Abstract
Introduction
The development of uterine transplantation (UTx) from deceased donors requires knowledge of the tolerance of the uterus to prolonged cold ischemia (CI). This can be evaluated through the use of biological parameters to assess degradation of the organ between its procurement and transplantation. The objective of this study was to analyze changes in the metabolic composition of the storage solution in cases of prolonged CI in uteri from ewes.
Methods
Eighteen uterine auto-transplantations were performed in ewes. CI time was 1 h (T1) or 24 h (T24). Samples of Celsior® were taken when the explanted uterus was flushed (T0) and at the end of CI. A dual approach to metabolic analyses was followed: targeted biochemical analyses targeting several predefined metabolites and non-targeted metabolomics analyses based on nuclear magnetic resonance (NMR).
Results
Metabolic analyses were performed on 16 explanted uteri. Metabolomic profiles differed significantly between T1 and T24 (p = 0.003). Hypoxia-associated degradation of the organ was demonstrated by the significantly higher lactate levels at T24 than at T1 (p < 0.05), accompanied by cell lysis, and significantly higher levels of creatine kinase activity in T24 than in T1 uteri (p < 0.05). Oxidative stress increased over time, with a significantly higher oxidized glutathione/glutathione ratio for T24 than for T1 uteri (p < 0.05).
Conclusion
The metabolic results indicate a significant degradation of the uterus during 24 h of CI. Metabolic analysis of the storage solution could be used as a non-invasive tool for evaluating uterine degradation during CI before transplantation.
Keywords: Uterus transplantation, Cold ischemia, Metabolic changes, Uterine transplantation
Introduction
For the estimated 1.5 million women worldwide with uterine infertility, uterine transplantation (UTx) currently offers the only possibility for becoming both the genetic mother and the legal bearer of their own children [1]. Most of the UTx resulting in births to date have used live donors [2]. Only one birth following UTx from a deceased donor has been reported, in Brazil [3]. Cold ischemia (CI) time in this case was 6 h and 20 min [3]. The use of deceased donors has the advantage of avoiding surgical risks for the donor and provides an alternative in the absence of available live donors [2, 4]. One of the main limitations of this option is that the associated CI time is much greater than for organs from live donors [5]. A number of studies have been performed, but there are still too few data to determine the maximum CI tolerated by the uterus [6].
By contrast to other regularly transplanted organs, such as the kidney or liver, no markers directly reflecting uterine functionality are currently available for use in UTx. There is, therefore, a need for biological parameters or for a panel of parameters reflecting the degradation of the uterus during its storage before transplantation. Tissue damage can result in structural alterations (cell lysis, vacuolization, etc.) or metabolic changes affecting sensitive biochemical pathways, such as the response to hypoxia. Indeed, the discrepancy of energy linked to the lack of oxygen supply induces a dramatic decrease of ATP production, promoting acidosis, edema, perturbation of cellular Na+ and Ca2+ homeostasis, and stimulating by enzyme activation, the burst of oxidative stress which appear during reperfusion phase. Thus, the severe lesions observed during reperfusion take the origin at the beginning of the ischemic period [7, 8]. Therefore, it is possible by appropriate techniques to highlight these metabolic changes in the cold ischemia period into the storage solution in which the graft is immersed before transplantation [8]. The concentration of these substances may reflect the degree of tissue injury, linked to the duration of ischemia. These endogenous substances can be analyzed with conventional biochemical approaches, through the targeted determination of metabolites or enzymatic activities or through non-targeted approaches involving a broader analysis of the metabolic content of the system without the need for the prior formulation of hypotheses. This metabolic content, also known as the metabolome, represents the ultimate response of an organism to the entire set of factors governing its homeostasis; it therefore reflects the state of the organism at a given moment, in a given situation [9]. Metabolomics is the study of metabolites, which are generally low-molecular weight molecules (< 1 kDa). It has been widely used in solid organ transplantation and has recently been used to identify biomarkers predicting long-term kidney viability after transplantation [10–13].
The objective of this study was to evaluate the metabolic consequences of prolonged CI on the uterus of ewes by analyzing the storage solution, with a view to identifying biomarkers indicative of the degradation of the organ during storage.
Materials and methods
Experimental design
We performed a prospective comparative study of the feasibility of evaluating the tolerance of ewes uteri to prolonged CI in a storage solution, based on a dual approach involving metabolic profiling (hypothesis-free analysis) and targeted biochemical analyses. We performed autologous uterine transplantations on 18 ewes: in nine cases, the CI time was 1 h (“1-h CI” group) and on the other nine cases, it was 24 h (“24-h CI” group).
Animals
We used Limousine ewes that had already lambed and weighed between 40 and 70 kg. The animals were anestrus before surgery and had the same nycthemeral cycle. Food was withdrawn 24 h before surgery and reintroduced the day after transplantation. The surgical interventions were carried out between November 2017 and July 2018 at the Analysis and Research Laboratory of Haute-Vienne (Limoges, France) (registration number: 87.797). The research protocol was accepted by the regional ethics committee for animal experimentation in the Limousin region (CREEAL) (approval number 06-2014-06). Animal welfare was ensured in accordance with EU Directive 2010-63. Our surgical protocol for autologous uterine transplantation, based on that described by the team of Pr. Brännström in Sweden, has been described elsewhere [13, 14]. However, the procedure was modified by ex vivo flushing with Celsior® solution. The anesthesia protocol and postoperative monitoring were identical to those previously reported. This large animal model with anatomical similarities with the woman uterus in terms of size and vascularization mimics surgical practice in human and offer preclinical data easily transfer to human.
Surgical technique
The ewe was placed in a supine position. Surgical disinfection of the abdomen was performed with dermal polyvidone iodine, and a sterile field was prepared. All surgical procedures were performed in sterile conditions. After median laparotomy from the pubis, the ewe was placed in the Trendelenburg position. The rumen, small intestine, and most of the colon were pushed towards the upper abdominal cavity or externalized and wrapped in wet surgical sheets to facilitate exposure of the pelvis. The procedure involved removal of the right uterine horn, the uterus itself, the cervix, the top of the vagina and a vaginal collar, and the right uterine appendages. Explantation began with the ligation and sectioning of the left oviduct, the uterine artery, and the left utero-ovarian pedicle. The left ovary was left in place. The bodies of the uterus, the cervix, and the upper part of the vagina were then dissected and isolated from their surrounding tissues. The two ureters were identified and attached to the side. The right ureter was isolated from the utero-ovarian vein on the inside and the uterine artery on the outside. The right ovary was also removed. The uterine artery and right utero-ovarian vein were dissected to their origin at the anterior branch of the internal iliac artery and vena cava. The umbilical artery, blind, and the vaginal artery, when present, were ligated and sectioned at their origin. The vagina was cut 1 to 2 cm below the cervix and closed by separate 2/0 absorbable multifilament sutures with a round-bodied needle. The left uterine horn was withdrawn with a surgical stapler. Once the uterus was attached only by the right uterine artery and the right utero-ovarian vein, a bolus of 12,500 IU heparin as injected intravenously. The posterior branch of the internal iliac artery, downstream from the uterine artery, was then clipped and sectioned. The hypogastric trunk upstream from the uterine artery was sectioned obliquely. The utero-ovarian vein was sectioned about 1 cm from the vena cava. The utero-ovarian vein stump was ligated, and the hypogastric trunk was closed with non-absorbable monofilament 4/0 sutures. Autologous transplantation and postoperative monitoring were performed according to the protocol described in our previous study [15]. The success of the transplantation has been defined by permeable and leaky anastomoses and a recolored and warmed uterus without any immediate sign of edema. We assessed the viability of uteri between day 8 (D8) and day 15 (D15) from surgery during a second-look laparotomy. The uterus was considered viable if it had normal staining, contractions to mechanical stimulation, and satisfactory venous and arterial Doppler flow.
Perfusion with storage solution and graft preservation
Once the uterus had been explanted, the uterine artery was catheterized with a feeding needle. It was perfused with Celsior® storage fluid (start of the CI) at a pressure of 100 mmHg, corresponding to systolic pressure in the pelvis of the ewe [16]. The uterus was lost its color and was cooled. The storage solution flowing through the utero-ovarian vein was collected in a sterile bowl. Washing was continued until the fluid collected contained no blood. The uterus was then placed in a sterile container filled with Celsior® at 4 °C. The container was placed in an insulated box filled with ice (Vital Pack®). Half of the uteri were stored in these conditions for 1 h (n = 9), and the other half were stored in these conditions for 24 h (n = 9).
Sampling of the storage solution
The T0 samples were collected at the end of ex vivo flushing; one completely clear liquid was obtained. The second samples were collected at the end of the CI time, after 1 h of CI (T1) or 24 h of CI (T24). Two types of samples were taken: from the Celsior® fluid in which the uterus was bathed during storage and after ex vivo flushing of the uterus at the end of the period of CI. For the collection of these samples after the period of CI, the uterus was removed from the Vital Pack® and was flushed again ex vivo with 10 mL Celsior®, taken from the solution in which the uterus was bathed during CI, as described above.
Metabolic profiling was performed on the samples taken from the Celsior® bathing the organ, and profiles were compared between sampling times. Targeted biochemical analyses were performed on both the sample taken from the Celsior® bathing the organ and the Celsior® sample recovered after uterine washing to compare the two sampling methods.
Macroscopic evaluation of the graft
Before transplantation, the change uterus weight during CI was evaluated by weighing at T0 and then at the end of CI (T1 or T24). Macroscopic and Doppler examinations of the graft were performed between D8 and D15 post-transplantation, as described in our previous study [15]. Ewes with viable grafts were left with rams during the reproductive period to obtain a pregnancy.
Metabolomic analyses
Metabolic profiling analyses were performed by proton magnetic resonance (H-NMR), with a Bruker® AVANCE III HD 600 MHz nuclear magnetic resonance (NMR) spectrometer (Briker Sadis, France). Samples were prepared by mixing 150 μL sample, 50 μL deuterated phosphate buffer, and 10 μL 3.2 mM TSP (trimethylsilylpropionate). The H-NMR spectra were processed using XWinNMR version 3.5 software (Brucker Daltonik, Germany). Spectral intensities were scaled to the total intensity, so that each sample was normalized for variable dilution. The abundance of sugars in the native preservation fluid limited the NMR analysis, and the method enabled the simultaneous measurement of only 15 metabolites. Each of them was quantified in the samples, providing a metabolic profile that corresponds to the qualitative and quantitative content of these metabolites in the sample.
Biochemical analyses
Determinations of amino acid concentrations (alanine, valine, glycine)
Amino acid levels were determined by liquid chromatography with a UV detector (Sykam, Germany) and staining of the amino acids with ninhydrin [17].
Determination of the GSSG/GSH ratio
Reduced and oxidized glutathione levels were performed with the Glutathione Assay Kit according to the manufacturer’s instructions (Cayman Chemical, USA).
Determination of 8-isoprostane F2a levels
Free 8-isoprostane (not esterified with phospholipids) levels were determined by with an ELISA kit, according to the manufacturer’s instructions (Cayman Chemical).
Determination of the enzyme activity associated with cell lysis
Creatine kinase (CK) and lactate dehydrogenase (LDH) activities were measured with a COBAS 6000 module c501 (ROCHE Diagnostics, France).
Determination of the lactate/pyruvate ratio
Pyruvate was determined by colorimetry, according to the kit manufacturer’s instructions (Biosentec, France). The lactate assay was performed on a COBAS 6000 Module c501 (ROCHE Diagnostics).
Statistical analysis
A supervised multivariate statistical analysis based on PLS-DA (partial least squares discriminant analysis) was performed on the metabolomic data generated by H-NMR. In this analysis, differences in overall metabolic content (several variables) between two conditions (Y, i.e., T0 vs T1, T0 vs T24 and T1 vs T24) were sought. If such differences were identified, the metabolites differing between conditions were identified on the basis of their variable importance on projection (VIP > 1), given by the model. The Q2cum, which is an overall measure of the quality of fit and predictive quality of the generated model; R2X, which corresponds to the correlations between components and variables; and R2Y, which corresponds to the correlations between the components and the Y variables were estimated for each comparison. PLS-DA was performed with SIMCA 13.0.3 software (Umetrics, Umea, Sweden). For each of the three evaluations, values greater than 0.5 or even 0.7 for model parameters were considered indicative of good model quality. Multigroup comparisons were performed with Fisher’s test. The results were considered to be significantly different when the α risk was ≤ 0.05.
Results from the targeted biochemical analyses are presented as the mean ± SD and were subjected to statistical analyses based on Kruskal–Wallis tests with Dunn’s post hoc tests, in GraphPad Prism software version 7.00 (La Jolla, CA, USA). Differences were considered significant if the α risk was ≤ 0.05. The uterine weights at the end of the CI time were compared using the Mann–Whitney test.
Results
Surgery
Seven of the 18 autologous transplants were successful (38.9%): four in the 1-h CI group and three in the 24-h CI group (Table 1). The exploratory laparotomy took place a mean of 9.6 days (± 3.9) after UTx. Two of the uteri in the 1-h CI group and one in the 24-h CI group were considered to be viable. The principal surgical data are described in Table 1. On removal from the storage fluid, the mean weight of the uteri was 98.8 g (± 20.6 g) for the 1-h CI group and 106.7 g (± 29.4 g) for the 24-h CI group. The uteri in the”24-h CI” group displayed a 12.2 g (± 11.1 g) increase in weight at the end of CI (p = 0.0105), whereas no change in weight was observed in the 1-h CI group.
Table 1.
Principal parameters of the surgical procedures
| “1-h CI” group (± standard deviation) (range) | “24-h CI” group (± standard deviation) (range) | |
|---|---|---|
| Number | 9 | 9 |
| Number of successful UTs | 4 | 3 |
| Mean duration of CI (min) | 86.1 (± 19.1) (69–130) | 1431.2 (± 23.6) (1428–1473) |
| Mean duration of the intervention to remove the uterus (min) | 143.6 (± 20.6) (110–180) | 135.8 (± 33) (86–190) |
Metabolomic profiling
We performed metabolomic profiling analyses for 16 explanted uteri (88.9%) (eight in each group).
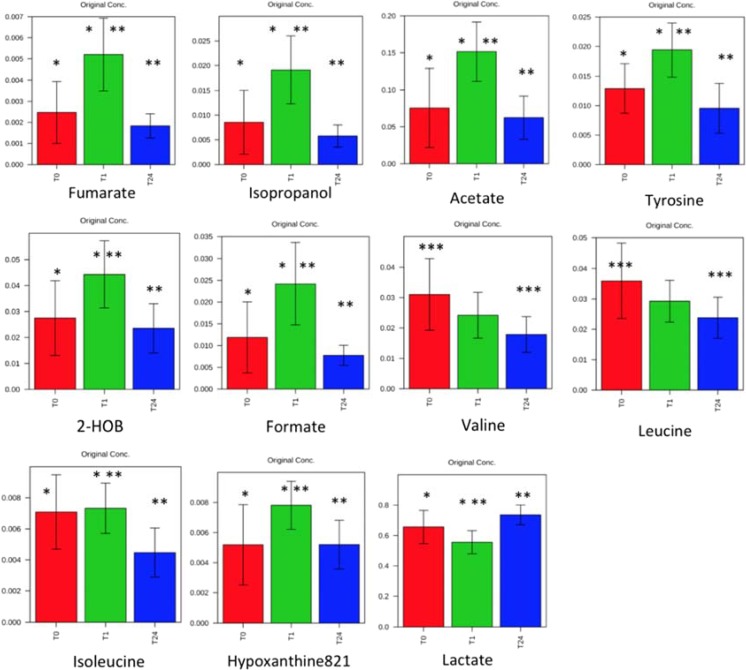
There was a significant difference between the metabolic profiles at T0 and T1 (R2Y = 0.74, Q2 = 0.68, CV-ANOVA p = 0.008). There was also a significant difference in metabolic profiles between T1 and T24 (R2Y = 0.81, Q2 = 0.74, CV-ANOVA p = 0.003). The temporal evolution of metabolites during ischemia is shown in Fig. 1. Between T0 and T1, there was a significant increase in some metabolites (fumarate, isopropanol, acetate, tyrosine, 2-hydroxybutyrate, formate, isoleucine, and hypoxanthine) and a decrease in lactate. After 24 h, all these metabolites regained their basal level, with the exception of valine and leucine, significantly decreased compared to T0. Lactate increased significantly between T1 and T24.
Fig. 1.
Evolution of different metabolites between T0, T1, and T24 (means ± standard). HOB hydroxybutyrate. Significantly different results between T0 and T1 (single asterisk), significantly different results between T1 and T24 (double asterisks), and significantly different results between T1 and T24 (triple asterisks)
Targeted biochemical analysis
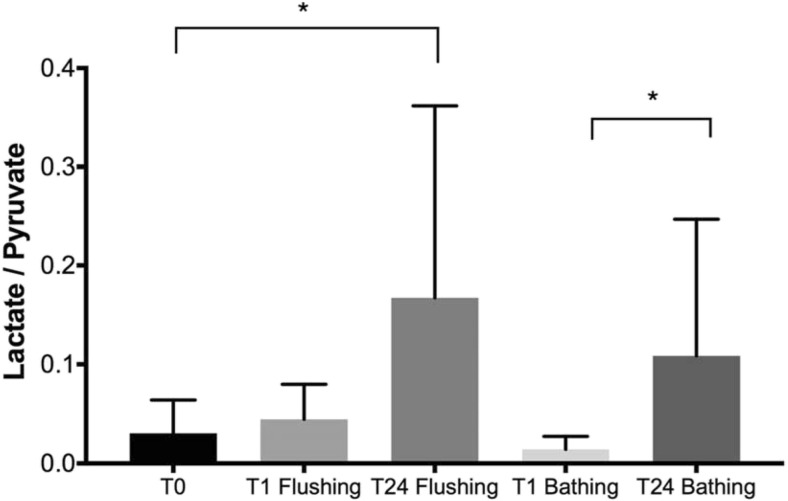
Biochemical analyses of the storage fluid used to bathe the organs were performed for 14 explanted uteri (77.8%) (seven in each group). For amino acids, glycine and alanine concentrations were significantly higher after 24 h of CI than after 1 h, whereas valine concentrations were similar in the two groups (Fig. 2). Evaluations of markers of cell lysis (CK and LDH activities) in the bathing solution revealed that CK activity levels were significantly higher after 24 h of CI than after 1 h, whereas there was a non-significant increase in LDH activity (Fig. 3). Oxidative stress marker determinations showed that the oxidized glutathione (GSSG)/reduced glutathione (GSH) ratio was significantly higher after 24 h than after 1 h of CI and that this was associated with a trend towards higher levels of 8-isoprostane (Fig. 4). The lactate/pyruvate ratio was also significantly higher after 24 h than after 1 h of CI (Fig. 5).
Fig. 2.
Comparison of the means ± standard deviations of alanine, valine, and glycine concentrations for each timepoint of the study with n = 7 for each CI time and n = 14 for T0. *p < 0.05. ns non-significant
Fig. 3.
Comparison of the means ± standard deviations of CK and LDH activities for each timepoint with n = 7 for each CI time and n = 14 for T0. *p < 0.05, **p < 0.01, ns non-significant
Fig. 4.
Comparison of the means ± standard deviations of the GSSH (oxidized glutathione)/GSH (reduced glutathione) ratio and isoprostane concentration for each timepoint with n = 7 for each CI time and n = 14 for T0. *p < 0.05, ns non-significant
Fig. 5.
Comparison of the means ± standard deviations of the lactate/pyruvate ratio for each timepoint with n = 7 for each. CI time and n = 14 for T0. *p < 0.05
The analysis of the solution used to rinse the uterus at the end of CI yielded results similar to those obtained for the bathing solution, with CK activity (p < 0.05) and the lactate/pyruvate ratio (p < 0.05) higher after 24 h of CI than after 1 h (Figs. 2, 3, 4, and 5). However, this analysis appeared to be less sensitive, because GSSG/GSH ratio, which was significantly higher after 24 h of CI in the bathing solution, was not significantly higher after this CI time in the graft flush solution (Fig. 4).
Discussion
This study demonstrates the feasibility of studying the tolerance of the ewe uterus to prolonged CI in a non-invasive manner compatible with transplantation, by analyzing the metabolic content of the storage solution in which the organ is bathed. An analysis of the metabolic profile of this solution made it possible to distinguish between two different pathophysiological states of the uterus. The observed changes in metabolite concentration are probably due to lesional or adaptive mechanisms related to hypoxia or hypothermia during the period of ex vivo storage. Thus, the intensity of the injuries exhibited by the organ during CI can be assessed by determining the concentration of markers of muscle lysis or metabolic acidosis in the bathing solution. This study focused on changes in the composition of the storage solution. The choice of this solution as the matrix for analysis is pertinent because it allows simple and non-invasive analysis methods that could be used in routine practice during uterine transplantation in women. In addition, the biomarkers highlighted in this study are easily quantify in a hospital clinical lab by automatic process and are already determined in blood to evaluate organ dysfunction, except for the amino acid assay, but new technologies will be offering this strategy. This sample-based metabolic analysis of the storage fluid makes it possible to assess graft viability rapidly in a non-traumatic matter and, in particular, to assess graft quality, an essential element guiding transplantation decisions.
The ewe is an animal model that has been widely used in UTx [6, 14]. Like the non-human primate, the sheep’s uterus has anatomical characteristics close to human uteri. Large animal studies limit the number of subjects that can be included for ethical reasons making the interpretation of results difficult. However, our study is to our knowledge the study with the largest number of ewes included. In our study, the rate of successful autologous transplantation was lower than that reported in previous studies, confirming the difficulty of performing such procedures in large animals [14–16, 18, 19]. Macroscopic analysis after transplantation identified no differences between the uteri of the two groups at the end of CI. However, the uteri displayed an increase in weight of more than 10% after 24 h of CI, reflecting the presence of cellular and interstitial edemas, despite the presence of mannitol and lactobionic acid in the storage solution. Edemas probably formed due to a dysfunction of the Na+/K+ ATPase pump of the membranes, which would lead to an increase in intracellular sodium concentration. In parallel, the increase in intracellular calcium levels triggers a pro-inflammatory process [8]. This edematous phenomenon during prolonged CI has already been observed and was confirmed by histological analyses [15]. The absence of an increase in uterine weight after 1 h of ischemia shows that this period is too short for the edema to become established or that the storage solution protects against graft weight gain during this period.
In this study, we used Celsior® as the storage solution because this solution is widely used in organ transplantation for hearts, kidneys, and pancreases. Its composition is suitable for prolonged organ preservation, as it protects against cellular and interstitial edema and acidosis and provides a substrate for energy production under anaerobic conditions, in the form of glutamate [20]. The storage solution used in the UTx that have resulted in births to date, proving its efficacy, was histidine–tryptophan–ketoglutarate (Custodiol, HTK) [3, 21, 22]. However, this choice of solution was not based on any scientific proof, as no study has yet compared storage solutions for UTx, partially due to the lack of pertinent biomarkers of graft viability. Biomarker identification in the preservation solution to assess uterine injury during cold ischemia offers new opportunity to test the different solutions used to graft preservation. For metabolomic profiling, the result was restricted to a limited spectral region due to the high sugar content of the storage solution. Despite the inclusion of only 15 metabolites, this analysis highlighted significant difference in metabolomic profiles between T0 and T1 and between T1 and T24. As NMR peaks were normalized to the global metabolite content, these results do not provide true quantitative measurement of each of them, but rather a relative content against all the metabolites. This enabled robust comparison of variations between sampling times but limits direct comparison of our results for the metabolites. Thus, metabolites were also analyzed using quantitative biochemical methods. The changes in metabolomic profiles during CI confirm that the uterus undergoes changes in cellular metabolism affecting several biochemical pathways.
Metabolomic analysis has already been performed on storage fluid in the context of renal transplantation to assess its relationship with graft quality and outcome [12, 13]. Bon et al. showed in pigs that the perfusion of kidney grafts for 22 h in a hypothermic perfusion machine led to an increase in the levels of lactate, choline, and several amino acids (valine, alanine, glutamate, and glycine), and a decrease in the level of the glutathione initially presents in the storage solution [13]. Guy et al. conducted a similar study with the human kidneys analyzed by NMR after 45 min and 4 h of storage [12]. In addition to the consumption of glutathione, the authors showed that 22 other metabolites not initially present in the medium were detectable after 45 min. The levels of 16 of these metabolites, including metabolites related to fatty-acid metabolism (3-hydroxybutyrate), anaerobic glycolysis (lactate), and the Krebs cycle (citrate and glutamate), increased significantly over time. Interestingly, the authors showed that decreases in gluconate, glucose, and inosine levels and increases in leucine levels were predictive of poor graft function recovery. However, the metabolism of organs like the kidney is different from that of the uterus, especially in terms of energy needs, as the kidney requires a large amount of energy for tubular exchange and the synthesis of numerous compounds. The lack of published data on markers of uterine metabolic activity and the absence of markers of uterine graft functionality make it difficult to interpret metabolomic data to determine the tolerance of the uterus to ischemia. Pregnancy remains the ideal parameter for evaluating uterine functionality, but it also depends on other parameters not strictly related to graft quality. Myometrial biopsies could be also a tool to evaluate graft tolerance to ischemia as previously described [15]. In this study, to avoid compromising the chances of pregnancy, myometrial biopsies are not performed linked to the invasively of this procedure, not used in current clinical practice. Thus, it is still difficult to correlate our biological findings with graft outcome due to the weak rate of uterine viability.
Our approach based on the determination of markers of uterine graft deterioration could facilitate the rapid diagnosis of uterine lesions. However, they are still preliminary results which offer new opportunity for further studies in order to correlate the biomarker levels in the preservation solution and the uterine viability. This study constitutes only one of the many steps towards understanding the parameters to be considered to improve uterine transplantation in women. However, our results are partly consistent with those of metabolomic studies conducted on storage media for other organs, demonstrating the pertinence of this approach.
Through targeted biochemical analyses, we were able us to focus on exploring the metabolic stress associated with oxygen deprivation and a lack of energy substrates in the graft during ischemia and on the development of oxidative stress. Oxidative stress is classically described as occurring during the reintroduction of oxygen during the reperfusion phase [8]. However, several studies have shown that it may originate during the ischemia phase in tissues [23]. In our study, only the concentrations of glycine and alanine increased significantly after 24 h of CI. These increases, associated with a trend towards an increase in valine concentration, are suggestive of cell lysis, as these amino acids are present in the intracellular medium or of the lysis of the fibrous structures of the graft.
In 2005, Wranning et al. showed an increase in total glutathione and oxidized glutathione levels during a comparison of storage solutions in a study assessing the resistance of the uterine myometrium to CI in humans [24]. In our study, the GSSG/GSH ratio increased significantly after storage in the solution for 24 h, bearing witness to the oxidative stress experienced by the graft during the ischemia phase. Celsior® solution includes a high concentration of reduced glutathione, explaining the high levels measured in the initial state. We also found a non-significant trend towards an increase in the concentration of 8-isoprostane in the storage solution after 24 h. Overall, these results for glutathione and 8-isoprostane assays suggest that this increase is indicative of oxidative stress after 24 h of CI.
LDH activity appeared to have increased after 24 h of CI, but this increase was non-significant. This marker appears to indicate greater cell lysis after 24 h of storage, which was confirmed by the study of CK activity. Overall, these results confirm the association of myometrial muscle cell lysis with CI time. However, in a previous study, we demonstrated the macroscopic and histological preservation of myometrial function after prolonged CI [15]. Thus, although myometrial function is preserved, the graft clearly presents cellular lesions.
There was also a significant increase in the concentration of lactate in the storage solution. This reflects cellular hypoxia and a dysfunction of mitochondrial oxidative metabolism associated with metabolic acidosis of the graft, which develops during storage and worsens over time.
This preliminary study based on extreme CI times has highlighted different metabolite levels related to uterine injury. This feasibility study is the first step to investigate the tolerance of uterus graft to cold ischemia times such as 3 h, 6 h, and 12 h. These results could be also used to test different organ preservation solutions and also pharmacological compounds such as compounds already tested in kidney transplantation as trimetazidine [25] or evaluate new donor conditions such as donors deceased after cardiac death. The limitation of this study is the lack of quantitative viability markers; however, qualitative evaluation was performed based on the graft coloration during reperfusion after transplantation, contractions to mechanical stimulation, and venous and arterial Doppler blood flows.
Conclusion
Overall, the metabolic results suggest that the uterus undergoes significant degradation after 24 h of CI. The targeted biochemical analysis provided information about markers of metabolic stress during prolonged CI and made it possible to identify biomarkers reflecting the metabolic changes occurring in the uterus during this period. The metabolic analysis obtained from preservation solution samples during CI of the graft could become a predictive tool to assess uterine injury. These markers could be used as reproducible outcome measures for assessing the uterine response to CI and useful for future comparative studies. Thus, these results should be compared with those of a study of uterine graft functionality based on the occurrence of pregnancy after UTx and prolonged CI.
Compliance with ethical standards
The research protocol was accepted by the regional ethics committee for animal experimentation in the Limousin region (CREEAL) (approval number 06-2014-06). Animal welfare was ensured in accordance with EU Directive 2010-63.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flyckt R, Kotlyar A, Arian S, Eghtesad B, Falcone T, Tzakis A. Deceased donor uterine transplantation. Fertil Steril. 2017;107:e13. doi: 10.1016/j.fertnstert.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Kisu I, Kato Y, Obara H, Matsubara K, Matoba Y, Banno K, Aoki D. Emerging problems in uterus transplantation. BJOG Int J Obstet Gynaecol. 2018;125:1352–1356. doi: 10.1111/1471-0528.15230. [DOI] [PubMed] [Google Scholar]
- 3.Ejzenberg D, Andraus W, Baratelli Carelli Mendes LR, Ducatti L, Song A, Tanigawa R, et al. Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet Lond Engl. 2019;392:2697–2704. doi: 10.1016/S0140-6736(18)31766-5. [DOI] [PubMed] [Google Scholar]
- 4.Kisu I, Mihara M, Banno K, Umene K, Araki J, Hara H, Suganuma N, Aoki D. Risks for donors in uterus transplantation. Reprod Sci Thousand Oaks Calif. 2013;20:1406–1415. doi: 10.1177/1933719113493517. [DOI] [PubMed] [Google Scholar]
- 5.Lavoué V, Vigneau C, Duros S, Boudjema K, Levêque J, Piver P, Aubard Y, Gauthier T. Which donor for uterus transplants: brain-dead donor or living donor? A systematic review. Transplantation. 2017;101:267–273. doi: 10.1097/TP.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 6.Brännström M. Uterus transplantation and beyond. J Mater Sci Mater Med. 2017;28:70. doi: 10.1007/s10856-017-5872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bon D, Chatauret N, Giraud S, Thuillier R, Favreau F, Hauet T. New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol. 2012;8:339–347. doi: 10.1038/nrneph.2012.83. [DOI] [PubMed] [Google Scholar]
- 8.Favreau F, Giraud S, Bon D, Chatauret N, Thuillier R, Hauet T. Med Sci MS. 2013;29:183–188. doi: 10.1051/medsci/2013292016. [DOI] [PubMed] [Google Scholar]
- 9.Fiehn O, Kopka J, Dörmann P, Altmann T, Trethewey RN, Willmitzer L. Metabolite profiling for plant functional genomics. Nat Biotechnol. 2000;18:1157–1161. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- 10.Bonneau E, Tétreault N, Robitaille R, Boucher A, De Guire V. Metabolomics: perspectives on potential biomarkers in organ transplantation and immunosuppressant toxicity. Clin Biochem. 2016;49:377–384. doi: 10.1016/j.clinbiochem.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Barin-Le Guellec C, Largeau B, Bon D, Marquet P, Hauet T. Ischemia/reperfusion-associated tubular cells injury in renal transplantation: Can metabolomics inform about mechanisms and help identify new therapeutic targets? Pharmacol Res. 2018;129:34–43. [DOI] [PubMed]
- 12.Guy AJ, Nath J, Cobbold M, Ludwig C, Tennant DA, Inston NG, Ready AR. Metabolomic analysis of perfusate during hypothermic machine perfusion of human cadaveric kidneys. Transplantation. 2015;99:754–759. doi: 10.1097/TP.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 13.Bon D, Billault C, Claire B, Thuillier R, Hebrard W, Boildieu N, et al. Analysis of perfusates during hypothermic machine perfusion by NMR spectroscopy: a potential tool for predicting kidney graft outcome. Transplantation. 2014;97:810–816. doi: 10.1097/TP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 14.Dahm-Kähler P, Wranning C, Lundmark C, Enskog A, Mölne J, Marcickiewicz J, el-Akouri RR, McCracken J, Brännström M. Transplantation of the uterus in sheep: methodology and early reperfusion events. J Obstet Gynaecol Res. 2008;34:784–793. doi: 10.1111/j.1447-0756.2008.00854.x. [DOI] [PubMed] [Google Scholar]
- 15.Tricard J, Ponsonnard S, Tholance Y, Mesturoux L, Lachatre D, Couquet C, Terro F, Yardin C, Marquet P, Piccardo A, Gauthier T. Uterus tolerance to extended cold ischemic storage after auto-transplantation in ewes. Eur J Obstet Gynecol Reprod Biol. 2017;214:162–167. doi: 10.1016/j.ejogrb.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Wranning CA, Marcickiewicz J, Enskog A, Dahm-Kähler P, Hanafy A, Brännström M. Fertility after autologous ovine uterine-tubal-ovarian transplantation by vascular anastomosis to the external iliac vessels. Hum Reprod Oxf Engl. 2010;25:1973–1979. doi: 10.1093/humrep/deq130. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler F, Le Boucher J, Coudray-Lucas C, Cynober L. Plasma amino-acid determinations by reversed-phase HPLC: improvement of the orthophthalaldehyde method and comparison with ion exchange chromatography. J Autom Chem. 1992;14:145–149. doi: 10.1155/S1463924692000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei L, Xue T, Yang H, Zhao G-Y, Zhang G, Lu Z-H, Huang YH, Ma XD, Liu HX, Liang SR, Yang F, Chen BL. Modified uterine allotransplantation and immunosuppression procedure in the sheep model. PLoS One. 2013;8:e81300. doi: 10.1371/journal.pone.0081300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wranning CA, Dahm-Kähler P, Mölne J, Nilsson UA, Enskog A, Brännström M. Transplantation of the uterus in the sheep: oxidative stress and reperfusion injury after short-time cold storage. Fertil Steril. 2008;90:817–826. doi: 10.1016/j.fertnstert.2007.07.1340. [DOI] [PubMed] [Google Scholar]
- 20.Karam G. Safety of the use of Celsior in kidney-pancreas transplantation. Progres En Urol J Assoc Francaise Urol Soc Francaise Urol. 2003;13:46–49. [PubMed] [Google Scholar]
- 21.Testa G, Koon EC, Johannesson L, McKenna GJ, Anthony T, Klintmalm GB, et al. Living donor uterus transplantation: a single center’s observations and lessons learned from early setbacks to technical success. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2017;17:2901–2910. doi: 10.1111/ajt.14326. [DOI] [PubMed] [Google Scholar]
- 22.Brännström M, Johannesson L, Dahm-Kähler P, Enskog A, Mölne J, Kvarnström N, Diaz-Garcia C, Hanafy A, Lundmark C, Marcickiewicz J, Gäbel M, Groth K, Akouri R, Eklind S, Holgersson J, Tzakis A, Olausson M. First clinical uterus transplantation trial: a six-month report. Fertil Steril. 2014;101:1228–1236. doi: 10.1016/j.fertnstert.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Favreau F, Petit-Paris I, Hauet T, Dutheil D, Papet Y, Mauco G, Tallineau C. Cyclooxygenase 1-dependent production of F2-isoprostane and changes in redox status during warm renal ischemia-reperfusion. Free Radic Biol Med. 2004;36:1034–1042. doi: 10.1016/j.freeradbiomed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Wranning CA, Mölne J, El-Akouri RR, Kurlberg G, Brännström M. Short-term ischaemic storage of human uterine myometrium--basic studies towards uterine transplantation. Hum Reprod Oxf Engl. 2005;20:2736–2744. doi: 10.1093/humrep/dei125. [DOI] [PubMed] [Google Scholar]
- 25.Faure JP, Petit I, Zhang K, Dutheil D, Doucet C, Favreau F, Eugene M, Goujon JM, Tillement JP, Mauco G, Vandewalle A, Hauet T. Protective roles of polyethylene glycol and trimetazidine against cold ischemia and reperfusion injuries of pig kidney graft. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2004;4:495–504. doi: 10.1111/j.1600-6143.2004.00365.x. [DOI] [PubMed] [Google Scholar]