We report xenodiagnosis results for 47 post-kala-azar dermal leishmaniasis (PKDL) and 15 visceral leishmaniasis (VL) patients. Skin parasite load was strongly associated with positive xenodiagnosis. Compared to VL (66.7%), nodular PKDL was more likely (86%) and macular PKDL less likely (35%) to result in positive xenodiagnosis.
Keywords: xenodiagnosis, post-kala-azar dermal leishmaniasis, visceral leishmaniasis, transmission
Abstract
Background
On the Indian subcontinent, visceral leishmaniasis (VL) incidence is on track to reach elimination goals by 2020 in nearly all endemic districts. Although not included in official targets, previous data suggest post-kala-azar dermal leishmaniasis (PKDL) patients can act as an infection reservoir.
Methods
We conducted xenodiagnosis on 47 PKDL patients and 15 VL patients using laboratory-reared Phlebotomus argentipes. In direct xenodiagnosis, flies were allowed to feed on the patient’s skin for 15 minutes. For indirect xenodiagnosis, flies were fed through a membrane on the patient’s blood. Five days later, blood-fed flies were dissected and examined by microscopy and/or polymerase chain reaction (PCR). A 3-mm skin snip biopsy (PKDL) or venous blood (VL) was processed by quantitative PCR.
Results
Twenty-seven PKDL patients (57.4%) had positive results by direct and/or indirect xenodiagnosis. Direct was significantly more sensitive than indirect xenodiagnosis (55.3% vs 6.4%, P < .0001). Those with positive xenodiagnosis had median skin parasite loads >1 log10 unit higher than those with negative results (2.88 vs 1.66, P < .0001). In a multivariable model, parasite load, nodular lesions, and positive skin microscopy were significantly associated with positive xenodiagnosis. Blood parasite load was the strongest predictor for VL. Compared to VL, nodular PKDL was more likely and macular PKDL less likely to result in positive xenodiagnosis, but neither difference reached statistical significance.
Conclusions
Nodular and macular PKDL, and VL, can be infectious to sand flies. Active PKDL case detection and prompt treatment should be instituted and maintained as an integral part of VL control and elimination programs.
Visceral leishmaniasis (VL), or kala-azar, shows prolonged fever, hepatosplenomegaly, wasting, and high mortality if not treated. Between 2005 and 2008, the estimated incidence on the Indian subcontinent ranged from 160000 to 315000 cases annually, representing 80% of the global burden [1]. There, Leishmania donovani is transmitted by a single sand fly species, Phlebotomus argentipes, and VL patients are the main infection reservoir during high incidence periods [2]. In 2005, India, Bangladesh, and Nepal announced a regional initiative aimed at eliminating VL as a public health problem, defined as VL incidence <1 case per 10000 population annually at the subdistrict level [3].
In Bangladesh, rapid diagnostic tests (RDTs) have been provided at health facilities since 2010, indoor residual spraying was introduced in 2012, and single-dose AmBisome was implemented in 2014 [4]. However, VL incidence, which peaked in 2006, had already declined 54% by 2009 and 80% by 2012, before vector control began [4]. The cyclic incidence curve in Bangladesh is consistent with a century of observations on the Indian subcontinent, where cycles of 5–10 years of high VL incidence are followed by 10–20 years of low incidence [2]. The fall in incidence is attributed to herd immunity, with resurgence occurring when the susceptible population is replenished through births, in-migration, and/or waning immunity in those previously infected [5, 6].
Post-kala-azar dermal leishmaniasis (PKDL) dermatosis occurs in 5%–15% of South Asian VL patients months to years after cure [7]. PKDL is even more frequent in East Africa, particularly in Sudan [8]. Lesions include hypopigmented macules, papules, and nodules. Since PKDL patients are rarely systemically ill, most do not seek treatment and will be missed by passive surveillance [9]. Their public health importance stems from their potential role as reservoir hosts, infecting sand flies [10].
The only accepted proof of reservoir infectiousness is xenodiagnosis, which consists of feeding laboratory-reared sand flies on the putative reservoir host and demonstrating subsequent infection in the fly [11]. Indian VL patients were first shown to be infectious in 1924, and findings were confirmed over the subsequent decade [12–16] (Supplemental Table S1). In 1928, a patient with nodular PKDL lesions was shown to be infectious to P. argentipes [17]. By 1933, additional experiments suggested that longer incubation and repeated feeding of flies on the same PKDL patient increased the yield; furthermore, both nodular and macular PKDL patients could infect sand flies [18, 19]. These studies were followed by more than 50 years without human xenodiagnosis publications.
In 1992, investigators in West Bengal described a community without previous VL which became an epidemic center in 1980 [10]. They postulated that the parasite was introduced by a single nodular PKDL patient in a naive population, leading to rapid transmission. This history, together with positive xenodiagnosis results from 4 PKDL patients, including the community index case, supported the long-standing hypothesis that PKDL patients constitute an important interepidemic reservoir [6, 10].
Despite the above evidence, no PKDL target is included in South Asian VL elimination validation requirements; moreover, a recent article questioned the role of PKDL as an infection reservoir [20]. With VL incidence at its lowest level in decades, elucidation of the role of PKDL patients in transmission is urgent. Here, we report the results of xenodiagnosis from 47 PKDL and 15 VL patients, the largest such study conducted to date. Our major aim is to provide quantitative data on the importance of PKDL patients as potential infection reservoirs in the context of regional VL elimination.
METHODS
The protocol was approved by the Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh (PR-14010). All patients provided written informed consent. Those with concurrent illness or a history of allergy to insect bites were excluded. Procedures were conducted at the Surya Kanta Kala-azar Research Centre (SKKRC), Mymensingh Medical College, Bangladesh, under the supervision of 2 physicians. There were no adverse events. Following xenodiagnosis, patients were referred for treatment following national protocols.
Procedures
Lacking previous PKDL data, we based our sample size calculations on experience in cutaneous leishmaniasis extrapolated to PKDL. Therefore, we assumed that 80% of probable PKDL cases would have parasites detectable by quantitative polymerase chain reaction (qPCR) in skin specimens and that at least 10% of confirmed PKDL cases would be infectous to sand flies (precision of ±5%). Considering these assumptions, we estimated that 44 confirmed PKDL cases would be needed for robust results. To allow for a stratified analysis by lesion type, we enrolled a similar number of macular and nodular patients. The 3 patients described in our previous proof-of-concept study were not included here [21]. Fifteen VL patients served as a comparison group.
PKDL patients aged ≥18 years were identified through active community searches and were eligible if confirmed by microscopy or PCR. Lesions were classified as macules, papules, or nodules, and the skin-affected area was quantified as published elsewhere [22]. Following antisepsis, a snip biopsy was collected by elevating a 3-mm diameter cone of an area of affected skin with a needle and shaving it off. One-half was used for molecular assays; the other half was used to prepare an impression smear for microscopy.
VL patients were enrolled at the time of presentation at SKKRC if they fulfilled diagnostic criteria in the national guidelines (>2 weeks fever, splenomegaly, and positive results by rK39 RDT [InBios, Seattle WA]). VL patients with a history of previous treatment were excluded. Collected blood was separated into serum, buffy coat, and red cells.
Sand Fly Colony
A colony was established starting with wild blood-fed female P. argentipes. Twenty randomly chosen first- and second-generation females were analyzed using real-time (RT) PCR to rule out flavivirus and phlebovirus infection; all were negative.
Xenodiagnosis
Direct xenodiagnosis was conducted as previously described [23]. The participant placed a hand into a cage for 15 minutes; the cage contained 20 to 25 seven-day-old female P. argentipes and 5 to 10 male flies. For patients without hand lesions, sand flies were placed in a 3-cm diameter tube topped with gauze, and held against a single lesion for flies to feed for 15 minutes. Male and unfed female flies were removed with an aspirator while blood-fed female flies were kept for 5 days at 27ºC, 85%–95% humidity, and fed on a 30% sucrose diet. Flies still living 5 days after the blood meal were anesthetized with carbon dioxide/chloroform, placed in a drop of sterile phosphate-buffered saline (PBS) on a microscope slide, and decapitated with needles. The midgut was drawn out and placed in another PBS drop, covered with a cover slip, and examined for promastigotes under a microscope. For each patient, flies that died prior to 5 days and microscopy-negative flies were processed in separate pools using qPCR. For indirect xenodiagnosis, sand flies were allowed to feed on patients’ venous blood in a membrane feeder, following published methods [24], with subsequent procedures as described above.
qPCR
DNA was extracted from tissue, buffy coat, and sand fly mid-gut specimens using Qiagen kits. RT-PCR was performed following published methods using Taqman primers and probes targeting the conserved region of Leishmania REPL repeats (L42486.1) [25]. To estimate parasite load, each run included a standard curve with DNA concentrations corresponding to 10000 to 0.1 parasites and 1 reaction with molecular-grade water as a negative control. Samples with cycle threshold >40 were considered negative.
Analyses
The major outcome was positive results by xenodiagnosis, defined as the detection of L. donovani promastigotes or DNA in at least 1 fly or pool of flies fed on that patient or their blood. Data were analyzed using SAS 9.0 and Stata 14.2. Univariate analyses utilized nonparametric tests as appropriate. Stepwise backward elimination procedures were used to construct multivariable logistic regression models with P = .05 for removal and addition. A receiver-operating-characteristic (ROC) curve was constructed to identify the skin parasite load threshold, with maximum sensitivity and specificity to differentiate PKDL patients positive and negative by xenodiagnosis (by maximizing Youden’s index, ie the sum of the sensitivity and specificity). Bias-corrected 95% confidence intervals (CIs) were computed for sensitivity and specificity and area under the ROC curve (AUC) by bootstrapping with 10000 replicates.
RESULTS
From July 2017 to November 2017, 47 PKDL patients were enrolled; 22 (46.8%) were previously treated and 17 (36.2%) were female (Table 1). Two-thirds came from the 2 highest-incidence subdistricts, Fulbaria and Trishal. All had been treated for VL prior to PKDL onset, 81% with pentavalent antimony. The median interval from VL treatment to PKDL onset was 3.9 years (interquartile range [IQR], 2.9, 5.8), and the median duration of lesions at the time of study was 3.5 years (IQR, 1.7, 5.8). Twenty-six (55.3%) patients had macular, papular, or maculopapular lesions, while 21 (44.7%) had nodules or a combination of nodules and macules. All PKDL patients were positive by skin biopsy qPCR, with median parasite load of 275.5 parasites/μg tissue (IQR, 41, 1232); 32 (68.1%) were also positive by microscopy. From August 2017 to December 2017, 15 VL patients were enrolled. VL patients were younger (P = .05) and more likely to come from subdistricts other than Fulbaria and Trishal compared with PKDL patients (P = .04 by 2-tailed Fisher exact test; Table 2). Thirteen (86.7%) VL patients had positive results by qPCR in venous blood, with median parasite load of 48 parasites/mL (IQR, 8.5, 137.6).
Table 1.
Characteristics of Post-Kala-Azar Dermal Leishmaniasis Patients Included in the Xenodiagnosis Study: Mymensingh, Bangladesh, 2017
| Characteristic | PKDL Patients N = 47 |
|---|---|
| Female N (%) | 17 (36.2) |
| Age (years) | |
| Mean [SD] | 35.3 [12.0] |
| Median [IQR] | 33 [26, 45] |
| Residence | |
| Fulbaria | 16 (34.0) |
| Trishal | 15 (31.9) |
| Other upazilas of Mymensingh | 12 (25.5)a |
| Outside Mymensingh District | 4 (8.5)b |
| Antecedent VL | 47 (100) |
| Initial VL treatment drug | |
| SSG monotherapy | 38 (80.9) |
| AmBisome monotherapy | 5 (10.6)c |
| Other | 4 (8.5)d |
| Treated more than once for VL | 6 (12.8)e |
| VL treatment to PKDL onset (years) | |
| Mean [SD] | 5.2 [4.0] |
| Median [IQR] | 3.9 [2.9, 5.8] |
| Duration of PKDL lesions (years) | |
| Mean [SD] | 4.7 [4.0] |
| Median [IQR] | 3.5 [1.7, 5.8] |
| PKDL score | |
| Mean [SD] | 146.4 [144.0] |
| Median [IQR] | 97 [14, 255] |
| PKDL lesion types | |
| Macular, papular, or maculopapular | 26 (55.3)f |
| Nodular or nodules plus macules | 21 (44.7)g |
| Body mass index (kg/m2) | |
| Mean [SD] | 20.8 [2.9] |
| Median [IQR] | 20.6 [18.7, 22.9] |
| Skin biopsy positive by microscopy | 32 (68.1) |
| Skin biopsy positive by quantitative polymerase chain reaction | 47 (100) |
| Parasite load (per microgram genomic DNA) | |
| Mean [SD] | 2164.7 [5636] |
| Median [IQR] | 275.5 [41, 1232] |
Abbreviations: IQR, interquartile range; PKDL, post-kala-azar dermal leishmaniasis; SD, standard deviation; SSG, sodium stibogluconate; VL, visceral leishmaniasis.
aBhaluka (6), Gaffargaon (1), Muktagachha (3), Mymensingh Sadar (2).
bKaliakoir/Gazipur (2), Sreepur/Gazipur (1), Modhupur/Tangail (1).
cAmBisome single dose (2), multiple doses (2), unspecified (1).
dMiltefosine (2), AmBisome-paromomycin (1), miltefosine-paromomycin (1).
eFive treated twice, SSG then AmBisome (2); SSG followed by miltefosine (1); miltefosine followed by SSG (2); 1 patient treated 3 times, 2 courses of SSG followed by AmBisome.
fMacular only (24), papules only (1), maculopapular (1).
gNodules only (1), mixed nodules and macules (20).
Table 2.
Characteristics of Visceral Leishmaniasis Patients Included in the Xenodiagnosis Study: Mymensingh, Bangladesh, 2017
| Characteristic | Visceral Leishmaniasis Patients N = 15 |
|---|---|
| Female N (%) | 7 (46.7) |
| Age (years) | |
| Mean [SD] | 28.7 [12.1] |
| Median [IQR] | 24 [19, 35] |
| Residence | |
| Fulbaria | 3 (20) |
| Trishal | 2 (13.3) |
| Bhaluka | 3 (20) |
| Other upazilas of Mymensingha | 2 (13.3) |
| Outside Mymensingh Districtb | 5 (33.3) |
| Body mass index (kg/m2) | |
| Mean [SD] | 17.0 [2.4] |
| Median [IQR] | 16.6 [15.8, 18.4] |
| Spleen size (centimeters below costal margin) | |
| Mean [SD] | 8.7 [6.6] |
| Median [IQR] | 6.0 [4, 12] |
| Liver size (centimeters below costal margin) | |
| Mean [SD] | 4.0 [4.2] |
| Median [IQR] | 3.0 [0, 8] |
| Blood positive by quantitative polymerase chain reaction | 13 (86.7) |
| Parasite load (per milliliter blood) | |
| Mean [SD] | 159.8 [270.9] |
| Median [IQR] | 48.0 [8.5, 137.6] |
Abbreviations: IQR, interquartile range; SD, standard deviation.
aGaffargaon (1), Mymensingh Sadar (2).
bAshuganj/Brahmanbaria (1), Gazipur Sadar/Gazipur (1), Gurudaspur/Natore (1), Dhonbari/Tangail (1), Gopalpur/Tangail (1).
Twenty-seven (57%) PKDL patients and 10 (66.7%) VL patients had positive results by xenodiagnosis. Supplementary Figures S1A–D show the median number of sand flies fed, surviving, and examined using microscopy and qPCR, as well as composite xenodiagnosis results by patient. Of 47 PKDL patients, 26 had positive results by direct xenodiagnosis (17 by both microscopy and qPCR, 5 by microscopy only, and 4 by qPCR only). Three PKDL patients had positive results by indirect xenodiagnosis (1 by both microscopy and qPCR, 2 by qPCR only); only 1 PKDL patient was positive by indirect but not direct xenodiagnosis. Among PKDL patients, direct xenodiagnosis was much more likely than indirect xenodiagnosis to yield positive results (26 [55.3%] vs 3 [6.4%], P < .0001). For VL patients, the difference in sensitivity was not significant (9 [60%] positive by direct vs 6 [40%] by indirect xenodiagnosis; P = .47).
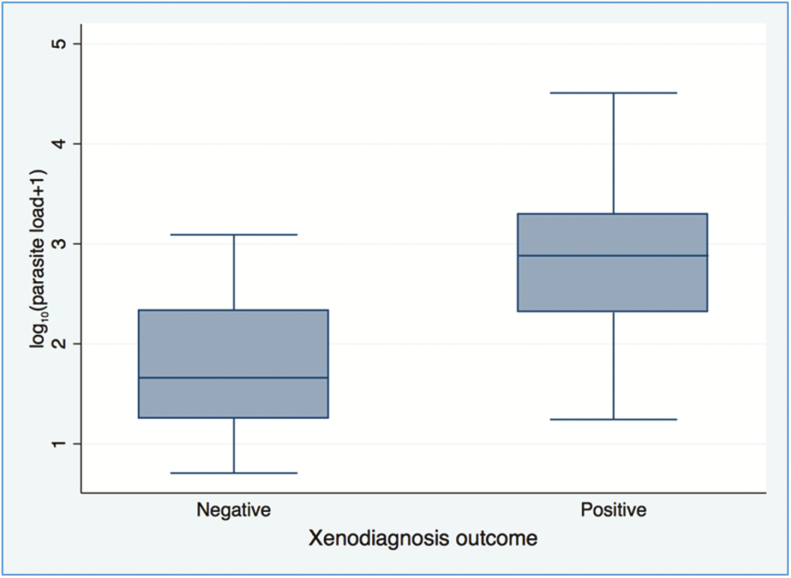
Among PKDL patients, factors associated with positive xenodiagnosis included nodular lesions, younger age, positive microscopy, and skin parasite load (Table 3). For patients with duration 3 to 12 months, 13 to 48 months, and longer than 48 months, positive xenodiagnosis results were found in 50% (4/8), 59% (13/22), and 50% (10/20) (P > .05 for all comparisons). Positive xenodiagnosis patients had median skin parasite loads >1 log10 unit higher than negative xenodiagnosis patients (2.88 vs 1.66, P < .0001) (Figure 1). Skin parasite load was significantly higher in nodular compared with maculopapular PKDL patients and in positive microscopy cases (Supplementary Table S2). In the multivariable model, skin parasite load, nodular PKDL, and positive microscopy showed significant associations with positive xenodiagnosis (Table 4). Among VL patients, blood parasite load was the strongest predictor of positive xenodiagnosis; Table 5 shows other factors with significant associations in univariable analyses. The small number of VL patients precluded multivariable modeling. Compared with VL, nodular PKDL was more likely and macular PKDL was less likely to result in positive xenodiagnosis (66.7% for VL, 85.7% for nodular [P = .24] and 34.6% for macular PKDL [P = .06]).
Table 3.
Factors Associated With Positive Xenodiagnosis Results Among 47 Post-Kala-Azar Dermal Leishmaniasis Patients: Mymensingh, Bangladesh, 2017
| Factor | Xenodiagnosis Resultsa | ||
|---|---|---|---|
| Negative | Positive | P Value | |
| PKDL patients | N = 20 | N = 27 | |
| Age | |||
| Mean [SD] | 40.0 [12.0] | 31.9 [11.0] | …b |
| Median [IQR] | 42.5 [28.5, 50] | 30 [12, 40] | .04 |
| Female sex n (%) | 9 (45.0) | 8 (30.0) | .36 |
| Previously treated PKDL | 8 (40) | 14 (51.9) | .55 |
| Duration of PKDL lesions (years) | |||
| Mean [SD] | 5.1 [4.5] | 4.4 [3.7] | …b |
| Median [IQR] | 3.6 [1.8, 8.9] | 3.5 [1.5, 5.4] | .84 |
| PKDL score | |||
| Mean [SD] | 153 [174] | 142 [121] | …b |
| Median [IQR] | 52 [11.5, 325] | 111 [44, 230] | .59 |
| PKDL lesion type n (%) | |||
| Macular, papular, or maculopapular | 17 (85.0) | 9 (33.3) | .0009 |
| Nodular or nodules plus macules | 3 (15.0) | 18 (66.7) | |
| BMI (kg/m2) | |||
| Mean [SD] | 21.2 [2.9] | 20.5 [3.0] | …b |
| Median [IQR] | 20.8 [18.6, 23.4] | 20.5 [18.7, 22.0] | .68 |
| Skin biopsy positive by microscopy n (%) | 10 (50.0) | 22 (81.5) | .03 |
| Positive qPCR in skin n (%) | 20 (100) | 27 (100) | 1.0 |
| Parasite load (per μg genomic DNA) | |||
| Mean [SD] | 177.4 [304] | 3636.8 [7130] | …b |
| Median [IQR] | 46.1 [19, 212.6] | 761.0 [205, 1958] | <.0001 |
| Log10 parasite loadc | |||
| Mean [SD] | 1.76 [0.69] | 2.92 [0.82] | …b |
| Median [IQR] | 1.66 [1.26, 2.33] | 2.88 [2.31, 3.29] | <.0001 |
We report both the median + IQR and mean + SD to provide some indication of the skew of the distribution.
Abbreviations: IQR, interquartile range; PKDL, post-kala-azar dermal leishmaniasis; SD, standard deviation.
aComposite results by microscopy and/or polymerase chain reaction in sand flies from any of the xenodiagnosis experiments.
bMean + SD shown to provide indication of skew of the distribution; P value based on Wilcoxon rank sum test
cParasite load per μg genomic DNA transformed as log10(parasite load +1) to account for the possibility of zero parasite load.
Figure 1.
Median log10 calculated parasites per microgram genomic DNA in skin biopsies from post-kala-azar dermal leishmaniasis patients by composite xenodiagnosis results. Box indicates interquartile range; whiskers indicate minimum and maximum.
Table 4.
Multivariable Logistic Regression Model for Factors Associated With Positive Xenodiagnosis Results in Post-Kala-Azar Dermal Leishmaniasis Patients
| Factor | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Log10 parasite load in skin | 7.25 | 1.78, 29.6 | .006 |
| Nodular post-kala-azar dermal leishmaniasisa | 11.7 | 1.37, 100.7 | .03 |
| Microscopy positive in skin | 7.04 | 1.02, 48.7 | .05 |
Based on stepwise backward elimination with 0.05 significance level for removal and addition.
aCompared to macular/maculopapular post-kala-azar dermal leishmaniasis.
Table 5.
Factors Associated With Positive Xenodiagnosis Results Among 15 Visceral Leishmaniasis Patients: Mymensingh, Bangladesh, 2017
| Factor | Xenodiagnosis Resultsa | ||
|---|---|---|---|
| Negative | Positive | P Value | |
| Visceral leishmaniasis patients | N = 5 | N = 10 | |
| Age | |||
| Mean [SD] | 22 [2.9] | 32.1 [13.7] | …b |
| Median [IQR] | 23 [20, 24] | 31.5 [19, 45] | .33 |
| Female Sex n (%) | 3 (60.0) | 4 (40.0) | .61 |
| BMI (kg/m2) | |||
| Mean [SD] | 15.0 [1.8] | 18.0 [2.0] | …b |
| Median [IQR] | 15.2 [13.8, 15.8] | 18.4 [16.4, 18.7] | .03 |
| Spleen size (cm below costal margin) | |||
| Mean [SD] | 12.0 [7.4] | 7.1 [5.9] | …b |
| Median [IQR] | 9.0 [7, 16] | 5.5 [3, 12] | .14 |
| Liver size (cm below costal margin) | |||
| Mean [SD] | 7.0 [3.1] | 2.5 [3.9] | …b |
| Median [IQR] | 6.0 [6, 9] | 0.75 [0, 3] | .04 |
| Blood positive by qPCR | 3 (60.0) | 10 (100) | .10 |
| Parasite load (per mL blood) | |||
| Mean [SD] | 13.5 [20.3] | 232.9 [310.1] | …b |
| Median [IQR] | 4.2 [0, 15.4] | 93.3 [40.5, 321.6] | .02 |
| Log10 parasite loadC | |||
| Mean [SD] | 0.72 [0.75] | 2.00 [0.64] | …b |
| Median [IQR] | 0.72 [0, 1.22] | 1.97 [1.61, 2.51] | .01 |
We report both the median + IQR and mean + SD to provide some indication of the skew of the distribution.
Abbreviations: IQR, interquartile range; SD, standard deviation.
aComposite results by microscopy and/or polymerase chain reaction in sand flies from any of the xenodiagnosis experiments.
bbMean + SD shown to provide indication of skew of the distribution; p value based on Wilcoxon rank sum test.
CParasite load per milliliter blood transformed as log10(parasite load + 1) to account for zero parasite loads.
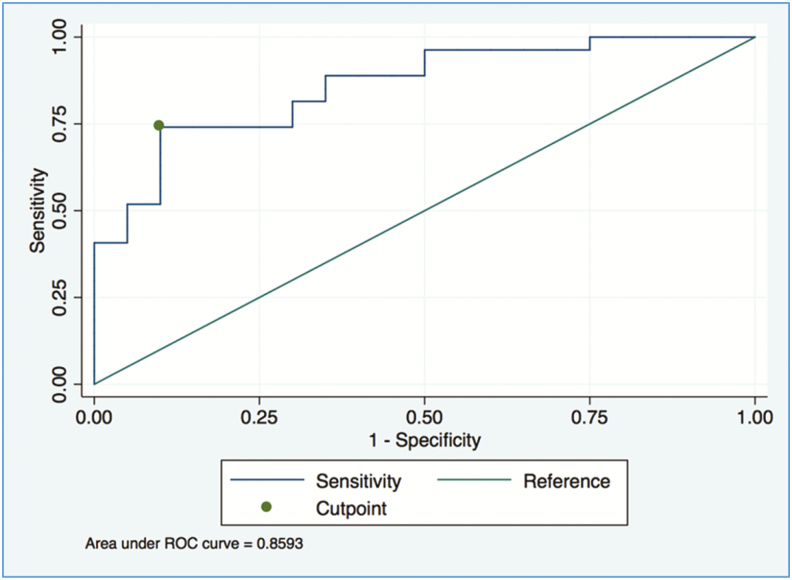
Figure 2 shows an ROC curve for skin parasite load as a classifier for positive xenodiagnosis in PKDL patients. The resulting threshold of 2.61 log10 parasites/μg genomic DNA showed sensitivity of 0.74 (95% CI, 0.44–0.92) and specificity of 0.90 (95% CI, 0.64–1), and appears to be a better-than-random classifier since the lower bound of the 95% CI for the AUC is >0.5 (AUC 95% CI, 0.70–0.90).
Figure 2.
Receiver-operating-characteristic curve for skin parasite load as the predictor of positive results by xenodiagnosis in post-kala-azar dermal leishmaniasis patients. The indicated threshold of 2.61 log10 parasites per microgram genomic DNA in skin biopsy shows sensitivity of 0.74 (95% confidence interval [CI], 0.44–0.92) and specificity of 0.90 (95% CI, 0.64–1). The threshold appears to be a better-than-random classifier because the lower bound of the 95% CI for the area under the curve (AUC) is >0.5 (AUC 95% CI, 0.70–0.90). (All CIs are bias-corrected CIs computed by bootstrapping with 10000 replicates.) Abbreviation: ROC, receiver-operating-characteristic.
DISCUSSION
This study positions PKDL as one of the central challenges to VL elimination on the Indian subcontinent. Our data provide proof and quantification of the infectious potential of nodular and macular PKDL patients, strongly supporting India’s and Bangladesh’s official policies that indicate treatment for all PKDL patients. However, without active case detection, most PKDL patients are never diagnosed and will continue to constitute a threat to sustained VL control [7, 9]. In our data, nodular PKDL and VL were both highly infectious, while macular PKDL patients were less so. However, PKDL patients go untreated for years. Hence, their cumulative transmission potential may be higher than that of VL patients. Finally, our molecular results suggest a way forward for epidemiological studies, an endpoint for clinical trials, and a more effective public health approach to PKDL.
For some years following peak VL incidence, herd immunity is high, especially within and close to households with previous VL cases [26]. Ninety percent of PKDL cases are themselves previous VL cases, so their households already have high immunity rates before onset of skin lesions [5]. Onward transmission from PKDL cases will therefore become detectable only after sufficient time has passed for local susceptibility to rise or when a PKDL patient migrates to a nonendemic community, as noted by Addy and Nandy [10]. These factors may explain the failure to demonstrate transmission from PKDL patients in locations where herd immunity is likely still very high [20].
Our PCR cutoff was not a perfect predictor of xenodiagnosis results, nor is xenodiagnosis a perfect reflection of infectiousness. Nevertheless, xenodiagnosis is still the best measure of infectiousness. Both measures have inherent biological variability. Recent animal models demonstrate that skin parasite distribution is heterogeneous [27]; no human data exist to address the relative load in skin with and without lesions or in different locations. Multiple skin biopsies from different locations would give a more nuanced picture of parasite load. In historical studies, repeated blood meals increased xenodiagnosis yield; in an experimental animal–sand fly model, a second blood meal caused a second cycle of parasite replication in flies and higher metacyclic promastigote production [18, 19, 28]. Serial xenodiagnoses, as in canine studies [29], would likely yield higher estimates of infectiousness. However, ethical considerations preclude repeated xenodiagnosis and multiple skin biopsies in humans.
Xenodiagnosis is not practical for field studies. Less invasive skin specimens, if validated for accurate parasite load quantification, could open the door to population-based studies of transmission dynamics [30, 31]. Although there may be some individual-level misclassification, our data suggest that at the population level, qPCR results are likely to provide a useful reflection of transmission potential. Such studies would provide crucial inputs to model the interventions for the prevention of VL resurgence when intensive elimination efforts are scaled back. For PKDL treatment trials, skin parasite load quantification could also provide a functional marker that is more relevant to disease control than, for example, repigmentation [7].
Xenodiagnosis has inherent challenges and biases. A sand fly colony is, by definition, a single population selected by captive breeding success, and different colonies of the same species show different susceptibility to infection [32]. Multiple colonies per region will provide more robust knowledge. Another limitation is that flies were forced to feed in tubes from lesions; ethical considerations preclude allowing flies to feed on any accessible site as in canine xenodiagnosis [33]. Importantly, we did not demonstrate transmission from the infected fly to a subsequent mammalian host. Onward transmission has been clearly demonstrated in human studies in India [34] and recently for other leishmanial species in experimental animals [35, 36]. Quantification of parasite loads and percentage of metacyclic promastigotes in individual flies infected via direct xenodiagnosis would be a first step toward assessing onward transmission potential and its relation to parasite load in infected humans [36].
In recent community-based studies in Bangladesh and India, 60%–95% of PKDL cases were macular or maculopapular, while fewer than 10% were nodular [5, 9, 20]. Nevertheless, 35% of macular cases also had positive xenodiagnosis results. Ignoring this form of the disease imperils the progress made in VL control on the Indian subcontinent. Our data, while generated in Bangladesh, have implications for other regions. No infectiousness data exist for East Africa, which now accounts for more than half the global VL disease burden [37]. In Sudan, PKDL occurs in more than 50% of all VL patients, and unlike South Asia, nodular forms are frequent [8]. Because many PKDL cases resolve spontaneously within 1 year, long-standing policy in Sudan mandates withholding treatment for the first year unless the disease is severe [8]. Our data show no difference in infectiousness based on PKDL duration, suggesting that waiting 12 months for resolution provides a substantial parasite reservoir to sustain ongoing transmission.
Our data indicate that all PKDL patients, regardless of lesion type or duration, should be treated promptly and that active PKDL case detection should be instituted and maintained to ensure comprehensive diagnosis and minimize the time that infectious patients go untreated. Great strides have been made in the control of VL on the Indian subcontinent. PKDL must be addressed in order to sustain elimination and perhaps eventually to permanently interrupt transmission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The funding sources had no role in the study design, collection, analysis, and interpretation of the data; preparation of the manuscript; or the decision to submit for publication.
Financial support. This work was supported by the World Health Organization Special Programme for Research and Training in Tropical Diseases, Switzerland; the Spanish Foundation for International Cooperation, Health and Social Affairs; and International Centre for Diarrhoeal Disease Research, Bangladesh core donors.
Potential conflicts of interest. C. B. reports personal fees from Drugs for Neglected Diseases initiative outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Alvar J, Vélez ID, Bern C, et al. ; WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012; 7:e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Courtenay O, Peters NC, Rogers ME, Bern C. Combining epidemiology with basic biology of sand flies, parasites, and hosts to inform leishmaniasis transmission dynamics and control. PLoS Pathog 2017; 13:e1006571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhattacharya SK, Sur D, Sinha PK, Karbwang J. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. Indian J Med Res 2006; 123:195–6. [PubMed] [Google Scholar]
- 4. WHO-Bangladesh & Kala-azar Elimination Program DGHS, Ministry of Health and Family Welfare, Government of Bangladesh. Annual Report of the Kala-azar Elimination Program Dhaka. Bangladesh: CDC/DGHS, 2015. [Google Scholar]
- 5. Islam S, Kenah E, Bhuiyan MA, et al. Clinical and immunological aspects of post-kala-azar dermal leishmaniasis in Bangladesh. Am J Trop Med Hyg 2013; 89:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Napier LE, Das Gupta CR. An epidemiological investigation of kala-azar in a rural area in Bengal. Indian J Med Res 1931; 19:295–341. [Google Scholar]
- 7. Zijlstra EE, Alves F, Rijal S, Arana B, Alvar J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: a threat to the South-East Asia region Kala-azar elimination programme. PLoS Negl Trop Dis 2017; 11:e0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis 2003; 3:87–98. [DOI] [PubMed] [Google Scholar]
- 9. Mondal D, Nasrin KN, Huda MM, et al. Enhanced case detection and improved diagnosis of PKDL in a Kala-azar-endemic area of Bangladesh. PLoS Negl Trop Dis 2010; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Addy M, Nandy A. Ten years of kala-azar in west Bengal, Part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas?Bull World Health Organ 1992; 70:341–6. [PMC free article] [PubMed] [Google Scholar]
- 11. Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009; 1–20. [DOI] [PubMed] [Google Scholar]
- 12. Knowles R, Napier LE, Smith ROA. On a Herpetomonas found in the gut of the sandfly, Phlebotomus argentipes, fed on kala-azar patients: a preliminary note. Ind Med Gaz 1924; 59:593–7. [PMC free article] [PubMed] [Google Scholar]
- 13. Christophers S, Shortt H, Barraud P. The development of the parasite of Indian kala-azar in the sandfly Phlebotomus argentipes Annandale and Brunetti. Indian J Med Res 1924; 12: 605–7. [Google Scholar]
- 14. Napier L, Smith R. The development of Leishmania donovani in the gut of the sand fly Phlebotomus papatasi. Indian J Med Res 1926; 14:713–6. [Google Scholar]
- 15. Shortt H, Barraud P, Craighead A. Transmission experiments in India kala-azar with Phlebotomus argentipes. Indian J Med Res 1926; 14:589–600. [Google Scholar]
- 16. Napier LE, Smith ROA. Further observations on the feeding of sandflies, Phlebotomus argentipes, on cases of kala-azar in Calcutta. Indian Med Res Memoirs 1926; 4:147–53. [Google Scholar]
- 17. Shortt H, Swaminath CS. Note on dermal leishmanoid. Indian J Med Res 1928; 16:239–40. [Google Scholar]
- 18. Napier L, Smith R, Das-Gupta C, Mukerji S. The infection of Phlebotomus argentipes from dermal leishmanial lesions. Indian J Med Res 1933; 21:173–7. [Google Scholar]
- 19. Christophers SR, Shortt HE, Barraud PJ. Development of the parasite of Indian kala-azar in the sandfly Phlebotomus argentipes: refed flies and further results of the feeding of sandflies on kala-azar cases. Indian Med Res Memoirs 1926; 4:141–5. [Google Scholar]
- 20. Das VN, Pandey RN, Siddiqui NA, et al. Longitudinal study of transmission in households with visceral leishmaniasis, asymptomatic infections and PKDL in highly endemic villages in Bihar, India. PLoS Negl Trop Dis 2016; 10:e0005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molina R, Lohse JM, Pulido F, Laguna F, López-Vélez R, Alvar J. Infection of sand flies by humans coinfected with Leishmania infantum and human immunodeficiency virus. Am J Trop Med Hyg 1999; 60:51–3. [DOI] [PubMed] [Google Scholar]
- 22. Mondal D, Hasnain MG, Hossain MS, et al. Study on the safety and efficacy of miltefosine for the treatment of children and adolescents with post-kala-azar dermal leishmaniasis in Bangladesh, and an association of serum vitamin E and exposure to arsenic with post-kala-azar dermal leishmaniasis: an open clinical trial and case-control study protocol. BMJ Open 2016; 6:e010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molina R, Ghosh D, Carrillo E, et al. Infectivity of post-kala-azar dermal leishmaniasis patients to sand flies: revisiting a proof of concept in the context of the kala-azar elimination program in the Indian subcontinent. Clin Infect Dis 2017; 65:150–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molina R, Cañavate C, Cercenado E, Laguna F, López-Vélez R, Alvar J. Indirect xenodiagnosis of visceral leishmaniasis in 10 HIV-infected patients using colonized Phlebotomus perniciosus. AIDS 1994; 8:277–9. [DOI] [PubMed] [Google Scholar]
- 25. Hossain F, Ghosh P, Khan MAA, et al. Real-time PCR in detection and quantitation of Leishmania donovani for the diagnosis of visceral leishmaniasis patients and the monitoring of their response to treatment. PLoS One 2017; 12:e0185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bern C, Haque R, Chowdhury R, et al. The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic Bangladeshi village. Am J Trop Med Hyg 2007; 76:909–14. [PubMed] [Google Scholar]
- 27.Doehl JSP, Bright Z, Dey S, et al. Skin parasite landscape determines host infectiousness in visceral leishmaniasis. Nat Commun 2017; 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serafim TD, Coutinho-Abreu IV, Oliveira F, Meneses C, Kamhawi S, Valenzuela JG. Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity. Nat Microbiol 2018; 3:548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Courtenay O, Carson C, Calvo-Bado L, Garcez LM, Quinnell RJ. Heterogeneities in Leishmania infantum infection: using skin parasite burdens to identify highly infectious dogs. PLoS Negl Trop Dis 2014; 8:e2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirstein OD, Abbasi I, Horwitz BZ, et al. Minimally invasive microbiopsies: a novel sampling method for identifying asymptomatic, potentially infectious carriers of Leishmania donovani. Int J Parasitol 2017; 47:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verma S, Bhandari V, Avishek K, Ramesh V, Salotra P. Reliable diagnosis of post-kala-azar dermal leishmaniasis (PKDL) using slit aspirate specimen to avoid invasive sampling procedures. Trop Med Int Health 2013; 18:268–75. [DOI] [PubMed] [Google Scholar]
- 32. Lawyer P, Killick-Kendrick M, Rowland T, Rowton E, Volf P. Laboratory colonization and mass rearing of phlebotomine sand flies (Diptera, Psychodidae). Parasite 2017; 24:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis 2002; 186:1314–20. [DOI] [PubMed] [Google Scholar]
- 34. Swaminath CS, Shortt HE, Anderson LAP. Transmission of Indian kala-azar to man by bites of Phlebotomus argentipes Ann and Brun India. Indian J Med Res 1942; 30:473–7. [PubMed] [Google Scholar]
- 35. Martín-Martín I, Jiménez M, González E, Eguiluz C, Molina R. Natural transmission of Leishmania infantum through experimentally infected Phlebotomus perniciosus highlights the virulence of Leishmania parasites circulating in the human visceral leishmaniasis outbreak in Madrid, Spain. Vet Res 2015; 46:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stamper LW, Patrick RL, Fay MP, et al. Infection parameters in the sand fly vector that predict transmission of Leishmania major. PLoS Negl Trop Dis 2011; 5:e1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. World Health Organization. Leishmaniasis in high-burden countries: an epidemiological update based on data reported in 2014. Releve Epidemiologique Hebdomadaire 2016; 91:287–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.