mice 6 weeks after BMT (B), and preinfected mice 6 weeks after BMT (C). (D–F) High magnification of the blue box in C showing an infiltrate predominantly composed of monocytes and lymphocytes in artery adventitia (D); the red box in C showing a mixed infiltrate of neutrophils, macrophages, eosinophils, and lymphocytes within a fibrin matrix in airway submucosa (E); and the green box in C showing a mixed infiltrate of neutrophils and macrophages in widened interstitium and in alveolar spaces (F). Scale bars, 50 μm. Images represent those of at least five mice per group in each of two independent experiments. (G–I) BAL fluid was collected from uninfected or preinfected mice at 8 weeks after BMT (n = 5), and assayed for the levels of total protein (G), tumor necrosis factor-α (H), and MRV genome copies (I). Dot plots are presented with mean ± SEM. *P < 0.05. allo = allogeneic; BALF = BAL fluid; TNF = tumor necrosis factor.
Although signs of diffuse alveolar injury, such as hyaline membranes, were not observed in the lungs of preinfected miHA-mismatched BMT mice, we found significantly increased levels of total protein (Figure 5G) and tumor necrosis factor-α (Figure 5H) in their BAL fluid, which indicates lung damage in these mice and is consistent with IPS pathology. Interestingly, most of the preinfected miHA-mismatched BMT mice had undetectable levels of viral genomic DNA in their BAL fluid by 8 weeks after BMT (Figure 5I), consistent with an occult infection defined as the “absence” of a pathogen in BAL fluid of patients with IPS (3, 8). Thus, reactivation of MRV is able to induce a moderate IPS-like phenotype in miHA-mismatched BMT mice.
MRV after miHA-mismatched BMT Reactivation Exacerbates aGVHD
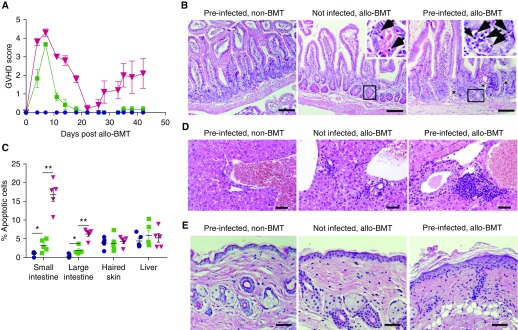
The B10.D2 → BALB/c miHA-mismatched BMT has been previously reported to cause sclerodermatous chronic GVHD in the recipients (24). We asked whether MRV reactivation after BMT can worsen signs of aGVHD as indicated in this and previous studies (29). Preinfected bone marrow recipients lost more body weight and developed higher aGVHD scores at 2 weeks after miHA-mismatched BMT and again at 4–6 weeks after BMT (Figures 4D and 6A, red lines). The small intestine histology of preinfected miHA-mismatched BMT recipients showed features of more severe aGVHD at 6 weeks after BMT than those of uninfected recipients, as evidenced by increased apoptotic cells in the crypt epithelium and infiltration of immune cells compared with BMT alone control animals (Figures 6B and 6C). Preinfected miHA-mismatched BMT mice also showed increased immune cell infiltration in the bile ducts, portal triads, and dermal layer (Figures 6D and 6E), and increased thickening of the epidermal layer and skin scaling (Figure 6E). Thus, latent MRV reactivation induced by miHA-mismatched BMT can exacerbate hallmark signs of gut, liver, and skin aGVHD.
Figure 6.
Murine roseolovirus reactivation exacerbates acute graft-versus-host disease (GVHD) after minor histocompatibility antigen–mismatched bone marrow transplant (BMT). Newborn BALB/c (H2d) mice were infected with murine roseolovirus via intraperitoneal injection within 24 hours after birth. Mice were then aged for 8–10 weeks to establish latent infection before receiving minor histocompatibility antigen–mismatched BMT by transplanting 5 × 106 BM cells and 5 × 106 splenocytes isolated from B10.D2 (H2d) mice. (A) Acute GVHD scores after minor histocompatibility antigen–mismatched BMT. Data representative of three replicate experiments with five mice per group. Error bars indicate SEM. Acute GVHD scores are based on weight loss, posture, activity status, fur texture, and skin integrity. (B, D, and E) Hematoxylin and eosin staining of tissue sections of small intestine (B), liver (D), or skin (E) at 6 weeks after BMT. Arrows point to apoptotic cells, and asterisks indicate areas of immune cell infiltration. Images represent those of at least five mice per group in each of two independent experiments. Scale bars, 50 μm. (C) Percentage of apoptotic cells in crypts of small or large intestines, skin epidermis cells, or hepatocytes. Percentage of apoptotic cells (mean ± SEM) of each cell type was estimated by the number of apoptotic cells divided by the total number of that cell type under a ×400 microscope field in five independent hematoxylin and eosin–stained slides. *P < 0.05 and **P < 0.01. allo = allogeneic.
Discussion
In summary, we report that first herpesviral infection is associated with an increased risk of developing post-HCT noninfectious lung injuries including IPS and BOS. FOVI with HHV-6 or EBV significantly associates with IPS, whereas FOVI with CMV significantly associates with BOS and aGVHD. After adjustment for year of HCT and other confounders, FOVI with HHV-6 was also significantly associated with aGVHD. We herein report for the first time a novel murine model of herpesvirus reactivation after BMT, demonstrating the casual association of viral reactivation and organ injuries and providing biologic plausibility for our human study observations.
Occult pathogens including HHV-6, CMV, EBV, and HSV were found in 56.5% of BAL fluid samples from patients previously diagnosed with IPS, suggesting a role for herpesvirus in the development of IPS (8). Post-HCT infection with CARV was also previously found to be a risk factor for IPS and BOS after allo-HCT (30, 31). These studies suggest that injuries to the lungs by virus may predispose HCT recipients to the development of IPS and BOS through dysregulated host and alloimmune responses (31). Our study supports a role for herpesvirus in the pathogenesis of IPS through clinical and preclinical observations. We did not see a significant association between FOVI with CARV and IPS or BOS, which may be attributed to our study only reporting the incidence of CARV infection as a first viral infection with clinical indications in an HCT recipient. As a result, the incidence of FOVI with CARV in this study is likely lower than previously reported for total early CARV infections in HCT recipients (31, 32).
HHV-6 is the most commonly reactivated virus that associated with the development of IPS in this study. HHV-6 collectively includes two species, HHV-6B and HHV-6A, and the combined seroprevalence of HHV-6B and HHV-6A is more than 90% in adults (33). The incidence of HHV-6 reactivation is reported at about 30–60% in HCT recipients (34–37). We have recorded a relatively low incidence of HHV-6 reactivations (7.6%) in this study, because we only reported the incidence of HHV-6 infection as a first viral infection with clinical indications. Nevertheless, like previous studies, our study has also determined cord blood transplant as a major risk factor for HHV-6 reactivation (Table E2) (36, 38, 39).
It has been established that HHV-6 reactivation can cause encephalitis (40), but causal associations between HHV-6 reactivation and other important outcomes, such as pneumonitis (8, 41, 42) and aGVHD (20, 29, 34, 37, 40, 43–46), are difficult to establish because of high rates of asymptomatic and transient HHV-6 reactivation, which may be ignored or undetected in end organs (47). Recent identification of MRV as a mouse homolog of HHV-6/HHV-7 (23) makes it possible to establish a small animal model for HHV-6 infection and reactivation with its natural pathogen. We have demonstrated that MRV can be reactivated by a miHA-mismatched BMT and the viral reactivation can cause moderate IPS-like lung injuries and aggravate signs of aGVHD.
FOVIs with EBV after HCT are also associated with the development of IPS. We previously reported that infection with a mouse homolog of EBV in engrafted syngeneic BMT mice causes severe pneumonitis and fibrosis, supporting a role for EBV infection in immunopathogenesis of IPS (10). Note that IPS-like lung injuries can also be induced by allo-BMT without viral infection (3). For example, complete major histocompatibility complex–mismatched allo-BMT mouse models best mimic acute, early-onset IPS (48), whereas isolated major histocompatibility complex I or II mismatch, and some of the miHA-mismatched allo-BMT models can reproduce the IPS pathology developed 3–6 weeks after HCT in humans (49).
In contrast to infections with HHV-6 or EBV, FOVIs with CMV do not increase the risk of the development of IPS after HCT, but increase the odds of BOS, which is usually diagnosed more than a year after HCT. An interesting difference between the CMV infection and HHV-6 or EBV infection is that whereas HHV-6 or EBV viremia is more likely to be transient, CMV viremia is often persistent and tissue invasive (47). As a consequence, CMV is often identified in end-organ diseases, such as pneumonia and enteritis. Current CMV preemptive therapy with antiviral drugs has significantly reduced CMV pneumonia, but once CMV pneumonia is developed after allo-HCT, death from CMV pneumonia still remains unacceptably high (50–52). Although preemptive therapy has dramatically reduced early CMV disease after allo-HCT, the incidence of late CMV infection and disease have increased (51). Furthermore, early CMV reactivation and prolonged antiviral therapy increase the odds of CMV recurrence as a late complication after HCT (53–55). CMV may contribute to the development of BOS by enhancing the dysregulated chronic inflammation during late recurrence. It is also possible that an altered immunologic tone, such as altered T-cell differentiation set by first CMV infection, manifests as BOS at a later time point. Note that in lung transplant recipients, it is generally accepted that in the antiviral treatment era, treated CMV pneumonia still increases the risk for BOS (56–58).
We also found that FOVIs with CMV are significantly associated with grade II–IV aGVHD. Previous studies on the relationship between CMV and aGVHD reported contradictory data (59–62). The discrepancies are possibly caused by the sensitivities of the assays for CMV surveillance among the studies. It seems that the less sensitive assays in the past, such as CMV culture or phosphoprotein 65 antigenemia, tend to link CMV infection with aGVHD (59, 60), whereas the recent highly sensitive real-time PCR assay coupled with preemptive therapy tend to dispute the association between CMV infection and aGVHD (61, 62). Because our study focused on CMV as a first infection but did not include CMV infections occurring after another infection, it suggests that the first episode of CMV infection may be the critical determinant in setting the development of aGVHD.
The mechanisms underlying the association between FOVI and pulmonary complications are not clear. Our previous preclinical studies of pneumonitis induced by MHV-68 after BMT suggested T-cell immunopathology (10, 63). MHV-68 viral infection–triggered immunopathologic type 17 responses that persisted even long after the virus reenters latency, and thus the subsequent pathology seems to be noninfectious. We suggest that many forms of noninfectious lung injury after HCT may actually represent “hit and run” insults by respiratory viruses. The MRV-miHA mismatched BMT model is the first animal model to allow such mechanistic investigations because attempts to reactivate MHV-68 after HCT have been unsuccessful. Our novel mouse model of MRV reactivation after allo-BMT provides opportunities to further investigate the mechanisms of multiorgan injuries caused by herpesviral reactivation after HCT.
This study included a large cohort of allo-HCT patients, used advanced statistical methods to adjust for multiple confounders and competing risks, and developed a novel animal allo-BMT model to interrogate possible causal associations of viral reactivation on pulmonary complications and aGVHD. However, it should be noted that this study has its limitations. First, the study is conducted in a single center with retrospective design. Second, besides routine monitoring for CMV status, the detection of the other viral infections was performed based on clinical indications. Thus, the number of reported viral reactivations may be underestimated because only cases that were symptomatic or suspected on clinical grounds were tested. Nevertheless, our results are in agreement with many published studies (20, 29, 36–38, 41, 43, 45, 47). Finally, although our cohort of allo-HCT patients is relatively large, the numbers with IPS and BOS remain small, and thus limits multivariate analyses related to IPS and BOS. In addition, analyses on the association of HSV and IPS were limited in size and statistical power.
In conclusion, our findings support a role for herpesviruses in the pathogenesis and progression of pulmonary complications and aGVHD after HCT. Among FOVIs, HHV-6 and EBV are associated with the development of IPS, whereas CMV is associated with the development of BOS and grade II–IV aGVHD. Taken together, our results demonstrate the crucial importance of identifying FOVIs after HCT and the potential of targeting FOVIs or their sequelae in the treatment of HCT-related organ injury.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Bone Marrow Transplantation Program at University of Michigan Medical Center for curating patients’ medical charts and the Unit for Laboratory Animal Medicine at the University of Michigan for technical support.
Footnotes
Supported by postdoctoral translational scholar grant MICHR 2 UL1TR000433 (X.Z.), HHV-6 Foundation Dharam Ablashi Research Fund Grant (X.Z.), and NIH grants HL127805 (B.B.M.) and K99HL139996 (D.N.O’D.).
Author Contributions: X.Z., G.A.Y., and B.B.M. designed the study. D.N.O’D. and G.A.Y. identified the cases. X.Z., D.N.O’D., M.X., S.M., and B.B.M. performed statistical analyses. X.Z., H.K.M., K.T., M.M.C., T.C.H., C.B., and K.S. reviewed medical charts. X.Z., P.R.C., M.M.C., C.A.W., S.J.P., and W.M.Y. designed and performed animal studies. X.Z. compiled data and wrote the initial manuscript. X.Z., D.N.O’D., G.A.Y., and B.B.M. wrote the manuscript. All other authors critically reviewed the manuscript drafts and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201809-1635OC on February 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lucena CM, Torres A, Rovira M, Marcos MA, de la Bellacasa JP, Sánchez M, et al. Pulmonary complications in hematopoietic SCT: a prospective study. Bone Marrow Transplant. 2014;49:1293–1299. doi: 10.1038/bmt.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170:22–48. doi: 10.1164/rccm.200309-1322SO. [DOI] [PubMed] [Google Scholar]
- 3.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, et al. American Thoracic Society Committee on Idiopathic Pneumonia Syndrome. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergeron A, Chevret S, Chagnon K, Godet C, Bergot E, Peffault de Latour R, et al. Budesonide/formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191:1242–1249. doi: 10.1164/rccm.201410-1818OC. [DOI] [PubMed] [Google Scholar]
- 6.Reid GE, Lynch JP, III, Weigt S, Sayah D, Belperio JA, Grim SA, et al. Herpesvirus respiratory infections in immunocompromised patients: epidemiology, management, and outcomes. Semin Respir Crit Care Med. 2016;37:603–630. doi: 10.1055/s-0036-1584793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185:273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 8.Seo S, Renaud C, Kuypers JM, Chiu CY, Huang ML, Samayoa E, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood. 2015;125:3789–3797. doi: 10.1182/blood-2014-12-617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Dwyer DN, Zhou X, Wilke CA, Xia M, Falkowski NR, Norman KC, et al. Lung dysbiosis, inflammation, and injury in hematopoietic cell transplantation. Am J Respir Crit Care Med. 2018;198:1312–1321. doi: 10.1164/rccm.201712-2456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, Loomis-King H, Gurczynski SJ, Wilke CA, Konopka KE, Ptaschinski C, et al. Bone marrow transplantation alters lung antigen-presenting cells to promote TH17 response and the development of pneumonitis and fibrosis following gammaherpesvirus infection. Mucosal Immunol. 2016;9:610–620. doi: 10.1038/mi.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domingo-Gonzalez R, Martínez-Colón GJ, Smith AJ, Smith CK, Ballinger MN, Xia M, et al. Inhibition of neutrophil extracellular trap formation after stem cell transplant by prostaglandin E2. Am J Respir Crit Care Med. 2016;193:186–197. doi: 10.1164/rccm.201501-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caddy SL, Wang M, Krishnamurthy P, Uttenthal B, Chandra A, Crawley C, et al. Characterization of innate immune viral sensors in patients following allogeneic hematopoietic stem cell transplantation. Innate Immun. 2018;24:112–121. doi: 10.1177/1753425918757898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coomes SM, Farmen S, Wilke CA, Laouar Y, Moore BB. Severe gammaherpesvirus-induced pneumonitis and fibrosis in syngeneic bone marrow transplant mice is related to effects of transforming growth factor-β. Am J Pathol. 2011;179:2382–2396. doi: 10.1016/j.ajpath.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauermann N, Burian J, von Garnier C, Dirnhofer S, Germano D, Schuett C, et al. Interferon-gamma regulates idiopathic pneumonia syndrome, a Th17+CD4+ T-cell-mediated graft-versus-host disease. Am J Respir Crit Care Med. 2008;178:379–388. doi: 10.1164/rccm.200711-1648OC. [DOI] [PubMed] [Google Scholar]
- 15.Varelias A, Gartlan KH, Kreijveld E, Olver SD, Lor M, Kuns RD, et al. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood. 2015;125:2435–2444. doi: 10.1182/blood-2014-07-590232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flamand L, Gosselin J, Stefanescu I, Ablashi D, Menezes J. Immunosuppressive effect of human herpesvirus 6 on T-cell functions: suppression of interleukin-2 synthesis and cell proliferation. Blood. 1995;85:1263–1271. [PubMed] [Google Scholar]
- 18.Gurczynski SJ, Zhou X, Flaherty M, Wilke CA, Moore BB. Bone marrow transplant-induced alterations in Notch signaling promote pathologic Th17 responses to γ-herpesvirus infection. Mucosal Immunol. 2018;11:881–893. doi: 10.1038/mi.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Döring M, Cabanillas Stanchi KM, Mezger M, Erbacher A, Feucht J, Pfeiffer M, et al. Cytokine serum levels during post-transplant adverse events in 61 pediatric patients after hematopoietic stem cell transplantation. BMC Cancer. 2015;15:607. doi: 10.1186/s12885-015-1616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki J, Numata A, Yamamoto E, Fujii E, Tanaka M, Kanamori H. Impact of human herpesvirus-6 reactivation on outcomes of allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:2017–2022. doi: 10.1016/j.bbmt.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, et al. Sixth European Conference on Infections in Leukemia; a joint venture of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation (EBMT-IDWP); the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer (EORTC-IDG); the International Immunocompromised Host Society (ICHS); the European Leukemia Net (ELN) Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–811. doi: 10.3324/haematol.2016.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Trulik K, Chadwick MM, Hoffman T, Bulte C, Bloye K, et al. Early post-transplant viral infections and the incidence of acute and chronic noninfectious pulmonary complications following hematopoietic stem cell transplantation (HSCT) Biol Blood Marrow Transplant 2017231–2.27865911 [Google Scholar]
- 23.Patel SJ, Zhao G, Penna VR, Park E, Lauron EJ, Harvey IB, et al. A murine herpesvirus closely related to ubiquitous human herpesviruses causes T-cell depletion. J Virol. 2017;91:e02463–16. doi: 10.1128/JVI.02463-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: cutaneous cytokines, chemokines, and immune cell activation. J Immunol. 2002;168:3088–3098. doi: 10.4049/jimmunol.168.6.3088. [DOI] [PubMed] [Google Scholar]
- 25.van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. J Stat Softw. 2011;43:1–23. [Google Scholar]
- 26.Robins JM. Marginal structural models versus structural nested models as tools for causal inference. New York, NY: Springer; 2000. pp. 95–133. [Google Scholar]
- 27.R Core Team. R: a language and environment for statistical computing. 3.3.2 ed. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 28.Rowe WP, Capps WI. A new mouse virus causing necrosis of the thymus in newborn mice. J Exp Med. 1961;113:831–844. doi: 10.1084/jem.113.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan TL, Carlin K, Ljungman P, Politikos I, Boussiotis V, Boeckh M, et al. Human herpesvirus-6B reactivation is a risk factor for grades II to IV acute graft-versus-host disease after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2018;24:2324–2336. doi: 10.1016/j.bbmt.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant. 2010;16:782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 32.Seo S, Xie H, Leisenring WM, Kuypers JM, Sahoo FT, Goyal S, et al. Risk factors for parainfluenza virus lower respiratory tract disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25:163–171. doi: 10.1016/j.bbmt.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxinger C, Polesky H, Eby N, Grufferman S, Murphy R, Tegtmeir G, et al. Antibody reactivity with HBLV (HHV-6) in U.S. populations. J Virol Methods. 1988;21:199–208. doi: 10.1016/0166-0934(88)90066-3. [DOI] [PubMed] [Google Scholar]
- 34.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:932–940. doi: 10.1086/428060. [DOI] [PubMed] [Google Scholar]
- 35.Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. 2015;28:313–335. doi: 10.1128/CMR.00122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inazawa N, Hori T, Hatakeyama N, Yamamoto M, Yoto Y, Nojima M, et al. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol. 2015;87:1427–1435. doi: 10.1002/jmv.24161. [DOI] [PubMed] [Google Scholar]
- 37.Verhoeven DH, Claas EC, Jol-van der Zijde CM, Thijssen JC, Lankester AC, Bredius RG, et al. Reactivation of human herpes virus-6 after pediatric stem cell transplantation: risk factors, onset, clinical symptoms and association with severity of acute graft-versus-host disease. Pediatr Infect Dis J. 2015;34:1118–1127. doi: 10.1097/INF.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 38.Chevallier P, Hebia-Fellah I, Planche L, Guillaume T, Bressolette-Bodin C, Coste-Burel M, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant. 2010;45:1204–1211. doi: 10.1038/bmt.2009.326. [DOI] [PubMed] [Google Scholar]
- 39.Jeulin H, Agrinier N, Guery M, Salmon A, Clément L, Bordigoni P, et al. Human herpesvirus 6 infection after allogeneic stem cell transplantation: incidence, outcome, and factors associated with HHV-6 reactivation. Transplantation. 2013;95:1292–1298. doi: 10.1097/TP.0b013e318289958b. [DOI] [PubMed] [Google Scholar]
- 40.Zerr DM. Human herpesvirus 6 (HHV-6) disease in the setting of transplantation. Curr Opin Infect Dis. 2012;25:438–444. doi: 10.1097/QCO.0b013e3283553362. [DOI] [PubMed] [Google Scholar]
- 41.Cone RW, Hackman RC, Huang ML, Bowden RA, Meyers JD, Metcalf M, et al. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N Engl J Med. 1993;329:156–161. doi: 10.1056/NEJM199307153290302. [DOI] [PubMed] [Google Scholar]
- 42.Carrigan DR, Drobyski WR, Russler SK, Tapper MA, Knox KK, Ash RC. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991;338:147–149. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- 43.Dulery R, Salleron J, Dewilde A, Rossignol J, Boyle EM, Gay J, et al. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol Blood Marrow Transplant. 2012;18:1080–1089. doi: 10.1016/j.bbmt.2011.12.579. [DOI] [PubMed] [Google Scholar]
- 44.Pichereau C, Desseaux K, Janin A, Scieux C, Peffault de Latour R, Xhaard A, et al. The complex relationship between human herpesvirus 6 and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:141–144. doi: 10.1016/j.bbmt.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Cirrone F, Ippoliti C, Wang H, Zhou XK, Gergis U, Mayer S, et al. Early human herpes virus type 6 reactivation in umbilical cord blood allogeneic stem cell transplantation. Leuk Lymphoma. 2016;57:2555–2559. doi: 10.3109/10428194.2016.1157873. [DOI] [PubMed] [Google Scholar]
- 46.Admiraal R, de Koning CCH, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017;140:1643–1650. doi: 10.1016/j.jaci.2016.12.992. [DOI] [PubMed] [Google Scholar]
- 47.Hill JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, et al. Kinetics of double-stranded DNA viremia after allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2018;66:368–375. doi: 10.1093/cid/cix804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panoskaltsis-Mortari A, Taylor PA, Yaeger TM, Wangensteen OD, Bitterman PB, Ingbar DH, et al. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogeneic recipients are due to donor T cell infusion and potentiated by cyclophosphamide. J Clin Invest. 1997;100:1015–1027. doi: 10.1172/JCI119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yousem SA. The histological spectrum of pulmonary graft-versus-host disease in bone marrow transplant recipients. Hum Pathol. 1995;26:668–675. doi: 10.1016/0046-8177(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 50.Erard V, Guthrie KA, Seo S, Smith J, Huang M, Chien J, et al. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin Infect Dis. 2015;61:31–39. doi: 10.1093/cid/civ215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 52.Ison MG, Fishman JA. Cytomegalovirus pneumonia in transplant recipients. Clin Chest Med. 2005;26:691–705. doi: 10.1016/j.ccm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 54.Einsele H, Hebart H, Kauffmann-Schneider C, Sinzger C, Jahn G, Bader P, et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000;25:757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 55.Ozdemir E, Saliba RM, Champlin RE, Couriel DR, Giralt SA, de Lima M, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40:125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 56.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010;181:1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas L, Hachem R. Bronchiolitis obliterans syndrome (BOS) following lung transplant. Am J Respir Crit Care Med. 2016;193:19–20. doi: 10.1164/rccm.19311P19. [DOI] [PubMed] [Google Scholar]
- 58.Paraskeva M, Bailey M, Levvey BJ, Griffiths AP, Kotsimbos TC, Williams TP, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11:2190–2196. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 59.Miller W, Flynn P, McCullough J, Balfour HH, Jr, Goldman A, Haake R, et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood. 1986;67:1162–1167. [PubMed] [Google Scholar]
- 60.Cantoni N, Hirsch HH, Khanna N, Gerull S, Buser A, Bucher C, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:1309–1314. doi: 10.1016/j.bbmt.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127:2427–2438. doi: 10.1182/blood-2015-11-679639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giménez E, Solano C, Piñana JL, Amat P, Vinuesa V, Navarro D. Re-examining the relationship between active cytomegalovirus (CMV) infection and acute graft-versus-host disease in allogeneic stem cell transplant recipients in the era of real-time PCR CMV assays. Transpl Int. 2016;29:126–128. doi: 10.1111/tri.12689. [DOI] [PubMed] [Google Scholar]
- 63.Coomes SM, Wilke CA, Moore TA, Moore BB. Induction of TGF-beta 1, not regulatory T cells, impairs antiviral immunity in the lung following bone marrow transplant. J Immunol. 2010;184:5130–5140. doi: 10.4049/jimmunol.0901871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.