Abstract
Purpose
Our purpose was to study whether application of femtosecond laser pulses for alphanumeric code marking in the volume of zona pellucida (ZP) could be effective and reliable approach for direct tagging of preimplantation embryos.
Methods
Femtosecond laser pulses (wavelength of 514 nm, pulse duration of 280 fs, repetition rate of 2.5 kHz, pulse energy of 20 nJ) were applied for precise alphanumeric code engraving on the ZP of mouse embryos at the zygote stage for individual embryo marking and their accurate identification. Embryo quality assessment every 24 h post laser-assisted marking as well as immunofluorescence staining (for ICM/TE cell number ratio calculation) were performed.
Results
Initial experiments have started with embryo marking in a single equatorial plane. The codes engraved could be clearly recognized until the thinning of the ZP prior to hatching. Since embryo may change its orientation during the ART cycle, multi-plane code engraving seems to be more practical for simplifying the process of code searching and embryo identification. We have marked the ZP in three planes, and no decrease in developmental rates as well as no morphological changes of embryos post laser-assisted engraving have been observed as compared to control group embryos.
Conclusions
Our results demonstrate the suitability of femtosecond laser as a novel tool for noninvasive embryo tagging, enabling embryo identification from day 0.5 post coitum to at least early blastocyst stage. Thus, the versatility and the potential use of femtosecond lasers in the field of developmental biology and assisted reproduction have been shown.
Keywords: Laser microsurgery, Femtosecond laser, Laser-assisted embryo marking, Embryo identification, Embryo tagging, Zona pellucida
Introduction
Since the invention of the laser just over 50 years ago, many laser applications in life sciences have been developed. The field of assisted reproduction is not an exception. Today, lasers are commonly employed in assisted reproduction treatment [1] for opening the zona pellucida (ZP, a glycoprotein layer surrounding the plasma membrane of the oocyte) in assisted hatching or prior to cell extraction from the developing embryo for preimplantation genetic diagnosis (embryo biopsy). Lasers are utilized not only for oocytes, but also for spermatozoa treatment and manipulations [2, 3]. Thus, for example, lasers have been in use for human sperm immobilization prior to ICSI (intracytoplasmic sperm injection) [4] or for sperm irradiation in order to improve spermatozoa motility and overall fertilizing potential [5, 6]. Studies regarding laser-assisted improvement of sperm motility are still underway [7]. As far as optical tweezers have proved to be an essential tool for noncontact manipulating single cells [8], the possibilities of their application in the field of assisted reproduction have been also extensively studied. Optical tweezers have been applied for quantitative analysis of sperm motility [9], for in vitro fertilization by noncontact laser-mediated sperm insertion into the perivitelline space of oocytes [10], and for trapping and delicate removal of polar bodies for their genetic analysis [11, 12].
Nowadays, 1.48-um diode lasers with milli- to microsecond pulse durations are the most popular lasers applied in the field of assisted reproduction for microdissection. The main danger of pointing a laser at an embryo is thermal damage. Infrared diode lasers seem to be an effective and safe tool, nevertheless strong recommendations regarding optimum regimes of embryo exposure should be taken into account in order to eliminate possible laser-related thermal risks [13–15]. According to this, application area of infrared diode lasers is commonly limited to zona pellucida dissection and spermatozoa immobilizing prior to use.
At the same time, there are many papers reporting new approaches to assisted reproduction problems based on a novel, more accurate and effective laser systems generating laser pulses with shorter durations. In recent years, femtosecond laser pulses have emerged as a promising tool for noninvasive microsurgery, micromanipulation, and even optical modification (transfection [16]) of living biological objects. Using ultrashort laser pulses, both whole cells and intracellular organelles can be treated with high spatial and temporal precision. Femtosecond lasers have been successfully applied for fully noncontact optical microinjection and trapping of developing embryos [17], for blastomere fusion [18, 19], oocyte enucleation [20], and trophectoderm cells dissection during embryo biopsy [21].
In this study, we demonstrate the versatility and the potential use of femtosecond lasers in the field of developmental biology and assisted reproduction by developing a novel technique for individual labeling of preimplantation embryos, based on femtosecond laser microsurgery of zona pellucida. Femtosecond laser pulses were applied for marking alphanumeric characters on zona pellucida. This technique may be useful not only in the field of developmental biology for studying characteristics of embryo development during their co-culture in groups, but also in assisted reproductive technologies for preventing medical accidents relating to mix-ups. Although such errors are rare, several cases of mix-up in IVF laboratories have been reported [22–25]. Such events can cause serious legal consequences and prolonged emotional distress for the patients and may directly affect the health of parents and babies. According to the latest research [25], 90.4% of the respondents (patients undergoing IVF treatment in a single private infertility center in Europe) expressed significant concerns relating to biological sample mix-up.
In order to prevent mismatching and eliminate the risk of mistake during the entire ART procedure, various strategies and safety policies have been implemented. First of all, strong recommendations and protocols by leading ART-related organizations (ESHRE and HFEA (Europe), FLASEF (Latin America)) have been developed [26, 27]. Their guidelines mandate accurate labelling of all labware for correct patient identification and “double-witnessing” procedure. Recently, electronic witness (EW) systems have been developed. Among them are systems based on radio-frequency identification technology [28, 29], barcode labels [30], and even direct embryo tagging system based on silicon barcode injection into zygotes/embryos [31]. However, some of the EW approaches proposed are at an early stage of development or have limitations to be addressed in the future. Thus, for example, additional equipment is required (such as label printer or code reader) when using safety systems based on QR (quick response) code generation and recognition [32]; volatile organic components in the printing and adhesive materials should be selected carefully so as not to be toxic to embryo development [32]. Moreover, possible effects of polysilicon barcodes proposed in [31] on fetal growth and development should be studied in the future. Thus, optimization of existing methods aimed at preventing biological sample mix-up and development of new alternative devices and techniques is still required. By using femtosecond laser pulses relatively fast, precise, and delicate microsurgery can be performed with a minimal risk of thermal damage.
Materials and methods
Animals
Mice of the strain C57BL/6J were purchased from animal house of Federal Medical Biological Agency, Branch “Andreevka”. Animals were maintained under controlled room conditions (22–24 °C and 14 L:10D photoperiod). Mice were given ad libitum access to food and water. The animal care and procedures employed in this study were performed according to protocols approved by the Faculty of Biology of the Lomonosov Moscow State University.
Embryo collection, culture, and monitoring
Female mice 6 to 8 weeks old were mated with males late in the evening and checked the following morning for a presence of a copulation plug. Later, the same day mice were sacrificed by cervical dislocation. Oviducts were removed and placed on previously equilibrated in the CO2-incubator (5%CO2/humidified air) HEPES-containing medium (Human Tubal Fluid Medium, Irvine Scientific). Then, oviducts were dissected using insulin gauge needles and zygotes were collected. Zygotes were collected from fresh oviducts according to standard protocol [33] with slight modifications. Briefly, to remove cumulus cells, cumulus-oocyte complexes (COCs) were placed into the HEPES-buffered solution containing 30 IU/ml hyaluronidase (COOK Sydney IVF) for 2 min and placed into the CO2-incubator. Then, zygotes were removed from cumulus cells by gentle pipetting and washed for three times in fresh HEPES-containing medium.
Embryos were collected and divided into three groups: group A (experimental embryos), group B (embryos kept out of the incubator for the same time as group A, but not exposed to laser radiation), and group C (fully intact control embryos stored in a CO2 incubator at 37 °C). Group A and B embryos were transferred to fresh drops of human tubal fluid medium in Petri dishes (glass-bottomed 170 μm thick, 5 embryos per dish) under mineral oil balanced with the medium. After the procedure, embryos of groups A and B were placed into 4-well plate (4–5 embryos per well), returned to the incubator, and cultured in global total medium (LifeGlobal Group) in the CO2 incubator until they hatched. Group C embryos after retrieval from mice were directly placed to the global total medium and cultured in the 4-well plate in the CO2 incubator. Embryos from the experimental group were treated with laser radiation in order to create the code in the zona pellucida. Evaluation of embryonic development was performed and readability of laser engraved codes was checked every 24 h post laser processing using AxioObserver.Z1 (Zeiss) or Olympus IX-71 microscopes.
Immunofluorescence staining
The differential staining of inner cell mass cells (ICM) in the embryos was made with the anti-Oct3/4 antibodies (PA5-27438, ThermoFisher Scientific) according to the manufacturer’s instructions and as described by Yan et al. [34] with slight modifications. Briefly, embryos (4.5 dpc) were fixed in 4% paraformaldehyde for 30 min at room temperature. Embryos were then washed three times for 15 min in 0.1% PBST (1xPBS plus 0.1% Triton X-100) to remove the residual paraformaldehyde and for permeabilization of the membranes. After permeabilization, embryos were transferred to the blocking solution (3% serum and 0.1% PBST) for 1 h at room temperature. Then, they were incubated overnight at + 4 °C in the primary anti-Oct3/4 antibodies produced in rabbit (1:200). After washing four times in blocking solution (for 15 min each time) embryos were incubated for 2 h at room temperature in secondary antibodies (1:200, produced in goat, Alexa Fluor 568 conjugated, ab175470, Abcam). Then, the nuclei of the cells were stained with Hoechst 33342 (4 μg/ml) for 15 min at room temperature. After several washes, embryos were mounted on the glass slides and examined under the confocal microscope Olympus Fluoview FV10i. Cells with nuclear localization of OCT3/4 were identified as ICMs.
Data analysis
Immunofluorescence staining was used to determine the ICM/TE cell number ratio in blastocysts in order to assess the quality of embryos post laser-assisted code engraving. The number of cells of different types was scored using freely available image analysis software ImageJ (NIH, USA). The z-stacks of whole embryos were analyzed. The step size of the z-stack was set to 1 μm.
Statistical significance was calculated by the Kruskal-Wallace test using Statistica 7.0 software (Dell, Inc., USA). To verify the hypothesis of normal distribution, Shapiro-Wilk normality test was used (test was also performed using Statistica 7.0 software). The Kruskal-Wallace test was applied because no normal distribution was observed in all cases. Differences were considered statistically significant if p < 0.05.
Ethics statement
All manipulations with animals were performed according to the Moscow State University Bioethical Committee recommendations (Ethical approval documentation registration number 72-j; date of registration March 26, 2018).
Experimental set-up
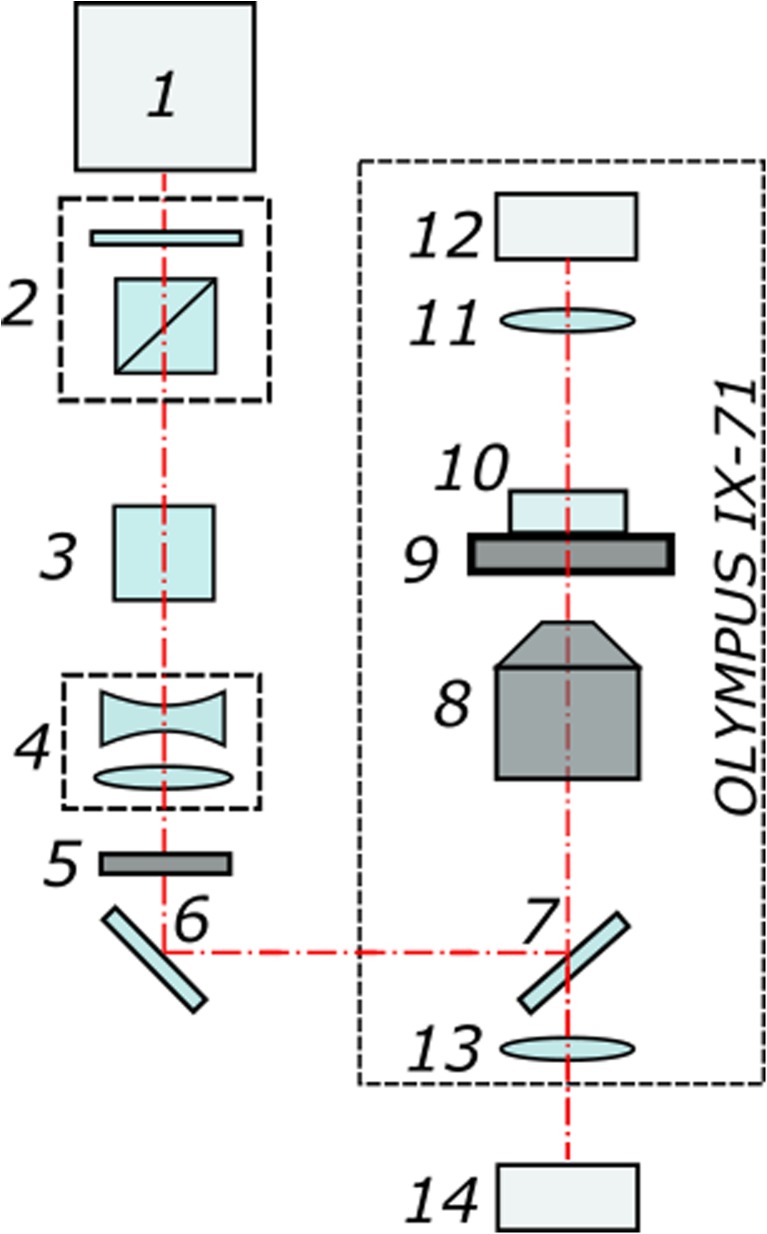
Figure 1 shows the schematic diagram of the experimental setup. The system utilizes a femtosecond ytterbium laser 1 (TETA, Avesta LLC) that operates at 280 fs with repetition rate of 2.5 kHz and wavelength of 1028 nm. The laser beam passes through a beam attenuator 2 consisting of half-wave plate and prism polarizer and then goes through a second-harmonic generator 3. We employ second-harmonic radiation (514 nm wavelength) to perform microdissections on ZP in the form of arbitrary alphanumeric characters. The radiation is then directed through a telescope 4 (with magnification 1:3) to fill the entrance aperture of the microscope objective 8. After passing through an elecro-mechanical shutter 5, laser beam enters the Olympus IX-71 microscope and is focused on the sample with a × 20, NA 0.5 UPLFLN objective. The focal spot diameter is measured to be about 1.9 um. The pulse energies were optimized to be slightly above the ablation threshold in order to create thin, well-defined cuts on the zona pellucida surface, while preventing (minimizing) the formation of multiple, relatively large cavitation bubbles. For 280 fs laser pulses at 514 nm with the energy of 20 nJ per pulse, we calculated the peak power and power density to be about 0.07·106 W and 2.5·1012 W cm−2.
Fig. 1.
Schematic diagram of the experimental set-up. (1) Femtosecond ytterbium laser. (2) Beam attenuator. (3) Second-harmonic generator. (4) Telescope. (5) Electro-mechanical shutter. (6) Mirror. (7) Dichroic mirror. (8) Microscope objective. (9) X-Y motorized stage. (10) Petri dish. (11) Condenser lens. (12) Microscope lamp. (13) Tube lens. (14) CMOS camera
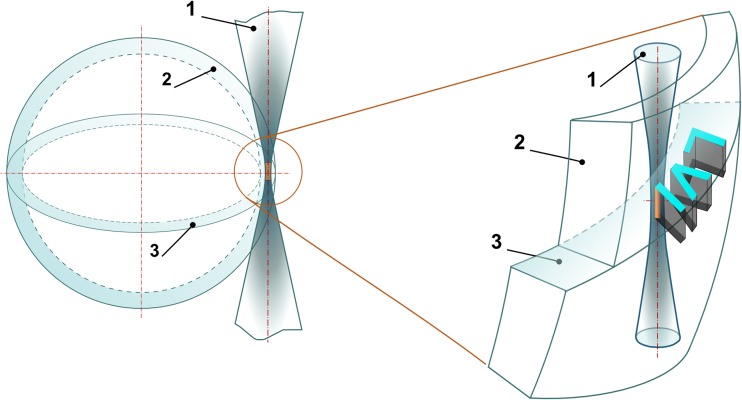
The Petri dishes with experimental embryos were placed on a motorized X-Y stage (Märzhäuser). Laser-assisted manipulations with embryos were recorded using a DFK 72AUC02 CMOS-camera (Imaging Source). Alphanumerical characters were overlaid in the custom-built software by simply drawing the combination of primitive elements (like lines, curves) on top of live image from the camera. Each drawn element was converted to a sequence of commands to the motorized stage. Schematic of the process of laser-assisted code marking in the volume of ZP is shown in Fig. 2. The equatorial plane—the plane of maximum embryo diameter—has been chosen for laser engraving. Figure 2 demonstrates relative position of the embryo’s ZP and the laser beam. Since ZP is transparent for laser radiation at chosen wavelength, the beam propagates freely within. When focusing, laser beam diameter decreases along beam axis, leading to laser intensity growth and reaching its maximum in the beam waist (the area of maximum intensity is colored in orange in Fig. 2). Laser-assisted engraving in the volume of zona pellucida occurs as soon as the incoming laser irradiance exceeds the threshold intensity. The excess of laser intensity over the threshold value determines the width (how bold the symbols look like) and the length (along beam axis) of the interaction area.
Fig. 2.
Schematic illustration of laser-assisted engraving of “LVI” code in the volume of the ZP. (1) Laser beam. (2) The zona pellucida. (3) The equatorial plane
Results
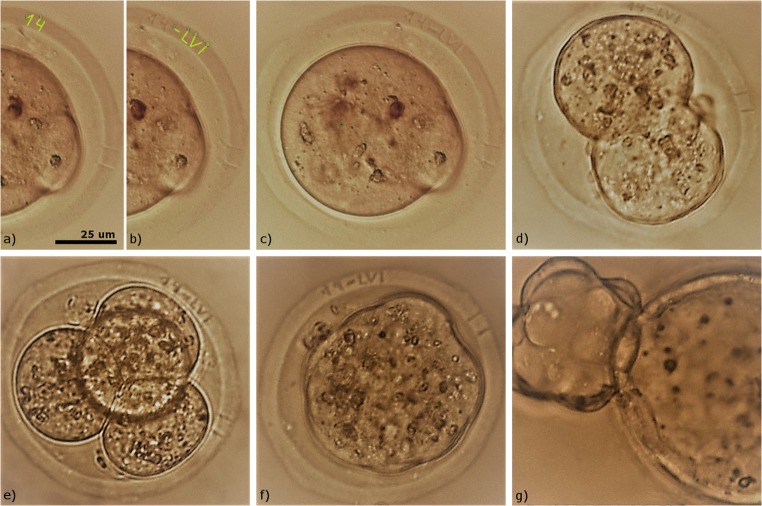
Thirty-three embryos were used in the group A in order to perform laser-assisted embryo tagging. Groups B and C included 38 and 43 embryos correspondingly. Embryos from the experimental group were treated with laser radiation only in the equatorial plane and one code per embryo was created. An example of alphanumeric code engraving on the zygote’s ZP is demonstrated in Fig. 3. At first, numeric characters “1” and “4” were created (Fig. 3a); then, the engraving procedure was started and fs-laser pulses with the energy of 20 ± 2 nJ were applied to replicate these characters in the volume of the ZP (Fig. 3b). Then, alphabetic code “LVI” was drawn (Fig. 3b) and engraved on the ZP (Fig. 3c). Normally, it takes no more than 30–40 s to create alphanumeric code in the software interface and only 8–13 s to mark each embryo with five alpha-numeric characters. Thus, laser-assisted engraving procedure takes about 2 min per embryo in current software version (including embryo snapshot pre- and post procedure, selection of plane along Z axis and a place where the code is to be engraved, the code drawing and engraving itself). The time required for manual code “drawing” using lines and multiline primitives can be minimized in the future by the automation of code creation procedure by using special templates.
Fig. 3.
Femtosecond laser-assisted tagging of preimplantation mouse embryos. a Drawing of numeric characters “1” and “4” in the software. b Laser engraving of numeric characters “1” and “4” on the zona pellucida and drawing of alphabetic characters “L, V, I” in the software. c Alphanumeric code engraved on the zygote’s zona pellucida. (d–f) Development of embryo with the engraved code on the 1.5, 2.5, 3.5 dpc. (g) Embryo hatching on the 4.5 dpc
Results on embryo development post laser-assisted single-code engraving in the experimental group A and untreated groups B and C are summarized in Table 1. The number of embryos reaching developmental stages on the 1.5, 2.5, and 3.5 dpc, as well as the number of embryos started hatching on the 4.5 dpc are shown. All of the treated embryos (group A) developed to the blastocyst stage, and 27 of 33 embryos (81.8%) started hatching at the time of their assessment. Eighty one percent of embryos (31 of 38) from the parallel control group B have also started hatching. Intact control group C demonstrated 25.6% development up to the blastocyst stage and 70% development up to the hatching stage.
Table 1.
Development of laser-exposed (group A) and untreated embryos (groups B and C)
| Groups | Group A (experimental) | Group B (parallel control) | Group C (intact control) | |||
|---|---|---|---|---|---|---|
| No. embryos (0.5 dpc)a | No. embryos at (BS)/(HS)b | No. embryos (0.5 dpc)a | No. embryos at (BS)/(HS)b | No. embryos (0.5 dpc)a | No. embryos at (BS)/(HS)b | |
| Single-plane code engraving | 33 |
BS = 6 (18.2%) HS = 27 (81.8%) |
38 |
BS = 5 (13.1%) HS = 31 (81.6%) |
43 |
BS = 11 (25.6%) HS = 30 (70%) |
| Three-plane code engraving | 18 |
BS = 4 (22.2%) HS = 14(77.7%) |
15 |
BS = 2 (13.3%) HS = 12 (80%) |
||
| No. of embryos in the group | 51 | 53 | 43 | |||
| Total no. of embryos | 147 | |||||
aNo. of embryos successfully marked with femtosecond laser pulses in the equatorial plane or in three different planes on the day 0.5 (zygote). bNo. of embryos achieved blastocyst (BS) or hatching stage (HS) at the time of their assessment on the day 4.5
It has been shown no decrease in the developmental rates of the embryos exposed to fs-laser radiation as compared to control embryos. As demonstrated in Fig. 3d–f, the code engraved on the zygote’s ZP (0.5 dpc) was also clearly recognizable on the 1.5–3.5 dpc.
We have also tested other codes engraving. As an example, in Fig. 4, we demonstrate that successful code recognition is still possible even if some symbols are not seen completely (due to embryo rotation). The code “07TEX” is clearly visible right after (0.5 dpc) and 1-day post (1.5 dpc) laser-assisted engraving procedure in Figs. 4a and b correspondingly. Embryo at 2.5 dpc and 3.5 dpc with codes “07TEX” slightly shifted outwards but still recognizable is shown in Figs. 4c and d.
Fig. 4.
Femtosecond laser-assisted engraving of code “07TEX” on the zona pellucida of preimplantation mouse embryos. a Embryo right after the code engraving (0.5 dpc). b Embryo on the following day (1.5 dpc) with clearly visible code. c–d Embryo at 2.5 dpc and 3.5 dpc with codes “07TEX” slightly shifted outwards but still recognizable
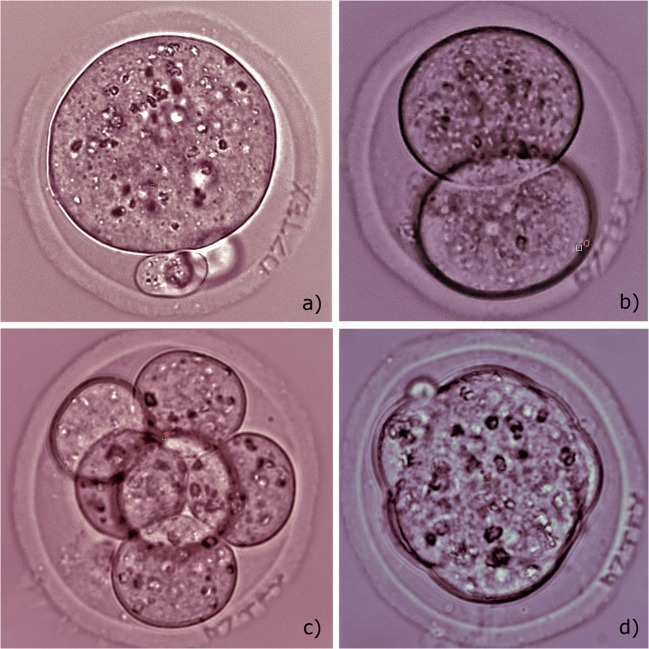
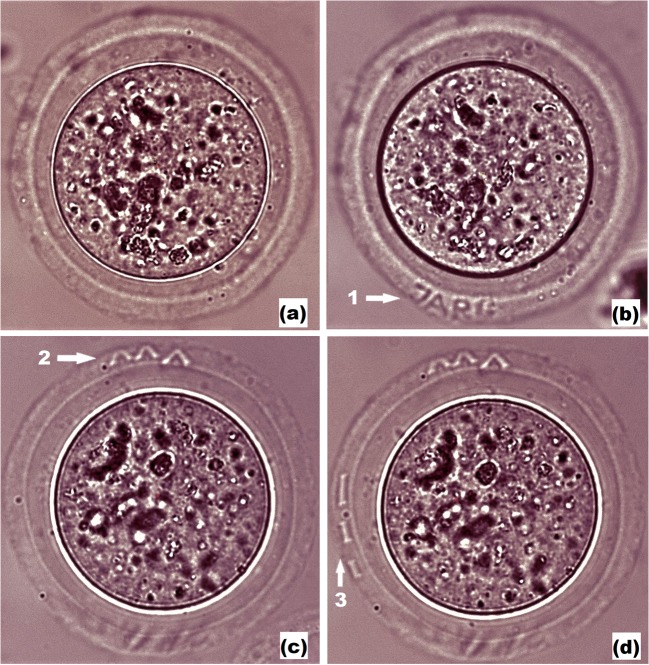
As far as embryo may change its orientation during the further ART cycle, we also tried to mark the egg coat of the embryo in several planes (typically, in three planes) in order to simplify the process of code searching and embryo identification. An additional 33 embryos were divided into group A (18 experimental embryos, that were subjected to laser radiation three times in order to create codes in three different planes) and group B (15 embryos kept out of the incubator for the same time as group A, but not exposed to laser radiation). Snapshots of the embryo with three different codes created in the zona pellucida in different planes are demonstrated. Embryo prior to laser engraving is shown in Fig. 5a. Figures 5b–d) demonstrate embryo after sequential engraving of three different codes in various planes (the code “JARG” is clearly seen in Fig. 5b, whereas it is blurred in Figs. 5c and d because codes “V V V” and “I I I” are formed in other planes). After first code was engraved (typically, in 5 embryos per dish), embryos were transferred back to the incubator and stored for 15 min, then, laser-assisted code marking was performed in other planes (with keeping embryos in the incubator between laser procedures). Results on embryo development post laser-assisted three-plane engraving in the experimental group A and untreated group B are also summarized in Table 1. It has been shown that the embryo development in both experimental (single-plane and three-plane code engraving) groups did not significantly differ from that in both (intact and parallel) control groups.
Fig. 5.
Femtosecond laser-assisted engraving of three codes in various planes. a Embryo prior to laser-assisted code engraving. b Laser-assisted engraving of code “JARG”. c, d laser-assisted engraving of codes “V V V” and “I I I” correspondingly
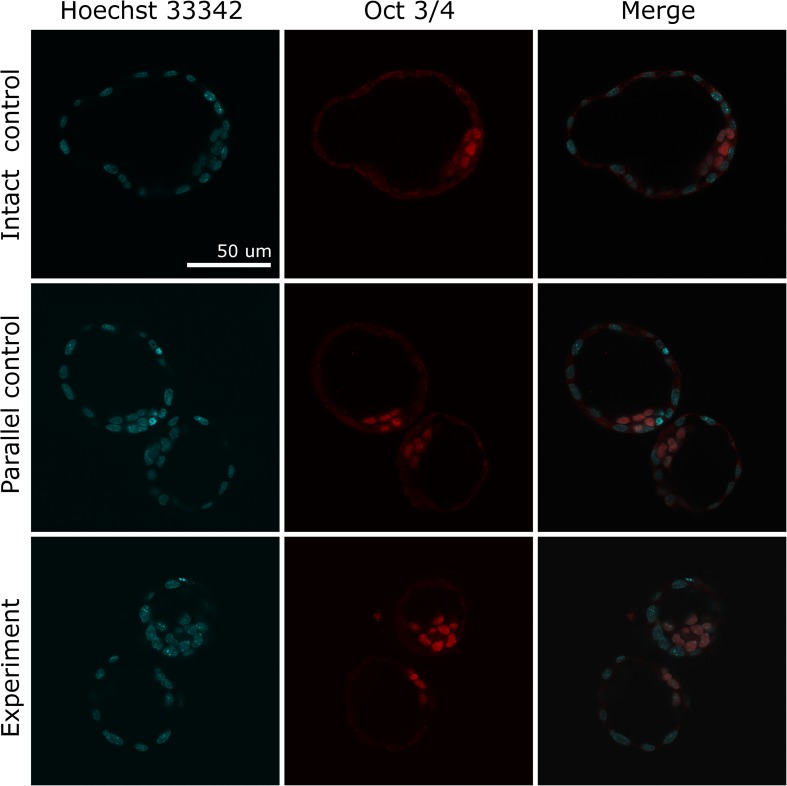
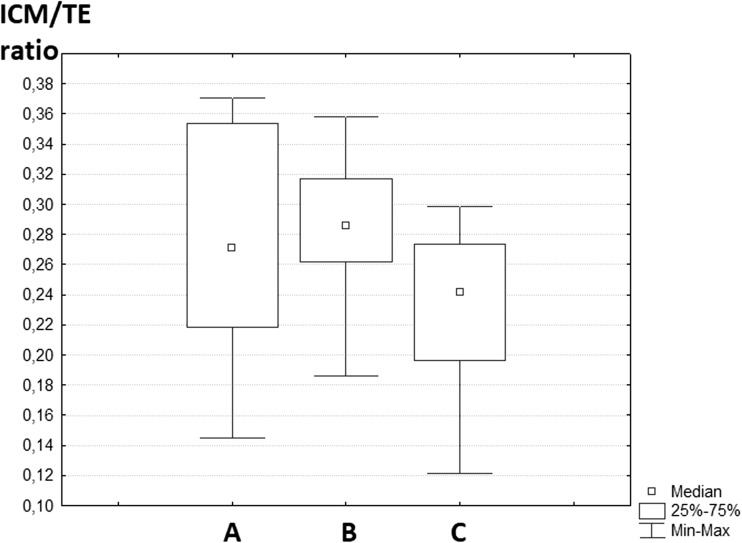
To analyze potential effect of laser pulses on embryo quality, the ratio of inner cell mass to trophectoderm (ICM/TE) cells in blastocysts from experimental and control groups was calculated. A total of 21 blastocysts were subjected to immunofluorescence staining. Figure 6 demonstrates stained cells in the equatorial plane of the embryos from experimental, parallel, and intact control groups (seven blastocysts per group). Embryos from three-plane code engraving group were selected since laser exposure was maximal compared to single-plane experimental group. It can be seen that TE and ICM cells can be easily distinguished and counted by means of sequential layer-by-layer scanning. No statistically significant differences (p > 0.05) in the ICM/TE ratio across three groups of samples were observed. The box-and-whisker-plot illustrating the values of ICM/TE ratio in experimental and control groups is given in Fig. 7.
Fig. 6.
Differential staining of inner cell mass and trophectoderm cells of mouse embryos. Row 1 is fully hatched blastocyst from intact control group, rows 2 and 3 are 8-shaped hatching blastocysts from parallel and experimental groups. All nuclei were stained with Hoechst 33342 (blue color), and ICM cells were stained with anti-Oct3/4 antibody conjugated with Alexa Fluor 568 (red color)
Fig. 7.
The box-and-whisker plot illustrating ICM/TE ratio in blastocysts from three different groups. (A) Experimental group (three-plane code engraving, samples n = 7). (B) Parallel control (n = 7). (C) Intact control (n = 7)
Discussion
Many studies have focused on utilization of lasers for oocyte, spermatozoa, and embryo treatment. Infrared diode lasers (wavelength of 1480 nm) are the most widely used lasers in clinical practice. While the majority of authors in their studies have noted relative safety of these laser systems [35, 36], certain authors have suggested that a heat produced during laser irradiation may have adverse effects on embryonic development [13, 14, 37]. When using laser pulses of microsecond duration and longer, one should carefully control the exposure time in relation to the thermal relaxation time of the tissue. If the exposure time is longer than the thermal relaxation time, the heat can diffuse within the tissue beyond the typical optical penetration depth [38]. According to the theory of laser-induced heating described in [13], the maximum temperature near the beam center (1480 nm wavelength, laser pulse duration 3 ms, laser power 100 mW, beam radius 3 um) may be as high as 200 °C. Several approaches have been proposed to avoid the risk of laser-induced thermal damage to embryos. Among them are artificial shrinkage of oocytes (aimed at increasing the perivitelline space) during the IVF with laser-assisted zona drilling by adding sucrose to the culture medium [39–41]. Moreover, drilling location should be selected rather thoroughly at an area with no cells touching the zona pellucida.
Using short and ultrashort laser pulses, whole cells and intracellular organelles can be altered with high spatial and temporal precision. It has been shown that zona pellucida drilling prior to in vitro fertilization and polar body biopsy could be successfully performed by means of nanosecond nitrogen laser with a wavelength of 337 nm [10, 11]. Inactivation of oocytes and two-cell mouse embryos as well as laser-assisted blastomere fusion using picosecond pulses from an infrared laser have been reported in [42].
Lasers with ultra-short pulse duration (~ 100–300 fs) allow delivery of nanojoules energy per pulse during laser exposure, thus minimizing possible risks of thermal damage to embryos. The advantages of femtosecond laser-assisted microsurgery compared to milli-/microsecond or even nano-/picosecond duration pulses for minimizing collateral damage have been discussed in [43–48]. Targeted high precision ablation of various types of tissue including bones [49, 50], cornea, and sclera [46, 47, 51], neural tissue [44, 45], skin [52] with femtosecond laser pulses have been demonstrated.
Biological tissues can be usually treated as a transparent dielectric [48]. Thus, water or fused silica are usually used to represent biological material. As a rule, nonlinear multiphoton absorption and ionization take place during the action of ultrashort laser pulses. The process of tissue ablation by ultra-short laser pulses is called photodisruption. The photodisruption significantly depends on light intensity and only occurs when it exceeds the threshold level (typically ~ 1011 W cm−2). When photodisruption occurs near the threshold value, most of the absorbed energy is used to create the plasma and only a small amount is dissipated by other mechanisms (shock wave and cavitation bubble formation, thermal diffusion) [51]. Laser energy is deposited in electrons much faster than it can be transferred to the bulk material, thus minimizing collateral damage. Plasma created by the ultrashort laser pulse acts as a mirror reflecting a portion of the incoming light. Femtosecond laser pulses, therefore, allow precise tissue microsurgery with almost no collateral damage. Targeted high precision photodisruption with femtosecond lasers has been successfully employed in ophthalmology for corneal refractive surgery [53, 54]. As far as femtosecond lasers offer several important advantages (such as high precision, minimal invasiveness, versatility) over conventional milli-/microsecond lasers, we believe that further advances in ultrafast laser technology aimed at reducing complexity, size, and high price of femtosecond lasers will help them gain popularity in the field of assisted reproduction also.
In this study, we aimed to develop a novel femtosecond laser-based technique for individual embryo tagging. By using fs-laser microsurgery, we demonstrate for the first time the possibility to create individual alphanumeric codes directly on embryo’s outer shell to simplify the process of embryo identification. Due to highly localized effect during the action of fs-laser pulses, which fades away for out-of-focus cellular structures, relatively low pulse energy, and ultrahigh intensity, fs-lasers could be used for precise and delicate microsurgery of ZP with minimal risk of thermal damage to the adjacent embryo cells. The technique proposed is relatively fast, reliable, and simple and can be fully automated in the future. It can be performed in a contactless mode under sterile conditions and does not require any additional equipment (except microscope) to visualize the code and to identify the embryo. Moreover, only the ZP is subjected to laser microsurgery while leaving the embryo cells intact.
The codes engraved have been proven to be readable up to the early blastocyst stage. Further thinning of the ZP due to expansion of the blastocyst may significantly reduce the code readability. Thus, we can say with confidence that the technique of femtosecond laser engraving on the ZP may be useful at least for embryo identification from the day 0.5 to day 3, when embryo transfer can be done [55–57]. However, we suppose that thorough selection of characters to be engraved may enable code recognition until a dramatic zona pellucida thinning takes place. Using alphabetic and numerical symbols that are different in writing is preferable in order to avoid possible recognition mistakes. Thus, the codes like “1OZHC” and “JQ2NG” are examples of improper symbol choice since they can be easily mixed up and should not be used for embryo tagging within one embryo group.
Initial experiments have started with marking in a single equatorial plane of the embryo. Since embryo may change its orientation during the ART cycle, multi-plane code engraving seems to be more practical for simplifying the process of code searching and embryo identification. We have marked the egg coat in three planes, and no decrease in developmental rates as well as no morphological changes of embryos subjected to three-plane laser-assisted engraving have been observed as compared to control group embryos.
In conclusion, we demonstrate that femtosecond lasers can be employed as precise and effective tools for embryo microsurgery. Ultrashort laser pulses were applied for creating alphanumeric code in the zona pellucida, thus making possible embryo labelling and identification during the first days (at least 3.5 days) of embryo development. Potential applications for this technique would not be limited to safety systems for preventing embryo mix-ups during the IVF treatment. The technique may be useful in the field of developmental biology for studying the peculiarities of embryo development during their culture in groups.
Absolute safety of the laser-assisted marking procedure needs to be confirmed by detailed statistical analysis of embryo developmental rates. Such an analysis will be the subject of further investigations as well as the optimization of laser radiation parameters (focused spot size, energy of laser pulses) for creating high-quality, clearly readable codes.
Acknowledgements
The work was conducted using Unique Facility “Terawatt Femtosecond Laser Complex” in the Center for Collective Usage “Femtosecond Laser Complex” of JIHT RAS.
Funding information
The reported study was funded by RFBR and Moscow city Government according to the research project no.19-32-70036.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving animals were in accordance with the ethical standards of the Moscow State University.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bedient C, Khanna P, Desai N. Laser pulse application in IVF. In: Jakubczak K, editor. Lasers - applications in science and industry: InTech; 2011. p. 193–214.
- 2.Karu TI. Lasers in infertility treatment: irradiation of oocytes and spermatozoa. Photomed Laser Surg. 2012;30:239–241. doi: 10.1089/pho.2012.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Salam Z, Harith MA. Laser researches on livestock semen and oocytes: a brief review. J Adv Res. 2015;6:311–317. doi: 10.1016/j.jare.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montag M, Rink K, Delacretaz G, van der Ven H. Laser induced immobilization and plasma membrane permeabilization in human spermatozoa. Hum Reprod. 2000;15:846–852. doi: 10.1093/humrep/15.4.846. [DOI] [PubMed] [Google Scholar]
- 5.Sato H, Landthaler M, Haina D, Schill WB. The effects of laser light on sperm motility and velocity in vitro. Andrologia. 1984;16:23–25. doi: 10.1111/j.1439-0272.1984.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 6.Lenzi A, Claroni F, Gandini L, Lombardo F, Barbieri C, Lino A, Dondero F. Laser radiation and motility patterns of human sperm. Syst Biol Reprod Med. 1989;23:229–234. doi: 10.3109/01485018908986845. [DOI] [PubMed] [Google Scholar]
- 7.Preece D, Chow KW, Gomez-Godinez V, Gustafson K, Esener S, Ravida N, Durrant B, Berns MW. Red light improves spermatozoa motility and does not induce oxidative DNA damage. Sci Rep. 2017;7:46480. doi: 10.1038/srep46480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Liu KK. Optical tweezers for single cells. J R Soc Interface. 2008;5:671–690. doi: 10.1098/rsif.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nascimento JM, Shi LZ, Meyers S, Gagneux P, Loskutoff NM, Botvinick EL, Berns MW. The use of optical tweezers to study sperm competition and motility in primates. J R Soc Interface. 2008;5:297–302. doi: 10.1098/rsif.2007.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clement-Sengewald A, Schütze K, Ashkin A, Palma GA, Kerlen G, Brem G. Fertilization of bovine oocytes induced solely with combined laser microbeam and optical tweezers. J Assist Reprod Genet. 1996;13:259–265. doi: 10.1007/BF02065947. [DOI] [PubMed] [Google Scholar]
- 11.Clement-Sengewald A, Buchholz T, Schütze K, Berg U, Berg FD. Noncontact, laser-mediated extraction of polar bodies for prefertilization genetic diagnosis. J Assist Reprod Genet. 2002;19:183–194. doi: 10.1023/A:1014894029099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilina IV, Rakityanskiy MM, Sitnikov DS, Ovchinnikov AV, Agranat MB, Khramova YV, Semenova ML. Biomedical and biotechnology applications of noncontact femtosecond laser microsurgery of living cells. AIP Conf Proc. 2012;1464:560–571. doi: 10.1063/1.4739909. [DOI] [Google Scholar]
- 13.Douglas-Hamilton DH, Conia J. Thermal effects in laser-assisted pre-embryo zona drilling. J Biomed Opt. 2001;6:205–213. doi: 10.1117/1.1353796. [DOI] [PubMed] [Google Scholar]
- 14.Tucker MJ, Ball GD. Assisted hatching as a technique for use in human in vitro fertilization and embryo transfer is long overdue for careful and appropriate study. J Clin Embr. 2009;12:5–8. [Google Scholar]
- 15.Taylor T, Gilchrist J, Hallowell S, Hanshew K, Orris J, Glassner M, Wininger J. The effects of different laser pulse lengths on the embryo biopsy procedure and embryo development to the blastocyst stage. J Assist Reprod Genet. 2010;27:663–667. doi: 10.1007/s10815-010-9461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson DJ, Gunn-Moore FJ, Campbell P, Dholakia K. Single cell optical transfection. J R Soc Interface. 2010;7:863–871. doi: 10.1098/rsif.2009.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres-Mapa ML, Antkowiak M, Cizmarova H, Ferrier DEK, Dholakia K, Gunn-Moore FJ. Integrated holographic system for all-optical manipulation of developing embryos. Biomed Opt Express. 2011;2:1564–1575. doi: 10.1364/BOE.2.001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilina IV, Ovchinnikov AV, Sitnikov DS, Rakityanskiy MM, Agranat MB, Khramova YV, Semenova ML. Application of femtosecond laser pulses in biomedical cell technologies. High Temp. 2013;51:173–178. doi: 10.1134/S0018151X13020089. [DOI] [Google Scholar]
- 19.Osychenko AA, Zalessky AD, Krivokharchenko AS, Shakhbazian AK, Ryabova AV, Nadtochenko VA. Fusion of blastomeres in mouse embryos under the action of femtosecond laser radiation. Efficiency of blastocyst formation and embryo development. Quantum Electron. 2015;45:498–502. doi: 10.1070/QE2015v045n05ABEH015767. [DOI] [Google Scholar]
- 20.Kuetemeyer K, Lucas-Hahn A, Petersen B, Lemme E, Hassel P, Niemann H, Heisterkamp A. Combined multiphoton imaging and automated functional enucleation of porcine oocytes using femtosecond laser pulses. J Biomed Opt. 2010;15:046006. doi: 10.1117/1.3463012. [DOI] [PubMed] [Google Scholar]
- 21.Ilina IV, Khramova YV, Filatov MA, Semenova ML, Sitnikov DS. Application of femtosecond laser scalpel and optical tweezers for noncontact biopsy of late preimplantation embryos. High Temp. 2015;53:804–809. doi: 10.1134/S0018151X15060103. [DOI] [Google Scholar]
- 22.Liebler R. Are you my parent? Are you my child? The role of genetics and race in defining relationships after reproductive technological mistakes. DePaul J Health Care L. 2002;5:15–56. [PubMed] [Google Scholar]
- 23.Spriggs M. 2003 IVF mixup: white couple have black babies. J Med Ethics. 2003;29:65. doi: 10.1136/jme.29.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bender L. ‘To err is human’. ART mix-ups: a labor-based, relational proposal. J Gender Race & Just. 2006;9:1–90. [Google Scholar]
- 25.Forte M, Faustini F, Maggiulli R, Scarica C, Romano S, Ottolini C, Farcomeni A, Palagiano A, Capalbo A, Ubaldi FM, Rienzi L. 2016 electronic witness system in IVF—patients perspective. J Assist Reprod Genet. 2016;33:1215–1222. doi: 10.1007/s10815-016-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de los Santos MJ, Ruiz A. Protocols for tracking and witnessing samples and patients in assisted reproductive technology. Fertil Steril. 2013;100:1499–1502. doi: 10.1016/j.fertnstert.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 27.The Practice Committe of the American Society for Reproductive Medicine and the Practice Committe of the Society for Assisted Reproductive Technology Revised guidelines for human embryology and andrology laboratories. Fertil Steril. 2008;90:S45–S59. doi: 10.1016/j.fertnstert.2008.08.099. [DOI] [PubMed] [Google Scholar]
- 28.Glew AM, Hoha K, Graves J, Lawrence H, Read S, Ah-Moye M. Radio frequency identity tags ‘RFID’ for electronic witnessing of IVF laboratory procedures. Fertil Steril. 2006;86:S170. doi: 10.1016/j.fertnstert.2006.07.454. [DOI] [Google Scholar]
- 29.Thornhill AR, Brunetti XO, Bird S. Measuring human error in the IVF laboratory using an electronic witnessing system // Proc. of 17th World Congress on Controversies in Obstetrics, Gynecology & Infertility (COGI). 2013;101–106.
- 30.Schnauffer K, Kingsland C, Troup S. Barcode labelling in the IVF laboratory. Hum Reprod. 2005;20(suppl.1):i79–i80. [Google Scholar]
- 31.Novo S, Nogues C, Penon O, Barrios L, Santalo J, Gomez-Martinez R, Esteve J, Errachid A, Plaza JA, Perez-Garcia L, Ibanez E. Barcode tagging of human oocytes and embryos to prevent mix-ups in assisted reproduction technologies. Hum Reprod. 2014;29:18–28. doi: 10.1093/humrep/det409. [DOI] [PubMed] [Google Scholar]
- 32.Hur YS, Ryu EK, Park SJ, Yoon J, Yoon SH, Yang GD, Hur CY, Lee WD, Lim JH. Development of a security system for assisted reproductive technology (ART) J Assist Reprod Genet. 2014;32:155–168. doi: 10.1007/s10815-014-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. New York: Cold Spring Harbor Lab; 2014. [Google Scholar]
- 34.Yan Z, Liang H, Deng L, Long H, Chen H, Chai W, Suo L, Xu C, Kuang Y, Wu L, Lu S, Lyu Q. Eight-shaped hatching increases the risk of inner cell mass splitting in extended mouse embryo culture. PLoS One. 2015;10:e0145172. doi: 10.1371/journal.pone.0145172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Germond M, Nocera D, Senn A, Rink K, Delacrétaz G, Fakan S. Microdissection of mouse and human zona pellucida using a 1.48-microns diode laser beam: efficacy and safety of the procedure. Fertil Steril. 1995;64:604–611. doi: 10.1016/S0015-0282(16)57800-5. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh YY, Huang CC, Cheng TC, Chang CC, Tsai HD, Lee MS. Laser-assisted hatching of embryos is better than the chemical method for enhancing the pregnancy rate in women with advanced age. Fertil Steril. 2002;78:179–182. doi: 10.1016/S0015-0282(02)03172-2. [DOI] [PubMed] [Google Scholar]
- 37.Malter HE, Schimmel T, Cohen J. Zona dissection by infrared laser: developmental consequences in the mouse, technical considerations, and controlled clinical trial. Reprod BioMed Online. 2001;3:117–123. doi: 10.1016/S1472-6483(10)61979-7. [DOI] [PubMed] [Google Scholar]
- 38.Fedele D, Fusi F. Thermal effects of NIR laser radiation in biological tissue: a brief survey. Energy for Health. 2010;6:10–15. [Google Scholar]
- 39.Sagoskin AW, Han T, Graham JR, Levy MJ, Stillman RJ, Tucker MJ. Healthy twin delivery after day 7 blastocyst transfer coupled with assisted hatching. Fertil Steril. 2002;77:615–617. doi: 10.1016/S0015-0282(01)03199-5. [DOI] [PubMed] [Google Scholar]
- 40.Li MW, Kinchen KL, Vallelunga JM, Young DL, Wright KDK, Gorano LN, Wasson K, Lloyd KCK. Safety, efficacy and efficiency of laser-assisted IVF in subfertile mutant mouse strains. Reproduction. 2013;145:245–254. doi: 10.1530/REP-12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anzai M, Nishiwaki M, Yanagi M, Nakashima T, Kaneko T, Taguchi Y, Tokoro M, Shin SW, Mitani T, Kato H, Matsumoto K, Nakagata N, Iritani A. Application of laser-assisted zona drilling to in vitro fertilization of cryopreserved mouse oocytes with spermatozoa from a subfertile transgenic mouse. J Reprod Dev. 2006;52:601–606. doi: 10.1262/jrd.18040. [DOI] [PubMed] [Google Scholar]
- 42.Karmenyan AV, Shakhbazyan AK, Sviridova-Chailakhyan TA, Krivokharchenko AS, Chiou AE, Chailakhyan LM. Use of picosecond infrared laser for micromanipulation of early mammalian embryos. Mol Reprod Dev. 2009;76:975–983. doi: 10.1002/mrd.21045. [DOI] [PubMed] [Google Scholar]
- 43.Vogel A, Noack J, Huttman G, Paltauf G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl Phys B Lasers Opt. 2005;81:1015–1047. doi: 10.1007/s00340-005-2036-6. [DOI] [Google Scholar]
- 44.Loesel FH, Fischer JP, Götz MH, Horvath C, Juhasz T, Noack F, Suhm N, Bille JF. Non-thermal ablation of neural tissue with femtosecond laser pulses. Appl Phys B Lasers Opt. 1998;66:121–128. [Google Scholar]
- 45.Suhm N, Gotz MH, Fischer JP, Loesel F, Schlegel W, Sturm V, Bille J, Schröder R. Ablation of neural tissue by short-pulsed lasers–a technical report. Acta Neurochir. 1996;138:346–349. doi: 10.1007/BF01411747. [DOI] [PubMed] [Google Scholar]
- 46.Juhasz T, Kastis GA, Suarez C, Bor Z, Bron WE. Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water. Lasers Surg Med. 1996;19:23–31. doi: 10.1002/(SICI)1096-9101(1996)19:1<23::AID-LSM4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 47.Oraevsky AA, Da Silva LB, Rubenchik AM, Feit MD, Glinsky ME, Perry MD, Mammini BM, Small WIV, Stuart BC. 1996 plasma mediated ablation of biological tissues with nanosecond-to-femtosecond laser pulses: relative role of linear and nonlinear absorption. IEEE J Sel Top Quantum Electron. 1996;2:801–809. doi: 10.1109/2944.577302. [DOI] [Google Scholar]
- 48.Feit MD, Rubenchik AM, Kim BM, da Silva LB, Perry MD. Physical characterization of ultrashort laser pulse drilling of biological tissue. Appl Surf Sci. 1998;127–129:869–874. doi: 10.1016/S0169-4332(97)00758-7. [DOI] [Google Scholar]
- 49.Schwab B, Hagner D, Muller W, Lubatschowski H, Lenarz T, Heermann R. Bone ablation using ultrashort laser pulses. A new technique for middle ear surgery. Laryngorhinootologie. 2004;83:219–225. doi: 10.1055/s-2004-814554. [DOI] [PubMed] [Google Scholar]
- 50.Emigh B, An R, Hsu EM, Crawford TH, Haugen HK, Wohl GR, Hayward JE, Fang Q. Porcine cortical bone ablation by ultrashort pulsed laser irradiation. J Biomed Opt. 2012;17:028001. doi: 10.1117/1.JBO.17.2.028001. [DOI] [PubMed] [Google Scholar]
- 51.Jiang F, Yang X, Dai N, Lu P, Long H, Cui L. An in vitro study of femtosecond laser photodisruption in rabbit sclera. Front Optoelectron China. 2008;1:162–167. doi: 10.1007/s12200-008-0022-4. [DOI] [Google Scholar]
- 52.Frederickson KS, White WE, Wheeland RG, Slaughter DR. Precise ablation of skin with reduced collateral damage using the femtosecond-pulsed, terawatt titanium-sapphire laser. Arch Dermatol. 1993;129:989–993. doi: 10.1001/archderm.1993.01680290061009. [DOI] [PubMed] [Google Scholar]
- 53.Mian SI, Shtein RM. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2007;18:295–299. doi: 10.1097/ICU.0b013e3281a4776c. [DOI] [PubMed] [Google Scholar]
- 54.Farid M, Steinert RF. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2010;21:288–292. doi: 10.1097/ICU.0b013e32833a8dbc. [DOI] [PubMed] [Google Scholar]
- 55.Coskun S, Hollanders J, Al-Hassan S, Al-Sufyan H, Al-Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: a controlled randomized trial. Hum Reprod. 2000;15:1947–1952. doi: 10.1093/humrep/15.9.1947. [DOI] [PubMed] [Google Scholar]
- 56.Bungum M, Bungum L, Humaidan P, Yding AC. Day 3 versus day 5 embryo transfer: a prospective randomized study. Reprod BioMed Online. 2003;7:98–104. doi: 10.1016/S1472-6483(10)61736-1. [DOI] [PubMed] [Google Scholar]
- 57.Hatirnaz S, Perkas MK. Day 3 embryo transfer versus day 5 blastocyst transfers: a prospective randomized controlled trial. Turk J Obstet Gynecol. 2017;14:82–88. doi: 10.4274/tjod.99076. [DOI] [PMC free article] [PubMed] [Google Scholar]