Abstract
Purpose
Proprotein convertase subtilisin/kexin type 9 (PCSK9) and lipoprotein (a) (Lp[a]) levels are associated with cardiovascular risk. To investigate PCSK9 and Lp(a) levels of children born after assisted reproduction technologies (ART) compared with naturally conceived (NC) controls.
Methods
In this exposure-matched cohort study, 73 racial-, sex-, and age-matched children (mean age 98 ± 35 months) of ART (intracytoplasmic sperm injection [ICSI] n = 33, classic in vitro fertilization [IVF] n = 40) and 73 NC children were assessed. Blood lipid profile, including PCSK9 and Lp(a) levels, was measured. Children were grouped according to age (< 8 years, 8–10 years, ≥ 10 years).
Results
In the overall population, PCSK9 levels were related to total cholesterol, low-density lipoprotein, and systolic blood pressure, while Lp(a) levels were related to age, apolipoprotein-B, birth weight, height, waist-to-hip ratio, insulin resistance, insulin, and high-sensitivity C-reactive protein. No significant differences were observed regarding lipid biomarkers between ART and NC children. However, a significant interaction was found between age groups and conception method (p < 0.001) showing that PCSK9 levels increase with age in ART children, while they decline with age in NC offspring. IVF children showed higher levels of adjusted mean Lp(a) than ICSI (13.5 vs. 6.8 mg/dl, p = 0.010) and NC children (12.3 vs. 8.3 mg/dl, p = 0.048).
Conclusions
We show that PCSK9 levels increase with age in ART children, indicating a gradual deterioration of lipidemic profile that could lead to increased cardiovascular risk. Moreover, our results indicate that ART method may be of importance given that classic IVF is associated with higher levels of Lp(a).
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01474-1) contains supplementary material, which is available to authorized users.
Keywords: PCSK9, In vitro fertilization, Assisted reproduction technologies, Lipoprotein (a), Cardiovascular risk, Age
Introduction
Since the introduction of assisted reproduction technologies (ΑRT) in clinical practice, including classic in vitro fertilization (IVF) and Intracytoplasmic sperm injection (ICSI), several studies have addressed concerns regarding the long-term health of the offspring and have revealed indications of an adverse cardiovascular/cardiometabolic (CV) outcome [1]. In specific, elevated arterial blood pressure, pulmonary hypertension, right ventricle systolic dysfunction, as well as abnormal arterial stiffness, endothelial dysfunction, and carotid atherosclerosis have been observed in apparently healthy children born by ART [2]. Several pathophysiologic mechanisms have been implicated in the CV abnormalities observed in the ART children, such as epigenetic alterations and environmental factors affecting early embryogenesis and intrauterine fetal development [2]. Intriguingly, the observed CV alterations have not been linked to an impaired lipid profile regarding classic lipid parameters, such as total cholesterol (TC), triglycerides (TGL), low-density lipoprotein (LDL-C), and high-density lipoprotein (HDL-C) [3–5].
Proprotein convertase subtilisin/kexin type 9 (PCSK9), an enzyme mainly produced by hepatocytes, plays a pivotal role in the regulation of lipid metabolism by affecting the degradation of LDL-C receptors expressed in hepatocyte surface [6]. PCSK9 levels are associated with metabolic markers, such as fasting glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), total cholesterol, LDL-C, triglycerides, HDL-C, apolipoproteins A1 and B, and body weight [7, 8]. Importantly, PCSK9 levels are markers of increased CV risk [9, 10]. Regarding children and adolescents, serum PCSK9 concentrations vary according to sex and age [7].
Elevated serum lipoprotein (a) (Lp[a]) values are the strongest inherited risk marker for premature CV events [11]. Lp(a) presents structural similarities with LDL-C and plasminogen and exhibits a strong proatherogenic and proinflammatory effect on the arterial wall [11]. Lp(a) levels are genetically determined and unrelated to birth weight [12], while they increase with age until adolescence [13].
Although gestational age affects both fetal Lp(a) and PCSK9 values [14, 15], no studies have investigated the effect of the reproductive process on PCSK9 levels, while limited and inconclusive findings regarding Lp(a) values are available. The purpose of this study was to explore whether ART methods affect plasma PCSK9 and Lp(a) levels by investigating children conceived by ART in comparison to naturally conceived (NC) children with normal birth.
Methods
Study population
In this prospective, exposure-matched cohort study, we measured plasma PCSK9 levels from 73 Greek Caucasian children (mean age 98 ± 35 months) of ART (intracytoplasmic sperm injection: n = 33, classic in vitro fertilization: n = 40) from the First Department of Obstetrics and Gynecology of the University of Athens and 73 racial-, sex-, and age-matched NC children among healthy children routinely examined at the “Aghia Sophia” Children’s Hospital, who served as controls. All children studied were healthy, receiving no medications. A detailed medical history of the participants was obtained from the files, the children’s health books, and their parents. The presence of parental dyslipidemia was self-reported. Results from the initial cohorts used in the study have been previously described [4, 5, 16] (see also Supplemental Methods). Inclusion criteria were age ≥ 2–≤ 16 years, stored plasma samples stored at − 80 °C, and available laboratory results on lipid parameters.
All children were included only after informed written consent was obtained from their parents or guardians. The study protocol was approved by the Institutional Research Ethics Committee and the Ethics Committee of the “Aghia Sophia” Children’s Hospital. The procedures followed were according to institutional guidelines and the Declaration of Helsinki.
Laboratory examination
After an overnight fast, a morning blood sample was withdrawn from children. Plasma or serum was separated by centrifugation at 4000 rpm for 10 min at 4 °C and immediately stored at − 80 °C for assay. Fasting glucose, insulin, total cholesterol, triglycerides, HDL-C, LDL-C, apolipoprotein-A1, apolipoprotein-B (apoB), Lp(a), high-sensitivity interleukin-6 (hsIL-6), and high-sensitivity C-reactive protein (hsCRP) were measured in all participants. Serum glucose, total cholesterol, triglycerides, HDL-C, and LDL-C were determined using the Siemens Advia 1650 Clinical Chemistry System (Siemens Healthcare Diagnostics, Erlangen, Germany), apolipoprotein-A1, apolipoprotein-B, Lp(a), and hs-CRP using latex particle–enhanced immunonephelometric assay on the BN ProSpec nephelometer (Siemens Healthcare Diagnostics, Liederbach, Germany) and serum insulin automated chemiluminescence Siemens ACS180 System Analyzer (Siemens Healthcare Diagnostics). The insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) with the following formula: fasting serum insulin (μU/l) times fasting plasma glucose (mg/dl) divided by 405. Measurements of the plasma PCSK9 concentrations were performed using a high-sensitivity, quantitative sandwich enzyme immunoassay (Quantikine ELISA, R&D Systems Europe Ltd.). The intra- and inter-assay coefficients of variation in our lab were not higher than 7.5% and 10%, respectively.
Statistical analyses
Numeric data are expressed as the mean ± standard deviation of the mean, whereas qualitative variables are presented as absolute and relative frequencies. All variables were tested for homogeneity of variance and normal distribution, using the Kolmogorov–Smirnov criterion. Non-normally distributed parameters that were significantly skewed before analysis were log-transformed and presented as the median (25th–75th percentiles). Specifically, PCSK9 levels, Lp(a), hsCRP, hsIL-6, glycemic measures, body mass index (BMI), blood pressures, and triglycerides were log-transformed due to their skewed distribution. Most skewed variables had a remarkably good fit to normal distribution after log transformation.
The role of age in the levels of PCSK9 and Lp(a) was also evaluated when age was considered as a categorical variable in the overall population. For the purpose of this analysis, subjects were classified according to their age in three groups (group 1 < 8 years [mean age = 5.7 ± 1.5 years], group 2 8–10 years [mean age = 8.9 ± 0.6 years], and group 3 ≥ 10 years [mean age = 11.9 ± 1.2 years]) based on previous studies that have shown a different progression rate over time in children [7].
For continuous variables, between-group comparisons were done by using Student t test or ANOVA. For categorical or non-continuous variables, between-group comparisons were done by the chi-square or Kruskal–Wallis rank tests. Linear correlations were evaluated by calculation of the Pearson’s (r) or Spearman’s (ρ) correlation coefficient. Preliminary analyses were performed to ensure no violation of the assumptions of normality, linearity, and homoscedasticity.
Multivariate analysis was performed by using analysis of covariance (ANCOVA). In ANCOVA, age, gender as well as a relevant lipid parameter (LDL-C for PCSK9 and apoB for Lp[a]) that were statistically significantly associated with each lipid biomarker were incorporated into the models as confounders. When ANOVA or ANCOVA yielded a significant difference between groups, the Bonferroni test for post-hoc comparisons was applied to compare each two of these subgroups. Exact p values < 0.05 were considered statistically significant. Data analyses were performed with SPSS software, version 24 (Chicago, IL).
Results
Baseline characteristics
The characteristics of the children included in the study, divided into groups according to the conception method, are shown in Tables 1 and 2. ART children were more often prematurely born with a lower birth-weight and birth length and had older parents compared to NC children. No significant differences were observed in most biochemical, lipid, and anthropometric parameters, including PCSK9 and Lp(a). There was only a non-significant trend for higher diastolic blood pressure (DBP), lower systolic blood pressure (SBP), and increased inflammatory status in ART children.
Table 1.
Characteristics of the study population
| Variable | Control (n = 73) | IVF–ICSI (n = 73) | p value |
|---|---|---|---|
| Age (months) | 98 ± 35 | 97 ± 35 | 0.867 |
| Boys, n (%) | 26 (36) | 28 (38) | 0.732 |
| Birth data | |||
| Gestational age (weeks n = 64/60) | 38.0 ± 2.1 | 36.0 ± 2.8 | < 0.001 |
| Preterm birth, n (%) | 14 (19) | 29 (40) | 0.006 |
| Birth weight (g, n = 70/61) | 3013.7 ± 609.8 | 2417.7 ± 672.2 | < 0.001 |
| SGA, n (%) | 13 (18) | 25 (34) | 0.024 |
| LGA, n (%) | 3 (4) | – | 0.08 |
| Birth length (cm, n = 68/47) | 49.8 ± 3.4 | 47.7 ± 3.7 | 0.003 |
| Primiparous, n (%) | 28 (38) | 50 (69) | < 0.001 |
| Singleton birth, n (%) | 60 (82) | 43 (59) | 0.002 |
| Parental characteristics | |||
| Maternal dyslipidemia, n (%) | 7 (10) | 8 (11) | 0.785 |
| Maternal age at birth (years, n = 73/58) | 31.7 ± 5.0 | 36.4 ± 5.3 | < 0.001 |
| Paternal dyslipidemia, n (%) | 12 (16) | 12 (16) | 1.000 |
| Paternal age at birth (years, n = 72/55) | 35.5 ± 5.1 | 39.1 ± 5.8 | < 0.001 |
| Children’s characteristics | |||
| Systolic blood pressure (mmHg, n = 70/65) | 105.0 (100.0–110.0) | 100.0 (90.0–110.0) | 0.064 |
| Diastolic blood pressure (mmHg, n = 70/65) | 60.0 (50.0–60.0) | 60.0 (50.0–70.0) | 0.067 |
| Height (cm) | 130.5 ± 19.3 | 131.0 ± 18.7 | 0.892 |
| BMI (kg/m2) (n = 73/71) | 17.7 (15.7–21.0) | 17.6 (15.4–21.0) | 0.923 |
| Waist-to-hip ratio (n = 71/72) | 0.93 ± 0.08 | 0.91 ± 0.09 | 0.163 |
| Prepubertal stage (Tanner I), n (%) | 13 (18) | 14 (19) | 0.831 |
Categorical variables are presented as absolute and relative frequencies, while continuous variables as mean value ± SD for normally distributed and median value (25th–75th percentiles) for skewed variables
SGA small for gestational age, LGA large for gestational age, BMI body mass index
Table 2.
Biochemical characteristics of the study population
| Variable | Control (n = 73) | IVF–ICSI (n = 73) | p value |
|---|---|---|---|
| Lipid biomarkers | |||
| Total cholesterol (mg/dl) | 169.0 ± 25.7 | 168.3 ± 24.0 | 0.871 |
| LDL-C (mg/dl) | 100.5 ± 21.0 | 100.3 ± 20.9 | 0.962 |
| HDL-C (mg/dl) | 56.7 ± 11.5 | 56.7 ± 11.0 | 0.988 |
| Τriglycerides (mg/dl) | 52.0 (39.0–71.5) | 50.0 (41.0–62.5) | 0.754 |
| ApoA1 (mg/dl) | 154.0 ± 19.2 | 150.2 ± 21.7 | 0.259 |
| ApoB (mg/dl, n = 73/72) | 72.6 ± 15.9 | 74.8 ± 15.1 | 0.411 |
| Lp(a) (mg/dl) | 6.5 (3.5–17.3) | 9.2 (2.6–24.8) | 0.277 |
| PCSK9 (ng/ml) | 184.4 (133.8–235.5) | 189.2 (148.7–226.8) | 0.515 |
| Glycemic biomarkers | |||
| Glucose (mg/dl) | 83.0 (77.5–88.0) | 84.0 (77.5–88.0) | 0.854 |
| Insulin (mU/l, n = 72/71) | 5.14 (4.32–8.55) | 6.20 (4.10–12.50) | 0.392 |
| HOMA-IR (n = 72/71) | 1.096 (0.856–1.839) | 1.274 (0.816–2.473) | 0.454 |
| Inflammatory biomarkers | |||
| hsCRP (mg/l, n = 32/64) | 0.39 (0.15–0.71) | 0.52 (0.21–1.40) | 0.086 |
| hsIL-6 (pg/ml, n = 43/68) | 1.09 (0.60–1.93) | 1.25 (0.72–1.99) | 0.443 |
Categorical variables are presented as absolute and relative frequencies, while continuous variables as mean value ± SD for normally distributed and median value (25th–75th percentiles) for skewed variables
LDL-C low-density lipoprotein, HDL-C high-density lipoprotein, ApoA1 apolipoproteinA1, ApoB apolipoproteinB, Lp(a) lipoprotein (a), PCSK9 proprotein convertase subtilisin/kexin type 9, HOMA-IR homeostasis model assessment of insulin resistance, hsCRP high-sensitivity C-reactive protein, hsIL-6 high-sensitivity interleukin-6
Associations of PCSK9 and Lp(a) with children’s characteristics
In the univariate model of the overall population, circulating PCSK9 levels were related to total cholesterol, LDL-C, and SBP. In the univariate model of the NC children, circulating PCSK9 levels were related to age, total cholesterol, LDL-C, HDL-C, apoB, birth length, height, and waist-to-hip ratio. In the univariate model of the ART children, circulating PCSK9 levels were related marginally to age and BMI and significantly to HOMA-IR and insulin. Levels of PCSK9 were not different based on fetal growth (either SGA or LGA) in both the control, the ART, and the overall population (p > 0.05) (Table 3).
Table 3.
Univariate relation of LogPCSK9 to relevant variables in the total cohort, control group and ART group
| Independent variable | Overall cohort | Controls | ART | |||
|---|---|---|---|---|---|---|
| Beta | p value | Beta | p value | Beta | p value | |
| Age (years) | − 0.405 | < 0.001 | 0.227 | 0.053 | ||
| Total cholesterol (mg/dl) | 0.186 | 0.025 | 0.388 | 0.001 | ||
| LDL-C (mg/dl) | 0.180 | 0.029 | 0.312 | 0.007 | ||
| HDL-C (mg/dl) | 0.249 | 0.034 | ||||
| ApoB (mg/dl) | 0.258 | 0.027 | ||||
| Birth length (cm) | − 0.332 | 0.006 | 0.225 | 0.059 | ||
| Body mass index (kg/m2) | ||||||
| Height (cm) | − 0.430 | p < 0.001 | ||||
| Waist-to-hip ratio | 0.279 | 0.019 | ||||
| HOMA-IR | 0.252 | 0.034 | ||||
| Insulin (mU/l) | 0.262 | 0.027 | ||||
| Systolic blood pressure (mmHg) | 0.199 | 0.021 | ||||
PCSK9 proprotein convertase subtilisin/kexin type 9, ART assisted reproductive technologies, LDL-C low-density lipoprotein, HDL-C high-density lipoprotein, ApoB apolipoproteinB, HOMA-IR homeostasis model assessment of insulin resistance
In the univariate model of the overall population, circulating Lp(a) levels were related to age, apoB, birth weight, height, waist-to-hip ratio, HOMA-IR, insulin, and hsCRP. In the univariate model of the NC children, circulating Lp(a) levels were related to age, birth weight, HOMA-IR, insulin, and waist-to-hip ratio. In the univariate model of the ART children, circulating Lp(a) levels were related to age, apoB, height, BMI, glucose, HOMA-IR, insulin, SBP, and waist to hip ratio. Levels of Lp(a) were not different based on fetal growth (either SGA or LGA) in both the control, the ART, and the overall population (p > 0.05) (Table 4).
Table 4.
Univariate relation of LogLp(a) to relevant variables in the total cohort, control group and ART group
| Independent variable | Overall cohort | Controls | ART | |||
|---|---|---|---|---|---|---|
| Beta | p value | Beta | p value | Beta | p value | |
| Age (years) | 0.269 | 0.001 | 0.240 | 0.041 | 0.301 | p = 0.010 |
| ApoB (mg/dl) | 0.214 | 0.010 | 0.290 | 0.014 | ||
| Birth weight (g) | − 0.183 | 0.037 | − 0.323 | 0.006 | ||
| Body mass index (kg/m2) | 0.298 | 0.012 | ||||
| Height (cm) | 0.263 | 0.001 | 0.324 | 0.005 | ||
| Waist-to-hip ratio | − 0.350 | < 0.001 | − 0.361 | 0.002 | − 0.331 | 0.004 |
| HOMA-IR | 0.319 | < 0.001 | 0.322 | 0.006 | 0.310 | 0.008 |
| Insulin (mU/l) | 0.316 | < 0.001 | 0.340 | 0.004 | 0.293 | 0.013 |
| Glucose (mg/dl) | 0.240 | 0.040 | ||||
| Systolic blood pressure (mmHg) | 0.262 | 0.035 | ||||
| hsCRP (mg/l) | 0.241 | 0.018 | ||||
Lp(a) lipoprotein (a), ApoB apolipoprotein B, HOMA-IR homeostasis model assessment of insulin resistance, hsCRP high-sensitivity C-reactive protein
The effect of ART method on PCSK9 and Lp(a) levels
A two-way between groups ANCOVA was conducted to explore the impact of the method of ART conception (ICSI vs. IVF) on levels of PCSK9 after adjusting for age (used as a continuous variable instead of a categorical variable because of the small number of children in each age group), gender, and LDL-C. The comparison of each method (ICSI or IVF) with NC did not show any statistically significant differences on PCSK9 levels (p = 0.565 and p = 0.255, respectively). Moreover, there were no statistically significant differences between each method (p = 0.922).
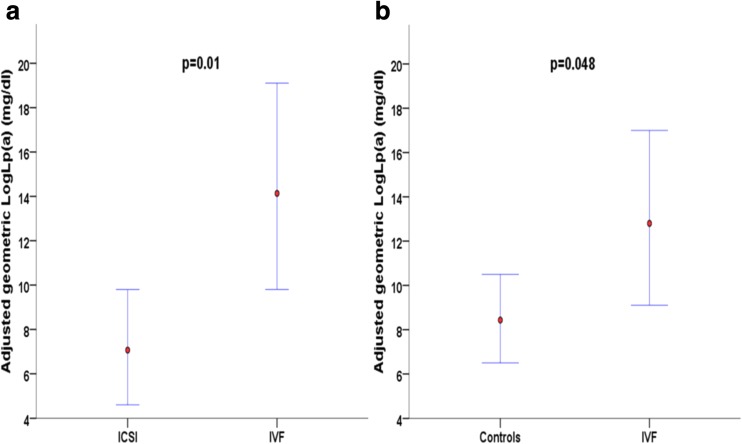
Further, we explored the impact of the method of ART conception (ICSI vs. IVF) on levels of Lp(a) after adjusting for age (as a continuous variable), gender, and apoB. Contrary to PCSK9 levels, levels of Lp(a) were statistically higher in classic IVF children compared to ICSI children (adjusted mean = 13.5 mg/dl with 95% CI [9.8–19.1] vs. adjusted mean = 6.8 mg/dl with 95% CI [4.6–9.8], p = 0.01; Fig. 1). Moreover, the main effects for age, gender (adjusted mean for girls = 12.0 mg/dl with 95% CI [8.9–16.2] vs. adjusted mean for boys = 6.3 mg/dl with 95% CI [4.3–9.5]), and apoB were all statistically significant (p = 0.018, p = 0.015 and p = 0.004, respectively) in the model (adjusted R2 of the model = 0.259). IVF children also had higher levels of Lp(a) compared to NC children (adjusted mean = 12.3 mg/dl with 95% CI [9.1–17.0] vs. adjusted mean = 8.3 mg/dl with 95% CI [6.5–10.5], p = 0.048). Furthermore, only the main effect for age was statistically significant (p = 0.003) in the model (adjusted R2 of the model = 0.103).
Fig. 1.
Adjusted geometric mean LogLp(a) concentrations and 95% confidence intervals for comparisons between children conceived with a classic in vitro fertilization and children conceived with intracytoplasmic sperm injection (a) and between children conceived with a classic in vitro fertilization and normally conceived children (b)
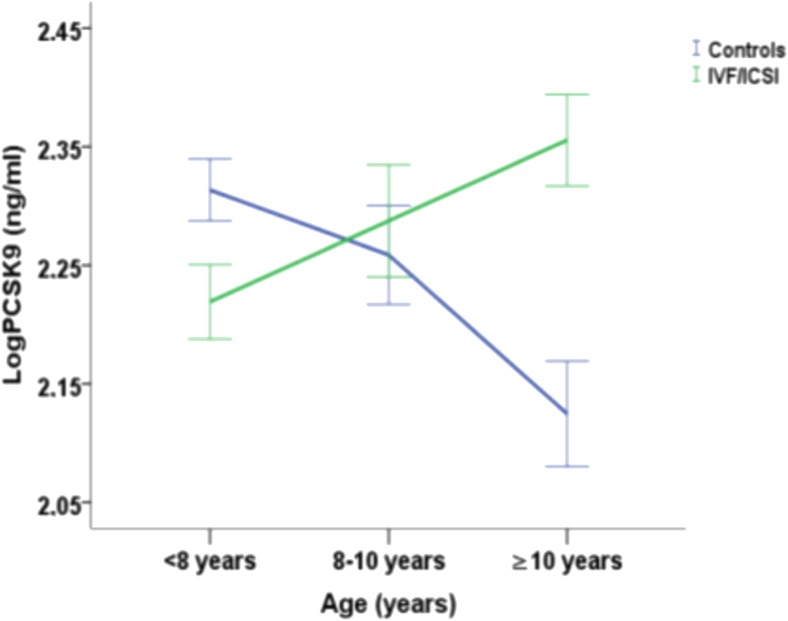
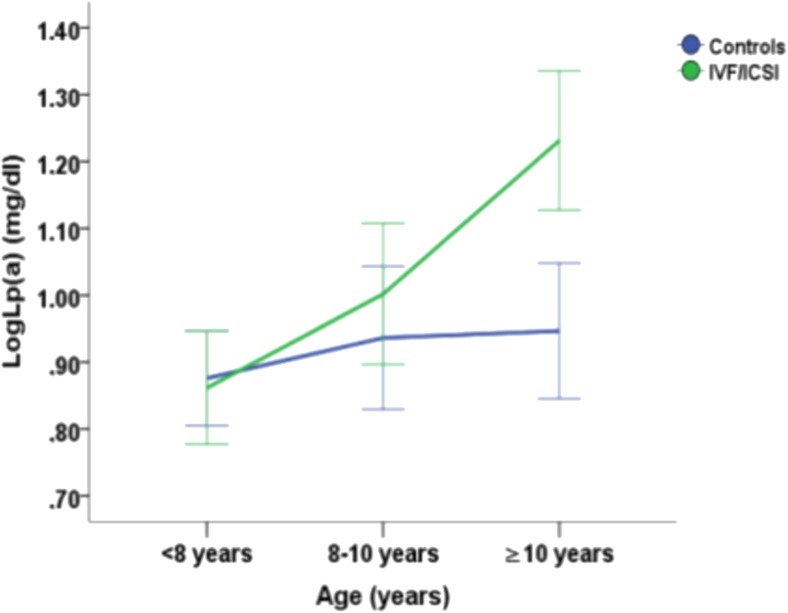
The effect of age and conception method on PCSK9 and Lp(a) levels (Figs. 2 and 3)
Fig. 2.
Mean LogPCSK9 concentrations with the standard error of the mean as error bars in age groups (< 8 years, 8–10 years and ≥ 10 years) according to the method of conception (blue line: normally conceived children and green line: children conceived with assisted reproduction technologies) and interpolation between these ages
Fig. 3.
Mean LogLp(a) concentrations with the standard error of the mean as error bars in age groups (< 8 years, 8–10 years and ≥ 10 years) according to the method of conception (blue line: normally conceived children and green line: children conceived with assisted reproduction technologies) and interpolation between these ages
A two-way between groups ANCOVA was conducted to explore the impact of age groups and method of conception (ART vs. NC) on levels of PCSK9 after adjusting for gender and LDL (adjusted R2 of the model = 0.118). The interaction effect between the method of conception and age groups was statistically significant, F (2, 138) = 8.66, p < 0.001; specifically, PCSK9 levels of ART children increased with age in contrast to NC children whose levels of PCSK9 declined with age (Fig. 2). There were also non-statistically significant trends for associations of LDL-C levels, F (1, 138) = 3.17, p = 0.077, and method of conception, F (1, 138) = 2.90, p = 0.091, with PCSK9 levels. The main effects of gender and age groups were not statistically significant (p = 0.218 and p = 0.785, respectively). The same analysis was conducted with age as a continuous variable confirming the results of our initial model showing statistically significant associations of PCSK9 levels (adjusted R2 of the model = 0.119) with the interaction between method of conception and age, F (1, 140) = 14.72, p < 0.001; LDL levels, F (1, 140) = 3.93, p = 0.049; and method of conception, F (1, 140) = 11.60, p = 0.001.
Lp(a) levels were significantly associated with age groups F (2, 137) = 4.07, p = 0.019 and with apoB levels, F (1, 137) = 8.73, p = 0.004. We also explored the impact of age groups and method of conception (ART vs. NC) on levels of LogLp(a) after adjusting for gender and apoB (adjusted R2 of the model = 0.082). The interaction effect between the method of conception and age groups was not statistically significant, F (2, 137) = 1.56, p = 0.214 (Fig. 3). The main effects of gender and method of conception were not statistically significant (p = 0.355 and p = 0.207, respectively). The same analysis was conducted with age as a continuous variable confirming the results of our initial model (adjusted R2 of the model = 0.114, data not shown).
Discussion
We demonstrated for the first time that PCSK9 levels increase with age in ART children in contrast to NC children in whom they decline and that Lp(a) values increase with age in both groups, albeit more abruptly in ART. Children conceived by classic IVF have significantly higher levels of Lp(a) than those conceived by ICSI. These changes occurred in the absence of differences in background lipid profile and may have implications for future CV risk.
PCSK9 and ART
Plasma PCSK9 concentrations were similar in ART and NC offspring in the present study that addressed the effect of the conception method (ART vs. NC) in humans. Interestingly, only one animal study has shown elevated expression of the sterol regulatory element-binding transcription factor 1 (SREBF1) gene product, a key molecule in the regulation of lipid metabolism and of PCSK9 expression, in the livers of IVF mice [17]. Data, yet inconclusive, exist for small for gestational age neonates. In one study, PCSK9 levels of children born after intrauterine growth restriction were lower than those of controls, while no significant differences were detected in the other studies [15].
One of the principal novel findings of our study was the increase of PCSK9 levels with age in ART children, in contrast to those of NC offspring, in whom we observed a decline of PCSK9 with age. Notwithstanding the conception method, previous studies had shown a significant association of circulating PCSK9 with age [7, 18]. Of note, in a study performed in white youth aged 9–16 years old, a reduction of PCSK9 levels was observed in boys, while the opposite was found in girls. This study was carried 10 years ago, and a non-validated across laboratories ELISA method was used [7].
The increase of PCSK9 values with age in ART children was independent of total cholesterol and LDL-C levels. Ample evidence suggests a modest correlation of PCSK9 concentrations with LDL-C [6]. While the modest sample of our study may account for this discrepancy, methodologic issues, such as the wide range of measured PCSK9 values and the simultaneous estimation of inactive forms of PCSK9, hamper comparison of findings within literature [6].
Mechanisms
The possible underlying mechanisms of a PCSK9 increase with age in ART children are multiple. First, methylation of the genes regulating lipid metabolism has been previously shown in ART offspring [19]. As these epigenetic alterations may remain during the life span [20], they may also induce modifications of PCSK9 concentrations with time. In addition, early postnatal intravenous lipid administration for the nutrition of newborns in order to improve their survival may lead to alterations of PCSK9 values [21]. ART-induced impaired hormonal activity might also explain the differential effect of puberty on PCSK9 levels according to the conception mode. Specifically, circulating dehydroepiandrosterone sulfate and luteinizing hormone values in IVF-conceived girls are elevated compared to controls [22]. It should also be noted that circulating PCSK9 depends on the physiologically occurring increases in estrogen and growth hormone secretion and insulin resistance during puberty [23, 24]. Finally, while Lp(a) levels are largely genetically determined [25], to what degree this is true for PCSK9, it is currently unknown. Accordingly, paternal characteristics could have played a significant role in our results. This should be addressed in future studies.
LP(a) and ART
There were no differences between ART and NC children regarding Lp(a), and these findings are in accordance with previous studies from our group [4, 5]. To the best of our knowledge, no other relevant data are available. It is, however, interesting, that in our study, Lp(a) levels increase with age and this increase was more prominent and abrupt in ART children than in NC children. Lp(a) levels are genetically regulated and ample evidence suggests that plasma Lp(a) levels increase from birth reaching their peak at the age of 2 years [26]. It should be noted that other investigators have shown an increase of Lp(a) values in adolescents aged 11–17 years and in adults [27].
Effect of different assisted reproduction techniques
No direct comparison between different ART methods had been previously performed regarding lipid parameters. In only one study, differences in the transcription of genes affecting lipid metabolism/catabolism in blastocysts obtained from ICSI vs. classic IVF were addressed [28]. We found that children born by IVF showed elevated Lp(a) levels compared to ICSI and NC offspring, while no difference was found with regard to serum PCSK9 levels. These are in line with our findings in a recent study from our group [4] that showed a trend for higher Lp(a) levels in IVF born children compared to NC children.
The differences between children born with IVF or ICSI may be explained preferentially by the difference in underlying maternal pathology. In specific, the ICSI method is usually used in case of paternal disease, so women who underwent ICSI are expected to have less often cardiometabolic disorders than those in the IVF method. The higher age of classic IVF than ICSI children included in our study might drive the significant difference of serum Lp(a) between classic IVF and ICSI offspring. In addition, since a significant independent association of low birth weight in children aged 6 to 9 years with increased Lp(a) concentrations has been previously demonstrated [29], the observed difference of Lp(a) values may stem from the lower birth weight of IVF children compared to NC conceived offspring.
Classic lipid profile and ART
In our study, there were no differences in classic lipid parameters between ART and NC children. Literature data are not conclusive. According to a recent meta-analysis, ART children show marginally lower values of LDL-C than NC children, while total cholesterol, HDL-C, and triglyceride levels were similar in the two groups [1]. On the other hand, a favorable metabolic profile consisting of higher HDL-C and lower triglycerides was revealed in another study [30]. Methodological limitations, such as a small overall sample of the ART children studied (332), difficulties in accurate matching of the comparison subgroups, and the participation of children at different stages of puberty, may account for these discrepancies [3].
Clinical implications
Since PCSK9 levels predict future cardiovascular risk [9, 10], our findings indicate that circulating PCSK9 levels could serve as a predictor of increased CV risk in ART children older than 10 years old. Due to the multiple factors affecting cardiovascular outcomes, it is not likely that the impact of the conception method is mediated by only one mechanism [22]. Accumulating evidence supports that the increasingly used ART predisposes to alterations of the cardiometabolic profile postnatally [2]. The increase of PCSK9 levels with age in ART children underpins a gradual deterioration of their lipidemic profile in the future. Further, additional impaired cardiometabolic manifestations that have been reported in ART children, such as elevated systolic and diastolic blood pressure and insulin resistance, might be partially mediated by elevated PCSK9 concentrations. Indeed, PCSK9 levels have been associated with blood pressure levels [18] and with metabolic indices, such as insulin and HOMA-IR [7]. This was evident in our study too.
Finally, the elevated circulating Lp(a) levels revealed in the classic IVF group compared to NC and ICSI offspring reveal a differential impact of this method on lipid parameters and consequently on cardiovascular risk, taking into account the strong correlation between Lp(a) levels and future cardiovascular events.
Limitations
Limitations and strengths of this study should be taken into account. First, a modest number of ART children were included. Due to the medium-sized groups, we have adjusted for the most clinically relevant covariates in the models. Despite this modest sample size and number of covariates included, statistically significant associations were revealed. Furthermore, factors which may alter the results such as the proportion from donor sperm and oocytes, as well as the ratio frozen/thawed embryos, were not available in the present study. Our study draws strength from assessing a large number of many pathophysiologic biomarkers that could have been affected by ART, especially those related to lipid parameters.
We cannot exclude that concentrations of PCSK9 may have changed during the years of storage before analysis. However, storage time did not differ between ART children and controls, and it seems unlikely that this factor would have a systematically different effect between them.
Conclusions
This study demonstrates for the first time that PCSK9 levels increase with age in ART, but not in NC, children, forecasting a gradual deterioration of their lipidemic profile that could progressively lead to increased cardiovascular risk in the future. Further, the method of ART may be of importance granted that IVF is associated with higher levels of Lp(a). The results of this study emphasize the role of novel lipid factors as early indices of latent cardiometabolic derangements according to the conception method.
Electronic supplementary material
(PDF 337 kb)
Compliance with ethical standards
All children were included only after informed written consent was obtained from their parents or guardians. The study protocol was approved by the Institutional Research Ethics Committee and the Ethics Committee of the “Aghia Sophia” Children’s Hospital. The procedures followed were according to institutional guidelines and the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ioanna Kosteria, Sophia Sakka and Alexandra Gkourogianni contributed equally to this work.
References
- 1.Guo XY, Liu XM, Jin L, Wang TT, Ullah K, Sheng JZ, Huang HF. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: a systematic review and meta-analysis. Fertil Steril. 2017;107(3):622–631. doi: 10.1016/j.fertnstert.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Scherrer U, Rexhaj E, Allemann Y, Sartori C, Rimoldi SF. Cardiovascular dysfunction in children conceived by assisted reproductive technologies. Eur Heart J. 2015;36(25):1583–1589. doi: 10.1093/eurheartj/ehv145. [DOI] [PubMed] [Google Scholar]
- 3.Yeung EH, Druschel C. Cardiometabolic health of children conceived by assisted reproductive technologies. Fertil Steril. 2013;99(2):318–326. doi: 10.1016/j.fertnstert.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakka SD, Loutradis D, Kanaka-Gantenbein C, Margeli A, Papastamataki M, Papassotiriou I, Chrousos GP. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil Steril. 2010;94(5):1693–1699. doi: 10.1016/j.fertnstert.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Gkourogianni A, Kosteria I, Telonis AG, Margeli A, Mantzou E, Konsta M, Loutradis D, Mastorakos G, Papassotiriou I, Klapa MI, Kanaka-Gantenbein C, Chrousos GP. Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI. PLoS One. 2014;9(4):e94001. doi: 10.1371/journal.pone.0094001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53(12):2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baass A, Dubuc G, Tremblay M, Delvin EE, O’Loughlin J, Levy E, Davignon J, Lambert M. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem. 2009;55(9):1637–1645. doi: 10.1373/clinchem.2009.126987. [DOI] [PubMed] [Google Scholar]
- 8.Filippatos TD, Liberopoulos E, Georgoula M, Tellis CC, Tselepis AD, Elisaf M. Effects of increased body weight and short-term weight loss on serum PCSK9 levels - a prospective pilot study. Arch Med Sci Atheroscler Dis. 2017;5(2):e46–e51. doi: 10.5114/amsad.2017.70502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Terentes-Printzios D, Georgiopoulos G, Skoumas I, Koutagiar I, Ioakeimidis N, Stefanadis C, Tousoulis D. Prediction of cardiovascular events with levels of proprotein convertase subtilisin/kexin type 9: a systematic review and meta-analysis. Atherosclerosis. 2016;252:50–60. doi: 10.1016/j.atherosclerosis.2016.07.922. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Bradwin G, Rose L. Plasma proprotein convertase subtilisin/kexin type 9 levels and the risk of first cardiovascular events. Eur Heart J. 2016;37(6):554–560. doi: 10.1093/eurheartj/ehv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsimikas S. A Test in Context: Lipoprotein (a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Obisesan TO, Aliyu MH, Adediran AS, Bond V, Maxwell CJ, Rotimi CN. Correlates of serum lipoprotein (A) in children and adolescents in the United States. The third National Health Nutrition and Examination Survey (NHANES-III) Lipids Health Dis. 2004;16(3):29. doi: 10.1186/1476-511X-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XL, Wang J. Lipoprotein (a) in children and adolescence. Pediatr Endocrinol Rev. 2003;1(2):109–119. [PubMed] [Google Scholar]
- 14.Kwiterovich PO, Jr, Virgil DG, Garrett ES, Otvos J, Driggers R, Blakemore K, et al. Lipoprotein heterogeneity at birth: influence of gestational age and race on lipoprotein subclasses and Lp (a) lipoprotein. Ethn Dis. 2004;14(3):351–359. [PubMed] [Google Scholar]
- 15.Pecks U, Rath W, Maass N, Berger B, Lueg I, Farrokh A, Farrokh S, Eckmann-Scholz C. Fetal gender and gestational age differentially affect PCSK9 levels in intrauterine growth restriction. Lipids Health Dis. 2016;15(1):193. doi: 10.1186/s12944-016-0365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosteria I, Tsangaris GT, Gkourogianni A, Anagnostopoulos A, Papadopoulou A, Papassotiriou I, et al. Proteomics of children born after intracytoplasmic sperm injection reveal indices of an adverse cardiometabolic profile. J Endocr Soc. 2017;1(4):288–301. doi: 10.1210/js.2016-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6(1):77–86. [PubMed] [Google Scholar]
- 18.Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen Q, Hu Y, Haas JV, Troutt JS, Pickard RT, Darling R, Konrad RJ, Zhou H, Cao G. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. 2010;213(2):632–636. doi: 10.1016/j.atherosclerosis.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Whitelaw N, Bhattacharya S, Hoad G, Horgan GW, Hamilton M, Haggarty P. Epigenetic status in the offspring of spontaneous and assisted conception. Hum Reprod. 2014;29(7):1452–1458. doi: 10.1093/humrep/deu094. [DOI] [PubMed] [Google Scholar]
- 20.Ingelfinger JR. Pathogenesis of perinatal programming. Curr Opin Nephrol Hypertens. 2004;13(4):459–464. doi: 10.1097/01.mnh.0000133977.09688.2f. [DOI] [PubMed] [Google Scholar]
- 21.Lewandowski AJ, Leeson P. Preeclampsia, prematurity and cardiovascular health in adult life. Early Hum Dev. 2014;90(11):725–729. doi: 10.1016/j.earlhumdev.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Pubertal development in children and adolescents born after IVF and spontaneous conception. Hum Reprod. 2008;23(12):2791–2798. doi: 10.1093/humrep/den309. [DOI] [PubMed] [Google Scholar]
- 23.Persson L, Cao G, Ståhle L, Sjöberg BG, Troutt JS, Konrad RJ, et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. 2010;30(12):2666–2672. doi: 10.1161/ATVBAHA.110.214130. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh M, Gälman C, Rudling M, Angelin B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J Lipid Res. 2015;56(2):463–469. doi: 10.1194/jlr.M055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kronenberg F. Human genetics and the causal role of lipoprotein(a) for various diseases. Cardiovasc Drugs Ther. 2016;30(1):87–100. doi: 10.1007/s10557-016-6648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlatohlávek L, Zídková K, Vrablík M, Haas T, Prusíková M, Svobodová H, Ceska R. Lipoprotein(a) and its position among other risk factors of atherosclerosis. Physiol Res. 2008;57(5):777–783. doi: 10.33549/physiolres.931133. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan SR, Dahlen GH, Jarpa RA, Webber LS, Berenson GS. Racial (black-white) differences in serum lipoprotein (a) distribution and its relation to parental myocardial infarction in children. Bogalusa Heart Study. Circulation. 1991;84(1):160–167. doi: 10.1161/01.cir.84.1.160. [DOI] [PubMed] [Google Scholar]
- 28.Bridges PJ, Jeoung M, Kim H, Kim JH, Lee DR, Ko C, Baker DJ. Methodology matters: IVF versus ICSI and embryonic gene expression. Reprod BioMed Online. 2011;23(2):234–244. doi: 10.1016/j.rbmo.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Moran M, Guerrero-Romero F. Low birthweight and elevated levels of lipoprotein(a) in prepubertal children. J Paediatr Child Health. 2014;50(8):610–614. doi: 10.1111/jpc.12598. [DOI] [PubMed] [Google Scholar]
- 30.Miles HL, Hofman PL, Peek J, Harris M, Wilson D, Robinson EM, Gluckman PD, Cutfield WS. In vitro fertilization improves childhood growth and metabolism. J Clin Endocrinol Metab. 2007;92(9):3441–3445. doi: 10.1210/jc.2006-2465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 337 kb)