Abstract
Rationale: CTSS (cathepsin S) is a cysteine protease that is observed at higher concentrations in BAL fluid and plasma of subjects with chronic obstructive pulmonary disease (COPD).
Objectives: To investigate whether CTSS is involved in the pathogenesis of cigarette smoke–induced COPD and determine whether targeting upstream signaling could prevent the disease.
Methods: CTSS expression was investigated in animal and human tissue and cell models of COPD. Ctss−/− mice were exposed to long-term cigarette smoke and forced oscillation and expiratory measurements were recorded. Animals were administered chemical modulators of PP2A (protein phosphatase 2A) activity.
Measurements and Main Results: Here we observed enhanced CTSS expression and activity in mouse lungs after exposure to cigarette smoke. Ctss−/− mice were resistant to cigarette smoke–induced inflammation, airway hyperresponsiveness, airspace enlargements, and loss of lung function. CTSS expression was negatively regulated by PP2A in human bronchial epithelial cells isolated from healthy nonsmokers and COPD donors and in monocyte-derived macrophages. Modulating PP2A expression or activity, with silencer siRNA or a chemical inhibitor or activator, during acute smoke exposure in mice altered inflammatory responses and CTSS expression and activity in the lung. Enhancement of PP2A activity prevented chronic smoke-induced COPD in mice.
Conclusions: Our study indicates that the decrease in PP2A activity that occurs in COPD contributes to elevated CTSS expression in the lungs and results in impaired lung function. Enhancing PP2A activity represents a feasible therapeutic approach to reduce CTSS activity and counter smoke-induced lung disease.
Keywords: cigarette smoke, cathepsin S, phosphatase, chronic obstructive pulmonary disease
At a Glance Commentary
Scientific Knowledge on the Subject
CTSS (cathepsin S), a lysosomal cysteine protease with elastase activity across a wide pH range, is elevated in chronic obstructive pulmonary disease (COPD) clinical samples, but its role in the disease process is unknown.
What This Study Adds to the Field
This study demonstrates that CTSS significantly contributes to cigarette smoke–induced loss of lung function in mice. CTSS expression is negatively regulated by PP2A (protein phosphatase 2A), but PP2A activity is inhibited by prolonged exposure to cigarette smoke. Chemical activation of PP2A reduces induction of CTSS expression in the lung and loss of lung function. Thus, these findings demonstrate a major role of CTSS and PP2A in smoke-induced COPD and identify a new potential therapeutic target to treat COPD. Finally, our results and approaches suggest that pharmacologic activation of important upstream signaling enzymes, such as phosphatases (PP2A), that negatively regulate key effectors associated with COPD progression, such as CTSS, may represent an alternative and possibly complementary approach to direct effector enzyme inhibition.
Lifelong cigarette smoke exposure decreases pulmonary function in susceptible smokers leading to the onset and progression of chronic obstructive pulmonary disease (COPD) (1). COPD is currently the third leading cause of death in the United States (2) and is a major global health problem. Exposure to cigarette smoke is the primary environmental factor associated with COPD formation in the developed world. Cellular responses triggered by cigarette smoke cause the release of inflammatory and proteolytic mediators that contribute to the pathogenesis of COPD (3). Although the role of proteases in COPD is well established, much of the research has focused on serine elastase and matrix metalloproteinases (MMP) (4, 5). In particular, the role of the CTS (cathepsin) family of enzymes, which are highly expressed in COPD, remains to be determined.
Several CTS are induced by smoke inhalation and are linked to emphysema development, including CTS E (6), G (7), K (8), and S (9). CTSS is a lysosomal cysteine protease that exerts elastase activity across a wide range of pH in alveolar macrophages, fibroblasts, and epithelial cells. CTSS activity is significantly elevated in the BAL fluid (BALF) (10) and plasma of patients with COPD (11). Altered CTSS levels are associated with a variety of pathologic conditions including cystic fibrosis, arthritis, cancer, and cardiovascular disease (12). CTSS has multiple functional roles, including major histocompatibility complex class II antigen presentation (13), and it can also cleave and inactivate key innate immunity proteins, such as β-defensins 2 and 3 (14), secretory leukocyte protease inhibitor (15), and lactoferrin (16). Unlike other CTS, CTSS has activity at a neutral pH (17) and increased levels of CTSS would have proteolytic activity in a healthy lung. Therefore, determining the stimuli that increase CTSS activity may provide key insights into the pathogenesis of lung diseases.
In view of the potential link between CTSS and COPD progression, we explored whether cigarette smoke alters CTSS signaling and determined whether CTSS impairs lung function and structure. Here we demonstrate that smoke exposure triggers robust Ctss expression and enhanced proteolytic activity in the lungs of mice. Using Ctss−/− mice, we determined that Ctss expression directly impacts cigarette smoke–induced changes in pulmonary physiology. One plausible mechanism for smoke induction of CTSS expression is inactivation of PP2A (protein phosphatase 2A), a phosphatase that regulates inflammatory and proteolytic responses (18–20). Chronic smoke exposure diminishes lung PP2A responses and coincides with airspace enlargement in response to smoke (19, 21). Inhibition of PP2A in mice before smoke exposure enhanced CTSS expression and lung inflammation. Equally, normalizing PP2A levels in mice or in human bronchial epithelial (HBE) cells isolated from subjects with COPD reduced CTSS expression and secretion. Chemical activation of PP2A prevents cigarette smoke–induced loss of lung function in mice and this study presents data showing PP2A regulation of CTSS that alters lung immune and proteolytic responses responsible for airway injury and function.
Methods
Detailed and expanded methodology is included in the online supplement.
Animal Models
Ctss−/− mice, on a C57BL/6J background, were exposed to cigarette smoke in a chamber (Teague Enterprises) for 4 hours daily, 5 d/wk at a total particulate matter concentration of 80–120 mg/m3 with the University of Kentucky reference research cigarettes 3R4F (Lexington). An additional group of wild-type mice were intraperitoneally injected with 2 μg/kg of okadaic acid (LC Labs) or intranasal delivery of 7.4 nmol PP2AA (mouse Ppp2r1a) silencer short, interfering RNA (Life Technologies). PP2A activity was enhanced in mice by oral administration of 50 mg/kg of a bioavailable SMAP (small molecule activator of PP2A) (see Reference 22) twice daily. All animal experiments were performed with approval from SUNY Downstate’s Institutional Animal Care and Use Committee and in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH and Institutional Animal Care and Use Committee guidelines and according to the Declaration of Helsinki conventions for the use and care of animals.
Forced Oscillation and Expiratory Measurements
Mice were anesthetized, tracheostomized, and connected via an endotracheal cannula to the SCIREQ flexiVent system (SCIREQ Inc.). Animals were paralyzed and pulmonary function measured (23). Airway responses to increasing doses of methacholine were assessed.
Histology and Lung Immune Cell Measurements
BALF and BALF cells were obtained from animals of each group and assessed by flow cytometry (24). Lungs underwent pressure fixation and morphometric analysis in accordance with the American Thoracic Society/European Respiratory Society issue statement (25). Mean linear intercept analysis was performed (26). Alveolar counts, boundary size, and ductal destructive measurements were performed (27). Sections from human bronchial tissue (28) and mouse lung tissue were stained for CTSS.
Cell Culture
HBE cells from nonsmokers and patients with COPD were isolated from human organ donor lungs rejected for transplant and fully redifferentiated at the air–liquid interface as previously described (29). Consent for research was obtained by the Life Alliance Organ Recovery Agency of the University of Miami. All consents were approved by the institutional review board and conformed to the Declaration of Helsinki. Cells were transfected with purified PP2A protein (Millipore) using Pro-Ject transfection reagent (Pierce) as per the manufacturers’ instructions (18). Cells were also transfected with PP2A or human antigen R (HuR)-specific siRNA. Alternatively, cell media were supplemented with 1 μM SMAP. Monocyte-derived macrophages were also examined for PP2A regulation of CTSS.
PP2A and CTSS Measurements
Immunoblots for ERK (extracellular signal–regulated kinase) phosphorylation (Thr202/Tyr204 and total ERK), the A subunit of PP2A and β-Actin (Cell Signaling Technologies), were performed. PP2A activity was determined using the Millipore PP2A activity assay (17–313; Millipore). Gene expression was performed by qPCR using Taqman probes (Applied Biosystems). CTSS concentrations were determined in BALF using a CTSS ELISA kit (R&D Systems) and immunoblots. CTSS activity was determined, as previously described (30).
Statistical Analyses
Data are expressed as mean ± SEM. Data were compared by Student’s t test (two-tailed) or by two-way ANOVA and Tukey post hoc test analysis, using Prism Software version 6.0h for Mac OS X (GraphPad).
Results
Cigarette Smoke Enhances CTS Expressions and CTSS Activity in Mouse Lungs
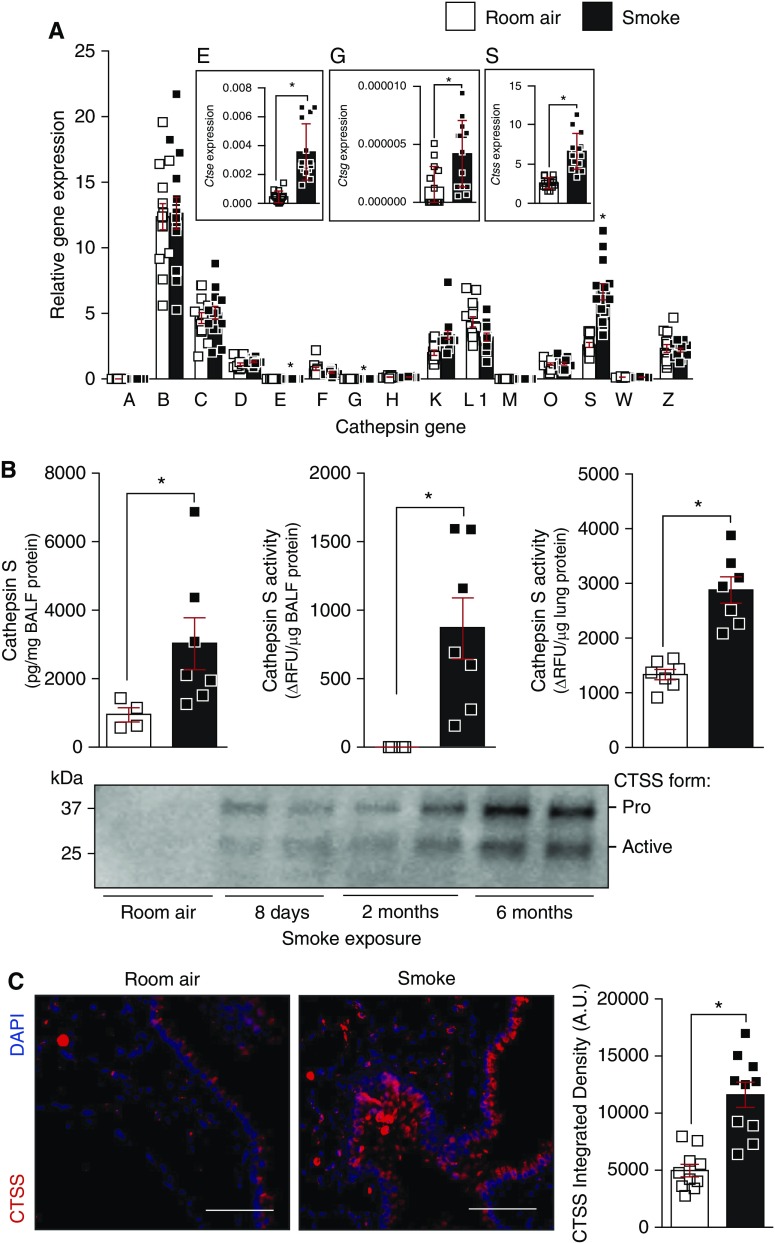
To investigate the impact of cigarette smoke on CTS expressions, C57BL/6J animals were exposed to cigarette smoke daily for several time points. Lung CTS expressions were determined by qPCR and CTSS was further analyzed by ELISA, substrate activity assays, and immunoblots. First, the gene expression of all CTS family members was examined in the lungs of mice exposed to smoke for 6 months, with gene expression relative to each other CTS gene. Ctse, Ctsg, and Ctss were significantly altered by smoke exposure in the lungs (Figure 1A; see Figure E1 in the online supplement). We primarily focused on CTSS because higher levels are observed in the BALF (10) and plasma of patients with COPD (11). Smoke exposure resulted in a significant increase in CTSS levels and activity in BALF (Figure 1B). Lung tissue analysis also confirmed that there is elevated CTSS activity within the tissue of smoke-exposed animals (Figure 1B). Western blot analysis confirmed elevated CTSS proteins levels in BALF from mice exposed to cigarette smoke, as early as 8 days postexposure and remained high throughout exposure (Figure 1B). Immunofluorescence evaluation demonstrated that CTSS is elevated in smoke-exposed mice and CTSS is located in immune and epithelial cells (Figure 1C). Therefore, smoke exposure elevates several CTS genes in the lungs.
Figure 1.
Smoke exposure enhances Ctss (cathepsin S) gene and protein expression in mice lungs. (A) CTS genes were quantified in C57BL/6J lung tissue, after 6 months exposure to room air and cigarette smoke, by qPCR and are shown as relative gene expression to each CTS gene. (B) CTSS protein and activity were quantified in the BAL fluid (BALF) of C57BL/6J mice, after 6 months exposure to room air and cigarette smoke, by ELISA and substrate activity assays, respectively. CTSS activity was also determined in total lung tissue protein. Immunoblots were also performed on BALF from C57BL/6J mice exposed to cigarette smoke for 0, 8 days, 2 months, or 6 months. The CTSS pro form is 37 kD, and the active form is 25 kD. Every lane represents an individual mouse. (C) Immunofluorescence was performed on lung tissue from room air– and smoke-exposed mice for CTSS and DAPI. Comparative images of the two mouse groups are presented here (scale bars, 150 μm). CTSS fluorescence intensity was determined and arbitrary units are shown here. Data are represented as mean ± SEM, with each measurement performed on 3 separate days from at least four animals/group. *P < 0.05, when comparing both treatments connected by a line, determined by Student’s t tests. AU = arbitrary units; RFU = relative fluorescence units.
Ctss Deficiency Prevents Smoke-induced Loss of Lung Function in Mice
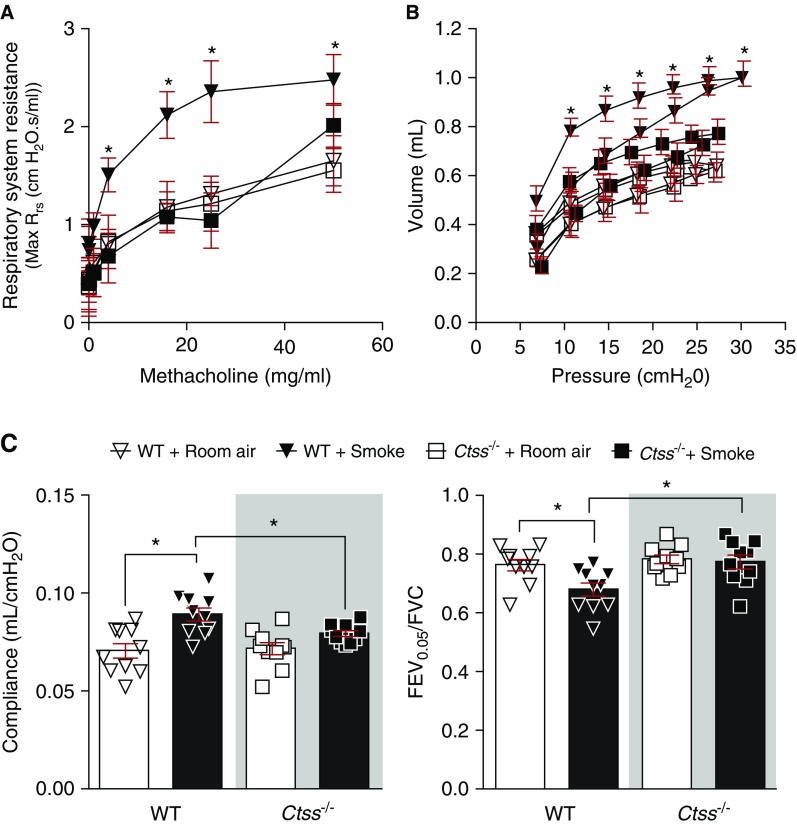
To determine whether Ctss expression impacted on airway resistance and lung function in mice, Ctss−/− mice and their wild-type littermates were exposed to cigarette smoke daily for 6 months. Airway resistance was assessed by methacholine challenge test. At every methacholine dose greater than or equal to 4 mg/ml, Ctss−/− mice exposed to cigarette smoke showed significantly lower respiratory resistance than wild-type mice exposed to cigarette smoke (Figure 2A). To examine how Ctss deficiency altered lung function in response to cigarette smoke, pressure–volume (PV) loops, compliance, and forced expiratory volume in 0.05 seconds (FEV0.05)/FVC were determined as previously described (23). A PV loop that shifts up and to the left, suggests an emphysematous lung as observed in wild-type mice exposed to smoke (Figure 2B). However, the PV loop from Ctss−/− mice exposed to smoke did not shift up. Lung compliance is a measure of the lung’s ability to stretch and expand, and FEV0.05/FVC is the proportion of the animal’s VC that is expired in the first one-twentieth of a second of forced expiration to the full VC. In mice, smoke inhalation typically enhances compliance and reduces FEV0.05/FVC levels (Figure 2C). Importantly, Ctss−/− mice developed less emphysematous changes after exposure to smoke compare with control animals, with reduced smoke-induced changes in lung function in all three parameters observed in these mice.
Figure 2.
Ctss (cathepsin S) deficiency prevents smoke-induced loss of lung function in mice. Wild-type and Ctss−/− mice were exposed to room air and cigarette smoke for 6 months. (A) Animals were challenged for airway resistance by a dose response of methacholine. (B and C) Negative pressure-driven forced expiratory and forced oscillation technique maneuvers were performed in all animal groups. (B) Pressure–volume loops and (C) compliance and forced expiratory volume in 0.05 seconds (FEV0.05)/FVC were determined in each animal. Data are represented as mean ± SEM, where n = 10 per group. *P < 0.05, when comparing both treatments connected by a line, determined by two-way ANOVA with Tukey post hoc test. WT = wild type.
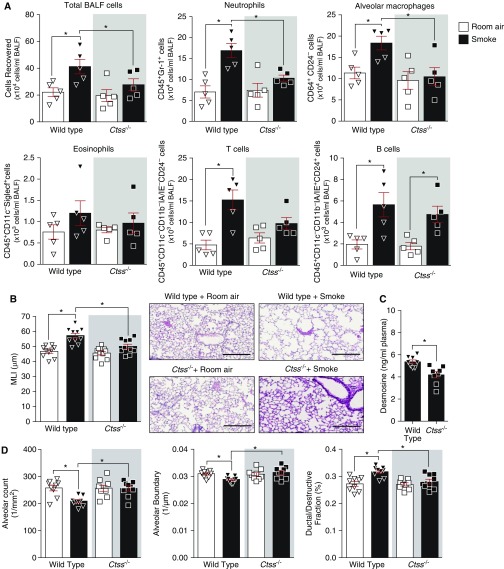
Immune cell infiltration is frequently observed in the lungs of patients with COPD (31). Total BALF immune cell counts were significantly increased in smoke-exposed wild-type mice but not in Ctss−/− mice (Figure 3A). Lung macrophages and neutrophils were reduced in Ctss−/− mice after smoke exposure compared with wild-type mice (Figure 3A). However, Ctss expression did not impact eosinophil, T-cell, or B-cell numbers in the lungs (Figure 3A). Smoke exposure did enhance T-cell and B-cell frequency in the airways, in a CTSS-independent manner (Figure 3A). Morphometric quantification demonstrated that the loss of Ctss expression prevented the increase in smoke-induced airspace enlargements, determined by mean linear intercept analysis (Figure 3B). Because CTSS is a potent elastase, elastin degradation was investigated by quantifying plasma levels of desmosine, an amino acid found in elastin. Smoke-exposed Ctss−/− mice had reduced desmosine in their plasma compared with wild-type mice (Figure 3C), indicating less elastin degradation. Parenchymal airspace profiling (27) was used to demonstrate that Ctss−/− mice had a higher alveolar count, reduced loss of alveolar boundary, and reduced ductal destruction compared with smoke-exposed wild-type mice (Figure 3D). Therefore, Ctss expression impacts on lung function, inflammation, elastin degradation, and lung tissue remodeling during chronic cigarette smoke exposure.
Figure 3.
Ctss (cathepsin S) deficiency prevents smoke-induced lung immune cell infiltration and airspace enlargements in mice. Wild-type and Ctss−/− mice were exposed to room air and cigarette smoke for 6 months. (A) BALF total immune cells, neutrophils, alveolar macrophages, eosinophils, T cells, and B cells were quantified in each group by flow cytometry. (B) Mean linear intercepts were measured in the lungs of the mice to assess air space size, and comparative histology images of the four mouse groups are presented here (scale bars, 40 μm). (C) Plasma desmosine levels were determined in smoke-exposed animals by ELISA. (D) Alveolar count, alveolar boundary, and ductal/destructive fractions were quantified in each animal by parenchymal airspace profiling. Data are represented as mean ± SEM, where n ≥ 5 per group. *P < 0.05, when comparing both treatments connected by a line, determined by two-way ANOVA with Tukey post hoc test or Student’s t test when comparing only two groups. BALF = BAL fluid; MLI = mean linear intercept.
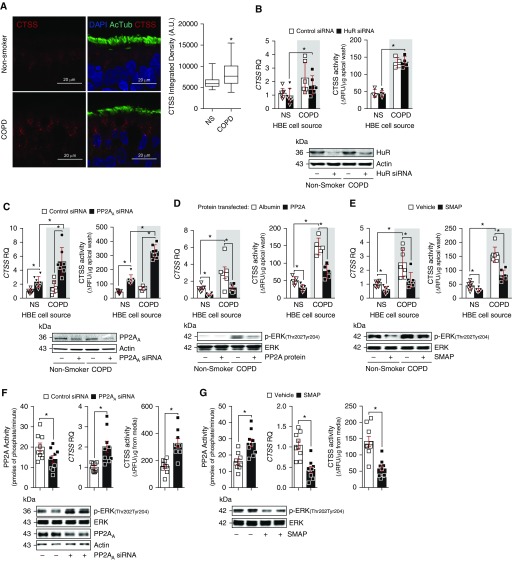
HBE Cells Isolated from Patients with COPD Express More CTSS Than Cells from Nonsmokers without COPD Partially Because of Altered PP2A Signaling
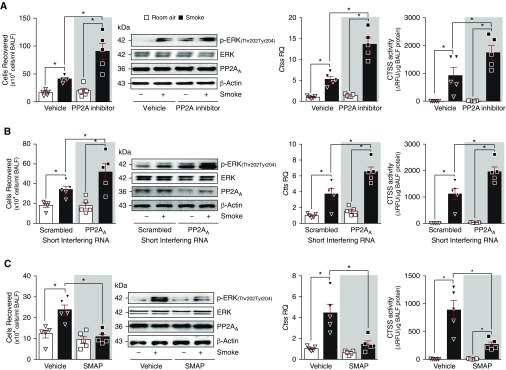
Previous work has identified airway epithelial cells as a source of pulmonary CTSS (32). Here, we further investigated CTSS levels in human bronchial tissue to confirm the presence of CTSS and elevated levels in COPD samples. Immunofluorescence analysis demonstrated that CTSS is expressed by bronchial tissue and is elevated in bronchial tissue from subjects with COPD (Figure 4A). To explore further the regulation of CTSS expression, we used HBE cells isolated from nonsmokers, and subjects with COPD. Cells isolated from subjects with COPD expressed and secreted more CTSS than cells from nonsmokers (Figures 4B–4E). The stabilizing RNA-binding protein HuR and the phosphatase, PP2A, have been linked to the regulation of CTSS expression in atherosclerosis (33) and Alzheimer disease/Down syndrome (34). Therefore, we examined CTSS gene expression and activity in HBE cells after modulation of HuR or PP2A signaling. Loss of HuR expression, with siRNA transfection, did not significantly alter CTSS signaling in HBE cells from nonsmokers or subjects with COPD (Figure 4B). However, transfecting siRNA specific for the A subunit of PP2A (PP2AA) (Figure 4C) or PP2A protein into HBE cells (Figure 4D) or the treatment of HBE cells with SMAP (Figure 4E) significantly altered CTSS expression and activity in both cell groups. Modulated PP2A signaling was confirmed by the regulation of ERK phosphorylation in these cells, with reduced ERK phosphorylation observed when PP2A is active (Figures 4D and 4E).
Figure 4.
Human bronchial epithelial (HBE) cells from patients with chronic obstructive pulmonary disease (COPD) have enhanced CTSS (cathepsin S) responses because of PP2A (protein phosphatase 2A) inhibition. (A) Bronchial tissue from nonsmokers and subjects with COPD were stained for CTSS (red), DAPI (blue), and acetylated tubulin (AcTub; green) and CTSS staining intensity was quantified. (B–E) HBE cells isolated from nonsmokers without COPD and individuals with COPD were transfected with scrambled or human antigen R (HuR) siRNA (B), scrambled or PP2AA siRNA (C), and albumin or active PP2A protein (D) or treated with SMAP (small molecule activator of PP2A) (E). Gene expression of CTSS was determined in all cells and CTSS activity quantified in media. Immunoblots were performed to confirm transfection efficiency for (B) HuR, (C and F) PP2AA, and (D–G) ERK (extracellular signal–regulated kinase) phosphorylation as a downstream readout of PP2A activity. (F and G) Macrophages derived from peripheral blood monocytes from nonsmokers were transfected with scrambled or PP2AA siRNA (F) or treated with SMAP (G). CTSS gene expression and PP2A and CTSS activities were determined, and immunoblots were performed. Data are represented as mean ± SEM, where each measurement was performed on 3 independent days and with n ≥ 3 subjects per group. *P < 0.05, when comparing both treatments connected by a line, determined by two-way ANOVA with Tukey post hoc test. AU = arbitrary units; NS = nonsmoker; RFU = relative fluorescence units; RQ = relative quantification.
Other cell types also express CTSS, such as macrophages (10). Human macrophages were derived from monocytes isolated from peripheral blood of nonsmokers. Similar to HBE cells, silencing PP2AA enhanced CTSS expression and activity in these macrophages (Figure 4F). Alternatively, SMAP treatment enhanced PP2A activity and reduced ERK and CTSS responses (Figure 4G). Therefore, loss of PP2A activity seems to result in enhanced CTSS expression and enzyme activity, possibly contributing to disease development.
Triggering PP2A Responses Prevents Smoke-induced CTSS Expression in Mice
To examine PP2A modulation and acute smoke effects on Ctss expression, wild-type mice were exposed to cigarette smoke daily for 3 days while they were administered daily injections of the phosphatase inhibitor okadaic acid, intranasal delivery of PP2AA silencer siRNA, or twice daily oral administration of SMAP (18, 19, 22). Mice treated with okadaic acid had significantly higher infiltrating immune cells into the lung after smoke exposure compared with control animals (Figure 5A). Okadaic acid treatment also enhanced lung ERK phosphorylation. In response to cigarette smoke, lung Ctss gene expression and BALF CTSS activity were significantly increased in okadaic acid–treated mice. Similarly, silencing PP2AA in the lungs enhanced inflammation, ERK phosphorylation, and CTSS responses in mice (Figure 5B). Alternatively, administration of SMAP to mice reduced smoke-induced immune cell infiltration, ERK phosphorylation, and CTSS expression and enzyme activity (Figure 5C).
Figure 5.
Modulating PP2A (protein phosphatase 2A) signaling alters acute smoke-induced lung Ctss expression. Mice were exposed to room air and cigarette smoke and either (A) daily injections of okadaic acid (2 μg/kg, i.p.), (B) intranasally administered scrambled or PP2AA silencer siRNA, or (C) two oral administrations of small molecule activator of PP2A daily for 3 days. Mice were killed 24 hours after the last exposure (n = 5 for each group). BAL fluid (BALF) cellularity levels were examined in each mouse. Immunoblots were performed for ERK (extracellular signal–regulated kinase) phosphorylation as a downstream readout of PP2A and total levels of PP2AA and β-actin were included as controls. Lung Ctss gene expression and BALF cathepsin S activity were examined by qPCR and substrate activity assays, respectively. *P < 0.05, when comparing both treatments connected by a line, determined by two-way ANOVA with Tukey post hoc test. CTSS = cathepsin S; RQ = relative quantification; SMAP = small molecule activator of PP2A.
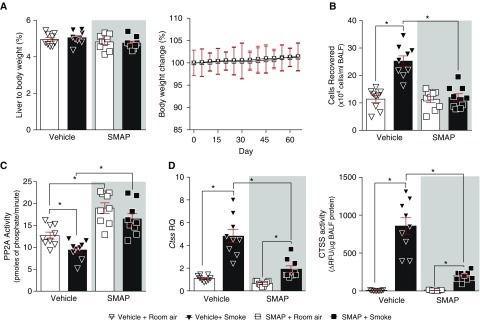
To determine the long-term effects of SMAP treatment on lung function, wild-type mice were administered SMAP twice daily during 2-month exposures to cigarette smoke. A/J mice were chosen because they are more sensitive to cigarette smoke–induced emphysema-like symptoms than other mouse backgrounds (35). Animal weight was recorded throughout the study and liver-to-body-weight ratio was measured at the end, as indicators of chemically induced changes to organs. No significant changes in weight were observed between groups (Figure 6A). Treatment with SMAP reduced smoke-induced immune cell infiltration into the airways (Figure 6B) and prevented smoke-induced inhibition of PP2A activity within the lungs (Figure 6C), which coincided with reduced lung Ctss gene and protein release into the airways during smoke exposure (Figure 6D). As expected, SMAP treatment was not able to completely block smoke-induced CTSS responses (Figure 6D). Nevertheless, it showed the importance of PP2A in regulating CTSS.
Figure 6.
Activating PP2A (protein phosphatase 2A) signaling alters long-term smoke-induced lung Ctss expression. A/J mice were exposed to room air and cigarette smoke and two oral administrations of small molecule activator of PP2A daily for 2 months. Mice were killed 24 hours after the last exposure (n = 9 for each group). (A) Liver-to-body-weight ratios and whole-body weights were recorded in each group. (B) BAL fluid (BALF) cellularity levels were examined in each mouse. (C) Lung PP2A activity, (D, left) lung Ctss gene expression, and (D, right) BALF CTSS activity were examined by substrate activity assays, qPCR, and substrate activity assays, respectively. *P < 0.05, when comparing both treatments connected by a line, determined by two-way ANOVA with Tukey post hoc test. CTSS = cathepsin S; RQ = relative quantification; SMAP = small molecule activator of PP2A.
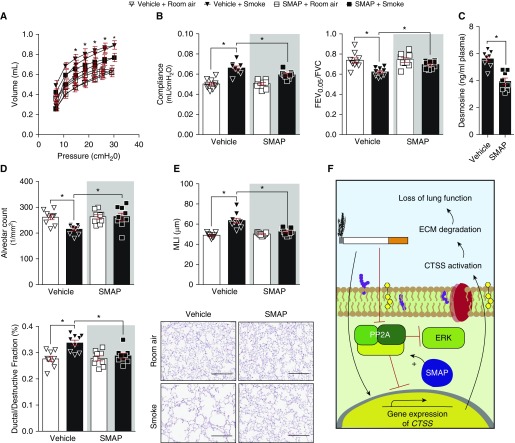
To examine whether SMAP treatment prevents the alteration of lung function in response to cigarette smoke, we examined PV loops, compliance, and FEV0.05/FVC. The PV loop analysis from SMAP-treated mice were lower compared with vehicle treated animals, when exposed to smoke (Figure 7A). SMAP-treated mice developed less emphysematous changes after exposure to smoke compared with control animals, with reduced smoke-induced changes in lung function in compliance and FEV0.05/FVC (Figure 7B). SMAP administration reduced desmosine levels in their plasma compared with vehicle-treated animals (Figure 7C). SMAP-treated animals had higher alveolar counts and reduced ductal destruction compared with smoke-exposed vehicle treated mice (Figure 7D). SMAP administration also prevented the increase in smoke-induced airspace enlargements, determined by mean linear intercept analysis (Figure 7E). These SMAP-mediated changes in CTSS levels were observed without changes in inflammation, such as IL-1β (36), IFN-γ (32), and tumor necrosis factor-α (see Figures E2A and E2B). Equally, SMAP administration did not impact smoke-induced Ctse or Ctsg (see Figure E2C). Therefore, SMAP treatment impacts on lung function, inflammation, elastin degradation, and lung tissue remodeling during chronic cigarette smoke exposure.
Figure 7.
Activating PP2A (protein phosphatase 2A) signaling prevents smoke-induced loss of lung function. Mice were exposed to room air and cigarette smoke and two oral administrations of small molecule activator of PP2A daily for 2 months. (A and B) Negative pressure-driven forced expiratory and forced oscillation technique maneuvers were performed in all animal groups. (A) Pressure–volume loops and (B) compliance and forced expiratory volume in 0.05 seconds (FEV0.05)/FVC were determined in each animal. (C) Plasma desmosine levels were assessed in smoke-exposed animals by ELISA. (D) Alveolar count and ductal/destructive fractions were quantified in each animal by parenchymal airspace profiling. (E) Mean linear intercepts were measured in the lungs of the mice to assess air space size, and comparative histology images of the four mouse groups are presented here (scale bars, 40 μm). Data are represented as mean ± SEM, where n = 9 per group. *P < 0.05, when comparing both treatments connected by a line, determined by two-way ANOVA with Tukey post hoc test. (F) Illustration of the possible signaling mechanism for PP2A regulation of CTSS (cathepsin S). Evidence presented in this study indicates that PP2A prevents signaling leading to CTSS gene expression, but after smoke exposure, CTSS expression is enhanced and the phosphatase activity of PP2A is diminished. This enhancement of CTSS directly impacts lung function. ECM = extracellular matrix; ERK = extracellular signal–regulated kinase; MLI = mean linear intercept; SMAP = small molecule activator of PP2A.
Discussion
Here, we establish that cigarette smoke enhances CTSS levels and activity, at least partly, because of a reduction in PP2A activity. Furthermore, CTSS contributes to cigarette smoke–induced COPD (Figure 7F). Ctss−/− mice were resistant to cigarette smoke–induced loss of lung function. Elevated levels of CTSS are observed in the lungs of mice from 8 days after the initiation of smoke inhalation and persisted throughout exposure. Expression of CTSS in the airway epithelium seems to be regulated by PP2A and not HuR. However, it is possible that HuR stabilizes CTSS mRNA in other cell types, as previously reported (33). Therefore, we propose that CTSS promotes the loss of lung function in COPD and also modulates pulmonary inflammatory responses. Either directly targeting CTSS activity or enhancing PP2A activity to decrease CTSS expression may represent a plausible means to counter COPD progression. Importantly, pharmacologic reactivation of the endogenous enzyme, PP2A, negatively regulates CTSS expression and prevented smoke-induced loss of lung function.
Neutrophil elastase and MMPs are the most frequent proteases implicated in the pathogenesis of COPD. SNPs in MMP1, MMP9 (37), and MMP12 (38) are associated with COPD. However, of the numerous protease inhibitory molecules tested, only one elastase inhibitor, Sivelestat (ONO-5046), is currently approved for the treatment of acute lung injury but not COPD because of toxicity issues (39). In recent years, CTSS has received more attention as a target for multiple diseases (12) and our data here outline the potential importance of inhibiting CTSS to reduce progression of COPD. Because CTSS activity is elevated in COPD patient samples (10, 11) and CTSS is activated at a neutral pH (17), increased levels of CTSS would have proteolytic activity in a healthy lung and may be a critical step in establishing early stage COPD. We and others have demonstrated that CTSE and CTSG are also enhanced by smoke exposure (6, 9). Both CTSE (6) and CTSG (7) play important roles in disease progression but seem not to be regulated at the transcriptional level by SMAP treatment. Our results establish the role of CTSS in early disease development and suggest that targeting this protease could be an effective therapeutic strategy in COPD.
We explored several mechanisms to determine how smoke exposure enhanced CTSS expression in the lungs. Inflammatory mediators can influence CTSS expression, with IFN-γ (32), tumor necrosis factor-α, and IL-1β (36) all linked to CTSS expression. However, we did not observe significant changes in these inflammatory mediators after SMAP administration but cannot rule out these or other unidentified factors regulating CTSS levels in COPD. We also explored HuR and PP2A as potential regulators of CTSS. Editing of RNA integrity is associated with the progression of multiple diseases, including cardiovascular disease (33). Recruitment of the stabilizing RNA-binding protein HuR, to the 3′ UTR of the CTSS transcript, enhances CTSS mRNA stability and expression (33). HuR expression did not impact CTSS expression in HBE cells in this study. However, we cannot completely rule out the possibility of HuR or other RNA stabilizing proteins playing a role on Ctss expression in smoke-exposed lungs. Cigarette smoke extract alters HuR expression to modulate SNAIL signaling in small airway epithelial cells (40). It is conceivable that HuR could exert similar effects to stabilize and enhance CTSS expression in the COPD lung.
Investigating how the mRNA stability of key COPD-associated genes alters the initiation and progression of this disease is an important future area of study. In our findings, however, PP2A seems to be the primary factor responsible for changes in CTSS expression. We previously observed increased Ctss expression and reduced PP2A activity in mice exposed to smoke while infected with respiratory syncytial virus (9). In this current study, we directly show that the loss of PP2A signaling is responsible for elevated CTSS expression in mice and HBE cells. This is important, because inhibition of PP2A coincides with multiple changes in the lungs, including immune responses (19), mucus production (41), protease expression (18), and corticosteroid sensitivity (42). The SMAP compound used in this study inhibits tumor formation via activation of PP2A (22, 43). The SMAP compound activates PP2A by binding to the A subunit of PP2A, promoting conformational changes, which increase cellular phosphatase activity (22) and promoting PP2A holoenzyme (ABC subunit) assembly and perturbs interactions with endogenous PP2A inhibitors. Other compounds, such as FTY-720, erlotinib, and analogous synthetic sphingolipids, also activate PP2A (21, 44, 45) by binding the endogenous PP2A inhibitors, inhibitor 2 of PP2A (I2PP2A/SET) or cancerous inhibitor of PP2A (CIP2A), and derepressing PP2A activity. These could also be possible therapeutic candidates for the treatment of COPD. Our data with smoke exposure in combination with SMAP suggests that this class of compounds could be considered for the treatment of smoke-associated diseases and warrant further preclinical investigations.
In addition, direct CTSS enzyme inhibitors are currently being investigated in multiple disease models. For example, RO5459072, a CTSS inhibitor, suppresses systemic and peripheral disease-associated mechanisms of autoimmune tissue injury in mice (46). RO5459072 also reduced CD4 T-cell and dendritic cell activation, and autoantibody production in a preclinical model of spontaneous systemic lupus erythematosus and lupus nephritis (47). CTSS inhibition also reduces the inflammatory responses of macrophages by causing these cells to secrete less proinflammatory cytokines and express less MHC class II and CD80 (48). Thus, the therapeutic benefits of reducing CTSS activity may be achieved in two ways: upstream by exploiting the negative regulation of CTSS transcription via PP2A activation, as shown in the present study; and directly by inhibiting CTSS enzyme activity. Combination therapy potential of SMAPs and CTSS inhibitors may be beneficial in several ways (i.e., allow reduced dosing of CTSS inhibitors to minimize its potential toxicity and targeting the neutrophil pool of CTSS) (46). Advancing our current studies, we will focus on combinational therapy potential, including the use of CTSS inhibitors and other therapeutic agents.
Together, our data identify PP2A’s negative regulation of CTSS as an important factor in smoke-induced COPD, because reduction in CTSS expression prevents loss of lung function, reduces inflammation, and slows the degradation of elastin and lung tissue remodeling. Indeed, our work highlights that targeting the PP2A/CTSS pathway may limit smoke-induced COPD.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Prof. Chris Scott from the School of Pharmacy at Queen’s University Belfast for supplying the Ctss−/− mice. The authors also thank the Pulmonary Division of SUNY Downstate Medical Centre for their support and the tissue and blood donors and their families who participated in this study.
Footnotes
Supported by Flight Attendant Medical Research Institute (YCSA113380 and CIA160005, P.G.; CIA160011 and CIA13033, M.S.), Alpha-1 Foundation (493373, P.G.), Medical Research Council (C.T.), James and Esther King Biomedical Program of the State of Florida (#5JK02, M.S.), and Partnership for New York City/BioAccelerate award (M.O.).
Author Contributions: Performed experiments, D.F.D., S.N., J.P., M.O., A.J.D., N.B., M.D.K., S.W., and P.G. Conception and design, R.F.F., M.O., C.T., and P.G. Analysis and interpretation, D.F.D., S.N., J.P., R.F.F., M.O., M.S., M. Birrell, M. Belvisi, N.B., M.D.K., S.W., C.T., and P.G. Drafting the manuscript for important intellectual content, D.F.D., M.O., M.S., N.B., C.T., and P.G.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201808-1518OC on January 14, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Soriano JB, Rodríguez-Roisin R. Chronic obstructive pulmonary disease overview: epidemiology, risk factors, and clinical presentation. Proc Am Thorac Soc. 2011;8:363–367. doi: 10.1513/pats.201102-017RM. [DOI] [PubMed] [Google Scholar]
- 2.Miniño AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. Natl Vital Stat Rep. 2010;59:1–52. [PubMed] [Google Scholar]
- 3.Rovina N, Koutsoukou A, Koulouris NG. Inflammation and immune response in COPD: where do we stand? Mediators Inflamm. 2013;2013:413735. doi: 10.1155/2013/413735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lomas DA. Does protease-antiprotease imbalance explain chronic obstructive pulmonary disease? Ann Am Thorac Soc. 2016;13:S130–S137. doi: 10.1513/AnnalsATS.201504-196KV. [DOI] [PubMed] [Google Scholar]
- 5.Abboud RT, Vimalanathan S. Pathogenesis of COPD: part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis. 2008;12:361–367. [PubMed] [Google Scholar]
- 6.Zhang X, Shan P, Homer R, Zhang Y, Petrache I, Mannam P, et al. Cathepsin E promotes pulmonary emphysema via mitochondrial fission. Am J Pathol. 2014;184:2730–2741. doi: 10.1016/j.ajpath.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brehm A, Geraghty P, Campos M, Garcia-Arcos I, Dabo AJ, Gaffney A, et al. Cathepsin G degradation of phospholipid transfer protein (PLTP) augments pulmonary inflammation. FASEB J. 2014;28:2318–2331. doi: 10.1096/fj.13-246843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golovatch P, Mercer BA, Lemaître V, Wallace A, Foronjy RF, D’Armiento J. Role for cathepsin K in emphysema in smoke-exposed guinea pigs. Exp Lung Res. 2009;35:631–645. doi: 10.3109/01902140902822304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foronjy RF, Dabo AJ, Taggart CC, Weldon S, Geraghty P. Respiratory syncytial virus infections enhance cigarette smoke induced COPD in mice. PLoS One. 2014;9:e90567. doi: 10.1371/journal.pone.0090567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geraghty P, Greene CM, O’Mahony M, O’Neill SJ, Taggart CC, McElvaney NG. Secretory leucocyte protease inhibitor inhibits interferon-gamma-induced cathepsin S expression. J Biol Chem. 2007;282:33389–33395. doi: 10.1074/jbc.M706884200. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima T, Nakamura H, Owen CA, Yoshida S, Tsuduki K, Chubachi S, et al. Plasma cathepsin S and cathepsin S/cystatin C ratios are potential biomarkers for COPD. Dis Markers. 2016;2016:4093870. doi: 10.1155/2016/4093870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson RD, Williams R, Scott CJ, Burden RE. Cathepsin S: therapeutic, diagnostic, and prognostic potential. Biol Chem. 2015;396:867–882. doi: 10.1515/hsz-2015-0114. [DOI] [PubMed] [Google Scholar]
- 13.Riese RJ, Wolf PR, Brömme D, Natkin LR, Villadangos JA, Ploegh HL, et al. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 14.Taggart CC, Greene CM, Smith SG, Levine RL, McCray PB, Jr, O’Neill S, et al. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol. 2003;171:931–937. doi: 10.4049/jimmunol.171.2.931. [DOI] [PubMed] [Google Scholar]
- 15.Taggart CC, Lowe GJ, Greene CM, Mulgrew AT, O’Neill SJ, Levine RL, et al. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem. 2001;276:33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- 16.Rogan MP, Taggart CC, Greene CM, Murphy PG, O’Neill SJ, McElvaney NG. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J Infect Dis. 2004;190:1245–1253. doi: 10.1086/423821. [DOI] [PubMed] [Google Scholar]
- 17.Chapman HA, Riese RJ, Shi GP. Emerging roles for cysteine proteases in human biology. Annu Rev Physiol. 1997;59:63–88. doi: 10.1146/annurev.physiol.59.1.63. [DOI] [PubMed] [Google Scholar]
- 18.Wallace AM, Hardigan A, Geraghty P, Salim S, Gaffney A, Thankachen J, et al. Protein phosphatase 2A regulates innate immune and proteolytic responses to cigarette smoke exposure in the lung. Toxicol Sci. 2012;126:589–599. doi: 10.1093/toxsci/kfr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geraghty P, Hardigan AA, Wallace AM, Mirochnitchenko O, Thankachen J, Arellanos L, et al. The glutathione peroxidase 1-protein tyrosine phosphatase 1B-protein phosphatase 2A axis: a key determinant of airway inflammation and alveolar destruction. Am J Respir Cell Mol Biol. 2013;49:721–730. doi: 10.1165/rcmb.2013-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geraghty P, Eden E, Pillai M, Campos M, McElvaney NG, Foronjy RF. α1-Antitrypsin activates protein phosphatase 2A to counter lung inflammatory responses. Am J Respir Crit Care Med. 2014;190:1229–1242. doi: 10.1164/rccm.201405-0872OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nath S, Ohlmeyer M, Salathe MA, Poon J, Baumlin N, Foronjy RF, et al. Chronic cigarette smoke exposure subdues PP2A activity by enhancing expression of the oncogene CIP2A. Am J Respir Cell Mol Biol. 2018;59:695–705. doi: 10.1165/rcmb.2018-0173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangodkar J, Perl A, Tohme R, Kiselar J, Kastrinsky DB, Zaware N, et al. Activation of tumor suppressor protein PP2A inhibits KRAS-driven tumor growth. J Clin Invest. 2017;127:2081–2090. doi: 10.1172/JCI89548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shalaby KH, Gold LG, Schuessler TF, Martin JG, Robichaud A. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir Res. 2010;11:82. doi: 10.1186/1465-9921-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu YR, O’Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, et al. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS One. 2016;11:e0150606. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsia CCW, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, et al. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1149–L1157. doi: 10.1152/ajplung.00481.2007. [DOI] [PubMed] [Google Scholar]
- 27.Xiao R, Goldklang MP, D’Armiento JM. Parenchymal airspace profiling: sensitive quantification and characterization of lung structure evaluating parenchymal destruction. Am J Respir Cell Mol Biol. 2016;55:708–715. doi: 10.1165/rcmb.2016-0143OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid A, Sailland J, Novak L, Baumlin N, Fregien N, Salathe M. Modulation of Wnt signaling is essential for the differentiation of ciliated epithelial cells in human airways. FEBS Lett. 2017;591:3493–3506. doi: 10.1002/1873-3468.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, et al. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem. 2011;286:19830–19839. doi: 10.1074/jbc.M110.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weldon S, McNally P, McAuley DF, Oglesby IK, Wohlford-Lenane CL, Bartlett JA, et al. miR-31 dysregulation in cystic fibrosis airways contributes to increased pulmonary cathepsin S production. Am J Respir Crit Care Med. 2014;190:165–174. doi: 10.1164/rccm.201311-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 32.Storm van’s Gravesande K, Layne MD, Ye Q, Le L, Baron RM, Perrella MA, et al. IFN regulatory factor-1 regulates IFN-gamma-dependent cathepsin S expression. J Immunol. 2002;168:4488–4494. doi: 10.4049/jimmunol.168.9.4488. [DOI] [PubMed] [Google Scholar]
- 33.Stellos K, Gatsiou A, Stamatelopoulos K, Perisic Matic L, John D, Lunella FF, et al. Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nat Med. 2016;22:1140–1150. doi: 10.1038/nm.4172. [DOI] [PubMed] [Google Scholar]
- 34.Lemere CA, Munger JS, Shi GP, Natkin L, Haass C, Chapman HA, et al. The lysosomal cysteine protease, cathepsin S, is increased in Alzheimer’s disease and Down syndrome brain: an immunocytochemical study. Am J Pathol. 1995;146:848–860. [PMC free article] [PubMed] [Google Scholar]
- 35.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, et al. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med. 2004;170:974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 36.Memmert S, Damanaki A, Nogueira AVB, Eick S, Nokhbehsaim M, Papadopoulou AK, et al. Role of cathepsin S in periodontal inflammation and infection. Mediators Inflamm. 2017;2017:4786170. doi: 10.1155/2017/4786170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minematsu N, Nakamura H, Tateno H, Nakajima T, Yamaguchi K. Genetic polymorphism in matrix metalloproteinase-9 and pulmonary emphysema. Biochem Biophys Res Commun. 2001;289:116–119. doi: 10.1006/bbrc.2001.5936. [DOI] [PubMed] [Google Scholar]
- 38.Joos L, He JQ, Shepherdson MB, Connett JE, Anthonisen NR, Paré PD, et al. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet. 2002;11:569–576. doi: 10.1093/hmg/11.5.569. [DOI] [PubMed] [Google Scholar]
- 39.Ohbayashi H. Neutrophil elastase inhibitors as treatment for COPD. Expert Opin Investig Drugs. 2002;11:965–980. doi: 10.1517/13543784.11.7.965. [DOI] [PubMed] [Google Scholar]
- 40.Gu XM, Wang XG, Sun J, Wang N, Jiang SJ. The role of HuR in mediating snail expression in human small airway epithelium induced by cigarette smoke extract [in Chinese] Zhonghua Jie He Hu Xi Za Zhi. 2017;40:515–519. doi: 10.3760/cma.j.issn.1001-0939.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Nair PM, Starkey MR, Haw TJ, Liu G, Horvat JC, Morris JC, et al. Targeting PP2A and proteasome activity ameliorates features of allergic airway disease in mice. Allergy. 2017;72:1891–1903. doi: 10.1111/all.13212. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi Y, Mercado N, Barnes PJ, Ito K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS One. 2011;6:e27627. doi: 10.1371/journal.pone.0027627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClinch K, Avelar RA, Callejas D, Izadmehr S, Wiredja D, Perl A, et al. Small-molecule activators of protein phosphatase 2A for the treatment of castration-resistant prostate cancer. Cancer Res. 2018;78:2065–2080. doi: 10.1158/0008-5472.CAN-17-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velmurugan BK, Lee CH, Chiang SL, Hua CH, Chen MC, Lin SH, et al. PP2A deactivation is a common event in oral cancer and reactivation by FTY720 shows promising therapeutic potential. J Cell Physiol. 2018;233:1300–1311. doi: 10.1002/jcp.26001. [DOI] [PubMed] [Google Scholar]
- 45.Rahman MM, Rumzhum NN, Hansbro PM, Morris JC, Clark AR, Verrills NM, et al. Activating protein phosphatase 2A (PP2A) enhances tristetraprolin (TTP) anti-inflammatory function in A549 lung epithelial cells. Cell Signal. 2016;28:325–334. doi: 10.1016/j.cellsig.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Tato M, Kumar SV, Liu Y, Mulay SR, Moll S, Popper B, et al. Cathepsin S inhibition combines control of systemic and peripheral pathomechanisms of autoimmune tissue injury. Sci Rep. 2017;7:2775. doi: 10.1038/s41598-017-01894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rupanagudi KV, Kulkarni OP, Lichtnekert J, Darisipudi MN, Mulay SR, Schott B, et al. Cathepsin S inhibition suppresses systemic lupus erythematosus and lupus nephritis because cathepsin S is essential for MHC class II-mediated CD4 T cell and B cell priming. Ann Rheum Dis. 2015;74:452–463. doi: 10.1136/annrheumdis-2013-203717. [DOI] [PubMed] [Google Scholar]
- 48.Thanei S, Theron M, Silva AP, Reis B, Branco L, Schirmbeck L, et al. Cathepsin S inhibition suppresses autoimmune-triggered inflammatory responses in macrophages. Biochem Pharmacol. 2017;146:151–164. doi: 10.1016/j.bcp.2017.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.