Abstract
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system (CNS). The present study explored the role of intestinal microbiota in the initiation and propagation of mice induced by experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. 48 C57BL/6 were randomly divided into control group and EAE group. The changes of body weight and the scores of neurological function were recorded. The mRNA expression of the receptor tyrosine kinase subfamily (AXL) was detected by real-time quantitative PCR. The levels of IL-17 and IFN-γ in blood samples were examined by ELISA. The intestinal microbial composition of mice at different time points during the EAE induction was analyzed by 16S rRNA gene-based sequencing. In EAE group, the body weight began to reduce at day 3 and neurological symptoms began to appear at day 7 after EAE induction. The levels of IL-17 and IFN-γ in EAE group reached the peak at day 21 and then decreased gradually. However, the expression of Axl and SOCS3 reached the lowest level at day 21 and then increased gradually. The microbiome analyses revealed that the abundances of Alistipes, Blautia, and Lachnospiraceae_NK4A136_group were significantly changed at day 14, whereas the abundances of Allobaculum, Eubacterium and Helicobacter were significantly changed at day 30 of EAE induction. The prevotellaceae_NK3B31_group may be key bacteria that contribute to the development of MS. Regulation of intestinal microbiota composition can become a new therapeutic target for the treatment of MS.
Keywords: experimental autoimmune encephalomyelitis, Gut microbiota, Inflammatory inhibitors, Receptor tyrosine kinase subfamily
Introduction
Multiple sclerosis (MS) is a devastating autoimmune disease leading to progressive deterioration of the central nervous system (CNS) [1]. The common symptoms of MS include blindness, paresis, and sensory disturbances, accompanied by many cognitive problems such as memory disturbances and inattention [2]. The incidence of MS in women is higher than that in men and it is the most important nervous system disease causing nervous dysfunction in young adults [3,4]. Although great efforts have been mode to elucidate the underlying mechanisms, the real cause of MS is still unknown. The pathogenesis of MS has been reported to be related to many genetic and environmental factors, such as virus infection, dietary salt intake, smoking, adolescent obesity, alcoholism, and vitamin D deficiency [5–7].
The mammalian intestine is colonized by a complex microbial community, which consists of numerous microorganisms such as viruses, fungi, and bacteria [8]. Bacteria are the most abundant organisms and play essential roles in modulating host physiology such as metabolizing dietary nutrients and defending against pathogen colonization [9]. Moreover, diverse bacteria in the intestine also provide rich sources of microbial-derived antigen that can drive and promote the development of host immune system [10]. Recently, accumulating evidences have showed that the gut microbiota is another important environmental factor that is associated with the development of MS [11–13]. For instance, relapsing-remitting mice can spontaneously develop experimental autoimmune encephalomyelitis (EAE), a murine disease model that recapitulates many aspects and features of MS [13,14]. Interestingly, the EAE incidence can only occur under specific pathogen-free conditions but not germ-free conditions [13]. Furthermore, mono-colonization with segmented filamentous bacteria into the germ-free mice is sufficient to induce the development of EAE, suggesting that the activation of immune system by microbial stimulation may be a key environmental component contributing to the onset of this disease [15].
Recently, human studies also provided evidences about the associations between altered gut microbiota and the pathogenesis of MS. For example, a small sample sizes (20 MS patients versus 40 healthy subjects) study has reported that decreased abundances of Faecalibacterium, Prevotella and Anaerostipes, and increased abundances of Bifidobacterium and Streptococcus were found in MS patients compared with healthy individuals [16]. In another study with a larger sample size (60 MS patients versus 43 healthy subjects), the relative abundance of Methanobrevibacter and Akkermansia was increased, while the relative abundance of Butyricimonas was decreased in MS patients compared with the healthy controls [12]. A recent study also found that the relative abundances of Akkermansia muciniphila and Acinetobacter calcoaceticus were enriched in MS patients. By exposing the peripheral blood mononuclear cells to the cultures of these bacteria, the authors observed that these bacteria could stimulate the differentiation into proinflammatory Th1 lymphocytes [17].
As mentioned above, EAE is an ideal animal model that can well simulate the occurrence, development and outcome of human MS [13,15]. EAE can be induced by immunization with antigens of CNS such as myelin oligodendrocyte glycoprotein (MOG) [18]. In the present study, we used MOG-induced EAE model to assess the dynamic relationships between the composition of gut microbiota and inflammatory factor levels in lymphocytes during the onset and development of MS. We hope these findings could provide new insights into the role of intestinal flora in the pathogenesis of MS and related immunotherapy for clinical practices.
Materials and methods
Experimental animals
Forty-eight female C57Bl/6J mice (6–8 weeks of age) were purchased from Animal research center of Gansu University of Traditional Chinese Medicine and maintained under standard specific-pathogen free facility. Mice were randomly divided into control group by using the method of random number table.
Establishment of EAE model
EAE was induced in mice as described previously [19]. Briefly, MOG35-55 (Synthetic Biomolecules) were diluted by 0.9% of normal saline into 10 mg/ml and then added with same volume of complete Freund’s adjuvant (CFA, Chondrex) and the same amount of tuberculin H37Ra (DIFCO, U.S.A.) to make the final concentration of tuberculin H37Ra as 4 mg/ml. Preparation of antigen emulsifier inducing EAE was made by pumping the reagent into a water-in-oil status. Mice were subcutaneously injected with 0.1 ml of emulsifier at 4 points on both sides of the spine, and then intraperitoneally injected with 0.5 ml of pertussis toxin (LBL, U.S.A.) as an immunopotentiator on the day of immunization and 48 h later, respectively.
The disease scores of neurobehavioral defects were measured with the following criteria: 0, no obvious symptoms; 1: tail paralysis; 2: hind legs weakness; 3: hindlimb paralysis; 4: limbs paralysis; 5: death.
Extraction of total genomic DNA from feces
Mice feces were collected from control group and EAE group on day 7, 14, 21, and 30 after immunization. Total DNA of fecal samples was extracted using a QIAamp-DNA stool mini kit (Qiagen, Germany) according to the manufacturer’s instructions. Electrophoresis in 1% agarose gels was used to examine the integrity of the DNA samples, which were then selected to perform the consequent PCR amplification by using the specific primers of V4 hyper-variable regions of 16S rRNA genes. Sequencing of the PCR amplification products was performed on an Illumina Miseq platform. High quality sequencing data were analyzed and were further classified into operational taxonomic units (OTUs) with a 97% similarity level. The comparison database was Silva_12816SrRNA database. Significant difference of bacterial composition in EAE group at different time points was analyzed by Metastats software. LEfSE online analysis software was used for comparisons among different time points.
ELISA
Cytokine (IL-17 and IFN-γ) levels in the serum of the control group and EAE group on day 7, 14, 21, and 30 after immunization were determined by ELISA kit (Neobio) according to the manufacturer’s instructions.
Real-time PCR
Total RNA of brain paraventricular tissues were extracted from the control group and EAE group on day 7, 14, 21, and 30 after immunization. Briefly, 70 mg brain tissues were added with 1 ml Trizol reagent to extract total RNA. DNA was synthesized and amplified by PCR on LightCycler (Roche). About 20 μl of the reaction system include: 10 μl of 2x All-on-One qPCR Mix, 2 μl of DNA template, 2 μl of All-in-one qPCR Primer (Fulengen), sterilized and distilled water supplemented to 20 μl.
Data analysis
Statistical analysis was performed through SPSS22.0. The difference between two groups was examined using un-paired Student’s t-test. Correlation analysis was conducted by Spearman correlation analysis. All the differences were considered to be significant at P < 0.05.
Results
Clinical symptom and body weight change
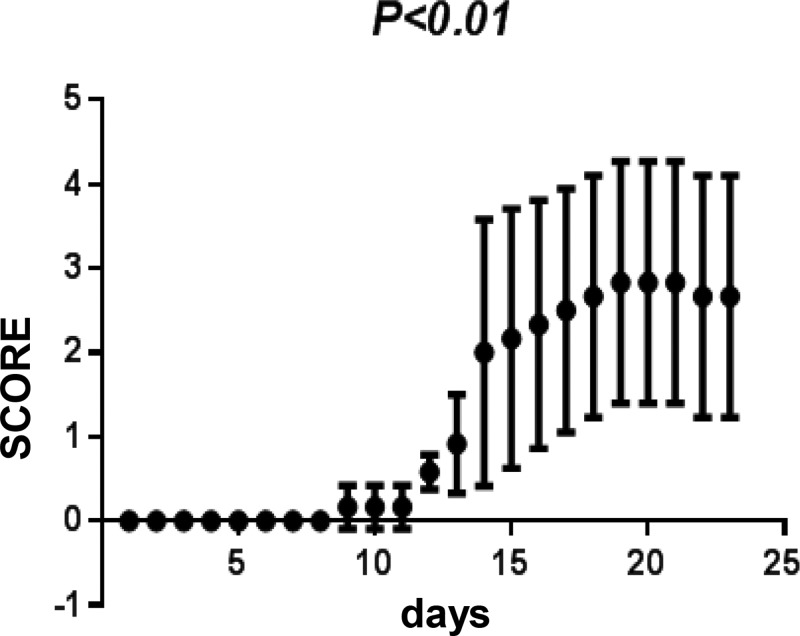
The average onset time of EAE was on day 7 and reached the most serious level on day 21. EAE animals developed a chronic disease with the loss of appetite and body weight, as well as other symptom such as rough fur. After EAE induction, animals also displayed neurological deficits such as tail weakness, limbs paralysis, and even death. In EAE group, the neurological function scores were 2 ± 1.58 on day 14 and 2.93 ± 1.43 on day 21. There were significant differences on neurological function scores and the body weight between control group and EAE group (Table 1 and Figure 1).
Table 1. The body weight between control group and EAE group (S).
| Group | Number | Day 14 | Day 21 | Day 30 |
|---|---|---|---|---|
| EAE | 24 | 16.28 ± 0.23 | 15.96 ± 0.45 | 18.28 ± 0.41 |
| Normal | 24 | 19.40 ± 0.46 | 20.86 ± 0.72 | 22.24 ± 0.50 |
Figure 1. Changes of neurological function scores in control group and experimental autoimmune encephalomyelitis (EAE) group.
Serum cytokine analysis
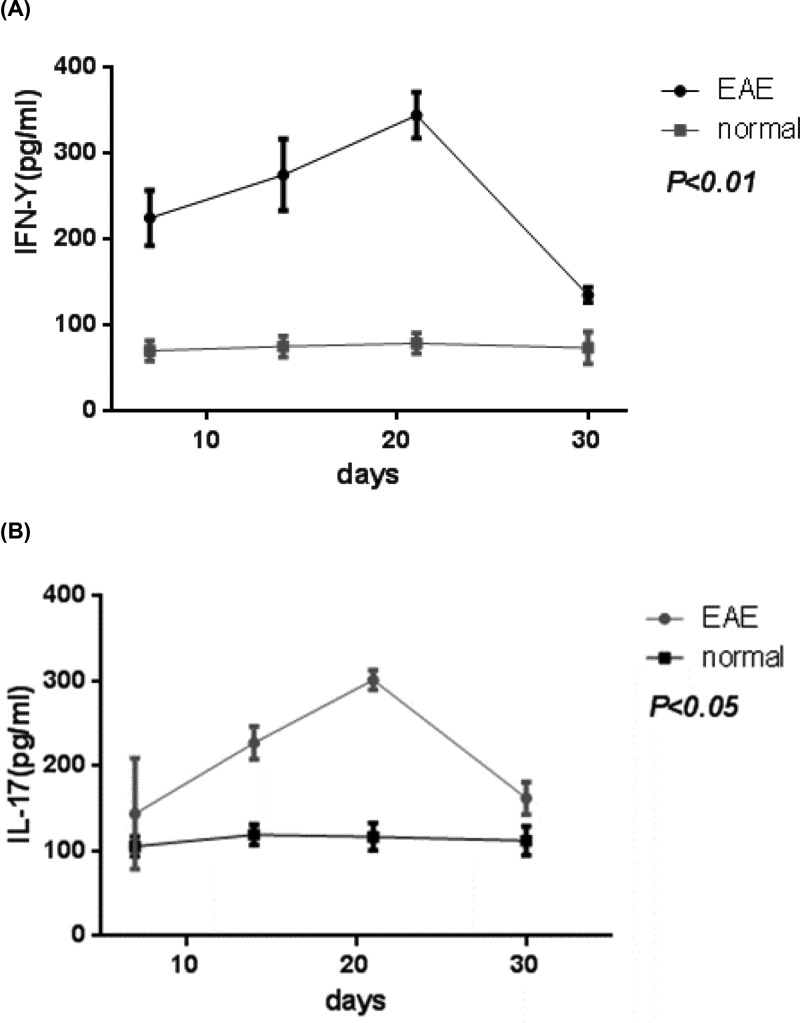
In EAE group, the serum levels of IL-17 on day21 were significantly higher than that on day 14 and 30. Similar trend was also observed for the concentration of and IFN-γ (Figure 2).
Figure 2. Serum cytokine analysis.
Serum levels of IL-17 (A) and IFN-γ (B) in control group and experimental autoimmune encephalomyelitis (EAE) group. P represents the comparison of day 12 and 30 with day 21 in EAE group.
qPCR analysis
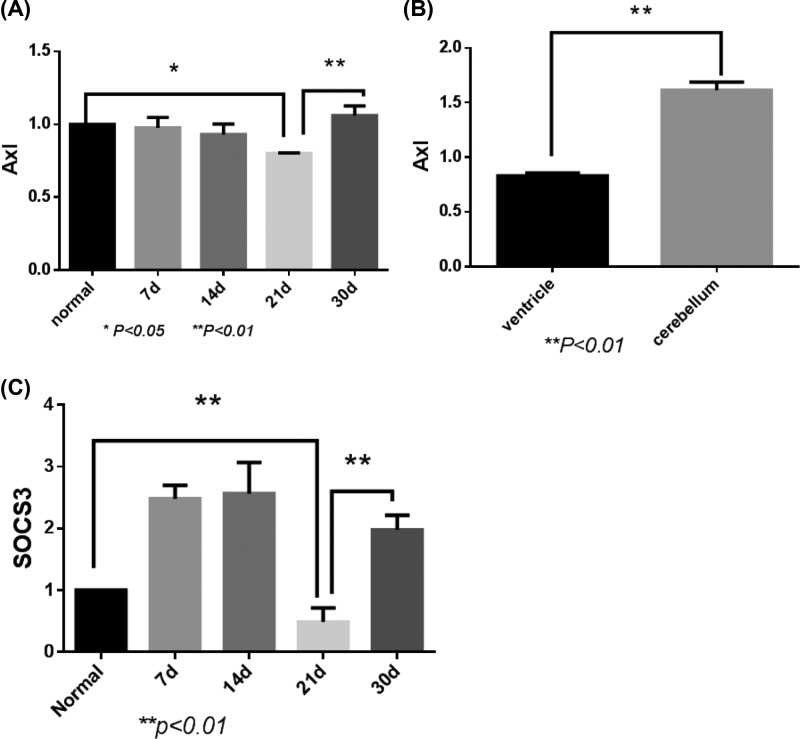
In EAE group, the expression of Axl in brain paraventricular tissues started to decrease on day 7 and reached the lowest level on day 21 (Figure 3A). On day 21 of EAE induction, Axl expression was significantly lower in the paraventricular compared with that in cerebellum (Figure 3B). However, the expression of SOCS3 increased on day 7 and 14 of EAE induction, and markedly decreased on day 21 (Figure 3C). The expressions of both Axl and SOCS3 were significant different between the control group and the EAE group (Figure 3).
Figure 3. Axl and SOCS3 expressions in ventricle of the control group and experimental autoimmune encephalomyelitis (EAE) group.
(A) The expression of Axl in brain paraventricular tissues on different days of EAE induction. (B) Axl expression in the paraventricular and cerebellum on day 21 of EAE induction. (C) The expression of SOCS3 on different days of EAE induction.
Gut microbiota composition analysis
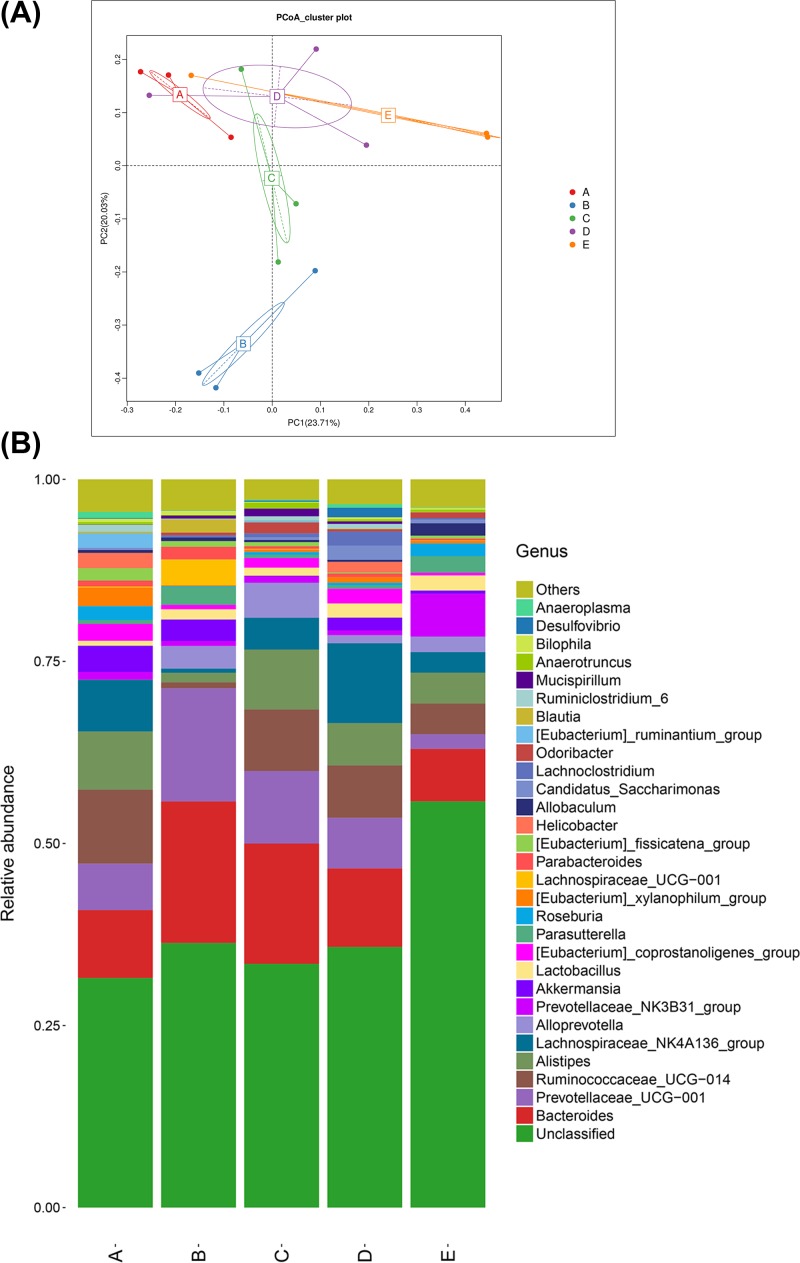
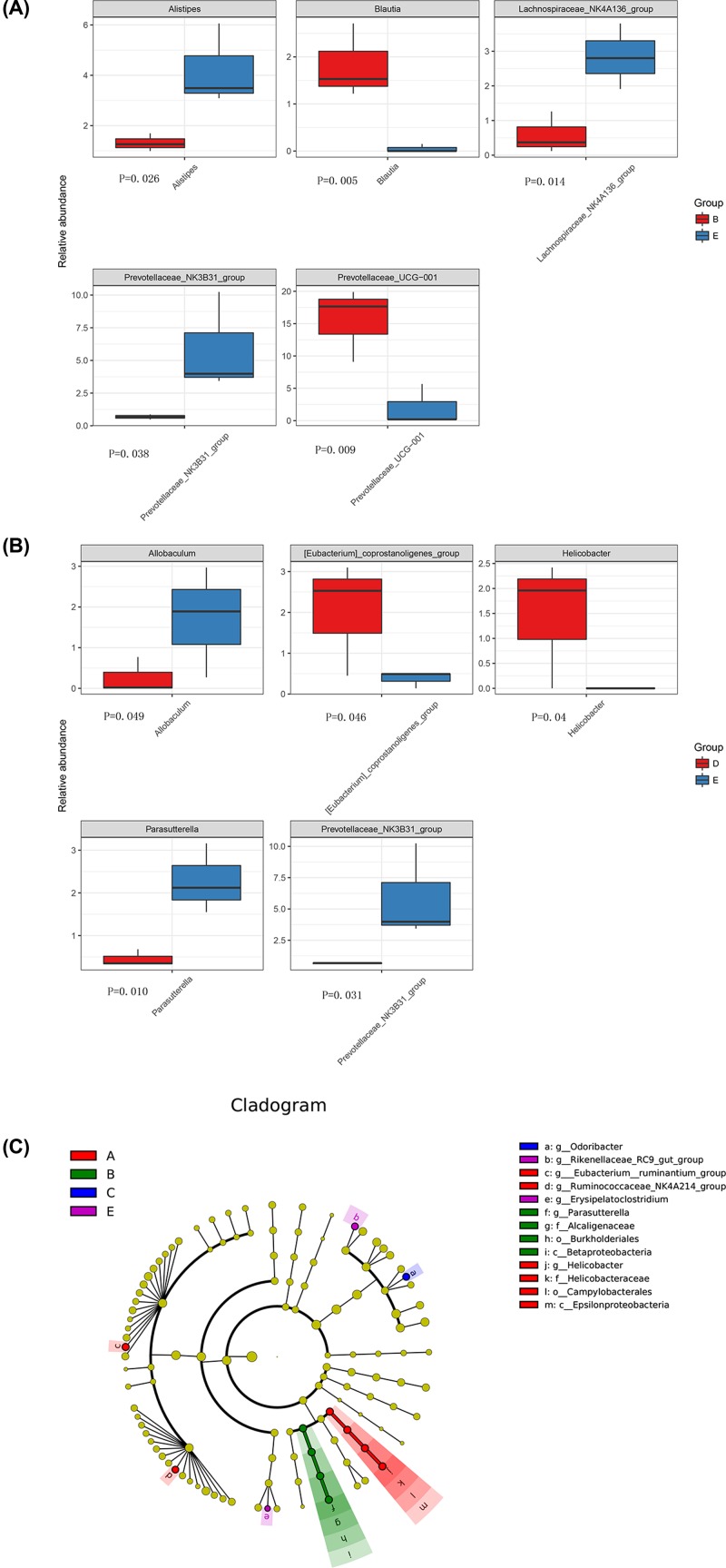
16S rDNA sequencing showed that a total of 221 OTUs with 97% similarity level were obtained from the control group and the EAE group. These OTUs included 15 kingdom, 125 phylum, 189 class, 189 order, 336 family, 688 genus, and 38 species. Principal component analysis (PCA) revealed that although individual difference existed in the EAE samples at the different time points, the bacterial composition between the EAE group and control group was still obvious (Figure 4A). At the genus level, Bacteroides, Prevotellaceae, Ruminococcaceae, Alistipes, Lachnospiraceae, and Alloprevotella were the most abundant bacteria in all samples (Figure 4B). Moreover, Alistipes, Blauti, Lachnospiraceae_NK4A136_group, prevotellaceae_NK3B31_group, and prevotellaceae_UCG-001 were the significantly changed bacteria between the control group and the day 14 of EAE group (Figure 5A). Allobaculum, Eubacterium, Helicobacter, Parasutterella, and Prevotellaceae were the significantly changed bacteria between the control group and the day 30 of EAE group (Figure 5B). LEfSE analysis further confirmed that Ruminococcaceae, Epsilonproteobacteria, Campylobacterales, Helicobacteraceae, and Helicobacter were the featured bacteria on day 7 of the EAE group. Burkholderiales, parasutterel, Betaproteobacteria, and Alcaligenaceae were the featured bacteria on day 14 of the EAE group. Odoribacter was the featured bacteria on day 30 of the EAE group (Figure 5C). It should be noted that the abundance of prevotellaceae_NK3B31_group, which was negatively correlated with serum IFN-γ level and positively correlated with Axl mRNA expression, was significantly reduced in the EAE group compared with that of the control group, suggesting that this bacteria may play an important role in the development and onset of EAE pathogenesis.
Figure 4. Analysis of principal component and bacterial composition differences.
(A) Principal component analysis (PCA) of the bacterial composition between the control group and experimental autoimmune encephalomyelitis (EAE) group. (B) The differences of bacterial composition at genus level. A–D represent day 7, 14, 21 and 30 after EAE induction in EAE group, respectively. E represents the control group.
Figure 5. The relative abundances and LEfSE analysis.
(A) The relative abundances of Alistipes, Blauti, Lachnospiraceae_NK4A136_group, prevotellaceae_NK3B31_group, and prevotellaceae_UCG-001 between the control group (blue-E) and the day 14 of EAE group (red-B). (B) The relative abundances of Allobaculum, Eubacterium, Helicobacter, Parasutterella, and Prevotellaceae between the control group (blue-E) and the day 30 of EAE group (red-D). (C) LEfSE analysis of the bacterial composition on day 7, 14, 21 and 30 of the EAE group. A–D represent day 7, 14, 21 and 30 after EAE induction in EAE group, respectively. E represents the control group.
Discussion
As the largest microbial colonization site in the human body, intestinal microbiota plays an important role in protecting intestinal barrier, inhibiting pathogenic colonization and promoting the formation of mucosa and systemic immune response [20]. There is growing evidence about the existence of Microbiome-Gut-Brin Axis and the role of gut microbiota in the pathogenesis of MS. It has been found that the abundances of Brevibacterium methanogens and Ekmania, which can activate the innate and adaptive immune system by stimulating the differentiation of T cells and monocytes, were significantly increased in the intestinal tract of MS patients [12]. However, disease-modifying treatment can inhibit the increase of prevotellaceae in MS patients [12]. These findings suggest that the increased pathogen and decreased beneficial bacteria can cause inflammatory status in the intestine and CNS, thereby leading to or aggravating the pathological changes of MS [21]. Thus, gut microbiota dysbiosis may play an important role in the course of multiple sclerosis.
It is believed that intestinal microbiota may be involved in the development of MS by regulating lymphocyte differentiation, metabolites and inflammatory factor production, as well as blood–brain barrier integrity. In the present study, we used MOG-induced EAE model to study the mechanism of how gut microbiota modulates inflammatory responses of MS onset. MOG35-55 specifically activates T lymphocytes, which may enter the CNS through damaged blood–brain barrier, causing brain lesions by releasing cytokines [22]. The mice under the induction of EAE showed chronic monophasic course and multiple different degrees of CNS lesions mainly located in white matter, cerebellum, brainstem, and spinal cord. Compared with the normal control group, HE staining of brain and spinal cord sections in EAE group at day 21 showed obvious inflammatory cell infiltration and the degree of inflammation is severed in the brain than the spinal cord. Moreover, vasodilation and ‘sleeve’ like infiltration of inflammatory cells can be seen in brain tissues, especially in subcortical and paraventricular areas. In the EAE group, the trend of body weight loss during the induction was consistent with the clinical scores.
Tyro3, Axl, and ER receptor tyrosine kinases (TAM receptors) belong to the receptor tyrosine kinase subfamily that can regulate innate immunity and mediate apoptotic cell clearance. The study found that Tyro3−/−, Axl−/-, and Mer−/− mice were suffered from chronic inflammation and autoimmune diseases [23]. However, the mechanism of action is not clear. TAM receptor can suppress innate immunity by down-regulating the JAK/STAT1 signaling pathway mediated by proinflammatory cytokines IFN-α [24]. Previous studies have found that the Axl protein can bind to INF-α and activate anti-inflammatory factors SOCS1 and SOCS3 [25]. The present study found that during the EAE induction the expression of Axl was lower on day 21 than that on day 7 and 14. Consistently, the expression of anti-inflammatory factor SOCS3 increased on day 7 and 14, but decreased to the lowest level on day 21 (P < 0.01). However, the expression of inflammatory factors IL-17 and IFN-γ reached the highest level on day 21. These results suggest that the inflammatory demyelination down-regulates the expression of Axl, which may result from the binding to INF-α or IFN-γ receptors, thereby mediating downstream JAK/STAT1 signaling pathway. As Axl expression decreased to the lowest level on day 21, when the SOCS3 expression also reached the lowest level, which eventually led to the peak of inflammatory demyelination after in 21 days of EAE induction. Therefore, it is suggested that IFN and IL-I7 are also important factors for Axl to inhibit immune responses through JAK/STAT1-SOCS3 signaling pathway [26]. Moreover, the expression of Axl in paraventricular tissues was significantly lower than that in cerebellum, indicating that inflammatory demyelination in MS was mostly found around lateral ventricles, where the low expression level of Axl diminished the inhibitory effect on inflammatory response.
The most abundant bacteria Bacteroides, Prevotellaceae, Ruminococcaceae, Alistipes, Lachnospiraceae, and Alloprevotella observed in EAE mice are in agreement with previous study about gut microbitoa composition in MS patients [12]. Compared with the normal control group, the bacterial composition in the EAE group were significantly different at different time points, suggesting that the disturbance of gut microbiota in EAE mice is closely related to the occurrence of inflammatory demyelination. Lachnospiraceae belongs to the Lachnospira [27]. In an experimental study of MS, it was found that after giving a high-vegetable or low-protein diet in patient with RRMS, the abundance of Lachnospiraceae in patients increased, and the production of IL-17 by CD4+ T cells significantly decreased (P = 0.04) [28], suggesting that Lachnospiraceae can reduce the inflammatory response. In the present study, in the EAE group over 7 days, the abundance of Lachnospiraceae_NK4A136_group was found to increase and the level of IL-17 increased that can activate CD4+ T cells and then promote inflammatory responses. The abundance of Helicobacter and Eubacterium in the EAE group increased compared with that in the normal control group at 30 days, suggesting that they may be opportunistic pathogens in the course of EAE. Both Blautia and Allobaculum can produce short-chain fatty acids [29]. The abundance of Blautia increased at 7 days, but decreased thereafter, and with no significant difference at 30 days compared with the normal control group. In EAE group, the abundance of Allobaculum at 30 days was significantly lower than that of the normal control group. One interesting finding of the present study is that the abundance of prevotellaceae_NK3B31_group was decreased on day 14 and 30 of the EAE group compared with the control group. Prevotellaceae is believed to be associated with the synthesis of short chain fatty acids (SCFAs) [30]. Haghikia et al. [31] found that SCFAs can induce the differentiation of Treg cells, indicating SCFAs play an important role in inhibiting the immune response in CNS diseases. IFN-γ is a typical Thl-type cytokine, which promotes the release of lymphotoxin or tumor necrosis factor and causes demyelination changes in MS patients [32,33]. IL-17 is an early inflammatory cell factor with a wide range of biological activities that is secreted by Th17 cells [15]. After the intestinal microbiota is disrupted, increased IL-17 in the blood system can directly cross through the blood–brain barrier by binding with the receptor on the endothelial cells on the barrier [34]. This suggests that gut microbiota and related inflammatory mediators may participate in the development of MS by directly crossing the blood–brain barrier, changing the integrity and permeability of the blood–brain barrier, or directly stimulating various neuroimmune substances [35]. Correlation analysis showed that prevotellaceae_NK3B31_group was negatively correlated with serum IFN-γ level and positively correlated with Axl mRNA expression, indicating the existence of microbiome–gut–brin axis [36]. Moreover, the LEfSE analysis showed that Odoribacter was the featured bacteria that was enriched on 21 of EAE induction. However, these were only few studies about Odoribacter and the specific mechanism of Odoribacter in MS development needs to be further studied.
In conclusion, the present study that focused on the role of Axl revealed the relationship between the gut microbiota and dynamic changes of important inflammatory factors for the immune-associated development of MS. The disorder of intestinal microbiota may play an important role in the differentiation of lymphocytes and the expression of inflammatory factors in EAE mice. The roles of specific intestinal bacteria such as prevotellaceae_NK3B31_group and Odoribacter in the pathogenesis of EAE need to be further studied to explore new ways of immunotherapy.
Abbreviations
- CNS
central nervous system
- EAE
encephalomyelitis
- MS
multiple sclerosis
Ethics Approval
The present study was carried out in accordance with the principles of the Basel Declaration and recommendations of the Ethic Committee of Animal Usage of Lanzhou University Second Hospital. The protocol was approved by the Ethic Committee of The Second Hospital of Lanzhou University.
Author Contribution
W.M.-X.: Guarantor of integrity of the entire study; L.X.-L.: Study design; Definition of intellectual content; Experimental studies; Data acquisition; Data analysis; Statistical analysis; Manuscript preparation; Manuscript editing; Manuscript review; Z.B.: Study design, Data analysis; Statistical analysis; Manuscript preparation; S.M.-J.: Experimental studies; B.C.-C.: Experimental studies; Manuscript review; X.Q.-F.: Statistical analysis; Y.B.-Y.: Manuscript review; W.L.-J.: Data acquisition. All authors have read and approved the final manuscript.
Funding
The author declares that there are no sources of funding to be acknowledged.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Wang Y., Cao L., Xu L.M.. et al. (2015) Celastrol ameliorates EAE Induction by suppressing pathogenic T cell responses in the peripheral and central nervous systems. J. Neuroimmune Pharmacol. 10, 506–516 10.1007/s11481-015-9598-9 [DOI] [PubMed] [Google Scholar]
- 2.Bhat R. and Steinman L. (2009) Innate and adaptive autoimmunity directed to the central nervous system. Neuron 64, 123–132 10.1016/j.neuron.2009.09.015 [DOI] [PubMed] [Google Scholar]
- 3.Dilokthornsakul P., Valuck R.J., Nair K.V.. et al. (2016) Multiple sclerosis prevalence in the United States commercially insured population. Neurology 86, 1014–1021 10.1212/WNL.0000000000002469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kingwell E., Marriott J.J., Jetté N.. et al. (2013) Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 13, 128. 10.1186/1471-2377-13-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao C., Simpson S. Jr, van der Mei I.. et al. (2016) Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 87, 1343–1349 10.1136/jnnp-2016-314013 [DOI] [PubMed] [Google Scholar]
- 6.Selmaj I., Mycko M.P., Raine C.S.. et al. (2017) The role of exosomes in CNS inflammation and their involvement in multiple sclerosis. J. Neuroimmunol. 306, 1–10 10.1016/j.jneuroim.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Brenton J.N. and Goldman MD. (2016) A study of dietary modification: Perceptions and attitudes of patients with multiple sclerosis. Mult. Scler. Relat. Disord. 8, 54–57 10.1016/j.msard.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 8.Hill D.A. and Artis D. (2010) Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 28, 623–667 10.1146/annurev-immunol-030409-101330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bäckhed F., Ley R.E., Sonnenburg J.L.. et al. (2005) Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- 10.McDermott A.J. and Huffnagle GB. (2014) The microbiome and regulation of mucosal immunity. Immunology 142, 24–31 10.1111/imm.12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joscelyn J. and Kasper LH. (2014) Digesting the emerging role for the gut microbiome in central nervous system demyelination. Mult. Scler. 20, 1553–1559 10.1177/1352458514541579 [DOI] [PubMed] [Google Scholar]
- 12.Jangi S., Gandhi R., Cox L.M.. et al. (2016) Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015. 10.1038/ncomms12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berer K., Mues M., Koutrolos M.. et al. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- 14.Stromnes I.M. and Goverman JM. (2006) Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 1, 1810–1819 10.1038/nprot.2006.285 [DOI] [PubMed] [Google Scholar]
- 15.Lee Y.K., Menezes J.S., Umesaki Y.. et al. (2011) Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 108, 4615–4622 10.1073/pnas.1000082107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyake S., Kim S., Suda W.. et al. (2015) Dysbiosis in the Gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV Clusters. PLoS One 10, e0137429. 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cekanaviciute E., Yoo B.B., Runia T.F.. et al. (2017) Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. U.S.A. 114, 10713–10718 10.1073/pnas.1711235114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefferl A., Brehm U. and Linington C. (2000) The myelin oligodendrocyte glycoprotein (MOG): a model for antibody-mediated demyelination in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neural Transm. Suppl. 57, 123–133 [DOI] [PubMed] [Google Scholar]
- 19.Yang L., Xing F., Han X.. et al. (2018) Astragaloside IV regulates differentiation and induces apoptosis of activated CD4+ T cells in the pathogenesis of experimental autoimmune encephalomyelitis. Toxicol. Appl. Pharmacol. 362, 105–115 10.1016/j.taap.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 20.Barreau F., Meinzer U., Chareyre F.. et al. (2007) CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS One 2, e523. 10.1371/journal.pone.0000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavasani S., Dzhambazov B., Nouri M.. et al. (2010) A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One 5, e9009. 10.1371/journal.pone.0009009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrill J.E. and Murphy SP. (1997) Inflammatory events at the blood brain barrier: regulation of adhesion molecules, cytokines, and chemokines by reactive nitrogen and oxygen species. Brain Behav. Immun. 11, 245–263 10.1006/brbi.1997.0496 [DOI] [PubMed] [Google Scholar]
- 23.Camenisch T.D., Koller B.H., Earp H.S.. et al. (1999) A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162, 3498–3503 [PubMed] [Google Scholar]
- 24.Rothlin C.V., Ghosh S., Zuniga E.I.. et al. (2007) TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136 10.1016/j.cell.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 25.Scutera S., Fraone T., Musso T.. et al. (2009) Survival and migration of human dendritic cells are regulated by an IFN-alpha-inducible Axl/Gas6 pathway. J. Immunol. 183, 3004–3013 10.4049/jimmunol.0804384 [DOI] [PubMed] [Google Scholar]
- 26.Weinger J.G., Brosnan C.F., Loudig O.. et al. (2011) Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. J. Neuroinflammation 8, 49. 10.1186/1742-2094-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekirov I., Russell S.L., Antunes L.C.M.. et al. (2010) Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- 28.Marina S., Laura M., Valentina R.. et al. (2017) Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: a pilot study. Front. Immunol. 8, 1391. 10.3389/fimmu.2017.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fengna C., Mingchao S. and Yue L. (2018) Gut microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: current applications and future perspectives. Mediators Inflamm. 2018, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Chia N., Kalari K.R.. et al. (2016) Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484. 10.1038/srep28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haghikia A., Jörg S., Duscha A.. et al. (2015) Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 43, 817–829 10.1016/j.immuni.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 32.Barreau F., Madre C., Meinzer U.. et al. (2010) Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut 59, 207–217 10.1136/gut.2008.171546 [DOI] [PubMed] [Google Scholar]
- 33.Steinman L. (2007) A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13, 139–145 10.1038/nm1551 [DOI] [PubMed] [Google Scholar]
- 34.Tzartos J.S., Friese M.A., Craner M.J.. et al. (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172, 146–155 10.2353/ajpath.2008.070690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan W., Banks W.A., Kennedy M.K.. et al. (1996) Differential permeability of the BBB in acute EAE: enhanced transport of TNT-alpha. Am. J. Physiol. 271, E636–E642 [DOI] [PubMed] [Google Scholar]
- 36.Dinan T.G. and Cryan JF. (2017) The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 46, 77–89 10.1016/j.gtc.2016.09.007 [DOI] [PubMed] [Google Scholar]