Abstract
Early life maltreatment by the caregiver constitutes a major risk factor for the development of later-life psychopathologies, including fear-related pathologies. Here, we used an animal model of early life maltreatment induced by the Scarcity-Adversity Model of low bedding (LB) where the mother is given insufficient bedding for nest building while rat pups were postnatal days (PN) 8–12. To assess effects of maltreatment on the expression of threat-elicited defensive behaviors, animals underwent odor-shock threat conditioning at three developmental stages: late infancy (PN18), adolescence (PN45) or adulthood (>PN75) and tested the next day with odor only presentations (cue test). Results showed that in typically developing rats, the response to threat increases with maturation, although experience with maltreatment in early infancy produced enhanced responding to threat in infancy and adulthood, but a decrease in maltreated adolescents. To better understand the unique features of this decreased threat responding in adolescence, c-Fos expression was assessed within the amygdala and ventromedial prefrontal cortex (vmPFC) associated with the cued expression of threat learning. Fos counts across amygdala subregions were lower in LB rats compared to controls, while enhanced c-Fos expression was observed in the vmPFC prelimbic cortex (PL). Correlational analysis between freezing behavior and Fos revealed freezing levels were correlated with CeA in controls, although more global correlations were detected in LB-reared rats, including the BA, LA, and CeA. Functional connectivity analysis between brain regions showed that LB reared rats exhibited more diffuse interconnectivity across amygdala subnuclei, compared the more heterogeneous patterns observed in controls. In addition, functional connectivity between the IL and LA switched from positive to negative in abused adolescents. Overall, these results suggest that in adolescence, the unique developmental decrease in fear expression following trauma is associated with distinct changes in regional function and long-range connectivity, reminiscent of pathological brain function. These results suggest that early life maltreatment from the caregiver perturbs the developmental trajectory of threat-elicited behavior. Indeed, it is possible that this form of trauma, where the infant’s safety signal or “safe haven” (the caregiver) is actually the source of the threat, produces distinct outcomes across development.
Keywords: infant, trauma, maltreatment, learned fear, expression threat, amygdala, medial prefrontal cortex
Introduction
In altricial species, such as humans and rodents, the brain continues to develop after birth and is quite sensitive to environmental programming that permits adaptation to diverse environments and cultures. However, this open system also leaves the brain vulnerable to programming by trauma, with programming that goes beyond adaptation to initiate a pathological developmental pathway. Childhood trauma experiences, especially when associated with the caregiver (as occurs in maltreatment), are associated with mental health issues, including PTSD, anxiety and other threat processing-related pathologies (De Bellis et al., 1999; Anda et al., 2006; Andersen et al., 2008; McEwen and Gregerson, 2019; Van Assche et al., 2019), which has been modeled in rodents and non-human primates (Harlow and Harlow, 1965; Brenhouse and Andersen, 2011; Drury et al., 2015; Perry et al., 2017; Callaghan et al., 2019). One challenge to understanding how maltreatment causes threat-associated pathologies is the protracted and dynamic maturation of the threat system, which morphs through childhood, adolescence and adulthood as life and environmental demands change to produce unique expression patterns across the lifespan (Anda et al., 2006; Moffitt et al., 2007; Gilbert et al., 2009; Green et al., 2010; Callaghan et al., 2019). Here, using a rat animal model, we assessed how responses to a learned threat (following threat conditioning) changes as pups begin the transition from dependence on the mother [postnatal day (PN) 18] to adolescence, and finally adulthood, following typical maternal care and early life maltreatment. To model maltreatment, we employ the Scarcity-Adversity Model of low bedding (LB, insufficient bedding for nest building to induce maltreatment of pups) from PN8-12 (Opendak and Sullivan, 2016; Walker et al., 2017; Perry et al., 2018; Watamura and Roth, 2018).
Exploring how trauma contributes to the pathogenesis of anxiety and other threat-related disorders relies on understanding the typically developing threat response system and how it morphs as the developing organism matures and changes ecological niches. The infant system is not an immature version of the adult threat system (Landers and Sullivan, 2012). Specifically, within the immature threat system, the young seek and approach the caregiver when threatened and the caregiver responds to the threat while protecting the infants. This is seen in children as they approach the attachment figure (i.e., parent, guardian) or “safe base” for protection when threatened (Kerns et al., 2015). The developmental transition to mature self-defense occurs gradually during maturation, with infants approaching independence sometimes engaging in defensive behaviors such as freezing, or more active responses such as escape or fleeing, as these skills develop and the infant is more frequently away from the caregiver (Callaghan et al., 2019). It is this age range in which we begin our neurobehavioral analysis in infant (PN18) rats. With further physical and cognitive maturation, pups leave the mother and responding to threat is completely independent: here, we explore adolescent (PN45) and adult responses to provide a lifespan assessment. This complements research from other labs, as well as our own, studying threat conditioning across development (Pattwell et al., 2011; Li et al., 2012; Cowan et al., 2013; Poulos et al., 2014; Hartley and Lee, 2015; Madsen and Kim, 2016; Bucci and Stanton, 2017; Tallot et al., 2017; Davis et al., 2018).
Animal models have shown that developmental behavioral changes are reflected in the dynamic changes in the neural substrates, including developmental changes in responding to threats. Specifically, the functional emergence of learned and innate threat-elicited behavior at PN10 (Sullivan et al., 2000; Wiedenmayer and Barr, 2001; Moriceau et al., 2004; Takahashi, 2014; Santiago et al., 2018) is dependent upon the developmental emergence of the amygdala (Sullivan et al., 2000). Through PN15, amygdala-dependent learning is suppressed if the mother is present (Moriceau et al., 2006; Upton and Sullivan, 2010). In rat pups PN16 and older, amygdala-dependent cue learning and expression occurs with or without the mother present. Similarly to younger pups, there is suppression of learning about threat if the mother is present, although adults and not pups use the PFC to reduce acquisition (Levine, 2001; Inagaki and Eisenberger, 2012; Hornstein et al., 2016; Opendak et al., 2019; Robinson-Drummer et al., 2019). In particular, the PFC subareas corresponding to the infralimbic (IL) and prelimbic (PL) cortices (Brodmann areas 25 and 32, respectively) have been shown to modulate amygdala in the expression and suppression of learned threat (Linnman et al., 2012; Motzkin et al., 2015; Corches et al., 2019). However, while basic features of threat conditioning appear similar over much of development, the neural substrates and key features of threat conditioning and its expression differ in infants and adults: both the PFC and hippocampus and supporting behaviors such as extinction and context learning functionally emerge around to the age of weaning, at around PN23 (Rudy and Morledge, 1994; Barnet and Hunt, 2005; Yap and Richardson, 2005; Kim et al., 2009; Raineki et al., 2010a; Chan et al., 2011; Callaghan and Richardson, 2012; Li et al., 2012; Poulos et al., 2014). We also focus on adolescence, a period of increased prevalence of psychopathology involving perturbation of emotion (Giedd et al., 1999; Spear, 2000; Teicher et al., 2003; Andersen and Teicher, 2008; Monk et al., 2008; Raineki et al., 2012; Pattwell et al., 2016). Furthermore, work in peri-adolescent rodents has uncovered unique behavioral patterns of threat learning and memory extinction, processes which have shown sensitivity to developmental events (Stanton et al., 1987; Kim et al., 2009; Pattwell et al., 2011; Callaghan and Richardson, 2012; Robinson-Drummer and Stanton, 2015; Pattwell and Bath, 2017; Bath, 2018). Here, we take a lifespan approach and question how the behavioral expression to a learned threat changes as the infant transitions across infancy, adolescence and adulthood. In particular, we explore how threat responses such as freezing, as well as more active defensive responses such as fleeing, changes across development.
Experiencing infant trauma and maltreatment from the caregiver has major effects on threat detection, as reflected in defensive behavior, and, on key structures within the defensive network, such as the amygdala and PFC, in both humans and animal throughout development (De Bellis et al., 1999; Gunnar et al., 2007; Lupien et al., 2009; Dannlowski et al., 2013; Gee et al., 2013b; Birn et al., 2014; Opendak and Gould, 2015; Fonzo et al., 2016; Opendak and Sullivan, 2016; Teicher et al., 2016; Heany et al., 2018; Lange et al., 2018; Roquet et al., 2018; Santiago et al., 2018; Watamura and Roth, 2018; Callaghan et al., 2019). Less well understood is how the trauma effects change during development. The effects of maltreatment can be seen in childhood but identifying these subtle effects can be challenging until early adolescence when psychiatric disorders, as well as fear pathologies, increasingly emerge, with the amygdala and PFC targeted (Demers et al., 2018; Roos et al., 2018; Torrisi et al., 2018). The protracted development of the brain likely contributes to these developmental transitions (Giedd et al., 1999; Lupien et al., 2009; Raineki et al., 2010a,b; Rincon-Cortes et al., 2015; Teicher et al., 2016; Opendak et al., 2017; Hagler et al., 2018; Heany et al., 2018; Hodel, 2018; Lange et al., 2018; VanTieghem and Tottenham, 2018; Botros et al., 2019).
Here, we use the rodent Scarcity-Adversity Model of low bedding (LB) to induce infant maltreatment by the mother by providing insufficient bedding for nest building. We target this treatment to PN8-12 in the rodent pup; though there is no consensus mapping rodent age onto human age, this developmental period corresponds roughly to toddlerhood (Callaghan et al., 2019). We use learning about threat (Pavlovian threat conditioning) following perturbed development using LB maltreatment or typical control rearing to explore transitions in threat expression. We focus on two brain areas critical for responses to threat, the amygdala and ventromedial prefrontal cortex (vmPFC), during adolescence to complement the existing literature on fear/threat development, amygdala and PFC function in infancy and adulthood (Rudy and Morledge, 1994; Fanselow and LeDoux, 1999; Sevelinges et al., 2007; Thompson et al., 2008; Moriceau et al., 2009a,b; Cowan et al., 2013; Marek et al., 2013; Maren et al., 2013; Spielberg et al., 2014; Takahashi, 2014; Almada et al., 2015; Do-Monte et al., 2015; Duits et al., 2015; Pattwell et al., 2016; Matsuda et al., 2018; Norrholm and Jovanovic, 2018).
Our specific goals were to chart the neurobehavioral expression of threat learning across pre-weaning age (PN18), adolescence (PN45) and adulthood in typical and perturbed development. We hypothesized that, due to the unique neural circuitry supporting threat learning across development, the effects of maltreatment would be distinct at each stage of development.
Materials and Methods
Subjects
Male and female Long-Evans rat pups were bred in the Institute’s animal care facilities and housed (polypropylene cages 34 × 29 × 17 cm, wood chips, ad libitum food and water) in a temperature (20°C) and light (06:00–18:00 h) controlled room. The birthdate was PN0. Litters were culled to 12 pups on PN1 and only 1 male and 1 female per litter was used in any conditioning/test condition. For pre-weaning analyses (PN18) males and females were used; sex differences in the response to threat have not been shown at this age (Robinson-Drummer et al., 2019 under revision). For analyses at PN45 and adulthood, only males were used (see Figure 1 for Experimental Timeline). Procedures were approved by the Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
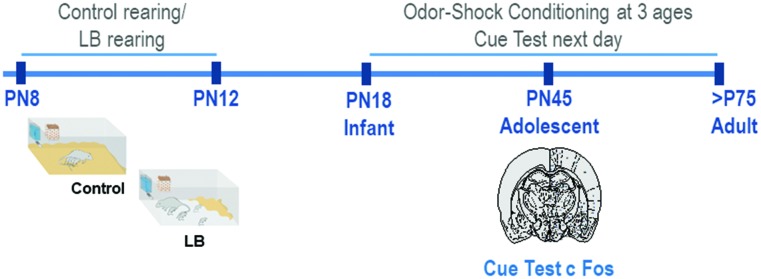
FIGURE 1.
Schematic of methodology and experimental timeline. From PN8-12, pups were exposed to either Scarcity-Adversity Model of low bedding (LB) rearing or control rearing from the mother. For LB rearing, the mother was provided with insufficient bedding for nest building, which resulted in maltreatment of pups but growth similar to controls. Pups are odor-shock conditioned at one age, either during infancy (PN18) adolescence (PN45) or adulthood (>PN75). All animals were tested in a cue test the next day; in adolescents, neural responses following cue testing were assessed with c-Fos.
Scarcity-Adversity Model of Low Bedding (LB)
We used a naturalistic stressed mother paradigm to induce maltreatment of pups over 5 days (from PN8 to PN12) by providing the mother with insufficient bedding: 1.3 cm layer compared to 0–0.5 cm layer covering the bottom of the cage (Roth and Sullivan, 2005; Walker et al., 2017). This limited bedding environment decreased the mother’s ability to construct a nest, which resulted in frequent nest building, and transporting/rough handling of pups (see Figure 2). Twice daily 15 min observation periods were undertaken on all 5 days of the manipulations to verify maltreatment in LB mothers and not in controls.
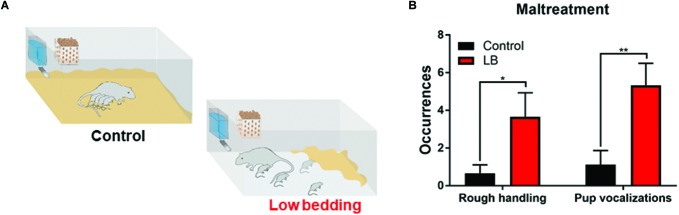
FIGURE 2.
Low bedding induces maternal maltreatment of pups. (A) Pups were exposed to either Scarcity-Adversity Model of low bedding (LB) rearing or control rearing from postnatal days (PN)8-12. LB rearing involved providing the mother with insufficient bedding for nest building, which produces maltreatment of pups and increased pup vocalizations (B) but growth indistinguishable from controls. Maltreatment was verified by repeated observations of maternal behaviors categorized as rough handling, such as stepping on pups, transporting pups by a body area other than the nape of the neck (i.e., leg), or roughly moving pups within the nest.
Odor-Shock Conditioning
Learning was induced using a classical Pavlovian odor-shock conditioning protocol using a standard conditioning apparatus (Coulbourn Instruments, Whitehall, PA) (LeDoux et al., 1988; Fanselow and LeDoux, 1999; Maren, 2001; Johansen et al., 2011). Although this protocol is typically known as “fear” conditioning, we refer to this as threat conditioning to avoid the implication that a subjective state of fear is the result of conditioning (LeDoux, 2014). 8–10 animals were used per rearing condition/learning condition and each animal was only used at one age. Adult and adolescent animals were acclimated to the conditioning apparatus for 5–10 min. Un-weaned pups (i.e., infant PN18) were not handled so as not to disturb mother-infant interaction and potentially inadvertently stress pups. On the conditioning day, a 30s consecutive odor (peppermint) presentation co-terminated with a 1s shock. The conditioned stimulus (CS) odor was delivered by an olfactometer (2 L/min flow rate at a concentration of 1:10). The unconditioned stimulus (US) foot shock was delivered by a grid at an age-specific intensity (PN18: 0.5 mA; adolescent: 0.55–6 mA; adult: 0.65–0.7 mA). CS-US pairings had a 4–5 min intertrial interval (ITI). Three training conditions were used: paired (as noted above), unpaired and odor only (Sevelinges et al., 2008; Tallot et al., 2016; Opendak et al., 2018).
Conditioning occurred in chambers constructed of aluminum and Plexiglas with metal stainless steel rod flooring attached to a shock generator (Wilensky et al., 2006). Each chamber had a top-mounted camera for videotaping and later analysis of the behaviors of individual rats during training and was enclosed within a sound-isolated, and ventilated cubicle to ensure odor withdrawal.
The cue test, consisting of three 30 s odor presentations (same parameters as conditioning), was given 24 h after conditioning in a novel context (attenuation chamber, Med Associates VT) in a beaker (PN18 glass beaker 2000 ml, 4″d; adolescent glass beaker 4000 ml, 6″d; adult plastic 8500 ml, 8.5″d). We measured time spent freezing and brains were removed 90 min after this test.
c-Fos Immunohistochemistry
As a global measure of neural activity, we assessed regional expression of the immediate early gene c-Fos, a proxy for neuronal activation. Brains were harvested 90 min after the cue test to assess the role of the amygdala, PL and IL during behavioral expression during a threat presentation using c-Fos (5–6 brains per condition were analyzed). Animals were decapitated, brains were removed within 3 min, frozen in isopentane and stored at -70°C until further processing using standard techniques (Sevelinges et al., 2007; Leuner et al., 2012). In the regions of interest (ROI), every 3rd coronal section (20 μm) was collected on pretreated slides (Fisherbrand Plus). Slices were post-fixed with 4% paraformaldehyde/100 mM phosphate buffered saline (PBS) and rinsed twice in 50 mM tris-HCl and 150 mM NaCl (TBS), and twice in TBS containing 0.05% Tween 20 (TBST). To eliminate endogenous peroxidase activity, sections were incubated in 70% Methanol, containing 1% hydrogen peroxide (H2O2). Following TBS and TBST rinse, unspecific epitopes of the brain slices were blocked by incubation in 2% Normal goat serum (Jackson ImmunoResearch) and 3% bovine serum albumin (Sigma Aldrich) in TBST for 2 h. Slides were further incubated overnight at 4°C in 2% Normal goat serum, 3% bovine serum albumin and primary c-Fos antibody (EMD Biosciences) diluted 1:5000 in TBST. Next they were rinsed in TBS and TBST and further incubated with a biotinylated secondary antibody (Jackson ImmunoResearch) for 2 h at room temperature and then incubated for 90 min in avidin-biotin-peroxidase (ABC) complex solution (Vectastain). Following washing in TBST, slides were treated with 2% 3,3′-diaminobenzidine and 0.06% H2O2 for 20 min. Brain slices were dehydrated in 70%, 95% and 100% ethanol solutions and Xylene (Thermo Fisher Scientific) and cover-slipped. Image acquisition was done at 10× using an Olympus BX51 microscope and the Neurolucida software (MBF Bioscience). c-Fos positive cells were counted using Image J software (NIH). Amygdala nuclei were outlined using a stereotactic atlas (Paxinos and Watson, 2013) and all c-Fos positive cells were counted bilaterally blind to treatment conditions. c-Fos positive cells were distinguished from the background by the density of staining, the shape and the size of the cell. The mean number of c-Fos positive cells per amygdala and prefrontal cortex nuclei for each animal was determined by averaging the total counts (both hemispheres) from three different sections.
Functional Connectivity Analysis
For functional connectivity modeling, bivariate correlation matrices were created by computing ratios of mean c-Fos uptake for all pairwise combinations of brain regions. For quantitative analyses, each group’s correlation matrices were transformed into z-scores using the Fisher transformation and group differences between modules were analyzed by ANOVA and Bonferroni-corrected pairwise tests.
Statistical Analysis
For behavioral conditioning data, 2-way repeated measured ANOVA was computed using CS-US pairing (three pairings) and rearing (LB vs. Control). Testing data was analyzed with 2-way ANOVA, comparing learning condition (paired, unpaired, odor only) and rearing (LB vs. Control). Fos data was analyzed in adolescents using 2-way ANOVA, comparing amygdala/vmPFC subnuclei and rearing condition; amygdala Fos and vmPFC Fos were analyzed in separate ANOVAs. To compare correlations, Pearson r values were converted into z-scores using the Fisher transformation.
Results
Scarcity-Adversity Model of Low Bedding (LB) Induced Maltreatment From PN8-12
During the bi-daily 15 min observation periods, maternal behaviors were scored and maltreatment verified (Raineki et al., 2010b). As illustrated in Figure 2, LB mothers show significantly more time stepping on and roughly handling pups and pups vocalized more (two-way ANOVA (rearing condition × behavior), ME of rearing [F(1,31) = 13.58, p = 0.001]). Weight was continuously monitored at the different ages studied through development and no differences between groups were observed, consistent with previous work: at weanling, average weight of controls was 62.71 ± 2.31 g (n = 14) and LB average weight was 57.88 ± 2.94 g (n = 16); at PN45, average weight of controls was 196.8 ± 4.72 g (n = 36) and LB average weight was 198.8 ± 3.74 g (n = 40); at adulthood, average weight for controls was 371.3 ± 4.19 g (n = 14) and average weight for LB pups was 366.7 ± 8.08 g (n = 14).
Odor-Shock Conditioning Across Development
Overall, we observed that pups across development showed increased freezing to the paired CS-US cues over the course of training (Figure 3A–C). This was observed across ages and conditions [Infant: ME of cue presentation (F(2,32) = 44.7, p < 0.000); Adolescent: ME of cue presentation (F(2,56) = 71.17, p < 0.000); Adult: ME of cue presentation (F(2,26) = 35.36, p < 0.000)]. However, previous maltreatment impaired acquisition at adolescence (Figure 3B), with decreased freezing during conditioning in the abused group [t(84) = 2.151, p = 0.032].
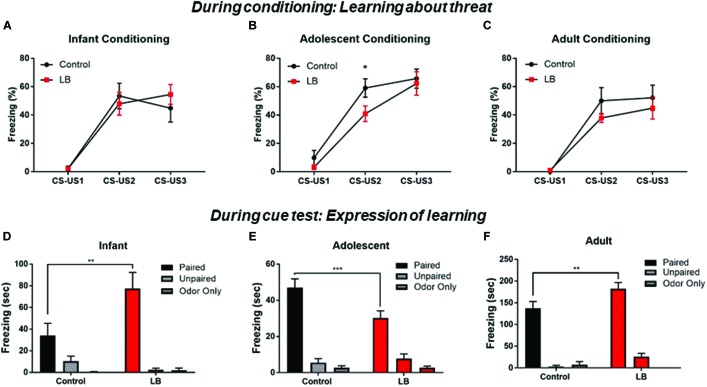
FIGURE 3.
Acquisition and expression of learned threat across development. During conditioning, responses to the CS increased over consecutive CS-US pairings, a pattern indicative of learning. No statistical difference was seen between LB and control rearing as suggested by rate of learning for infants and adults (A,C), although maltreated adolescents showed some retardation of learning (B). Cue testing 24 h later in a novel environment was analyzed for percent time spent freezing to the CS at the three ages. All paired animals (control and LB) were significantly higher than unpaired and odor-only controls, although freezing to cues following maltreatment increased in infancy (D), decreased in adolescence (E) and increased in adults (F), relative to control-reared animals. ∗∗p < 0.01, ∗∗∗p < 0.001.
Expression of Learned Threat Throughout Development
CS cue testing was done 24 h after conditioning in a different context (3 CSs, odor and ITI same as conditioning). Overall, all paired animals at all ages and in both rearing conditions showed increased freezing to the CS relative to controls, indicating a learned association between the odor and the shock (Figure 3D–F).
For infants, an ANOVA revealed a significant interaction [F(2,41) = 5.76; p < 0.006], no main effect of rearing condition [F(1,41) = 3.449; p = 0.07] and a main effect of conditioning [F(2,41) = 27.72; p = 0.0001]. Post hoc tests revealed that the Paired groups (LB and control) were always each significantly higher than unpaired and odor only groups (all p’s < 0.05). Freezing was higher in LB reared pups than controls in the paired conditioning groups [t(41) = 3.82, p = 0.004].
For adolescents, an ANOVA revealed a significant interaction [F(2,52) = 4.47; p = 0.016], no main effect of rearing [F(1,52) = 2.582; p = 0.11] and a main effect of conditioning [F (2,52) = 97.36; p < 0.001]. Post hoc tests revealed that the Paired groups (LB and control) were always each significantly higher than unpaired and odor only groups (all p’s < 0.05). Freezing levels were lower in LB reared pups than control-reared pups trained in the paired condition [t(52) = 3.53, p < 0.001].
For adults, an ANOVA revealed no significant interaction [F(2,31) = 2.307; p < 0.1164], no main effect of rearing [F(1,31) = 3.925; p = 0.056] and a main effect of conditioning [F(2,31) = 112.7; p = 0.0001]. Post hoc tests revealed that the Paired groups (LB and control) were each always significantly higher than unpaired and odor only groups (all p’s < 0.05). Freezing levels were higher in the LB pups compared to controls in the Paired groups [t(31) = 2.976, p = 0.005].
Amygdala Subnuclei and vmPFC IL and PL Cue Test Activation During Adolescence
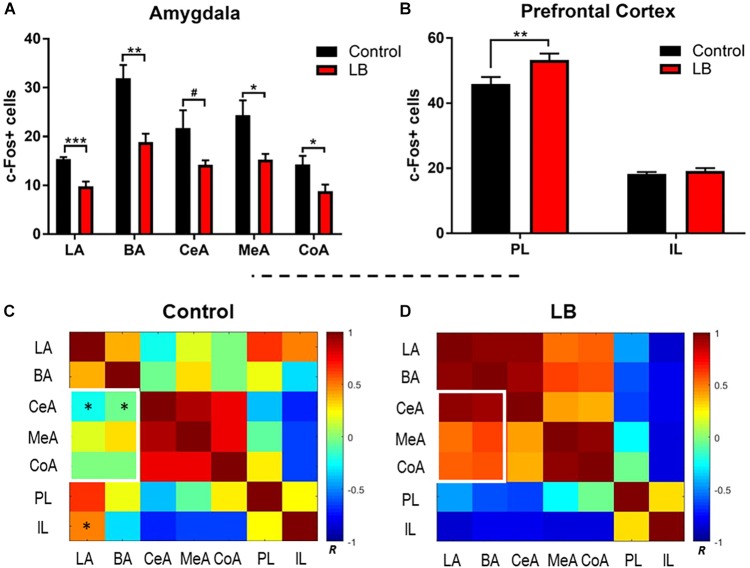
Our behavioral data indicate that adolescence represents an inflection point in the effects of maltreatment on the response to threat. To examine the neural substrates of this adolescent decrease in the fear response following maltreatment, c-Fos-positive cells were counted bilaterally in ROI, including the lateral, basal, central, medial and cortical nuclei of the amygdala, as well as the IL and PL of the prefrontal cortex, due to an established role for the vmPFC in the fear circuit (Figure 4). Laterality effects were not observed [three-way ANOVA (rearing × region × hemisphere), amygdala: no ME of hemisphere (F(1,80) = 0.621, p = 0.433) or significant interactions; PFC: no ME of hemisphere (F(1,40) = 0.821, p = 0.37)] and counts were therefore collapsed across hemispheres for further analysis. Within the amygdala, counts were analyzed via two-way ANOVA (rearing × region), which revealed main effects of both factors [region: F(4,40) = 15.51, p < 0.0001; rearing: F(1,40) = 40.36, p < 0.0001]. In accordance with the behavioral observations showing reduced freezing to the paired CS-US cue, amygdala subnuclei showed reduced activation in abused animals [Figure 4A; LA: t(40) = 1.941, p = 0.059; BA: t(40) = 4.568, p < 0.001; CeA: t(40) = 2.634, p = 0.012; MeA: t(40) = 3.157, p = 0.003; CoA: t(40) = 1.909, p = 0.064].
FIGURE 4.
Amygdala and prefrontal cortex activation following memory retrieval at 24 h in adolescent animals. (A) Amygdala subnuclei activation following fear retrieval at 24 h in both control (black) and abused (red) animals; LA: lateral nucleus, B: basal nucleus, CeA: central nucleus, CoA: cortical nucleus, and MeA: medial nucleus. (B) Prefrontal cortex activation following fear memory retrieval at 24 h in both normal (black) and abused (red) animals; PL: prelimbic cortex, IL: infralimibic cortex. (C,D) Bivariate correlation matrices were contructed across regions assessed for c-Fos levels after cue test during adolescents following control or low bedding rearing. White squares denote example nodules (groups of regions) that show dramatic changes in connectivity across condition. Color bar shows Pearson’s r values with positive correlations in red and negative correlations in blue. For statistical comparisons, r values were converted to z scores via Fisher transform and compared using ANOVA. Asterisks indicate pairwise correlations that significantly differed between LB and control. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and #p = 0.05.
We observed that early life abuse had a significant effect on the PL but not the IL (Figure 4B). Two way ANOVA (rearing × subregion) revealed an interaction [F(1,20) = 4.434, p = 0.0481] as well as main effects of subregion [F(1,20) = 401.3, p < 0.0001] and rearing [F(1,20) = 7.191, p = 0.0143]. Post hoc tests revealed that c-Fos counts within the PL were significantly higher in the abused pups [t(20) = 3.385, p = 0.002].
Functional Connectivity Within the Amygdala-Prefrontal Network
Next, we compared how activity between subnuclei assessed in the ROI analysis above changed with rearing, using functional connectivity analysis (Perry et al., 2017; Opendak et al., 2019). This type of data representation provides an overview of patterns of change in the brain across broad networks of interconnected regions. Fos counts across individual animals for each brain region was used to construct bivariate correlation matrices for all pairwise combinations of brain regions analyzed for rearing condition. A single data point represents the correlation between Fos counts in a given brain region across all animals in a given condition with uptake in a different region in the same animals. Brain network matrices for the control-reared and LB maltreated-reared groups are shown in Figure 4C,D. Pairwise comparisons show several group differences: abused pups show higher connectivity between the BA-CeA (p = 0.015) and LA-BA (p = 0.043) than controls (Figure 4C, asterisks). In contrast, controls show higher connectivity between the IL-LA (p = 0.038) than abused adolescents. Finally, we observed that inter-connectivity between all amygdala subnuclei was higher in abused adolescents than controls (p = 0.027) and connectivity between the basolateral complex and other amygdala subnuclei was higher after abuse (Figure 4C,D, white rectangles; p = 0.004).
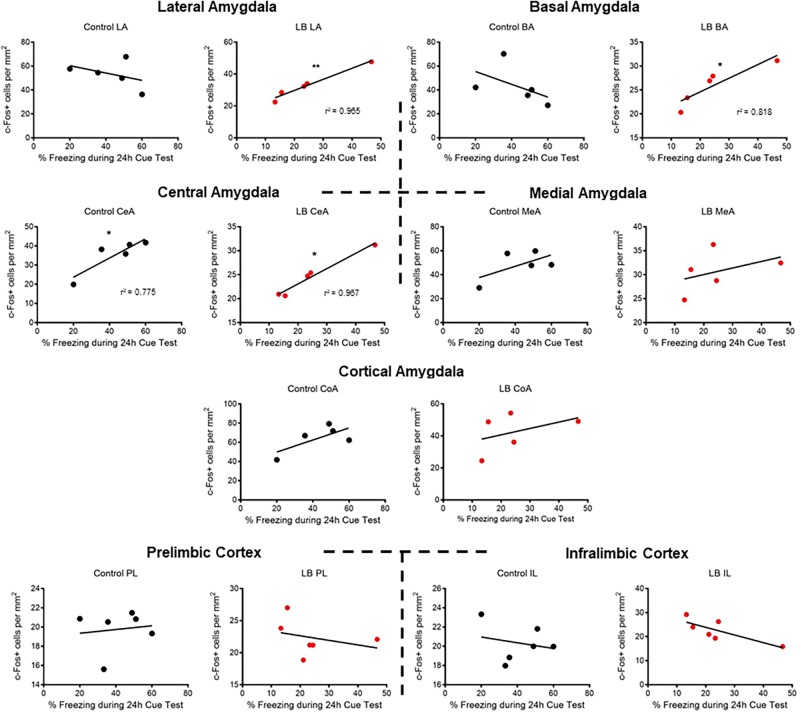
Adolescent Correlational Analysis Between Amygdala-Freezing and vmPFC-Freezing
Lastly, correlational analyses were performed between freezing behavior and amygdala-PFC ROIs in adolescent animals (Figure 5). In control-reared adolescent rats, we only observed a significant correlation between freezing levels and central amygdala c-Fos levels (p = 0.0487). In contrast, LB-reared pups showed significant correlations between freezing and activation of several amygdala subnuclei, including lateral, basal and central (LA: p = 0.003; BA: p = 0.039; CeA: p = 0.003).
FIGURE 5.
Correlations between cued threat response and ROI activation during adolescence. Correlations were computed between time spent freezing to CS and c-Fos levels for control-reared and maltreated (LB) pups. Pearson’s r values statistically different from 0 are. ∗p < 0.05, ∗∗p < 0.01.
Discussion
Here, we showed that response to a learned threat increased from infancy through adolescence into adulthood, which was modified in a non-linear fashion by experience with LB maltreatment during early infancy. As summarized in Table 1, threat expression increased significantly across development, but this trajectory was altered by experience with infant maltreatment: relative to controls, freezing to a cue is increased in older maltreated infants (soon to be independent), which dips below control levels in adolescence before increasing above control levels in adulthood. The decreased response to the threat at adolescence may be due, at least in part, to reduced acquisition during conditioning. However, reduced response of the amygdala during cue testing may also contribute to adolescent reduced threat responding. To our knowledge, this is the first demonstration of these repeated dynamic developmental shifts on threat responding across development.
Table 1.
Summary of the developmental neurobehavioral response to threat to a across development after experiencing infant trauma.
| PN18 late infancy | PN45 adolescence | >PN75 adults | |
|---|---|---|---|
| Behavioral response to threat | Increased | Decreased | Increased |
| Integrating our adolescent neural results with existing literature | |||
| Amygdala | Increased | Decreased | Increased |
| PFC PL | NA | Increased | Increased |
Infants experienced PN8-12 maternal maltreatment with Scarcity-Adversity LB Model induced by providing mothers with insufficient bedding for nest building. Summarizing the behavioral data presented here, maltreatment produced a decrease response to threat while this same procedure produced a development switch to a maltreatment induced decrease followed by a developmental switch to adult increase. Next we integrate our adolescent amygdala and PL results with the existing literature on infants (Bath, 2018; Santiago et al., 2018; Opendak et al., 2019) and extensive adult literature (Barbosa Neto et al., 2012; Dannlowski et al., 2013; Jovanovic et al., 2014; Hartley and Lee, 2015; van Rooij et al., 2017; Demers et al., 2018; Norrholm and Jovanovic, 2018). Overall, the amygdala activity tracks trauma-induced changes in threat expression across the lifespan, while the PL does not.
As noted in Table 1, the literature has already documented that amygdala-dependent learning occurs in late infancy, adolescence and adulthood (Pare et al., 2004; Pattwell et al., 2012; Hartley and Lee, 2015). The literature also documents its protracted development: amygdala dependent cue learning developmentally emerges at PN10 (Sullivan et al., 2000) and is functional in PN18 pups studied here (Opendak et al., 2018, 2019). Our lab has shown that early life maltreatment causes precocious incorporation of the amygdala into the threat learning circuit by a few days (PN7 rather than PN10) and the amygdala is hyperactive within a threat conditioning paradigm at PN18 to support enhanced learning shown in our previous rodent work (Moriceau et al., 2009a; Opendak et al., 2019).
At adolescence, following early infancy LB maltreatment, decreased responses to threat were accompanied by decreased c-Fos amygdala activation compared to controls across amygdala nuclei. This suggests widespread disruption of amygdala function. Specifically, the lower LB c-Fos in the cortical nucleus, considered the olfactory input area of the amygdala (Iurilli and Datta, 2017), suggests altered processing of the threat odor. The LB rats also exhibited lower lateral and basal nuclei c-Fos levels: this area is considered key for CS and US convergence and plasticity, suggesting learned association between the odor and the shock was also compromised (Romanski et al., 1993; Fanselow and LeDoux, 1999). Finally, the LB rats’ lower central nucleus (CeA) c-Fos suggests output is impaired (McKernan and Shinnick-Gallagher, 1997; Rogan et al., 1997; Phelps and LeDoux, 2005; Wilensky et al., 2006; Sah et al., 2008).
Using correlational analysis between freezing levels and amygdala nuclei (Figure 5), we observed that typically reared adolescents’ freezing was only correlated with the amygdala central nucleus, a brain area typically associated with output (LeDoux et al., 1988; LaLumiere, 2014). However, maltreated adolescents exhibited widespread correlations between freezing levels and specific amygdala nuclei, including the central amygdala and basolateral complex. Thus, while LB adolescent rats had lower c-Fos activation than controls, LB amygdala nuclei appeared to converge with the freezing response during the cue test.
Further analysis with functional connectivity between brain regions suggests that, within the amygdala, subnuclei exhibited enhanced communication following LB maltreatment, suggesting an expanded, diffuse response of the amygdala to a threat. Functional connectivity analysis within maltreated adolescents also suggests a switch from positive to negative connectivity between the amygdala-PFC, most notably between the IL and lateral amygdala. A similar pattern of transitioning positive to negative connectivity between the PFC and amygdala has been observed in developing children (Gee et al., 2013a). Furthermore, these patterns of altered interconnectivity are reminiscent of patterns observed in humans exhibiting pathology following early trauma (Yu et al., 2019).
LB maltreated adolescents exhibited enhanced PFC PL c-Fos expression, while IL levels were similar to controls. We interpret these results within the context of PFC function as a regulator of fear behavior by modulation of the amygdala and other brain areas (Pare et al., 2004). The vmPFC subdivisions IL and PL generally appear to have opposing effects on fear in adult animals: the PL promotes the expression of fear, and the IL inhibits it (Vidal-Gonzalez et al., 2006; Corcoran and Quirk, 2007). During adolescence, inactivation of the PL but not the IL was found to have an effect on freezing behavior during a predator odor (Chan et al., 2011). While we found enhanced PL activation in maltreated animals compared to controls, no correlation was found between freezing and IL or PL c-Fos activity for either typically reared and maltreated reared adolescents. In humans, the engagement of the vmPFC (PL and IL are not typically individually assessed in human fMRI) during threat and its enhanced function and functional connectivity has been seen in clinical populations (Phelps et al., 2004; Gee et al., 2013b; Birn et al., 2014; Schubert et al., 2015; Fonzo et al., 2016; Demers et al., 2018; Goetschius et al., 2019).
The literature also indicates that the PFC is functional during adolescent threat conditioning, although PFC development has been suggested to accelerate following early life stress (Yap and Richardson, 2005; Kim et al., 2009; Callaghan and Richardson, 2012; Li et al., 2012; Cowan et al., 2013). Specifically, in rat pups, the PFC does not seem to be functionally incorporated into the rat threat circuit until around PN23 (around weaning age) for innate threat responses (Chan et al., 2011; Takahashi, 2014) and learning about threat in a threat conditioning paradigm, behavioral expression, and during extinction in both humans and animal models (Nair et al., 2001; Kim et al., 2009; Ball and Slane, 2012; Li et al., 2012; Shechner et al., 2014; Almada et al., 2015; Heroux et al., 2017), as well as in other learning paradigms potentially at an earlier age (Lilliquist et al., 1999; Nair et al., 2001). It should be noted that the rodent and human PFC continues to develop though adolescence and appears to support unique features of adolescent extinction (Nair et al., 2001; Kim et al., 2009; Jovanovic et al., 2014; Lu et al., 2019).
Conclusion
Our results suggest that experiencing maltreatment in early life sets in motion a complex developmental trajectory of neurobehavioral responses to threat that transitions across infancy, adolescence and into adulthood. These results complement research on children, where caregiver maltreatment is well-known to alter the threat system throughout development. Indeed, it is possible that this unique form of trauma in which the infant’s safety signal or “safe haven” (the caregiver) is actually the source of the threat (Kerns et al., 2015; Hornstein et al., 2016) produces distinct outcomes. Understanding unique trajectories following this specific developmental perturbation associated with maltreatment from the caregiver can help inform development of age-appropriate and trauma-specific treatments and interventions.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Procedures were approved by the Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
Author Contributions
AJ and RS designed and conducted the research. AJ, MO, and RS analyzed the data and wrote the manuscript. JL contributed to the data interpretation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the NIH F32MH112232 and BBRF NARSAD Young Investigator Award (to MO), T32MH019524 (to MO), and NIH R37HD083217 (to RS).
References
- Almada R. C., Coimbra N. C., Brandao M. L. (2015). Medial prefrontal cortex serotonergic and GABAergic mechanisms modulate the expression of contextual fear: intratelencephalic pathways and differential involvement of cortical subregions. Neuroscience 284 988–997. 10.1016/j.neuroscience.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Anda R. F., Felitti V. J., Bremner J. D., Walker J. D., Whitfield C., Perry B. D., et al. (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 256 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. L., Teicher M. H. (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 31 183–191. 10.1016/j.tins.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Andersen S. L., Tomada A., Vincow E. S., Valente E., Polcari A., Teicher M. H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 20 292–301. 10.1176/appi.neuropsych.20.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K. T., Slane M. (2012). Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psychopharmacology 224 377–385. 10.1007/s00213-012-2762-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa Neto J. B., Tiba P. A., Faturi C. B., de Castro-Neto E. F., da Graça Naffah-Mazacoratti M., de Jesus Mari J., et al. (2012). Stress during development alters anxiety-like behavior and hippocampal neurotransmission in male and female rats. Neuropharmacology 62 518–526. 10.1016/j.neuropharm.2011.09.011 [DOI] [PubMed] [Google Scholar]
- Barnet R. C., Hunt P. S. (2005). Trace and long-delay fear conditioning in the developing rat. Learn. Behav. 33 437–443. [DOI] [PubMed] [Google Scholar]
- Bath K. G. (2018). “Early life stress is associated with precocious amygdala development and an unexpected dip in threat-associated freezing,” in Proceedings of the International Society for Developmental Psychobiology, San Diego, CA. [Google Scholar]
- Birn R. M., Patriat R., Phillips M. L., Germain A., Herringa R. J. (2014). Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety 31 880–892. 10.1002/da.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botros M., Hodgins G., Nemeroff C. B. (2019). The long-lasting neurobiological scars of early-life stress: implications for the neurobiology of depression. Neurobiol. Depress. 111–121. Available at: https://www.sciencedirect.com/science/article/pii/B9780128133330000111 [Google Scholar]
- Brenhouse H. C., Andersen S. L. (2011). Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 35 1687–1703. 10.1016/j.neubiorev.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci D., Stanton M. (2017). The ontogeny of learning and memory. Neurobiol. Learn. Mem. 143:iv. [DOI] [PubMed] [Google Scholar]
- Callaghan B., Meyer H., Opendak M., Van Tieghem M., Harmon C., Li A., et al. (2019). Using a developmental ecology framework to align fear neurobiology across species. Annu. Rev. Clin. Psychol. 15 345–369. 10.1146/annurev-clinpsy-050718-095727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B. L., Richardson R. (2012). Early-life stress affects extinction during critical periods of development: an analysis of the effects of maternal separation on extinction in adolescent rats. Stress 15 671–679. 10.3109/10253890.2012.667463 [DOI] [PubMed] [Google Scholar]
- Chan T., Kyere K., Davis B. R., Shemyakin A., Kabitzke P. A., Shair H. N., et al. (2011). The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J. Neurosci. 31 4991–4999. 10.1523/JNEUROSCI.5216-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corches A., Hiroto A., Bailey T. W., Speigel J. H., Pastore J., Mayford M., et al. (2019). Differential fear conditioning generates prefrontal neural ensembles of safety signals. Behav. Brain Res. 360 169–184. 10.1016/j.bbr.2018.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran K. A., Quirk G. J. (2007). Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 12 200–206. [DOI] [PubMed] [Google Scholar]
- Cowan C. S., Callaghan B. L., Richardson R. (2013). Acute early-life stress results in premature emergence of adult-like fear retention and extinction relapse in infant rats. Behav. Neurosci. 127 703–711. 10.1037/a0034118 [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Kugel H., Huber F., Stuhrmann A., Redlich R., Grotegerd D., et al. (2013). Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum. Brain Mapp. 34 2899–2909. 10.1002/hbm.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. M., Rice M., Rudlong J., Eaton V., King T., Burman M. A. (2018). Neonatal pain and stress disrupts later-life pavlovian fear conditioning and sensory function in rats: evidence for a two-hit model. Dev. Psychobiol. 60 520–533. 10.1002/dev.21632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M. D., Keshavan M. S., Clark D. B., Casey B. J., Giedd J. N., Boring A. M., et al. (1999). A.E. bennett research award developmental traumatology. Part II: brain development. Biol. Psychiatry 45 1271–1284. [DOI] [PubMed] [Google Scholar]
- Demers L. A., McKenzie K. J., Hunt R. H., Cicchetti D., Cowell R. A., Rogosch F. A., et al. (2018). Separable effects of childhood maltreatment and adult adaptive functioning on amygdala connectivity during emotion processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 116–124. 10.1016/j.bpsc.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte F. H., Manzano-Nieves G., Quinones-Laracuente K., Ramos-Medina L., Quirk G. J. (2015). Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 35 3607–3615. 10.1523/JNEUROSCI.3137-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S. S., Sanchez M. M., Gonzalez A. (2015). When mothering goes awry: challenges and opportunities for utilizing evidence across rodent, nonhuman primate and human studies to better define the biological consequences of negative early caregiving. Horm. Behav. 77 182–192. 10.1016/j.yhbeh.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P., Cath D. C., Lissek S., Hox J. J., Hamm A. O., Engelhard I. M., et al. (2015). Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety 32 239–253. 10.1002/da.22353 [DOI] [PubMed] [Google Scholar]
- Fanselow M. S., LeDoux J. E. (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23 229–232. [DOI] [PubMed] [Google Scholar]
- Fonzo G. A., Huemer J., Etkin A. (2016). History of childhood maltreatment augments dorsolateral prefrontal processing of emotional valence in PTSD. J. Psychiatr. Res. 74 45–54. 10.1016/j.jpsychires.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D. G., Gabard-Durnam L. J., Flannery J., Goff B., Humphreys K. L., Telzer E. H., et al. (2013a). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U.S.A. 110 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D. G., Humphreys K. L., Flannery J., Goff B., Telzer E. H., Shapiro M., et al. (2013b). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 33 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J. N., Blumenthal J., Jeffries N. O., Castellanos F. X., Liu H., Zijdenbos A., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2 861–863. [DOI] [PubMed] [Google Scholar]
- Gilbert R., Widom C. S., Browne K., Fergusson D., Webb E., Janson S. (2009). Burden and consequences of child maltreatment in high-income countries. Lancet 373 68–81. 10.1016/S0140-6736(08)61706-7 [DOI] [PubMed] [Google Scholar]
- Goetschius L. G., Hein T. C., Mattson W. I., Lopez-Duran N., Dotterer H. L., Welsh R. C., et al. (2019). Amygdala-prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. Neuroimage 191 278–291. 10.1016/j.neuroimage.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. G., McLaughlin K. A., Berglund P. A., Gruber M. J., Sampson N. A., Zaslavsky A. M., et al. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry 67 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M., Quevedo K. M., Ronald De Kloet M. S. O. A. E. V. E. (2007). Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog. Brain Res. 167 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D. J., Jr., Hatton S. N., Makowski C., Cornejo M. D., Fair D. A., Dick A. S., et al. (2018). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. bioRxiv [Preprint]. 10.1101/457739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow H. F., Harlow M. K. (1965). “The affectional systems,” in Behavior of Nonhuman Primates Vol. 2 (New York, NY: Academic Press; ), 287–344. [Google Scholar]
- Hartley C. A., Lee F. S. (2015). Sensitive periods in affective development: nonlinear maturation of fear learning. Neuropsychopharmacology 40 50–60. 10.1038/npp.2014.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heany S. J., Groenewold N. A., Uhlmann A., Dalvie S., Stein D. J., Brooks S. J. (2018). The neural correlates of childhood trauma questionnaire scores in adults: a meta-analysis and review of functional magnetic resonance imaging studies. Dev. Psychopathol. 30 1475–1485. 10.1017/S0954579417001717 [DOI] [PubMed] [Google Scholar]
- Heroux N. A., Robinson-Drummer P. A., Sanders H. R., Rosen J. B., Stanton M. E. (2017). Differential involvement of the medial prefrontal cortex across variants of contextual fear conditioning. Learn. Mem. 24 322–330. 10.1101/lm.045286.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel A. S. (2018). Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Dev. Rev. 48 113–144. 10.1016/j.dr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein E. A., Fanselow M. S., Eisenberger N. I. (2016). A safe haven: investigating social-support figures as prepared safety stimuli. Psychol. Sci. 27 1051–1060. 10.1177/0956797616646580 [DOI] [PubMed] [Google Scholar]
- Inagaki T. K., Eisenberger N. I. (2012). Neural correlates of giving support to a loved one. Psychosom. Med. 74 3–7. 10.1097/PSY.0b013e3182359335 [DOI] [PubMed] [Google Scholar]
- Iurilli G., Datta S. R. (2017). Population coding in an innately relevant olfactory area. Neuron 93 1180–1197.e7. 10.1016/j.neuron.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. P., Cain C. K., Ostroff L. E., LeDoux J. E. (2011). Molecular mechanisms of fear learning and memory. Cell 147 509–524. 10.1016/j.cell.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Nylocks K. M., Gamwell K. L., Smith A., Davis T. A., Norrholm S. D., et al. (2014). Development of fear acquisition and extinction in children: effects of age and anxiety. Neurobiol. Learn. Mem. 113 135–142. 10.1016/j.nlm.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns K. A., Mathews B. L., Koehn A. J., Williams C. T., Siener-Ciesla S. (2015). Assessing both safe haven and secure base support in parent-child relationships. Attach. Hum. Dev. 17 337–353. 10.1080/14616734.2015.1042487 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Hamlin A. S., Richardson R. (2009). Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J. Neurosci. 29 10802–10808. 10.1523/JNEUROSCI.0596-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere R. T. (2014). Optogenetic dissection of amygdala functioning. Front. Behav. Neurosci. 8:107. 10.3389/fnbeh.2014.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers M. S., Sullivan R. M. (2012). The development and neurobiology of infant attachment and fear. Dev. Neurosci. 34 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I., Goossens L., Bakker J., Michielse S., van Winkel R., Lissek S. (2018). Neurobehavioural mechanisms of threat generalization moderate the link between childhood maltreatment and psychopathology in emerging adulthood. J. Psychiatry Neurosci. 44 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. E. (2014). Coming to terms with fear. Proc. Natl. Acad. Sci. U.S.A. 111 2871–2878. 10.1073/pnas.1400335111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. E., Iwata J., Cicchetti P., Reis D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B., Caponiti J. M., Gould E. (2012). Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus 22 861–868. 10.1002/hipo.20947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. (2001). Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol. Behav. 73 255–260. [DOI] [PubMed] [Google Scholar]
- Li S., Kim J. H., Richardson R. (2012). Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behav. Neurosci. 126 217–225. 10.1037/a0027151 [DOI] [PubMed] [Google Scholar]
- Lilliquist M. W., Nair H. P., Gonzalez-Lima F., Amsel A. (1999). Extinction after regular and irregular reward schedules in the infant rat: influence of age and training duration. Dev. Psychobiol. 34 57–70. [PubMed] [Google Scholar]
- Linnman C., Zeidan M. A., Furtak S. C., Pitman R. K., Quirk G. J., Milad M. R. (2012). Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am. J. Psychiatry 169 415–423. 10.1176/appi.ajp.2011.10121780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K., Jing X. B., Xue Q., Song X. J., Wei M. D., Wang A. G. (2019). Impaired fear memory extinction during adolescence is accompanied by the depressive-like behaviors. Neurosci. Lett. 699 8–15. 10.1016/j.neulet.2019.01.039 [DOI] [PubMed] [Google Scholar]
- Lupien S. J., McEwen B. S., Gunnar M. R., Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Madsen H. B., Kim J. H. (2016). Ontogeny of memory: an update on 40 years of work on infantile amnesia. Behav. Brain Res. 298(Pt. A), 4–14. 10.1016/j.bbr.2015.07.030 [DOI] [PubMed] [Google Scholar]
- Marek R., Strobel C., Bredy T. W., Sah P. (2013). The amygdala and medial prefrontal cortex: partners in the fear circuit. J. Physiol. 591 2381–2391. 10.1113/jphysiol.2012.248575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. (2001). Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 24 897–931. [DOI] [PubMed] [Google Scholar]
- Maren S., Phan K. L., Liberzon I. (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14 417–428. 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Matsuzawa D., Ishii D., Tomizawa H., Shimizu E. (2018). Development of the fear regulation system from early adolescence to young adulthood in female mice. Neurobiol. Learn. Mem. 150 93–98. 10.1016/j.nlm.2018.03.007 [DOI] [PubMed] [Google Scholar]
- McEwen C. A., Gregerson S. F. (2019). A critical assessment of the adverse childhood experiences study at 20 years. Am. J. Prev. Med. 56 790–794. [DOI] [PubMed] [Google Scholar]
- McKernan M. G., Shinnick-Gallagher P. (1997). Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390 607–611. [DOI] [PubMed] [Google Scholar]
- Moffitt T. E., Caspi A., Harrington H., Milne B. J., Melchior M., Goldberg D., et al. (2007). Generalized anxiety disorder and depression: childhood risk factors in a birth cohort followed to age 32. Psychol. Med. 37 441–452. [DOI] [PubMed] [Google Scholar]
- Monk C. S., Telzer E. H., Mogg K., Bradley B. P., Mai X., Louro H. M., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch. Gen. Psychiatry 65 568–576. 10.1001/archpsyc.65.5.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Raineki C., Holman J. D., Holman J. G., Sullivan R. M. (2009a). Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front. Behav. Neurosci. 3:22. 10.3389/neuro.08.022.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Roth T. L., Okotoghaide T., Sullivan R. M. (2004). Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int. J. Dev. Neurosci. 22 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Shionoya K., Jakubs K., Sullivan R. M. (2009b). Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J. Neurosci. 29 15745–15755. 10.1523/JNEUROSCI.4106-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D. A., Levine S., Sullivan R. M. (2006). Dual Circuitry for Odor–Shock Conditioning during Infancy: corticosterone Switches between Fear and Attraction via Amygdala. J. Neurosci. 26 6737–6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin J. C., Philippi C. L., Wolf R. C., Baskaya M. K., Koenigs M. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry 77 276–284. 10.1016/j.biopsych.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H. P., Berndt J. D., Barrett D., Gonzalez-Lima F. (2001). Metabolic mapping of brain regions associated with behavioral extinction in preweanling rats. Brain Res. 903 141–153. [DOI] [PubMed] [Google Scholar]
- Norrholm S. D., Jovanovic T. (2018). Fear processing, psychophysiology, and PTSD. Harv. Rev. Psychiatry 26 129–141. 10.1097/HRP.0000000000000189 [DOI] [PubMed] [Google Scholar]
- Opendak M., Gould E. (2015). Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn. Sci. 19 151–161. 10.1016/j.tics.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Opendak M., Gould E., Sullivan R. (2017). Early life adversity during the infant sensitive period for attachment: programming of behavioral neurobiology of threat processing and social behavior. Dev. Cogn. Neurosci. 25 145–159. 10.1016/j.dcn.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M., Robinson-Drummer P. A., Blomkvist A., Zanca R. M., Lundstrom J., Serrano P., et al. (2019). Neurobiology of maternal regulation of infant fear: the role of mesolimbic dopamine and its disruption by maltreatment. Neuropsychopharmacology 8:14679. 10.1038/s41386-019-0340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M., Sullivan R. M. (2016). Unique neurobiology during the sensitive period for attachment produces distinctive infant trauma processing. Eur. J. Psychotraumatol. 7 31276–31276. 10.3402/ejpt.v7.31276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M., Zanca R. M., Anane E., Serrano P. A., Sullivan R. M. (2018). Developmental transitions in amygdala PKC isoforms and AMPA receptor expression associated with threat memory in infant rats. Sci. Rep. 8:14679. 10.1038/s41598-018-32762-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D., Quirk G. J., Ledoux J. E. (2004). New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 92 1–9. [DOI] [PubMed] [Google Scholar]
- Pattwell S. S., Bath K. G. (2017). Emotional learning, stress, and development: an ever-changing landscape shaped by early-life experience. Neurobiol. Learn. Mem. 143 36–48. 10.1016/j.nlm.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S. S., Bath K. G., Casey B. J., Ninan I., Lee F. S. (2011). Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc. Natl. Acad. Sci. U.S.A. 108 1182–1187. 10.1073/pnas.1012975108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S. S., Duhoux S., Hartley C. A., Johnson D. C., Jing D., Elliott M. D., et al. (2012). Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. U.S.A. 109 16318–16323. 10.1073/pnas.1206834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S. S., Liston C., Jing D., Ninan I., Yang R. R., Witztum J., et al. (2016). Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat. Commun. 7:11475. 10.1038/ncomms11475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (2013). The Rat Brain in Stereotaxic Coordinates, 7th Edn. Boston, MA: Academic Press. [Google Scholar]
- Perry R. E., Blair C., Sullivan R. M. (2017). Neurobiology of infant attachment: attachment despite adversity and parental programming of emotionality. Curr. Opin. Psychol. 17 1–6. 10.1016/j.copsyc.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. E., Finegood E. D., Braren S. H., Dejoseph M. L., Putrino D. F., Wilson D. A., et al. (2018). Developing a neurobehavioral animal model of poverty: drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Dev. Psychopathol. 31 399–418. 10.1017/S095457941800007X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. A., Delgado M. R., Nearing K. I., LeDoux J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43 897–905. [DOI] [PubMed] [Google Scholar]
- Phelps E. A., LeDoux J. E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48 175–187. [DOI] [PubMed] [Google Scholar]
- Poulos A. M., Reger M., Mehta N., Zhuravka I., Sterlace S. S., Gannam C., et al. (2014). Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biol. Psychiatry 76 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Holman P. J., Debiec J., Bugg M., Beasley A., Sullivan R. M. (2010a). Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus 20 1037–1046. 10.1002/hipo.20702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Moriceau S., Sullivan R. (2010b). Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol. Psychiatry 67 1137–1145. 10.1016/j.biopsych.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Rincón-Cortés M., Belnoue L., Sullivan R. M. (2012). Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J. Neurosci. 32 7758–7765. 10.1523/JNEUROSCI.5843-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M., Barr G. A., Mouly A. M., Shionoya K., Nunez B. S., Sullivan R. M. (2015). Enduring good memories of infant trauma: rescue of adult neurobehavioral deficits via amygdala serotonin and corticosterone interaction. Proc. Natl. Acad. Sci. U.S.A. 112 881–886. 10.1073/pnas.1416065112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer P., Opendak M., Blomkvist A., Chan S., Tan S., Delmer C., et al. (2019). Infant trauma alters social buffering of threat learning: emerging role of prefrontal cortex in adolescence. Front. Behav. Neurosci. 9:132. 10.3389/fonc.2019.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer P. A., Stanton M. E. (2015). Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol. Behav. 148 22–28. 10.1016/j.physbeh.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan M. T., Staubli U. V., LeDoux J. E. (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390 604–607. [DOI] [PubMed] [Google Scholar]
- Romanski L. M., Clugnet M. C., Bordi F., LeDoux J. E. (1993). Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav. Neurosci. 107 444–450. [DOI] [PubMed] [Google Scholar]
- Roos L. E., Horn S., Berkman E. T., Pears K., Fisher P. A. (2018). Leveraging translational neuroscience to inform early intervention and addiction prevention for children exposed to early life stress. Neurobiol. Stress 9 231–240. 10.1016/j.ynstr.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquet R. F., Seo D. O., Jones C. E., Monfils M. H. (2018). Differential effects of predictable vs. unpredictable aversive experience early in development on fear memory and learning in adulthood. Behav. Neurosci. 132 57–65. 10.1037/bne0000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. L., Sullivan R. M. (2005). Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry 57 823–831. [DOI] [PubMed] [Google Scholar]
- Rudy J. W., Morledge P. (1994). Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav. Neurosci. 108 227–234. [DOI] [PubMed] [Google Scholar]
- Sah P., Westbrook R. F., Luthi A. (2008). Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann. N. Y. Acad. Sci. 1129 88–95. 10.1196/annals.1417.020 [DOI] [PubMed] [Google Scholar]
- Santiago A. N., Lim K. Y., Opendak M., Sullivan R. M., Aoki C. (2018). Early life trauma increases threat response of peri-weaning rats, reduction of axo-somatic synapses formed by parvalbumin cells and perineuronal net in the basolateral nucleus of amygdala. J. Comp. Neurol. 526 2647–2664. 10.1002/cne.24522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Martens G. J., Kolk S. M. (2015). Molecular underpinnings of prefrontal cortex development in rodents provide insights into the etiology of neurodevelopmental disorders. Mol. Psychiatry 20 795–809. 10.1038/mp.2014.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y., Moriceau S., Holman P., Miner C., Muzny K., Gervais R., et al. (2007). Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol. Psychiatry 62 1070–1079. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Sullivan R. M., Messaoudi B., Mouly A. M. (2008). Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learn. Mem. 15 649–656. 10.1101/lm.998508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Hong M., Britton J. C., Pine D. S., Fox N. A. (2014). Fear conditioning and extinction across development: evidence from human studies and animal models. Biol. Psychol. 100 1–12. 10.1016/j.biopsycho.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24 417–463. [DOI] [PubMed] [Google Scholar]
- Spielberg J. M., Olino T. M., Forbes E. E., Dahl R. E. (2014). Exciting fear in adolescence: does pubertal development alter threat processing? Dev. Cogn. Neurosci. 8 86–95. 10.1016/j.dcn.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M. E., Wallstrom J., Levine S. (1987). Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev. Psychobiol. 20 131–145. [DOI] [PubMed] [Google Scholar]
- Sullivan R. M., Landers M., Yeaman B., Wilson D. A. (2000). Good memories of bad events in infancy. Nature 407 38–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi L. K. (2014). Olfactory systems and neural circuits that modulate predator odor fear. Front. Behav. Neurosci. 8:72. 10.3389/fnbeh.2014.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallot L., Diaz-Mataix L., Perry R. E., Wood K., LeDoux J. E., Mouly A. M., et al. (2017). Updating of aversive memories after temporal error detection is differentially modulated by mTOR across development. Learn. Mem. 24 115–122. 10.1101/lm.043083.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallot L., Doyere V., Sullivan R. M. (2016). Developmental emergence of fear/threat learning: neurobiology, associations and timing. Genes Brain Behav. 15 144–154. 10.1111/gbb.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M. H., Andersen S. L., Polcari A., Anderson C. M., Navalta C. P., Kim D. M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 27 33–44. [DOI] [PubMed] [Google Scholar]
- Teicher M. H., Samson J. A., Anderson C. M., Ohashi K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 17 652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Thompson J. V., Sullivan R. M., Wilson D. A. (2008). Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res. 1200 58–65. 10.1016/j.brainres.2008.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi R., Arnautovic E., Vital M., Manini A., Suardi F., Gex-Fabry M., et al. (2018). Developmental delay in communication among toddlers and its relationship to caregiving behavior among violence-exposed, posttraumatically stressed mothers. Res. Dev. Disabil. 82 67–78. 10.1016/j.ridd.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Upton K. J., Sullivan R. M. (2010). Defining age limits of the sensitive period for attachment learning in rat pups. Dev. Psychobiol. 52 453–464. 10.1002/dev.20448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche L., Van de Ven L., Vandenbulcke M., Luyten P. (2019). Ghosts from the past? The association between childhood interpersonal trauma, attachment and anxiety and depression in late life. Aging Ment. Health. 10.1080/13607863.2019.1571017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- van Rooij S. J. H., Cross D., Stevens J. S., Alexander Vance L., Kim Y. J., Bradley B., et al. (2017). Maternal buffering of fear-potentiated startle in children and adolescents with trauma exposure. Soc. Neurosci. 12 22–31. 10.1080/17470919.2016.1164244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem M. R., Tottenham N. (2018). Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Top. Behav. Neurosci. 38 117–136. 10.1007/7854_2016_42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I., Vidal-Gonzalez B., Rauch S. L., Quirk G. J. (2006). Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 13 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. D., Bath K. G., Joels M., Korosi A., Larauche M., Lucassen P. J., et al. (2017). Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress 20 421–448. 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watamura S. E., Roth T. L. (2018). Looking back and moving forward: evaluating and advancing translation from animal models to human studies of early life stress and DNA methylation. Dev. Psychobiol. 61 323–340. 10.1002/dev.21796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmayer C. P., Barr G. A. (2001). Developmental changes in responsivity to threat are stimulus-specific in rats. Dev. Psychobiol. 39 1–7. [DOI] [PubMed] [Google Scholar]
- Wilensky A. E., Schafe G. E., Kristensen M. P., LeDoux J. E. (2006). Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 26 12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap C. S., Richardson R. (2005). Latent inhibition in the developing rat: an examination of context-specific effects. Dev. Psychobiol. 47 55–65. [DOI] [PubMed] [Google Scholar]
- Yu M., Linn K. A., Shinohara R. T., Oathes D. J., Cook P. A., Duprat R., et al. (2019). Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc. Natl. Acad. Sci. U.S.A. 116 8582–8590. 10.1073/pnas.1900801116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.