From the Authors:
We read with great interest the letter by Stanislav Tatkov and thank him for his interest in our work. Dr. Tatkov’s thoughts are interesting, and the figure he provides is insightful.
The figure shows that two distinct combinations of respiratory rate and FiO2 (which may reflect two different clinical situations in terms of disease severity)—a respiratory rate of 20 with FiO2 of 0.8, and a respiratory rate of 40 with FiO2 of 0.5—provide the same ROX index. The figure further shows that the ROX index is unlikely to drop below 4.88 with FiO2 up to 0.5. Although the construction of the curves is unquestionable, the combination Dr. Tatkov highlights is more than unlikely. It is indeed quite exceptional to encounter a patient with a respiratory rate of 40 breaths/min 12 hours after admission to an ICU for acute respiratory failure (which is the time at which the ROX yielded the best value). For example, in the FLORALI (Clinical Effect of the Association of Noninvasive Ventilation and High Flow Nasal Oxygen Therapy in Resuscitation of Patients with Acute Lung Injury) study, the mean respiratory rate of patients under high-flow nasal cannula (HFNC) treatment at 12 hours was only 27 breaths/min. In addition, only 6.5% of the patients had a respiratory rate ≥40 (minimum, 40; maximum, 44). Similarly, in the ROX validation cohort, only one patient had a respiratory rate of 40 breaths/min at 12 hours of HFNC, and the mean respiratory rate at this time point was 24 breaths/min. The second component of the combination is the FiO2. Once again, it is rare to encounter a patient with FiO2 below 0.5 within the first 12 hours of treatment. This observation is supported by solid data. For example, in the validation cohort, only 5.2%, 9.2%, and 21.3% of the patients after HFNC onset needed a FiO2 < 0.5 after 2 hours, 6 hours, and 12 hours of treatment, respectively (1). Even lower percentages were observed in the FLORALI cohort: 3.9%, 7.1%, and 15.8% at 1 hours, 6 hours, and 12 hours, respectively (2).
The second point raised by Dr. Tatkov is the fact that the ROX index would get under 4.88 when the FiO2 was >0.8 for respiratory rates of ≥20 breaths/min. It is our opinion that the initial settings should favor high levels of FiO2 in patients with more severe disease. However, most of the patients who needed FiO2 > 0.8 presented respiratory rates of ≥20 breaths/min (83.1%, 79.6%, and 85.7% after 2 hours, 6 hours, and 12 hours of treatment in the validation cohort). It should also be noted that the ROX index measured after 12 hours of treatment predicted better HFNC outcomes than the FiO2 value (area under the receiver operating characteristic curve [AUROC], 0.752 [0.664–0.840] vs. 0.672 [0.574–0.770]; P = 0.008). Therefore, even though mathematically the figure is totally valid, from a practical standpoint, most patients may benefit from ROX estimation rather than direct use of the set FiO2.
On the other hand, we also provided other cutoff points for predicting HFNC failure: 2.85, 3.47, and 3.85 at 2 hours, 6 hours, and 12 hours after initiation of HFNC therapy. We suggest that the ROX index should be monitored over time. In fact, if the value of the ROX index is between 3.85 and 4.88 after 12 hours of treatment, the ROX index could be reassessed in 1 or 2 hours. Then, if the score has increased, the patient may have a greater likelihood of success. In contrast, if it has decreased, the patient may be more likely to fail. Finally, if the score is unchanged, another reassessment could be performed after one more hour.
We also thank Drs. Karim and Esquinas for their interest in our paper. We fully agree that oxygen saturation as measured by pulse oximetry (SpO2)/FiO2 does not always correlate well with PaO2/FiO2. In fact, when this correlation was assessed in patients with acute respiratory distress syndrome, measurements with SpO2 values > 97% were excluded from the analysis because the oxyhemoglobin dissociation curve is flat above these levels (3). Therefore, as the authors pointed out, theoretically, one might expect that the diagnostic accuracy of the ROX index using PaO2/FiO2 in predicting HFNC outcomes would be better than the one observed with SpO2/FiO2. However, two important issues should be taken into account. First, in the validation cohort, less than half of the patients had a PaO2 value available, which suggests that patients undergoing HFNC treatment are mainly monitored noninvasively using SpO2. Second, no significant differences were observed when the diagnostic accuracy of the ROX index constructed with PaO2/FiO2 was compared with the same index constructed with SpO2/FiO2 at any time point (P = 0.652, P = 0.122, and P = 0.407 at 2 hours, 6 hours, and 12 hours of HFNC treatment, respectively). Thus, according to these data, it seems that a more practical and feasible approach for measuring the ROX index would be to use the SpO2, as it will be available in all patients treated with HFNC at the bedside and it does not predict worse than the modified ROX index using the PaO2.
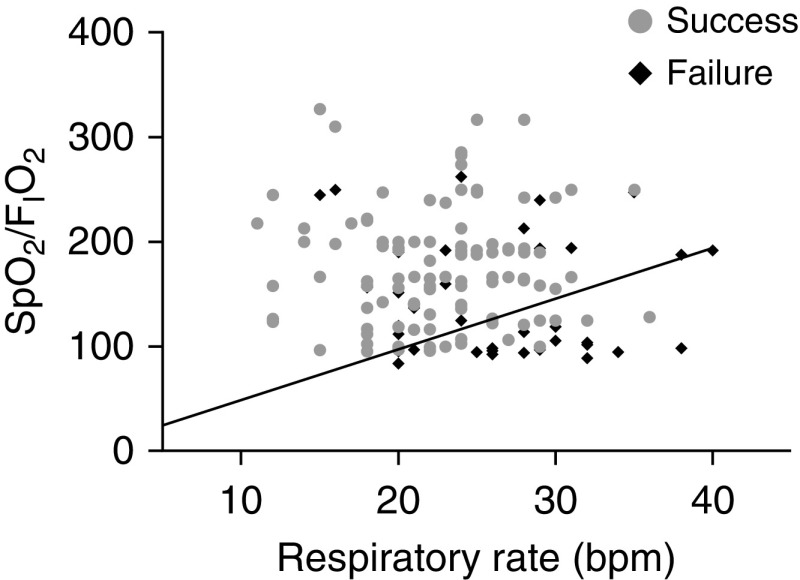
Finally, we thank Dr. Tulaimat for his interest in our manuscript. As he suggested, we provided the scatterplot with the respiratory rate on the x-axis and the SpO2/FiO2 on the y-axis, with the successes and failures in different colors and the isopleth with a slope of 4.88 marked in black (Figure 1). As the range of the respiratory rate is narrow, we have also tested the diagnostic accuracy of the ROX index with the respiratory rate squared. However, no differences in the AUROC values between the ROX index and the squared ROX index were observed (AUROC, 0.752 [0.664–0.840] vs. 0.753 [0.665–0.840]; P = 0.968). Therefore, as the use of a squared respiratory rate for the calculation of the ROX index does not provide any additional benefit in terms of prediction of HFNC success or failure, the use of the normal ROX index may be recommended.
Figure 1.
Scatterplot of respiratory rate and SpO2/FiO2 with the isopleth corresponding to a ROX index value of 4.88. SpO2 = oxygen saturation as measured by pulse oximetry.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201903-0571LE on March 21, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high flow therapy. Am J Respir Crit Care Med. doi: 10.1164/rccm.201803-0589OC. [online ahead of print] 21 Dec 2018; DOI: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 2.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 3.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]