Abstract
Purpose
To better understand the characteristics of patients who returned to thaw their frozen eggs to attempt conception and their outcomes.
Methods
A retrospective analysis of clinical records for all own egg thaw patients in two UK fertility clinics across 10 years, 2008–2017.
Results
There were 129 patients who returned to thaw their eggs, of which 46 had originally frozen their eggs for social reasons and 83 for a variety of clinical, incidental, and ethical reasons (which we have called “non-social”). Women who had frozen their eggs for social reasons were single at time of freeze, with an average age of 37.7. They kept their eggs in storage for just under 5 years, returning to use them at the average age of 42.5. 43.5% were single at time of thaw, and 47.8% used donor sperm to fertilise their eggs. Women whose eggs were frozen for non-social reasons were almost all (97.6%) in a relationship at both time of freeze and thaw. They had an average age of 37.2 at first freeze and 37.6 at thaw, having kept their eggs in storage for an average of 0.4 years. Overall, there was a 20.9% success rate among women attempting conception with frozen-thawed eggs.
Conclusions
Despite widespread assumptions, many women attempting conception with thawed eggs had not initially frozen them for social reasons. Women who froze their eggs for social reasons presented distinctly and statistically different characteristics at both time of freeze and thaw to women whose eggs were frozen for non-social reasons.
Keywords: Egg freezing, Cryopreservation, Fertility preservation, Egg thaw, Sociology, Ethics
Introduction
Since its development during the first decade of the new millennium, the technology of oocyte vitrification, or egg freezing as it is more commonly known, has firmly established its place in both popular discourses and media representations [1]. However, it has taken longer to establish the empirical scholarship on this topic and to develop a rigorous understanding of the impact of the “egg freezing revolution” [2] on society, relationships, and our notions of reproductive ageing. Strikingly, although it was only in 2012, a mere 7 years ago, that the American Society for Reproductive Medicine (ASRM) lifted the “experimental” label from the practice of egg freezing [3], some have argued that we are now witnessing “the dawn of a new ice age” [4], in which a significant proportion of young women consider themselves to be “potential social oocyte freezers” [5, 6]. No doubt, the demonstrated successes of egg freezing, including a series of randomised controlled trials, which have suggested comparable fertilisation and pregnancy rates for in vitro fertilisation (IVF) cycles using fresh and frozen-thawed eggs [7–9], have been a key factor in the growth of this technology. In addition to these reassurances, there has also been a growing body of clinical and medical data exploring the obstetric and perinatal outcomes associated with vitrification, which suggest that there is no significant increase in congenital abnormalities for children born from vitrified eggs [10–12]. However, to date, much of the clinical research has looked at outcomes of frozen eggs from young egg donors that have no fertility impairments [13], without necessarily enough attention being directed at how and in what ways women electing to freeze and subsequently use their own eggs might differ from egg donor populations. It is these women that we focus on in this paper.
Until recently, we have had only very limited demographic information about women who have frozen their own eggs [14, 15], with our team conducting the first UK-based large-scale quantitative analysis of the characteristics women who had frozen their eggs [16]. In that paper, we were able to give details of the number of women freezing their eggs each year, as well as their age at egg freeze, relationship status, average number of cycles undertaken, and average number of eggs frozen. We were also able to identify different categories of egg freezing based on an analysis of 514 freezing cycles, although (due our methodology, which involved analysing clinic records as opposed to speaking with women themselves) we were unable to provide details of women’s experiences and motivations as they would have articulated them. Such qualitative and much needed insights into women’s own perspectives on and assessments of egg freezing have only recently begun to emerge, thanks to the efforts of medical anthropologists [17–19] and sociologists [20–23]. Currently, however, we remain mostly in the dark regarding how egg freezing impacts women’s lives and circumstances and have no long-term follow-ups that explore the reasons why women may never use their eggs, nor indeed the decision-making or circumstances of women who return to use their frozen-thawed eggs to attempt conception. Indeed, studies from both US [24] and UK [16] clinics suggest that around 95% of women who froze their eggs for social reasons still have them in storage several years later. While many more women may choose to use their eggs in the future, we simply do not know at this stage what these women’s clinical, social, and emotional experiences will be. This not only creates a fundamental gap in our understandings of the social uses and impacts of egg freezing technology but also has direct consequences for providing accurate information for women considering the procedure and challenges the possibilities for informed consent [25].
For example, while much of the public discussion around egg freezing has focused solely on women freezing their eggs for “medical” or so-called “social” reasons, egg freezing actually takes place in a variety of clinical situations used, for example, to manage the IVF treatment of low-responder patients [26, 27] or in cases of sperm unavailability or risk of ovarian hyperstimulation syndrome [28]. Indeed, our team’s prior analysis of all own egg freezing cycles at the London Women’s Clinic over a 5-year period revealed the importance of noting these “non-social” reasons for freezing eggs, which we conceptualised as distinct categories with statistically significant differences between them (from one another [16]). We were particularly struck to note that while social egg freezing (SEF) accounted for 75.7% of the freezing cycles in that dataset, it only corresponded with 14.9% of cycles in which eggs had already been thawed to attempt conception. This suggest that the information we have (for example from the HFEA) about women who have thawed their frozen eggs concerns mainly women who originally froze their eggs for non-social reasons and may therefore not be appropriate in counselling or informing women who are considering freezing their eggs for social reasons about their future success rates and outcomes. We would therefore argue that it is not only imperative that women receive accurate information about the potential success rates of a technology like egg freezing [29–31], but are able to discern which findings apply to them (or women in their situation), in order to make informed decisions.
In the UK, the Human Fertilisation and Embryology Authority (HFEA), the statutory body that regulates and inspects all clinics providing ARTs, has allowed the use of frozen eggs in fertility treatments since 2000 [32], but only began publishing national data pertaining to egg freezing in 2016 [33]. Their two reports released last year, in March and October 2018 [34, 35], expand on the information available by providing broad figures for egg freezing practice in the UK up to 2016. Although the HFEA data provides the most comprehensive information about egg freezing in the UK, the reports contain some important inconsistencies [36] and leave many questions regarding the practice of egg freezing and the characteristics of British women who freeze their eggs completely unanswered. For example, while the HFEA’s March 2018 report provides information on the age distribution of women who have frozen their eggs, there is no information on their relationship status [34]. In the October 2018 report, we are told that 53% of women freezing their eggs in 2016 were registered with a male partner, but are not told anything about the reasons why these partnered women decided to freeze their eggs [35]. Perhaps even more importantly, while we have some information about thaw cycles that have taken place in the UK and the age of women thawing their eggs, we do not have any information regarding, for example, the length of time women kept their eggs in storage for, or whether they used partner sperm or donor sperm to fertilise their thawed eggs [34, 35]. In general, only a small percentage of the thaw cycles reported in the latest HFEA report [35] are linked with corresponding freeze cycles, making it impossible to test on a larger scale our findings regarding the differing thaw rates of eggs corresponding to different categories of egg freezing (RBMO). Although there has already been some US-based discussions of the difficulties of reporting banking cycles, considering the increase of (both embryo and egg) banking cycles, it has now become imperative to address these difficulties [27, 37, 38]. Moreover, since the UK context has a reputation for being well regulated and thoroughly documented, these data omissions are both surprising and detrimental to our understanding of egg freezing practice.
Thus, in the current study, we aim to address some of these important gaps in knowledge, in particular regarding egg freezing and egg thawing in the UK. Our primary question concerns the characteristics of patients who have returned to thaw their eggs to attempt conception and the outcomes of these thaw cycles. Our analysis provides, for the first time, the complete demographic characteristics (e.g. age at freeze and thaw; relationship status at freeze and thaw) of all the women who thawed their eggs at the London Women’s Clinic (LWC) or the Bridge Centre over a 10-year period, from the first own egg thaw case, which occurred in 2008 to the end of 2017. We also provide a comparative exploration of the characteristics of women who froze their eggs for social and other non-social reasons, building on our previous claim [16] that it is crucial to record the “category of egg freezing” in order to adequately understand the different characteristics and trajectories of women who freeze their eggs in different circumstances. While we can only provide data from two clinics, since these clinics between them have carried out a large proportion of egg freeze and egg thaw cycles in the UK over the past 10 years, we believe the results to be illuminating and informative.
It is important to clarify at the outset precisely what we mean when we refer to “social” and “non-social” egg freezing in this paper. Throughout the existing literature, SEF has been the category most commonly associated with the phenomenon of egg freezing. This refers to women who have chosen to freeze their eggs for so-called “social reasons,” concerned primarily with age-related fertility decline and wishing to increase their chances of motherhood in the future. Although there are valuable debates regarding whether “social” is the best or most appropriate terminology for this type of egg freezing [15, 19], in this paper, we have decided to stay with it in order to differentiate between this and other forms of “elective” (i.e. non-medical) egg freezing.
Indeed, as discussed in our analysis of clinical egg freezing (CEF) data [16], there were several other categories of “elective” egg freezing within our sample. In this paper, we refer to them collectively as “non-social egg freezing” (in order to differentiate these from the better known category of “social egg freezing”). Within this “non-social” group, there were in fact three different categories, which we have termed CEF, IEF, and EEF. We explain the specificities of each of these categories below.
CEF refers to cycles in which eggs were frozen for “clinical” reasons, as an intentional part of the IVF treatment of certain patients. These patients were attempting conception through IVF, but had been advised to undergo egg freezing to “batch” eggs, either because they were expected to produce a low number of eggs per cycle or because they had a high likelihood of producing eggs with chromosomal abnormalities. As such, these patients were not using egg freezing to postpone conception per se, but to increase their chances of a healthy pregnancy through IVF (see also [26]).
IEF refers to the “incidental” freezing of eggs during what was intended to be a routine IVF cycle, when, for some reason, there was no sperm to enable fertilisation on the day of egg collection. In our sample, this included both patients whose male partners were unable to reach the clinic that day, as well as patients whose partner’s semen sample proved—unexpectedly—unusable. In these cases, the eggs were frozen (as opposed to discarded) with the intention to fertilise them as and when a viable sperm sample could be provided.
And finally, EEF referred to one egg freezing cycle in our sample in which the patient undergoing IVF wished to freeze her eggs because she found it ethically objectionable to create and store embryos.
It is interesting to note that there were no instances of medical egg freezing (MEF) within our sample of women who had come to thaw their eggs, even though this is the second most recognised category of egg freezing in the literature. The explanation for this is the fact that we were looking at records from two private clinics, and in the UK, most women who wish to access MEF are likely to do so through NHS clinics with public funding.
Materials and methods
Data for this paper were gathered using a retrospective evaluation of the LWC’s and the Bridge Centre’s existing documentation regarding past patients. Patient records and laboratory data were used to create a comprehensive database of all own frozen egg thaw cycles undertaken at the LWC and the Bridge Centre.
First, all treatment cycles logged as “own egg thaw” at the LWC and the Bridge Centre were compiled in a spreadsheet. Then, the entries were double-checked manually to ensure that all were instances of own egg thaws, and any cycles of frozen donor eggs were excluded. (For the rest of this paper, for convenience, “egg freezing” and “egg thaw” refer exclusively to “own egg” cycles and exclude any freezing or thaw cycles that were part of egg donation programmes.) These cycles were then linked with the original corresponding egg freeze cycles of the same patients. This left us with 151 thaw cycles over a 10-year period, beginning with the very first egg thaw cycle in 2008 and ending at the end of 2017. In total, the study sample represents 151 cycles of egg thaw undertaken by 129 patients, who had frozen their eggs in a total of 220 original egg freezing cycles. The difference between the number of freeze and thaw cycles can be accounted for by the fact that some women underwent several cycles of egg freezing (particularly those in the CEF category, as we have descried above), but thawed all of their eggs in one thaw cycle.
Researchers reviewed and cross-checked the clinical entries and lab records for each of the 129 patients identified as having thawed their eggs and created an extensive excel database containing all relevant information with regard to these patients’ egg freeze and egg thaw cycles. In this way, for each patient, we are able to compile data on: patient ID; date of birth; age at first egg freeze cycle; total number of egg freezing cycles undertaken; dates for each egg freezing cycle undertaken; number of eggs frozen per cycle; total number of eggs frozen; reason for egg freezing; relationship status at time of egg freeze; location where eggs were frozen; years eggs were in storage; number of thaw cycles undertaken; dates of thaw cycle(s); number of eggs thawed (total and per cycle); age at thaw cycle(s); relationship status at time of thaw; sperm source at thaw (partner or donor sperm); outcome; and current status (regarding any additional eggs or embryos remaining in storage).
Once the above data were recorded, they were examined and analysed. Building on our previous analysis of egg freezing data [16], we explored the differences in this dataset between patients who had originally frozen their eggs for social reasons with patients who had frozen for other, non-social, reasons (including CEF, IEF, and EEF as described above).
An in-depth numerical analysis was undertaken on various aspects of the data using Excel workbooks. This included a range of measures, including for example, calculating the average numbers of eggs frozen per cycle by patients in each category and the average numbers of freezing cycles undertaken by each patient; determining the age ranges and average ages of patients in each category at both the time of freeze and thaw; and working out the comparative percentages of patients who were in a relationship and patients who were single at both time of freeze and time of thaw. These calculations were used to create result tables and figures to illustrate specific properties of this complex dataset as clearly as possible.
Statistical analyses
Statistical analyses using the chi-squared test were applied as appropriate, in particular to determine the statistical significance of the observed differences between the thaw cycles of eggs originating from social versus non-social egg freezing cycles.
Freezing and thawing techniques
Almost all of the thaw cycles (145/151) included in our sample refer to the thawing of eggs, which were originally frozen either at the LWC or the Bridge Centre using the vitrification method. These eggs were denuded of cumulus cells with hyaluronidase solution (Irvine, USA) approximately 2 h after egg collection. Throughout the years, different vitrificvation protocols have been used. The majority of them have occurred with Kitazato, but some older ones were done with COOK, Medicult, or SAGE Media. The vitrification protocol is in most cases the same with slight modifications. The eggs once denuded were then vitrified in two steps at room temperature (24–26 °C). They are firstly moved for 12–15 min in an equilibrium solution, which contained 7.5% DMSO and 7.5% EG. Eggs showing full recovery to normal size were then transferred to the vitrification solution, which contained 15% DMSO and EG plus 0.5-m sucrose for 70–80 s. Using a minimum of vitrification solution, eggs were loaded in Cryotop straws (Kitazato, Japan) as quickly as possible and plunged into liquid nitrogen for storage. The remaining six thaw cycles refer to eggs that were moved to the LWC Harley Street laboratory after being frozen at other clinics (four in Croydon; one at Bourn Hall; one at Kent).
For all cycles of egg thawing, three welled plates from Cryotech were used. The wells contained warming solutions at 37 °C, with declining concentrations of sucrose (1.0, 0.5, and 0.0 m). The Cryotop straws containing the vitrified eggs were removed from storage, moved to liquid nitrogen, and then moved to 0.7 ml of pre-warmed (37 °C) 1.0 M of sucrose solution. The eggs were then moved through the prepared sucrose enriched solutions (50 μl each) with each step lasting 3–5 min. Eggs that showed signs of full recovery were transferred for (2–3 h) culture before commencing ICSI.
Ethical approval
This retrospective analysis did not require ethical or institutional review board approval, as it assessed laboratory and clinical records from previously validated and approved procedures, practised under licence from the HFEA.
Results
As can be seen in Table 1, our dataset included 151 thaw cycles undertaken by 129 patients across the 10 years. Many of the patients originally undertook multiple cycles of egg freezing, and some batched eggs frozen across multiple cycles to be thawed in one go.
Table 1.
Number of thaw cycles, patients, and corresponding freeze cycles 2008–2017
| Thaw cycles n (%) |
Patients n (%) |
Corresponding freeze cycles n (%) |
|
|---|---|---|---|
| All | 151 (100%) | 129 (100%) | 220 (100%) |
| Social egg freezing | 55 (36.4%) | 46 (35.7%) | 69 (31.4%) |
| Non-social egg freezing | 96 (63.6%) | 83 (64.3%) | 151 (68.6%) |
| Clinical | 58 | 49 | 117 |
| Incidental | 33 | 34 | 33 |
| Ethical | 5 | 1a | 1 |
aThe one patient who froze her eggs for ethical reasons froze 12 eggs following a single egg retrieval (hence, one cycle of egg freezing) and thawed these in five different egg thaw cycles. The second thaw cycle led to a live birth; the remaining four were unsuccessful
In terms of categories of egg freezing, 55 of the thaw cycles, undertaken by 46 patients, refer to 69 SEF cycles. The remaining 96 thaw cycles correspond to 83 patients who had originally frozen their eggs for “non-social” reasons, including CEF, IEF, and EEF. Brief descriptions of each of these categories have been provided above. Although we have provided a breakdown of our data in these various categories, for the purposes of this article, we are primarily interested to note the differences and distinctions between patients (both at time of freeze and at time of thaw) who originally froze their eggs for social reasons (SEF) versus those whose eggs were frozen for non-social reasons (non-SEF). This is to clearly demonstrate that egg freezing does not refer to a homogenous practice that is synonymous with “social egg freezing.” SEF accounts for approximately a third of our sample, and non-SEF makes up two thirds.
Number of treatment cycles and number of eggs frozen
Table 2 presents details of the average number of egg freezing cycles undertaken by patients in the different categories, the average number of eggs frozen per cycle, and the average number of total eggs frozen by patients. As can be seen, while the average number of eggs frozen in one cycle (across all patients) is 7.2, the average total number of eggs per patient is 12.2, because many patients underwent more than one cycle of egg freezing. Women who froze their eggs for social reasons froze on average a total of 14 eggs.
Table 2.
Number of egg freezing treatment cycles undertaken and number of eggs frozen
| Average number of freezing cycles undertaken per patient | Average number of eggs frozen per cycle | Average number of total eggs frozen by patient | |
|---|---|---|---|
| All | 1.7 (Range 1–8) | 7.2 (Range 0–27) | 12.2 (Range 1–55) |
| Social egg freezing | 1.5 | 9.3 | 14.0 |
| Non-social egg freezing | 1.8 | 6.1 | 11.2 |
| Clinical | 2.4 | 5.3 | 12.7 |
| Incidental | 1 | 9.1 | 9.1 |
| Ethical | 1 | 12 | 12 |
Age at freeze and thaw
Looking at the average age of patients across our sample (see Table 3), we note that while there is only a minimal difference in age at first freeze between SEF and non-SEF (37.7 vs. 37.2, respectively), there is a great difference between these groups when we look at age at first thaw (42.5 vs. 37.6). This is because while SEF patients have kept their eggs in storage for an average of 4.8 years before thawing them to attempt conception, across the non-SEF sample, eggs were thawed on average 0.4 years after freezing.
Table 3.
Age at freeze vs. age at thaw (by patient)
| Average age at first freeze | Average age at first thaw | Average number of years between first freeze and first thaw | |
|---|---|---|---|
| All | 37.4 (range 25–45) | 39.4 (range 25–50) | 2.0 (range 0–10) |
| Social egg freezing | 37.7 | 42.5 | 4.8 |
| Non-social egg freezing | 37.2 | 37.6 | 0.4 |
| Clinical | 38.9 | 39.2 | 0.3 |
| Incidental | 34.8 | 35.5 | 0.7 |
| Ethical | 30.0 | 31.0 | 1 |
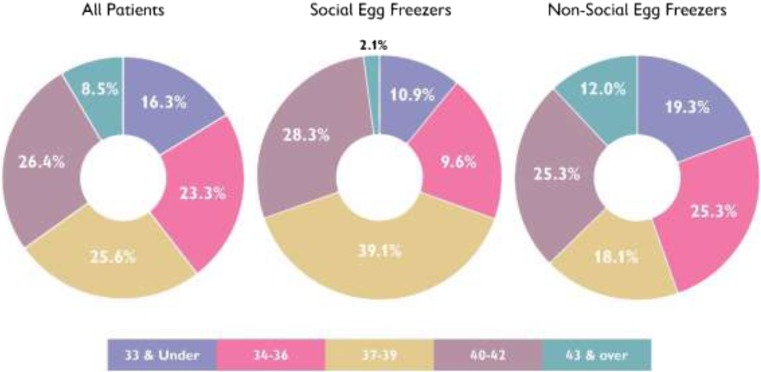
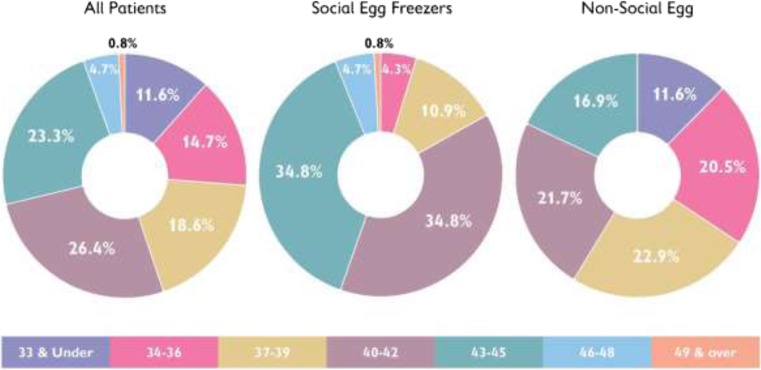
Moreover, we can see the different distribution of ages for SEF and non-SEF at both time of first freeze (Fig. 1) and at time of first thaw (Fig. 2). Looking at Fig. 2 in particular, we note that while 75.1% of SEF patients were aged 40 or over at time of first thaw, this was only true for 38.6% of non-SEF patients who tended to thaw their eggs at a younger age.
Fig. 1.
Age at first freeze. Comparisons between all patients, social egg freezers, and non-social egg freezers
Fig. 2.
Age at first thaw. Comparisons between all patients, social egg freezers, and non-social egg freezers
Relationship status at freeze and thaw
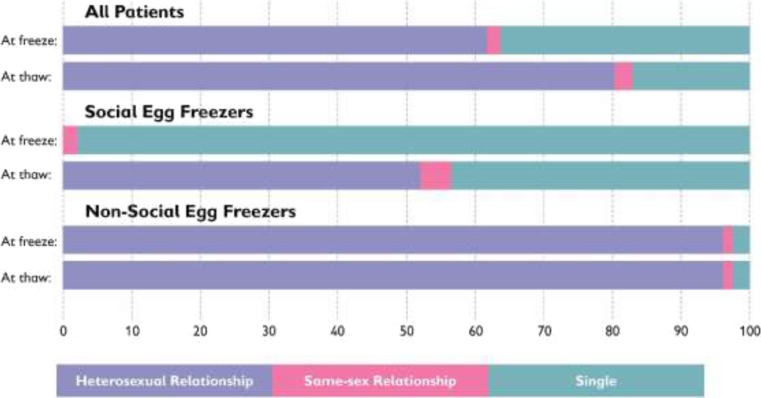
One of the most striking differences between SEF and non-SEF patients related to their relationship status at both time of freeze and time of thaw is shown in Table 4. Almost all of the SEF in our sample (97.8%) were single at time of first freeze, and while 52.2% were in heterosexual relationships when they came back to thaw their eggs, a substantial 43.5% were still single. Non-SEF on the other hand was almost all in heterosexual relationships (96.4%) at both time of freeze and time of thaw. As can be seen clearly in Fig. 3, while there is a noteworthy difference between relationship at freeze and thaw among SEF patients, there is no difference at all in the relationship status of patients who were non-SEF. The difference between the relationship status of SEF and non-SEF patients was statistically significant at both time of freeze (x2 = 112.3, P < 0.01) and time of thaw (x2 = 32.4, P < 0.01).
Table 4.
Relationship status at freeze vs relationship status at thaw (by patient)
| Relationship status at freeze n (%) |
Relationship status at thaw n (%) |
|||||
|---|---|---|---|---|---|---|
| Heterosexual relationship | Same-sex relationship | Single | Heterosexual relationship | Same-sex relationship | Single | |
| All | 80 (62.0%) | 2 (1.6%) | 47 (36.4%) | 104 (80.6%) | 3 (2.3%) | 22 (17.1%) |
| Social egg freezing | 0 | 1 (2.1%) | 45 (97.8%) | 24 (52.2%) | 2 (4.3%) | 20 (43.5%) |
| Non-social egg freezing | 80 (96.4%) | 1 (1.2%) | 2 (2.4%) | 80 (96.4%) | 1 (1.2%) | 2 (2.4%) |
| Clinical | 46 | 1 | 2 | 46 | 1 | 2 |
| Incidental | 33 | 0 | 0 | 33 | 0 | 0 |
| Ethical | 1 | 0 | 0 | 1 | 0 | 0 |
Fig. 3.
Relationship status at a time of freeze and thaw
Sperm source for fertilisation
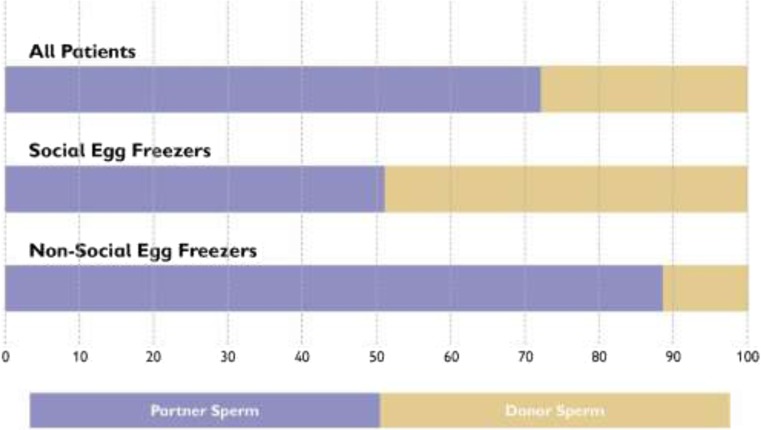
Related to the question of relationship status at time of thaw, we can see in Fig. 4 that almost half of SEF patients (47.8%) used donor sperm to fertilise their thawed eggs, compared with a very small minority (10.8%) of non-SEF patients, representing a statistically significant difference between the two groups (x2 = 20.2, P < 0.01). All but two of the SEF patients who used donor sperm were single; the other two were in same-sex relationships. Of the nine non-SEF patients who used donor sperm, two were single, one was in a same-sex relationship, and six were in heterosexual relationships but were not able to use their partner’s sperm. All of the latter six women had originally undertaken IEF, because sperm had not been available on the day of egg collection (Table 5).
Fig. 4.
Sperm source for fertilising thawed eggs
Table 5.
Sperm source for fertilisation (by patient)
| Partner sperm n (%) |
Donor sperm n (%) |
|
|---|---|---|
| All | 98 (76.1%) | 31 (24.0%) |
| Social egg freezing | 24 (52.2%) | 22 (47.8%) |
| Non-social egg freezing | 74 (89.2%) | 9 (10.8%)a |
| Clinical | 46 | 3 |
| Incidental | 27 | 6 |
| Ethical | 1 | 0 |
aOf the nine women who were in the non-social egg freezing category and used donor sperm, two were single, one was in a same-sex relationship, and six were in heterosexual relationships but partner sperm was not available
Outcome of thaw cycles
In this paper, we have chosen to report success rate outcomes per patient (rather than per thaw cycle or per transfer), including the result of cumulative thaw cycles where appropriate. This was considered to be the most meaningful way to represent the “outcome” of thaw cycles, since this (i.e. the percentage of women who successfully become pregnant and give birth following the thawing of their frozen eggs) is the information our patients most often request to help them make informed decisions about egg freezing.
In our analysis, as represented in Table 6, we designated the egg thaw outcome for each patient as falling into one of three categories: (1) successful, (2) unsuccessful, and (3) pending.
Table 6.
Outcome of egg thaw (by patient)
| Successful n (%) |
Unsuccessful n (%) |
Pending n (%) |
|
|---|---|---|---|
| All | 27 (20.9%) | 76 (58.9%) | 26 (20.2%) |
| Social egg freezing | 8 (17.4%) | 26 (56.5%) | 12 (26.1%) |
| Non-social egg freezing | 19 (22.9%) | 50 (60.2%) | 14 (17.0%) |
| Clinical | 10 | 27 | 12 |
| Incidental | 8 | 23 | 2 |
| Ethical | 1 | 0 | 0 |
The first category, comprising 20.9% of patients, refers to those women who had a successful outcome, meaning either a live birth or a continuing pregnancy at the time of analysis. Almost half of these women also had additional frozen eggs or embryos still in storage (potentially giving them the option to try for further children in the future).
The second category, comprising 58.9% of patients, refers to those women who had been unsuccessful in having a child from their frozen-thawed eggs, meaning that they had exhausted all of their frozen eggs without achieving a live birth (or a continuing pregnancy).
The third category, covering the final 20.2% of patients, we categorised as having a “pending” outcome, because even though they had thawed their eggs and had not yet been successful, they still had eggs or embryos (created from their thawed eggs) in storage, which could potentially lead to successful outcomes in the future. At the time of analysis, some of these women had thawed their eggs to create embryos but had not yet had an embryo transfer; others had had one or more (unsuccessful) embryo transfers already but still had the option to try for more. We would expect to see some further successes among this group as women use their remaining eggs, in time increasing the overall success rates among these 129 patients.
The success rates for non-SEF (22.9%) are higher than for SEF (17.4%), but since our numbers are small this is not a statistically significant difference (x2 = 0.3, P = 0.61).
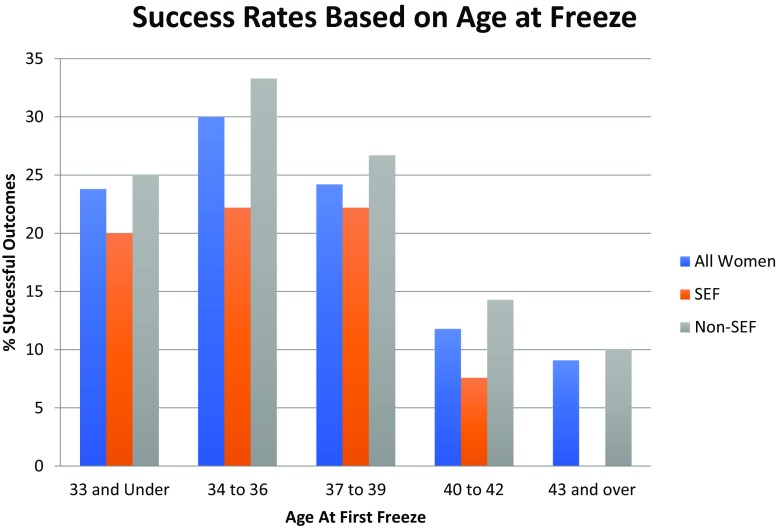
Figure 5 shows the relationship between age at freeze and outcome of the thaw cycle. Although the numbers are small, it is worth noting that this graph clearly shows the percentage of positive outcomes declining drastically as age at first freeze increases above 40.
Fig. 5.
Percentage of successful outcomes for women based on age of first freeze (per patient). Note for Fig. 5: “success” refers to live birth or ongoing pregnancy at time of data analysis
Discussion
The results of our analysis provide the most detailed information to date about the characteristics of women who return to use their frozen eggs for conception in the UK. It is relevant to note that while the majority of discussions around egg freezing assume that eggs have been frozen for “social” reasons, this was only true for approximately a third of our sample. The remaining two thirds of women had originally frozen their eggs for a variety of non-social reasons, including the categories CEF, IEF, and EEF. Building on our previous analysis of egg freezing data [16], this shows that different categories of egg freezing patients may make significantly different decisions following the storage of their eggs and that the subset who decide to subsequently attempt conception with their eggs may disproportionately represent some categories over others. In fact, there is now enough evidence to suggest that women who have thawed their eggs in the UK to date are a very specific sub-group of the women who have frozen their eggs, made up predominantly of those women whose eggs were frozen as part of an IVF cycle (either because this was a clinically indicated decision to “batch” multiple cycles of eggs, or because there was no sperm sample available on the day of egg collection) [16]. This should make us aware of our hitherto unexamined assumptions, namely that it is social egg freezers who have been coming back to thaw their eggs. Indeed, it seems that many women who froze their eggs for social reasons still have these eggs in storage, and we should be cautious both about predicting their “thawing” behaviour in the future and about how we present thaw information, aiming to delineate its applicability more clearly to the relevant categories of egg freezing patients.
As discussed, the average age of all groups in this sample was quite similar at time of freeze, at around 37 years old. This mean age at the time of egg freezing has also been reported by other studies in other geographical locations, such as Australia [39], and was interestingly calculated, using a decision-analysis model assessment by Mesen et al. [40] as precisely the age at which egg freezing provides the greatest improvement in probability of live birth compared with no action and is thus most cost-effective. Yet, it is important to note that although 37 may be the mean age for egg freezing and the age at which these treatments can be considered “most cost-effective,” it is undeniable that by 37, both the quality and quantity of a woman’s eggs have already diminished, making this—from a clinical and biological perspective—a suboptimal age for freezing one’s eggs. This is confirmed by our results showing success rates diminishing with increased age at freeze. As Mertes and Pennings [41] note, the diminished chance of potential success when eggs are frozen at 37 and above creates not only practical but also ethical issues for this technology. While women freezing for non-social reasons may have little leeway in terms of when they freeze their eggs (since they are already undergoing fertility treatment), women freezing for social reasons would benefit from better information and awareness about fertility decline and the diminished chances of conception using eggs frozen at later ages. However, it is also important to note that there is currently a 10-year storage limit on frozen eggs in the UK, which may act as a disincentive for British women to freeze their eggs when they are at their most fertile, for fear of having to discard their eggs before they are ready to use them [42] a tough choice that has already been faced by some of the pioneers of this technology [43]. We would thus also encourage the adoption of better institutional, structural, economic, and legal conditions that can enable women to freeze their eggs at a more clinically optimal age than the current average of 37, if they wish to do so.
While the average age at first freeze was similar for women in the different categories of egg freezing across our sample, it is striking that the average age at first thaw was very different. This is explained by the fact that while the women in the SEF category in our sample had stored their frozen eggs for an average of 4.8 years, women in the non-SEF category had used them almost immediately, after an average of 0.4 years. These findings are not surprising given the reason for freeze. Non-SEF (not including medical reasons in this sample) included women who were trying to conceive using IVF, whereas SEF included women who were wishing to bank their eggs to preserve their fertility for the future. Women who freeze their eggs for medical reasons, though not present in this study, may also keep their eggs in storage for longer periods because they are about to undergo a medical procedure which may take time to recover from and which may have occurred many years before their desired timing for conception [44]. These findings indicate that birth, pregnancy, and obstetrical outcome measures for women using their own frozen-thawed eggs must differentiate between women who intended to store their eggs for conception in the future, at a time when their fertility had declined (i.e. SEF or MEF), and women whose eggs were frozen as part of an IVF cycle in which they were seeking to conceive as soon as possible (e.g. CEF, IEF, and EEF). One way for the HFEA to do this in the UK would be to introduce a distinction between eggs that are (a) frozen for fertility preservation and future use and (b) cryopreserved as part of a treatment cycle with the intention to transfer an embryo within 12 months (a similar distinction between fertility preservation and short-term freezing has been identified by Kulak et al. (2016) to identify missing cycles from the Society for Assisted Reproductive Technologies (SART) database).
Since the age at thaw is different between different egg freezing groups, with women in the SEF category being on average 5 years older at time of thaw, we could reasonably hypothesise that, as well as any differences in IVF treatment results, there may be some increase in adverse outcomes for women seeking conception with frozen eggs after SEF (and longer-term storage), who are more likely to thaw their eggs when they are over the age of 40, compared with women in the non-SEF category. Unfortunately, we did not have data on the pregnancy or birth experiences of women from this dataset; however, it would be extremely informative to include this type of data in future research. Especially as older motherhood becomes more common [2], we would recommend for obstetricians and gynaecologists to systematically study and report the pregnancy and birth experiences and outcomes for older women and particularly for women using their own frozen-thawed eggs and for their findings to inform future practice in fertility clinics as well as the decision-making processes of women considering egg freezing or fertility options more broadly.
It is also important to note that our data indicated significant differences between women in the SEF and non-SEF categories with regard to their relationship status. While the latter show no difference between their relationship status at time of freeze and time of thaw, with the great majority of those in the non-SEF category being in heterosexual relationships, the relationship status of women who froze their eggs for social reasons tells a completely different story. These women, as expected, are almost all single at the time of egg freezing, in line with findings from qualitative studies which report their main reasons for freezing as not being in a relationship, or not being in the right relationship and their motivations as a desire to “buy time” and to avoid “panic partnering” [15, 18–22]. These women hope to meet partners before starting their families and are “anticipating coupledom” in the future [17]. Meeting such expectations, 52.2% of the women undergoing a thaw cycle who had originally frozen their eggs for social reasons were indeed in a heterosexual relationship when they came back to use their eggs, fertilising them with their partner’s sperm to start their much-anticipated families. However, at this stage, 43.5% were still single and had returned to the clinic to fertilise their eggs using donor sperm. This subsequent foray into solo motherhood—for almost half of the women in this category—is an important social reality, but one that remains currently invisible in media discourses or imaginaries surrounding social egg freezing. Lacking qualitative data on the experiences and views of these women, it is not possible for us to know exactly why they returned to pursue solo motherhood; in some cases this may represent the disappointment of partnering hopes, while others may simply have decided that the time is right to become a mother. It is important that the voices of these women be heard and that attention is paid to their experiences in future qualitative research.
The outcomes reported for this population of women who have thawed their frozen eggs at the LWC and the Bridge Centre, with a total success rate of 20.9%, are encouraging, particularly because our dataset includes all thaw cycles over the past 10 years. This figure is higher than the latest national data provided by the HFEA, which notes a birth rate of 18% for women using their own eggs in thaw cycles in 2016 [35] and suggests to us that specialist laboratories and clinics may, especially in recent years, be able to provide patients with success rates above the national average. However, despite this, we must note that, as others have argued, our figures also confirm that egg freezing must never be considered a “guarantee” of future conception. It is crucial that this message is clearly and repeatedly delivered to women considering freezing their eggs, so that they can make fully informed decisions about their future.
Since women interviewed in qualitative research report a desire for more detailed information about egg freezing and complain of a lack of sustained discussion regarding post freezing processes and outcomes [21], it is very important for data, such as reported in this paper, to be made more readily available to patients, both within clinics and by the HFEA. Moreover, since women report receiving most of their information about egg freezing from media sources [21, 45], it is also crucial for press and television reports to supplement opinion and human interest pieces on these issues with reliable empirical data whenever possible.
Strengths and limitations of the study
It is a central strength of the current study that all thaw cycles performed in two large clinics, the LWC and the Bridge Centre from the first case in 2008 to the end of 2017 (when data were gathered), were included in the analysis. This study is also the first instance, as far as we are aware, of detailed demographics being provided for patients who have thawed their eggs, incorporating a comparison between those that froze their eggs for social and non-social reasons.
The limitations of the study concern our relatively small sample (129 patients and 151 thaw cycles). Since the technology is still growing and since the numbers of women freezing their eggs in these clinics have multiplied threefold in the last 3 years [16], we would expect significantly higher numbers of women to thaw their eggs in the upcoming decade. It is important to note that as the UK has a 10-year storage limit for frozen eggs, we will be able to have final outcomes for all patients by the end of this period and will be able to note what percentage of women who freeze their eggs ever come back to use them. As of yet, since egg freezing is still a relatively new practice with small numbers of women thus far having thawed their eggs, we are unable to have conclusive and comprehensive data about the practice of egg freezing and thawing. It is also a limitation that our data cover only two UK clinics, albeit two clinics that are particularly active in egg freezing; we would not only urge other clinics to perform similar analyses on their data, but better still we hope to persuade the HFEA of the necessity and merits of analysing national data in this detailed manner.
It is notable that in this study, 20.2% of women who had not yet had successful outcomes still had eggs or embryos from thawed eggs in storage. We anticipate that some of these women will have successful outcomes, but in the meantime, they have been listed as pending. Such pending outcomes are a necessary component of research into fertility treatments, as it may take years for women to complete their fertility journeys and to use all of their frozen material; yet, it is important in the meantime to have data on successful outcomes, so that other women can make informed decisions about whether or not they want to freeze their eggs.
Future research
The details provided in this paper about women who are using their frozen-thawed eggs to attempt conception provides a brief snapshot of their characteristics, circumstances, and outcomes and has enabled us to arrive at the conclusions noted above. However, we feel that our data also give rise to myriad further questions, which could not be answered by the current research and analysis—for example, what have been the life trajectories of women who decide to freeze their eggs for social reasons and what are their experiences of relationships? What are their views about later motherhood or solo motherhood using donor sperm to conceive? How are their lives impacted when their thaw cycles result in successful pregnancies, or perhaps even more crucially, when they do not? These are questions that can only be answered with detailed qualitative research that seeks to elicit the thoughts, feelings, and opinions of women who have frozen and thawed their eggs and provides their narratives in their own words. It is the intention of the first author to work towards answering some of these questions in future research, aiming to flesh out the stories of these “social pioneers” of egg freezing beyond these figures.
Acknowledgements
The authors gratefully acknowledge the clinical work undertaken by a large team of clinicians, nurses, and embryologists that has enabled this data analysis to occur. We appreciate the suggestions made by Professor Susan Golombok on earlier drafts and are thankful to Elena Linara-Demakakou for her help with the statistical analyses. We would also like to thank the editor of JARG and the anonymous reviewers for their helpful comments during the peer review process.
Author contributions
ZG—designed concept and analysis; collected and analysed data; and wrote the paper.
LM—developed database and analysed data.
DO—helped with initial stage of data collection.
JW—provided access to data, as head of lab from which data were gathered.
KA—helped in concept design; provided overview; head of clinic from which data was gathered.
Funding information
There was no independent funding received for the study.
Compliance with ethical standards
Conflict of interest
There is no financial or commercial conflict of interest. During the period of research, all authors were working in the clinic from which the data was obtained, although some have now moved to other institutions.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zeynep B. Gürtin, Email: z.gurtin@ucl.ac.uk
Lucy Morgan, Email: z.gurtin@ucl.ac.uk.
References
- 1.Van de Wiel L. For whom the clock ticks: reproductive ageing and egg freezing in Dutch and British news media. Studies in the Maternal. 2014;6(1).
- 2.Inhorn, M.C. (2017) The egg freezing revolution? Gender, technology, and fertility preservation in the twenty-first century. In Emerging trends in the social and behavioural sciences. John Wiley & Sons, Inc.. Retrieved from: https://marciainhorn.com/wp-content/uploads/etrds0428.pdf.
- 3.American Society for Reproductive Medicine (2012) Mature oocyte cryopreservation: a guideline. https://www.scribd.com/document/339331836/ASRM-2012-Mature-Oocyte-Cryopreservation-A-Guideline
- 4.Jones BP, Saso S, Mania A, Smith JR, Serhal P, Ben Nagi J. The dawn of a new ice age: social egg freezing. Acta Obstet Gynecol Scand. 2018;97(6):641–647. doi: 10.1111/aogs.13335. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien Y, Martyn F, Glover LE, Wingfield MB. What women want? A scoping survey on women’s knowledge, attitudes and behaviours towards ovarian reserve testing and egg freezing. Eur J Obstet Gynecol Reprod Biol. 2017;217:71–76. doi: 10.1016/j.ejogrb.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Stoop D, Nekkebroeck J, Devroey P. A survey on the intentions and attitudes towards oocyte cryopreservation for non-medical reasons among women of reproductive age. Hum Reprod. 2011;26(3):655–661. doi: 10.1093/humrep/deq367. [DOI] [PubMed] [Google Scholar]
- 7.Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remohi J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89:1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 8.Parmegiani L, Cognigni GE, Bernardi S, Cuomo S, Ciampaglia W, Infante FE, Tabarelli de Fatic C, Arone A, Maccarini AM, Filicon M. Efficinetcy of aspetic open vitrification and hermetical cryostorage of human oocytes. Reprod BioMed Online. 2011;23:505–5012. doi: 10.1016/j.rbmo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Rienzi L, Romano S, Albricci L, Maggiuli R, Capalbo A, Baroni E, Colamaria S, Sapienza F, Ubaldi F. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;24:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chian R, Huang JYJ, Tan SL, Lucena E, Saa A, Rojas A, Castellón LAR, Amador MIG, Sarmiento JEM. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod BioMed Online. 2008;16(5):608–610. doi: 10.1016/S1472-6483(10)60471-3. [DOI] [PubMed] [Google Scholar]
- 11.Cobo A, Serra V, Garrido N, Olmo I, Pellicer A, Remohi J. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril. 2014;102(4):1006–1015. doi: 10.1016/j.fertnstert.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod BioMed Online. 2009;18(6):769–776. doi: 10.1016/S1472-6483(10)60025-9. [DOI] [PubMed] [Google Scholar]
- 13.Schattman GL. A healthy dose of reality for the egg-freezing party. Fertil Steril. 2016;105(2):307. doi: 10.1016/j.fertnstert.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin K, Culley L, Hudson N, Mitchell H, Lavery S. Oocyte cryopreservation for social reasons: demographic profile and disposal intentions of UK users. RBMOnline. 2015;31:239–245. doi: 10.1016/j.rbmo.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Inhorn MC, Birenbaum-Carmeli D, Westphal LM, Doyle, Joseph G, Norbert M, Dirnfeld M, Seidman D, Kahane A, Patrizio P. Elective egg freezing and its underlying socio-demography: a binational analysis with global implications. Reprod Biol Endocrinol. 2018;16:70. doi: 10.1186/s12958-018-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.XXX Authors (2018) Reconceiving egg freezing: insights from an analysis of five years of data from a UK clinic. RBMOnline, [DOI] [PubMed]
- 17.Carroll K, Kroløkke C. Freezing for love: enacting “responsible” reproductive citizenship through egg freezing. Cult Health Sex. 2018;20(9):992–1005. doi: 10.1080/13691058.2017.1404643. [DOI] [PubMed] [Google Scholar]
- 18.Inhorn MC, Birenbaum-Carmeli D, Westphal LM, Doyle J, Gleicher N, Dirnfeld M, Seidman D, Kahane A, Meirow D, Patrizio P (2017) Gender and educational disparities underlying elective egg freezing: results from the first major qualitative study of oocyte cryopreservation in the United States and Israel. Poster presented at: 33rd Annual Meeting of the European Society of Human Reproduction and Embryology. 2–5 July 2017: Geneva, Switzerland.
- 19.Inhorn MC, Birenbaum-Carmeli D, Westphal LM, Doyle, Joseph G, Norbert M, Dirnfeld M, Seidman D, Kahane A, Patrizio P. Ten pathways to elective egg freezing: a binational analysis. J Assist Reprod Genet. 2018;35(11):2003–2011. doi: 10.1007/s10815-018-1277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldwin K. ‘I suppose I think to myself, that’s the best way to be a mother’: how ideologies of parenthood shape women’s use of social egg freezing technology. Sociol Res Online. 2017;22(2):2. doi: 10.5153/sro.4187. [DOI] [Google Scholar]
- 21.Baldwin K, Culley L. Women’s experience of social egg freezing: perceptions of success, risks, and ‘going it alone’. Hum Fertil. 2018;1:1–7. doi: 10.1080/14647273.2018.1522456. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin K, Culley L, Hudson N, Mitchell H. Running out of time: exploring women’s motivations for social egg freezing. J Psychosom Obstet Gynecol. 2018;12:1–8. doi: 10.1080/0167482X.2018.1460352. [DOI] [PubMed] [Google Scholar]
- 23.Martin LJ. Anticipating infertility: egg freezing, genetic preservation, and risk. Gend Soc. 2010;24(4):526–545. doi: 10.1177/0891243210377172. [DOI] [Google Scholar]
- 24.Myers C, Daily Z, Jain J. Why do so few women return to utilize cryopreserved oocytes?, qualitative insights into elective oocyte cryopreservation. Fertil Steril. 2015;103(2):e30–0.
- 25.Jackson E. The ambiguities of “social” egg freezing and the challenges of informed consent. BioSocieties. 2017;13:1–20. [Google Scholar]
- 26.Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a news strategy for managing low-responder patients. Reprod BioMed Online. 2012;23:824–829. doi: 10.1016/j.rbmo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Doody KJ. Cryopreservation and delayed embryo transfer- assisted reproductive technology registry and reporting implications. Fertil Steril. 2014;102:27–31. doi: 10.1016/j.fertnstert.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Cobo A, Garcia-Velasco JA. Why all women should freeze their eggs. Curr Opin Obstet Gynecol. 2016;28(3):206–210. doi: 10.1097/GCO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 29.Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update. 2016;22(4):440–449. doi: 10.1093/humupd/dmw007. [DOI] [PubMed] [Google Scholar]
- 30.Dondorp W, de Wert G, Pennings G, Shenfield F, Devroey P, Tarlatzis B, Barri P, Diedrich K, ESHRE Task Force on Ethics and Law Oocyte cryopreservation for age-related fertility loss. Hum Reprod. 2012;27(5):1231–1237. doi: 10.1093/humrep/des029. [DOI] [PubMed] [Google Scholar]
- 31.Lockwood GM. Social egg freezing: the prospect of reproductive ‘immortatlity’ or a dangerous delusion? RBMOnline. 2011;23:334–340. doi: 10.1016/j.rbmo.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Wise J. UK lifts ban on frozen eggs. 2000. p. 334. [PMC free article] [PubMed] [Google Scholar]
- 33.HFEA (2016) Fertility treatment 2014—trends and figures. Human fertilisation and embryology authority. https://www.hfea.gov.uk/media/1783/fertility-treatment-2014-trends-and-figures.pdf
- 34.HFEA (2018) Fertility treatment 2014–2018—trends and figures. Human fertilisation and embryology authority. https://www.hfea.gov.uk/media/2544/hfea-fertility-treatment-2014-2016-trends-and-figures.pdf
- 35.HFEA (2018) Egg freezing in fertility treatment. Trends and figures: 2010–2016. Human fertilisation and embryology authority. https://www.hfea.gov.uk/media/2656/egg-freezing-in-fertility-treatment-trends-and-figures-2010-2016-final.pdf
- 36.Gurtin ZB (2018) Unscrambling HFEA data on egg freezing: where are the missing frozen eggs? BioNews, 943. https://www.bionews.org.uk/page_135010
- 37.Doody KJ. Public reporting of assisted reproductive technology cycle outcomes is not simple. Fertil Steril. 2016;105:893–894. doi: 10.1016/j.fertnstert.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Kulak D, Jindal SK, Oh C, Morelli SS, Kratka S, McGovern PG. Reporting in vitro fertilization cycles to the Society for Assisted Reproductive Technology database: where have all the cycles gone? Fertil Steril. 2016;105:927–931. doi: 10.1016/j.fertnstert.2015.12.128. [DOI] [PubMed] [Google Scholar]
- 39.Hammerberg K, Kirkman M, Pritchard N, Hickey M, Peate M, McBain J, Agresta F, Bayly C, Fisher J. Reproductive experiences of women who cryopreserved oocytes for non-medical reasons. Hum Reprod. 2017;32(3):575–581. doi: 10.1093/humrep/dew342. [DOI] [PubMed] [Google Scholar]
- 40.Mesen TB, Mersereau JE, Kane JB, Steiner AZ. Optimal timing for elective egg freezing. Fertil Steril. 2015;103(6):1551–1556. doi: 10.1016/j.fertnstert.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mertes H, Pennings G. Social egg freezing: for better, not worse. RBMOnline. 2011;23(7):824–829. doi: 10.1016/j.rbmo.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Jackson E. Social egg freezing and the UK’s statutory storage time limits. J Med Ethics. 2016;42(11):738–741. doi: 10.1136/medethics-2016-103704. [DOI] [PubMed] [Google Scholar]
- 43.Gurtin ZB, Smith V, and Ahuja K (2018) The social pioneers of egg freezing are facing tough choices. Bionews, 960.
- 44.Inhorn MC, Birenbaum-Carmeli D, Patrizio P. Medical egg freezing and cancer patients’ hopes: fertility preservation at the intersection of life and death. Soc Sci Med. 2017;195:25–33. doi: 10.1016/j.socscimed.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson EL, Gispanski L, Fields K, Cappadora M, Hurt M. Knowledge and decision making about future fertility and oocyte cryopreservation among young women. Hum Fertil. 2019;9:1–10. doi: 10.1080/14647273.2018.1546411. [DOI] [PubMed] [Google Scholar]