Abstract
Industrial treatments of commercial honeys during extraction and storage affect the quality of honey. One of the most common treatments in the honey industry is thermal treatment which could make some changes in the physicochemical and antioxidant properties of honey. This study was conducted to determine the effect of thermal treatment at 63 °C for 10, 20 and 30 min on physicochemical and antioxidant properties of lotus, thyme, and multifloral honeys. Samples were analyzed for pH, free acidity, moisture, hydroxymethylfurfural (HMF), color, total phenolic content, DPPH° radical-scavenging activity and ferric reducing antioxidant power (FRAP). Changes were more or less observed in all the physicochemical characteristics of honeys during the thermal treatment. However, among the physicochemical characteristics, increase in HMF content and decrease in total phenolic contents were more evident. Considering the antioxidant capacity of honeys, decreases in DPPH° radical scavenging activity of thyme honeys and FRAP values of thyme and lotus honeys during the thermal treatment were observed. Changes made in physicochemical characteristics of honeys during the thermal treatment are merely important from the standpoint of compliance with national and international legal limits. However, from the nutritional point of view, decrease in the antioxidant capacity of honeys is of particular importance and may affect the nutritional and health benefits of honey.
Keywords: Food science, Food analysis, Food processing, Honey, Physicochemical, Antioxidant capacity, Thermal treatment
1. Introduction
Honey, as a natural product, contains a complex mixture of simple sugars (mainly fructose and glucose) and other less frequent substances, such as amino acids, minerals, organic acids, proteins, lipids, vitamins, aroma compounds, pigments, waxes, pollen grains and other phytochemicals. In addition, honey serves as a source of natural antioxidants which play an important role in human health by reducing the risk of heart disease, cancer, cataracts, immune-system decline and different inflammatory processes [1, 2, 3, 4, 5]. Serum antioxidant capacity was significantly increased in humans after consumption of buckwheat honey in water [6]. Honey consumption was also effective in increasing total plasma antioxidant capacity as well as the total plasma reducing capacity in humans [7].
All over the world, the quality of honeys varies greatly depending on many factors including the source plant, climate and environmental condition and bees’ species. Moreover, industrial treatments of commercial honeys during extraction and storage affect the quality of honey. One of the most common treatments in the honey industry is thermal treatment which is done for several reasons. Thermal treatment facilitates filling the bottles by reducing the viscosity of honey [8], reduces the water content in honey to prevent fermentation [9], homogenizes honey color for the preference of consumers [10], dissolves the sugar crystal nuclei to retard granulation and destroys the sugar tolerant osmophilic yeasts to prolong the shelf life of honey [11, 12].
Thermal treatment may alter the physicochemical and antioxidant properties of honey. Changes made in some of these properties are only important from the standpoint of compliance with standard limits, e.g. pH, acidity, HMF content, etc. However, from the nutritional point of view, change in the antioxidant capacity of honey is of particular importance and may affect the health benefits of honey. Although this issue has been addressed in a limited number studies, there is no information about lotus, thyme, and multifloral honeys. Hence, this study was conducted to determine the effect of thermal treatment on physicochemical and antioxidant properties of lotus, thyme, and multifloral honeys from Iran.
2. Materials and methods
2.1. Honey samples
Three types of Iranian honey, lotus (Ziziphus lotus), thyme (Zataria multiflora), and multifloral honeys, were used in this study. For each type of honey, 4 different raw batches were obtained directly from local beekeepers. Each batch was divided into 4 parts; one was analyzed in its raw state (unheated) and the other 3 were analyzed following heat treatment at 63 °C for 10, 20 and 30 min. Thermal treatments were performed in a temperature-controlled water bath and samples were quickly cooled in the ice bath.
2.2. Physicochemical analysis
2.2.1. pH
Honey pH was measured using a pH-meter (Sartorius Ag, Goettingen, Germany) in a solution prepared with 10 g of honey in 75 ml of distilled water [3].
2.2.2. Free acidity
Ten grams of honey were dissolved in 75 ml of distilled water in a 250 ml beaker, and 100 μl of 1 % alcoholic solution of phenolphthalein added. The solution was titrated with 0.1 M NaOH. Free acidity was determined as 10 times the volume of NaOH used in titration and expressed as milliequivalent of acid/kg of honey [3].
2.2.3. Moisture
The moisture content was determined by measuring refractive index using an Abbe refractometer (2WAJ Abbe refractometer, Bluewave Industry Co., Ltd., China) and obtaining the corresponding % moisture (g/100 g honey) by consulting the standard table [13].
2.2.4. Hydroxymethylfurfural (HMF)
The standard AOAC method was used to determine hydroxymethylfurfural content of the honey samples [13]. Briefly, 5 grams of honey were dissolved in 25 ml of distilled water, treated with 500 μl of Carrez I and 500 μl of Carrez II solutions and then the volume made up to 50 ml. The solution was filtered, and the first 10 ml discarded. The absorbance of the filtered solution was measured by UV/VIS spectrophotometer (CECIL CE 2040 spectrophotometer 2000 series from CECIL instruments, Cambridge, England) at 284 and 336 nm against an aliquot of the filtered solution treated with NaHSO3. HMF was determined using the following equation:
| HMF (mg/kg) = [(A284 ˗ A336) × 149.7 × 5] / Weight of sample |
2.2.5. Color intensity
Honey samples were diluted to 50 % (w/v) with warm (50 °C) distilled water; in order to dissolve sugar crystals. The absorbance was measured using a spectrophotometer at 635 nm and the color intensity was determined using the Pfund scale: (2)
| Pfund (mm) = (371.39 × Abs) ˗ 38.70 |
2.2.6. Total phenolics
The total phenolic content was determined using the Folin- Ciocalteu method, and the results were expressed as mg tannic acid/kg honey. 100 μl of 50 % honey solution was mixed with 1.5 ml 10 % Folin–Ciocalteu reagent and 1.4 ml 7.5 % sodium carbonate solution. After incubation at room temperature for 30 min, the absorbance of the reaction mixture was measured at 750 nm against a blank of distilled water (CECIL CE 2040 spectrophotometer 2000 series from CECIL instruments, Cambridge, England). Tannic acid (100–1000 mg/kg) was used as standard to produce the calibration curve [14].
2.3. Antioxidant capacity
2.3.1. DPPH° radical-scavenging activity
The DPPH° radical scavenging activity of honey samples was determined according to the method of Brand-Williams modified by Miliauskas [15, 16, 17]. The DPPH° solution in methanol (0.06 mM) was prepared daily, and 2.7 ml of this solution was mixed with 0.3 ml of 50 % (w/v) honey solution. The mixture was shaken vigorously and left to stand for 60 min in the dark (until stable absorption values were obtained). The reduction of the DPPH° radical was determined by measuring the absorption at 517 nm. The experiment was carried out in triplicate. Radical scavenging activity (RSA) was calculated as a percentage of DPPH° discoloration using the equation:
| Radical scavenging activity (%) = [(ADPPH° ˗ AS) / ADPPH°] × 100 |
where AS is the absorbance of the sample solution and ADPPH° is the absorbance of the DPPH° solution.
2.3.2. Ferric reducing antioxidant power (FRAP)
The procedure described by Benzie and Strain (1996) was used with minor modification. Briefly, 0.2 ml of 50 % (w/v) honey solution was mixed with 2.8 ml of daily-prepared FRAP reagent containing 2.5 ml of a 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, 2.5 ml of 20 mM FeCl3 and 25 ml of 0.3 M acetate buffer, pH 3.6. The absorbance of the reaction mixture was measured spectrophotometrically at 593 nm after incubation at 37 °C for 15 min. Aqueous standard solutions of FeSO4.7H2O (100–1000 μM) were used for the calibration curve and the results were expressed as the FRAP value (μM Fe(II)) of the 50 % honey solution [18].
2.4. Statistical analysis
All analyses were carried out in triplicate, and the data were expressed as mean ± standard deviation (SD). In each type of honey, the changes made in the measured parameter during the thermal treatment were analyzed using the Repeated Measure ANOVA (SPSS 20). The significance levels are expressed at a 95% confidence level (P ≤ 0.05) throughout.
3. Results and discussion
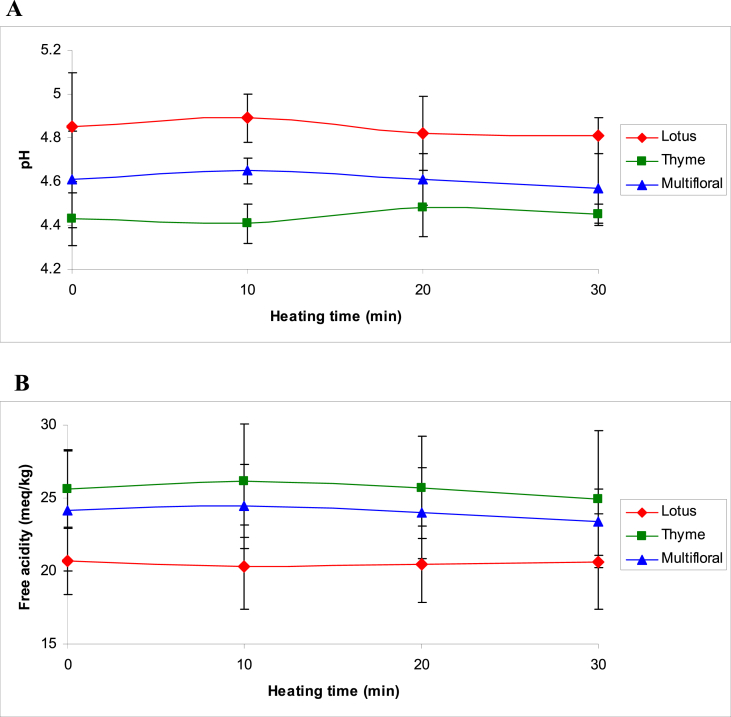
pH is a parameter that is correlated with honey storage and with microorganism growth that could change the texture and honey stability [19]. According to the Iranian national standards organization (INSO), the lowest acceptable pH of honey samples is 3.5 [20]. The initial pH values for studied honey samples were in the range of 4.43 ± 0.12 (thyme honeys) to 4.85 ± 0.25 (lotus honeys), which were acceptable values and comparable with those obtained in other works [3, 20, 21]. Furthermore, the initial levels of free acidity for studied honey samples were in the range of 20.7 ± 2.3 meq/kg (lotus honeys) to 25.6 ± 2.7 meq/kg (thyme honeys), which were acceptable values (below 40 meq/kg) and comparable with those obtained in other works [21]. The free acidity of honey may be explained by taking into account the presence of organic acids in equilibrium with their corresponding lactones, or internal esters, and some inorganic ions, such as phosphate, sulfate and chloride [23]. As shown in Figs. 1A and 1B, in all three types of honey, thermal treatment did not affect significantly the pH and free acidity (P ≥ 0.05).
Fig. 1.
Changes in pH (A) and free acidity (B) of honeys during thermal treatment.
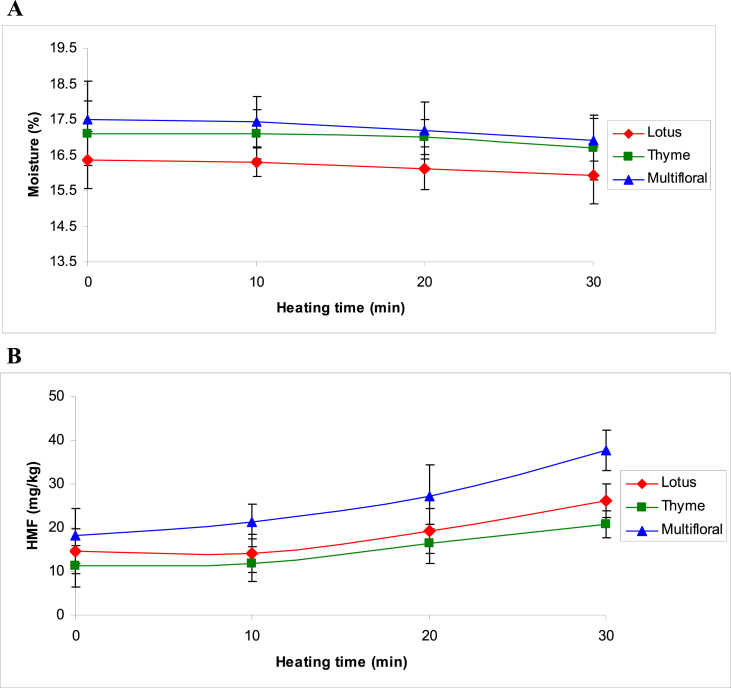
Honey moisture content depends on the environmental conditions such as the temperature and relative humidity in the geographical origin during honey producing in honey colonies, and also the manipulation from beekeepers at the harvest period [3]. Moisture content of honey is a limiting factor for determining its quality, stability and spoilage resistance against yeast fermentation. In the present study, the initial moisture contents of three types of honey samples were in the range of 16.4 ± 0.8% in lotus honeys to 17.5 ± 1.1% in multifloral honeys, which were well below the maximum acceptable limit (20 %) determined by national and international standards [20, 24]. As shown in Fig. 2A, slight decreases were observed in the moisture contents of all three types of honey, where, after 30 min thermal treatment, a decrease of 0.4–0.56 % was observed in the moisture content of all types of honey (P ≥ 0.05).
Fig. 2.
Changes in moisture (A) and HMF content (B) of honeys during thermal treatment.
HMF, naturally present in honey as a consequence of the action of normal honey acidity on reducing sugars, is recognized as a marker of honey freshness and quality deterioration. The HMF content tends to increase during processing and/or aging of the product. Several factors influence the levels of HMF, such as temperature and time of heating, storage conditions, pH and floral source, thus it provides an indication of overheating and storage in poor conditions [21, 22, 25]. In this study, the mean values of initial HMF content in studied honeys were below the national and international legal limit (≤40 mg/kg), indicating freshness of the honey and good storage condition. Multifloral honeys showed the highest initial level of HMF (18.1 ± 6.3 mg/kg) and thyme honeys showed the lowest one (11.2 ± 4.7 mg/kg).
As expected, HMF content in studied honeys increased gradually with increasing heating time, where after 30 min heating at 63 °C, HMF content increased by 81.3 %, 85.6 % and 108.3 % in lotus, thyme and multifloral honeys, respectively (P < 0.05). Nevertheless, after 30 min heating at 63 °C, the mean values for HMF content in three types of studied honey were still below the maximum acceptable limit (Fig. 2B).
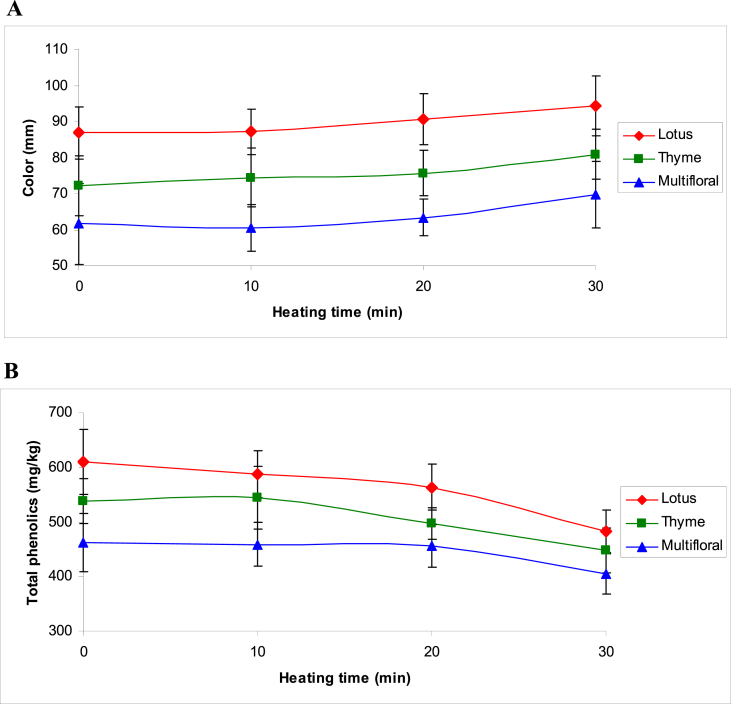
The color of honey usually ranges from pale yellow to dark amber and sometimes even green or red tinge [26]. In this study, multifloral honeys were yellow to brown, with Pfund value of 61.6 ± 11.2 mm. The color of thyme honeys was pale brown to light amber with Pfund value of 72.2 ± 7.3 mm. Lotus honeys were amber, more or less dark, with the highest Pfund value (86.82 ± 5.1 mm). In all types of honey, Pfund values increased gradually with increasing heating time (Fig. 3A). Color enhancement could be due the reduction in the moisture content and consequently increase in the concentration of the components responsible for honey's color, such as minerals.
Fig. 3.
Changes in color (A) and total phenolic content (B) of honeys during thermal treatment.
The method of Folin–Ciocalteu's is largely used to evaluate total phenolics in many different samples, including honey. Phenolic compounds include different subclasses (flavonoids, phenolic acids, stilbenes, lignans, tannins and oxidised polyphenols) displaying a large diversity of structures, some of which may interfere in the assay. For instance, ascorbic acid is a widespread reducing agent that can interfere in the Folin–Ciocalteu reaction. Other reducing substances such as some sugars and amino acids could also interfere [27]. Honey samples usually contain some of these compounds which can lead to an increase in the absorbance values and therefore overestimation of the phenolic compounds.
Results of the present study showed that the total phenolic content (mg tannic acid/kg honey) varied among the honey types. The lowest initial value was determined in multifloral honeys, where the average result of the samples was 462 ± 53 mg/kg, rising further in thyme (538 ± 41 mg/kg) and lotus (609 ± 60 mg/kg) honeys.
Changes in phenolic contents of honeys during the first 20-min of thermal treatment was not significant. However, after 30 min of heating, total phenolic contents in all types of honey were significantly reduced. As shown in Fig. 3B, total phenolic contents in lotus, thyme and multifloral honey samples decreased to 482 ± 39, 447 ± 40 and 404 ± 36 mg/kg, respectively; which were significantly lower than the initial levels.
In the recent years, there has been an increasing interest in determination of the antioxidant activity of honey. The quantity of the components responsible for antioxidant activity of honey varies widely according to the floral and geographical origin of honey. Processing, handling and storage of honey affect antioxidant activity of honey only to a minor degree [28, 29, 30]. Many methods have been used for determining the antioxidant activity of honey, e.g., the DPPH° (2,2-diphenyl-1-picrylhydrazyl) method, the FRAP (ferric-reducing/antioxidant power) assay, the ORAC (oxygen radical absorbance capacity) assay, and TEAC (Trolox equivalent antioxidant activity) assay.
In the present study, we used the DPPH° method to determine the percent of radical scavenging activity (% RSA) of honey samples. This means that the higher is the % RSA value of the honey, the higher is its antioxidant activity.
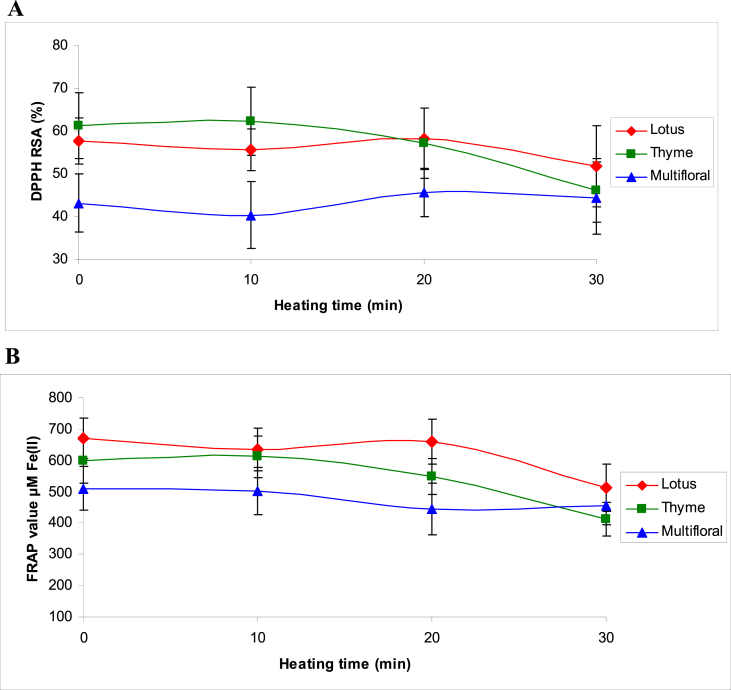
The lowest initial RSA values were 43.1 ± 6.8% for multifloral honey samples and the highest was 61.3 ± 7.6% for thyme honey. During a 30-min thermal treatment, changes in % RSA did not follow the same trend in different types of honey. As shown in Fig. 4A, at the end of 30-min thermal treatment, a slight and insignificant increase in % RSA of multifloral honeys and a slight and insignificant decrease in % RSA of lotus honeys were observed compared to initial % RSA levels (P > 0.05). However, at the same time, % RSA was decreased significantly in thyme honeys and reached 46.1 ± 7.5% (P < 0.05).
Fig. 4.
Changes in DPPH° radical scavenging activity (A) and FRAP value (B) of honeys during thermal treatment.
Since the DPPH° assay procedure reflects only the activity of water-soluble antioxidants [31], FRAP assay was used for the evaluation of the total antioxidant activity. Results of the present study showed that, the initial FRAP value for different types of honey increased in the order: multifloral < thyme < lotus honey. Multifloral honeys had an average FRAP value of 510 ± 69 μM Fe(II), while the highest FRAP value was 671 ± 65 μM Fe(II) in lotus honeys. During the thermal treatment, FRAP value did not change significantly in multifloral honeys, while at the end of 30 min heating, FRAP value reached 455 ± 60 μM Fe(II). In contrast, FRAP value decreased significantly in thyme and lotus honeys during the thermal treatment and reached 412 ± 63 and 513 ± 74 μM Fe(II) (Fig. 4B). According to Nayik and Nanda (2016), during thermal treatment, honey losses most of its natural antioxidants which can be compensated by the formation of non-nutrient antioxidants like Maillard reaction products [32]. Although we do not deny the role of Maillard reaction products in compensating the reduced antioxidant activity during thermal treatment, the rate of this compensation is practically unknown.
4. Conclusion
This study aimed to evaluate the changes in physicochemical and antioxidant properties of honey during thermal treatment at 63 °C for 30 min. During the thermal treatment, physicochemical and antioxidant properties of different types of honeys were altered. Among the physicochemical characteristics, increase in HMF content and decrease in total phenolic contents were more obvious. Considering the antioxidant capacity of honeys, % RSA decreased significantly during the thermal treatment in thyme honeys, however, in two other types of studied honey, changes in % RSA were not significant. In addition, FRAP value decreased significantly in thyme and lotus honeys during the thermal treatment. From the nutritional point of view, decrease in the antioxidant capacity of honeys is of particular importance and could affect the health benefits of honey.
Declarations
Author contribution statement
Mehdi Zarei: Conceived and designed the experiments; Wrote the paper.
Ali Fazlara: Contributed reagents, materials, analysis tools or data.
Noushin Tulabifard: Performed the experiments; Analyzed and interpreted the data.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
No additional information is available for this paper.
Acknowledgements
This study was supported by the research grant provided by Shahid Chamran University of Ahvaz. The authors would like to thank Mrs. P. Esfahani for her kind assistance.
References
- 1.Bertoncelj J., Dobersek U., Jamnik M., Golob T. Evaluation of the phenolic content, antioxidant activity and colour of slovenian honey. Food Chem. 2007;105:822–828. [Google Scholar]
- 2.Ferreira I.C.F.R., Aires E., Barreira J.C.M., Estevinho L.M. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. [Google Scholar]
- 3.Gomes S., Dias L.G., Moreira L.L., Rodrigues P., Estevinho L. Physicochemical, microbiological, and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010;48(2):544–548. doi: 10.1016/j.fct.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Nayik G.A., Nanda V. A chemometric approach to evaluate the phenolic compounds, antioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT - Food Sci Tech. 2016;74:504–513. [Google Scholar]
- 5.National Honey Board . Vol. 28. National Honey Board, Longman; 2003. (Honey: Health and Therapeutic Qualities). [Google Scholar]
- 6.Gheldof N., Wang X.-H., Engeseth N.J. Buckwheat honey increases serum antioxidant capacity in humans. J. Agric. Food Chem. 2003;51:1500–1505. doi: 10.1021/jf025897t. [DOI] [PubMed] [Google Scholar]
- 7.Schramm D.D., Karim M., Schrader H.R., Holt R.R., Cardetti M., Keen C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003;51:1732–1735. doi: 10.1021/jf025928k. [DOI] [PubMed] [Google Scholar]
- 8.Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998;63(4):549–562. [Google Scholar]
- 9.Subramanian R., Hebbar H.U., Rastogi N.K. Processing of honey: a review. Int. J. Food Prop. 2007;10(1):127–143. [Google Scholar]
- 10.Abu-Jdayil B., Ghzawi A.A., Al-Malah K.I.M., Zaitoun S. Heat effect on rheology of light- and dark-coloured honey. J. Food Eng. 2002;51:33–38. [Google Scholar]
- 11.Turhan I., Tetik N., Karhan M., Gurel F., Reyhan Tavukcuoglu H. Quality of honeys influenced by thermal treatment. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2008;41(8):1396–1399. [Google Scholar]
- 12.Escriche I., Visquert M., Juan-Borras M., Fito P. Influence of simulated industrial thermal treatments on the volatile fractions of different varieties of honey. Food Chem. 2009;112(2):329–338. [Google Scholar]
- 13.AOAC. seventeenth ed. Association of Official Analytical Chemists; Arlington, TX: 2000. Official Methods of Analysis. [Google Scholar]
- 14.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 15.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 1995;28:25–30. [Google Scholar]
- 16.Miliauskas G., Venskutonis P.R., Van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. [Google Scholar]
- 17.Dudonné S., Vitrac X., Coutiere P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH°, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 18.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘Antioxidant Power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 19.Feas X., Pires J., Iglesias A., Estevinho M.L. Characterization of artisanal honey produced on the Northwest of Portugal by melissopalynological and physico-chemical data. Food Chem. Toxicol. 2010;48:3462–3470. doi: 10.1016/j.fct.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 20.INSO. Iranian national standards organization; 2013. Honey: Specification And Test Methods, No: 92, 7th rev. [Google Scholar]
- 21.Habib H.M., Al Meqbali F.T., Kamal H., Souka U.D., Ibrahim W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014;153:35–43. doi: 10.1016/j.foodchem.2013.12.048. [DOI] [PubMed] [Google Scholar]
- 22.Truzzi C., Illuminati S., Annibaldi A., Finale C., Rossetti M., Scarponi G. Physicochemical properties of honey from marche, Central Italy: classification of unifloral and multifloral honeys by multivariate analysis. Nat Prod Commun. 2014;9(11):1595–1602. [PubMed] [Google Scholar]
- 23.Terrab A., Recalames A.F., Hernanz D., Heredia F.J. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004;88:537–542. [Google Scholar]
- 24.Codex Alimentarius Commission . FAO; Rome, Italy: 2001. Codex Standard for Honey. [Google Scholar]
- 25.Fallico B., Arena E., Verzera A., Zappala M. The European food legislation and its impact on honey sector. Accred Qual. Assur. 2006;11:49–54. [Google Scholar]
- 26.Bogdanov S., Ruoff K., Persano Oddo L. Physico-chemical methods for the characterisation of unifloral honey: a review. Apidologie. 2004;35:S4–S17. [Google Scholar]
- 27.George S., Brat P., Alter P., Amiot M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005;53:1370–1373. doi: 10.1021/jf048396b. [DOI] [PubMed] [Google Scholar]
- 28.Gheldof N., Engeseth N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002;50:3050–3055. doi: 10.1021/jf0114637. [DOI] [PubMed] [Google Scholar]
- 29.Turkmen N., Sari F., Poyrazoglu E.S., Velioglu Y.S. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2005;95:653–657. [Google Scholar]
- 30.Wang X.H., Gheldof N., Engeseth N.J. Effect of processing and storage on antioxidant capacity of honey. J. Food Sci. 2004;69:96–101. [Google Scholar]
- 31.Aljadi A.M., Kamaruddin M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518. [Google Scholar]
- 32.Nayik G.A., Nanda V. Effect of thermal treatment and pH on antioxidant activity of saffron honey using response surface methodology. J Food Meas Charact. 2016;10:64–70. [Google Scholar]