Abstract
A decreased lung diffusing capacity for carbon monoxide (DLCO) in systemic sclerosis (SSc) is considered to reflect losses of alveolar membrane diffusive conductance for CO (DMCO), due to interstitial lung disease, and/or pulmonary capillary blood volume (VC), due to vasculopathy. However, standard DLCO does not allow separate DMCO from VC. Lung diffusing capacity for nitric oxide (DLNO) is considered to be more sensitive to decrement of alveolar membrane diffusive conductance than DLCO. Standard DLCO and DLNO were compared in 96 SSc subjects with or without lung restriction. Data showed that DLNO was reduced in 22% of subjects with normal lung volumes and DLCO, whereas DLCO was normal in 30% of those with decreased DLNO. In 30 subjects with available computed tomography of the chest, both DLCO and DLNO were negatively correlated with the extent of pulmonary fibrosis. However, DLNO but not DLCO was always reduced in subjects with ≥ 5% fibrosis, and also decreased in some subjects with < 5% fibrosis. DMCO and VC partitioning and Doppler ultrasound‐determined systolic pulmonary artery pressure could not explain individual differences in DLCO and DLNO. DLNO may be of clinical value in SSc because it is more sensitive to DMCO loss than standard DLCO, even in nonrestricted subjects without fibrosis, whereas DLCO partitioning into its subcomponents does not provide information on whether diffusion limitation is primarily due to vascular or interstitial lung disease in individual subjects. Moreover, decreased DLCO in the absence of lung restriction does not allow to suspect pulmonary arterial hypertension without fibrosis.
Keywords: Interstitial lung disease, lung diffusing capacity for carbon monoxide, lung diffusing capacity for nitric oxide, systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a generalized fibrotic disease classified among the autoimmune connective‐tissue disorders (van den Hoogen et al. 2013). Widespread microangiopathy (Asano and Sato 2015) and interstitial lung disease (ILD) (Baldwin Ede et al. 1949; Schoenfeld and Castelino 2015) are commonly found, whereas pulmonary arterial hypertension (PAH) affects only 5–12% of patients (Khanna et al. 2013). The impairment of lung function is generally inferred from measurements of forced vital capacity (FVC) and lung diffusing capacity for carbon monoxide (DLCO) (Schneider et al. 1982). However, FVC is not an accurate measure of lung restriction, that is, decreased total lung capacity (TLC) (Aaron et al. 1999; Pellegrino et al. 2005), and decrement of DLCO in SSc may reflect loss of alveolar surface or thickening of blood‐gas barrier (Schoenfeld and Castelino 2015), but also changes in pulmonary microvasculature (Asano and Sato 2015).
Pulmonary CO uptake is mostly limited by its reaction rate with blood hemoglobin (Hb), thus DLCO is considered to be more sensitive to changes of pulmonary capillary blood volume (VC) than alveolar membrane diffusive conductance for CO (DMCO) (Guénard et al. 1987; Borland and Higenbottam 1989). Accordingly, the reduced DLCO in isolation or disproportionately to FVC seen in a minority of SSc subjects has been interpreted as a sign of PAH with limited or no ILD (Wilson et al. 1964; Steen et al. 1992), though the correlation between DLCO and mean pulmonary artery pressure is weak (Mukerjee et al. 2004).
Nitric oxide (NO) has a much greater affinity and faster reaction with free Hb than CO (Gibson and Roughton 1957), which makes the lung diffusing capacity for nitric oxide (DLNO) presumably more sensitive to alveolar membrane diffusive conductance than DLCO (Guénard et al. 1987; Borland and Higenbottam 1989). Thus, the analysis of combined DLNO and DLCO measurement has been proposed as a method to partition DMCO and VC subcomponents (Guénard et al. 1987) in various parenchymal (Phansalkar et al. 2004; Barisione et al. 2014, 2016) and vascular (Farha et al. 2013) disorders. By using this method in SSc, one study (Sivova et al. 2013) suggested that partitioning of DLCO might be of interest to detect PAH either with or without ILD, whereas two other studies (Guarnieri et al. 2015; Degano et al. 2017) concluded that partitioning of DLCO is of little use in distinguish the patients with only ILD from those with ILD complicated by PAH. Two studies (Overbeek et al. 2008; Pernot et al. 2012) using the classical multistep alveolar O2 partial pressure (PAO2) method (Roughton and Forster 1957) to calculate DMCO and VC also gave contrasting results. Although DLNO has been demonstrated to be more sensitive than DLCO in idiopathic pulmonary fibrosis and this was due to the prevailing DMCO impairment (Barisione et al. 2016), this information in SSc is lacking. Therefore, the added value of DLNO and the partitioning of diffusion subcomponents in the diagnostic workup of SSc is still unclear.
The aim of the present study was to investigate whether DLNO and standard DLCO can provide different information on the lung involvement in individuals with SSc that can be attributed to interstitial or vascular abnormalities by DMCO and VC partitioning.
Materials and Methods
Study subjects
We retrospectively collected data from 96 Caucasian consecutive subjects fulfilling the current diagnostic criteria for SSc with a total score ≥ 9 (van den Hoogen et al. 2013). They had pulmonary function and other diagnostic tests completed between March 2014 and October 2017, when they were in stable clinical conditions. Sixteen of them received long‐term intravenous iloprost, with various combinations of oral prednisone (n = 11), bosentan (n = 9), mofetil mycophenolate (n = 6), methotrexate (n = 4), and nifedipine (n = 3). Thirty‐nine healthy subjects, matched for anthropometric and life‐style data, were selected among health‐care professionals to serve as a control group (Table 1). The study was approved by the Regional Ethics Committee (Registry no.: P.R.170REG/2015) and written informed consent was obtained from each subject to use his/her personal data.
Table 1.
Subjects’ anthropometric characteristics and lung function data
| Controls | SSc‐N | SSc‐D | SSc‐R | P value | |
|---|---|---|---|---|---|
| Male/Female | 5/34 | 2/49 | 1/18 | 5/21 | 0.24 |
| Age (years) | 55 ± 19 | 64 ± 13*, † | 63 ± 10 | 54 ± 16† | 0.008 |
| BMI (kg·m−2) | 25 ± 5 | 24 ± 4 | 24 ± 5 | 26 ± 5 | 0.27 |
| Smoking habit (c/f/n) | 4/6/29 | 3/6/42 | 5/5/9 | 1/6/19 | 0.08 |
| [Hb] (g·dL−1) | 13.5 ± 0.66 | 13.1 ± 0.93 | 12.7 ± 1.09* | 12.9 ± 1.09 | 0.011 |
| FVC (L) | 3.53 ± 0.78 | 3.00 ± 0.54* | 2.83 ± 0.68* | 2.38 ± 0.63*, †, ‡ | <0.001 |
| (% predicted) | 112 ± 14 | 104 ± 14* | 96 ± 11*, † | 66 ± 11*, †, ‡ | <0.001 |
| (z‐score) | 0.72 ± 0.84 | 0.22 ± 0.89* | −0.31 ± 0.73*, † | −2.48 ± 0.93*, †, ‡ | <0.001 |
| FEV1 (L) | 2.81 ± 0.68 | 2.31 ± 0.43* | 2.17 ± 0.49* | 1.97 ± 0.56*, † | <0.001 |
| (% predicted) | 110 ± 13 | 102 ± 12* | 93 ± 12*, † | 68 ± 9*, †, ‡ | <0.001 |
| (z‐score) | 0.70 ± 0.83 | 0.10 ± 0.83* | −0.46 ± 0.78*, † | −2.23 ± 0.70*, †, ‡ | <0.001 |
| TLC (L) | 5.25 ± 0.90 | 4.84 ± 0.59* | 4.50 ± 0.99* | 3.60 ± 0.83*, †, ‡ | <0.001 |
| (% predicted) | 109 ± 10 | 103 ± 11* | 94 ± 10*, † | 67 ± 11*, †, ‡ | <0.001 |
| (z‐score) | 0.66 ± 0.74 | 0.20 ± 0.86* | −0.40 ± 0.77*, † | −2.79 ± 0.94*, †, ‡ | <0.001 |
| DLCO (mL·min−1·mmHg−1) | 25.5 ± 6.12 | 18.2 ± 2.90* | 12.4 ± 2.28*, † | 14.5 ± 4.15*, † | <0.001 |
| (% predicted) | 116 ± 14 | 96 ± 12* | 65 ± 10*, † | 65 ± 12*, † | <0.001 |
| (z‐score) | 0.87 ± 0.72 | −0.30 ± 0.70* | −2.73 ± 1.16*, † | −2.74 ± 1.17*, † | <0.001 |
| KCO (mL·min−1·mmHg−1·L−1) | 4.60 ± 0.77 | 3.96 ± 0.56* | 3.01 ± 0.53*, † | 4.17 ± 0.65‡ | <0.001 |
| (% predicted) | 104 ± 14 | 92 ± 12* | 70 ± 12*, † | 96 ± 14‡ | <0.001 |
| (z‐score) | 0.24 ± 0.87 | −0.52 ± 0.84* | −1.87 ± 1.35*, † | −0.31 ± 0.95‡ | <0.001 |
| DLNO (mL·min−1·mmHg−1) | 95.6 ± 26.0 | 71.7 ± 16.0* | 52.5 ± 11.4*, † | 55.9 ± 21.3*, † | <0.001 |
| (% predicted) | 88 ± 9 | 74 ± 11* | 53 ± 9†*, † | 46 ± 12*, † | <0.001 |
| (z‐score) | −0.69 ± 0.53 | −1.30 ± 0.66* | −2.35 ± 0.63*, † | −3.17 ± 0.88*, †, ‡ | <0.001 |
| KNO (mL·min−1·mmHg−1·L−1) | 18.8 ± 3.18 | 15.3 ± 2.38* | 12.7 ± 2.64*, † | 15.2 ± 3.40*, ‡ | <0.001 |
| (% predicted) | 86 ± 9 | 74 ± 8* | 61 ± 12*, † | 68 ± 12*, †, ‡ | <0.001 |
| (z‐score) | −1.05 ± 0.71 | −1.90 ± 0.56* | −2.91 ± 0.97*, † | −2.50 ± 0.98* | <0.001 |
Data are absolute numbers or mean ± SD.
SSc‐N, subjects with all standard lung function data > 5th percentile (LLN5); SSc‐D, subjects with DLCO < LLN5 but TLC > LLN5; SSc‐R, subjects with TLC < LLN5 with (n = 21) or without (n = 5) DLCO < LLN5; c/f/n, current/former/never; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; TLC, total lung capacity; DLCO, standard single‐breath (9‐11 s breath‐hold time) lung diffusing capacity for carbon monoxide; KCO, DLCO/alveolar volume; DLNO, lung diffusing capacity for nitric oxide; KNO, DLNO/VA.
Significantly different from control.
Significantly different from SSc‐N.
Significantly different from SSc‐D.
Standard lung function measurements
Spirometry (Miller et al. 2005) and lung volumes (Wanger et al. 2005) were measured with the subject sitting in a whole‐body plethysmograph (V62J, SensorMedics‐Viasys, CareFusion; Höchberg, Germany). FVC, forced expiratory volume in one second (FEV1), FEV1/FVC ratio, and TLC were determined and compared with available predicted values (Quanjer et al. 1993, 2012). Standard DLCO was measured (MasterScreen PFT System, Jaeger‐Viasys, CareFusion, Höchberg, Germany) by single‐breath technique with 9–11 breath‐hold time free of Valsalva or Müller maneuvers (Macintyre et al. 2005). CO back pressure was measured in expired gas prior to the inspiration of test mixture and compensated for analytically (Graham et al. 2002). Measured values were compared with reference values from Stanojevic et al. (2017) and adjusted for effective [Hb] in venous blood (Cotes et al. 1972).
DLNO measurement
At least 5‐10 min after standard DLCO, single‐breath DLNO‐DLCO were simultaneously measured in sitting position (MasterScreen PFT System, Jaeger‐Viasys, CareFusion, Höchberg, Germany) from the exponential disappearance rate of NO and CO with respect to helium (He) in exhaled air (Guénard et al. 1987). A gas mixture of 0.28% CO, 9.0% He, and 20.9% O2 balanced with N2 was blended with 450 ppm NO in N2 and inhaled from a plastic bag containing a final concentration of NO of 50.1 ± 4.8 ppm obtained ≤ 2 min before its use. The linearity of the electrochemical cell was checked by factory and the apparatus was calibrated for gas fractions using automated procedures. The subject was in a relaxed sitting position, wearing a nose‐clip and breathing quietly through a mouthpiece with filter connected to a screen‐type pneumotachograph. After breathing 4–5 stable tidal volumes, he/she was requested to perform a full expiration to RV. Then a valve was opened allowing the subject to forcefully inhale the gas mixture achieving an inspired volume ≥ 90% of inspiratory vital capacity in < 2.5 sec. A breath‐hold time of 4–6 sec duration, at near atmospheric intrapulmonary pressure and free of Valsalva and Müller maneuvers, was then requested followed by a rapid and smooth expiration to RV (Zavorsky et al. 2017). The total breath‐hold time was calculated from the beginning of inspiration, minus 30% of inspiratory time, to the middle of expiratory gas sampling (Jones and Meade 1961). The first 750 mL of expired gas was discarded and the following 750 mL was sampled from the bag to be analyzed for NO, CO, and He fractional concentration. When the subject's vital capacity was < 2.0 L, sample and washout volumes were reduced to 500 mL (Macintyre et al. 2005). Between successive tests, an interval of ≥ 4–5 min was allowed to ensure complete elimination of prior test gases from the lungs. DLNO‐DLCO values were accepted if two successive measurements were within 17.0 and 3.2 mL·min−1·mmHg−1, respectively, and the mean of two properly performed maneuvers was retained for analysis (Zavorsky and Murias 2006).
Derivation of diffusion subcomponents
DMCO and VC were derived by assuming a fixed value of blood conductance for NO (θ NO) of 4.5 mL (NO)·min−1·mmHg−1·mL (blood)−1 (Carlsen and Comroe 1958) which is largely independent of pulmonary blood [Hb] (van der Lee et al. 2005) and mean (expired) PAO2 (Borland and Cox 1991). By contrast, the value of blood conductance for CO (θ CO) is variable and strictly dependent on [Hb] (Cotes et al. 1972) and , due to the competitive binding of CO and O2 for Hb‐accessible sites (Crapo et al. 1988). This makes the inspired O2 fractional concentration (FIO2) critical to DLCO measurement (Crapo et al. 1988) and, in turn, the DMCO and VC partitioning (Roughton and Forster 1957; Forster 1987). Thus, θ CO values were calculated according to Guénard et al.(2016) in vivo equation:
| (1) |
where capillary O2 partial pressure () is equal to [] (Bohr 1909), with being the ratio of O2 uptake to lung diffusing capacity for O2 which was assumed as ≈DLCO·1.61 (Hsia et al. 2008). Moreover, [Hb] standard values were set at 14.6 and 13.4 g∙dL‐1 for males and females, respectively (Cotes et al. 1972). For the simultaneous DLNO‐DLCO measurement, due to an automated flushing procedure with 100% O2 preceding each test and specific to this apparatus (Munkholm et al. 2018), the actual mean FIO2 () in the inspiratory bag was 0.228 ± 0.01, thus resulting in a of 125.4 ± 8.5 mmHg instead of the expected 99.1 ± 3.3 mmHg. To account for this potential bias, the corresponding values of θ CO were calculated from Equation (1) to be 0.50 ± 0.04 and 0.55 ± 0.04 mL (CO)∙min−1∙mmHg−1∙mL (blood)−1, with of 125.4 ± 8.5 and 99.1 ± 3.3 mmHg, respectively. These values were used to derive the diffusion subcomponents in mild hyperoxia and normoxia from simultaneous DLNO‐DLCO and separate standard DLCO and DLNO measurements, respectively, as follows (Zavorsky et al. 2017):
| (2) |
where DMCO = DMNO/1.97 according to NO/CO physical diffusivity ratio (Wilhelm et al. 1977). Predicted values for DLNO and diffusion subcomponents were from Zavorsky et al.(2017).
Chest CT scanning
In 30 subjects, a thin‐section CT scan obtained within 3 months before or after pulmonary function measurements was available. Scans of the entire chest had been routinely obtained in a supine position, during breath‐hold at full inspiration by a multidetector row‐spiral scanner (SOMATOM Emotion 6, Siemens AG Medical, Forchheim, Germany). Images were acquired by 110 kVp tube voltage at 1.25 mm slice thickness and reconstructed at 1‐mm increments by using smooth (B41s) and sharp (B70s) convolution kernels. All CT scans were independently analyzed quantitatively as well as semi‐quantitatively by two experienced readers (G.B. and A.G.), with the latter being blind to clinical diagnosis and lung function data. For both analyses, the following six axial levels were considered: (1) aortic arch, (2) tracheal carina, (3) right pulmonary venous confluence, (4) midpoint between level three and level five, (5) 1 cm above the dome of the right hemi‐diaphragm, and (6) 2 cm below the dome of the right hemi‐diaphragm (Colombi et al. 2015). Mean lung density (g·mL−1) was calculated by adding CT numbers (HU) and parenchymal volume (Gattinoni et al. 2005) measured in 2D at the six axial levels. In 13 subjects, mean lung density could also be obtained by automatic quantitative 3D analysis of the entire lungs (VIDA's Lung Volume Analysis, Coralville, IA, US). At each axial level, ground glass opacities and fibrotic changes, that is, subpleural honeycombing, reticular opacities, and traction bronchiectasis, were visually bounded and their respective volumes computed by a three‐dimensional active contour segmentation software (ITK‐Snap 3.6.0, Philadelphia, PA, USA) (Yushkevich et al. 2006). In each patient, the total percentages of lung volume with ground glass attenuation and/or fibrotic changes were calculated in relation to the total parenchymal volume of the six axial levels (Barisione et al. 2016).
Doppler echocardiography
In all subjects, a transthoracic Doppler echocardiography (Vivid E90, GE Healthcare, Little Chalfont, UK) was routinely obtained within 3 months before or after pulmonary function testing. In subjects with measurable tricuspid regurgitation velocity (TRV), systolic pulmonary artery pressure (sPap) was calculated as sPap = (4·TRV2 + RAP), where RAP is right atrial pressure, estimated from the diameter and breath‐induced variability in the inferior vena cava (Hatle et al. 1981). Values of sPap ≥ 36 mmHg were considered as suggestive of likely PAH (Greiner et al. 2014).
Statistical analysis
Lung function data were expressed as z‐score, which indicates how many standard deviations a given measure differs from predicted, with a value −1.645, corresponding to the 5th percentile of the reference population, assumed as the lower limit of normal (LLN5). Categorical variables were compared by z‐test with Yates correction and continuous variables by ANOVA with Holm‐Sidak post hoc test for multiple comparisons. Associations between variables were determined by Spearman's rank correlation. Data are presented as mean ± SD or median with 25–75% interquartile range (IQR25–75%). In all analyses, the acceptable type I error was set at P < 0.05.
Results
Based on the standard lung function data, SSc subjects as the whole (n = 96) were divided into three groups, one with both TLC and DLCO above the LLN5 (SSc‐N) (n = 51), one with DLCO below the LLN5 but TLC above the LLN5 (SSc‐D) (n = 19), and one with TLC below the LLN5 with or without DLCO below the LLN5 (SSc‐R) (n = 26). Two SSc‐D subjects showed mild‐to‐moderate airflow obstruction, but no CT evidence of emphysema. Eighteen out of the 39 subjects with measurable TRV had CT scan analysis and nine of them showed sPap values > 36 mmHg. Although, by definition all subjects of SSc‐N group had standard lung function parameters above the LLN5, the group mean values were significantly (P < 0.001) lower than in the control group.
Considering individual data, DLNO was below the LLN5 in all restricted subjects, all but two SSc‐D, eight out of nine with sPap > 36 mmHg, and also 11 out of 51 SSc‐N subjects (Fig. 1). DLCO was below the LLN5 in 21 out of 26 SSc‐R subjects and six out of nine with sPap > 36 mmHg. DMCO was below the LLN5 in the majority but not all SSc‐R subjects and VC was below the LLN5 in the majority but not all SSc‐D subjects (Fig. 2). Overall, using LLN5 as cutoff value, neither DLNO nor DLCO provided falsely positive results (100% specificity) but their sensitivities to the presence of SSc were 56% and 42%, respectively.
Figure 1.
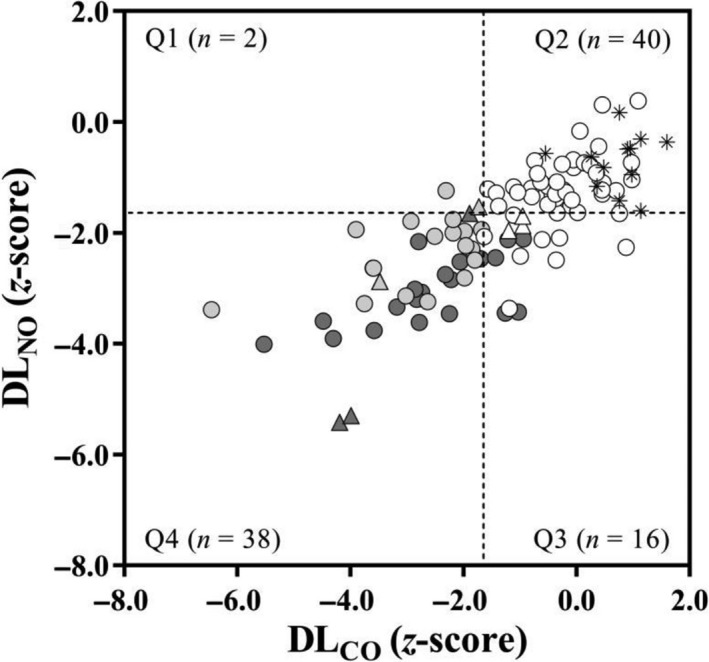
Relationships between the z‐scores of lung diffusing capacity for nitric oxide (DLNO) (y‐axis) and standard (9–11 sec breath‐hold time) lung diffusing capacity for carbon monoxide (DLCO) (x‐axis) in healthy controls (asterisks) and SSc subjects. Symbols indicate subjects with spirometry and total lung capacity (TLC) > LLN5 (white), DLCO < LLN5 (light grey), and TLC < LLN5 (dark grey). Triangles indicate subjects with systolic pulmonary artery pressure (sPap) > 36 mmHg. The dashed lines indicate the LLN5 (z‐score < −1.645). The absolute values indicate the number of the total 96 test results for DLNO that fall into each quadrant (Q1–Q4).
Figure 2.
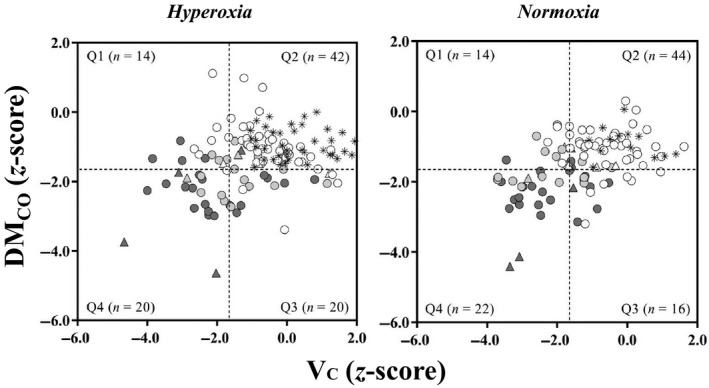
Relationships between the z‐scores of alveolar membrane diffusive conductance for CO (DMCO) (y‐axis) and pulmonary capillary blood volume (VC) (x‐axis) in mild hyperoxia (left panel) and normoxia (right panel) in healthy controls (asterisks) and SSc subjects. Symbols indicate subjects with spirometry and total lung capacity (TLC) > LLN5 (white), DLCO < LLN5 (light grey), and TLC < LLN5 (dark grey). Triangles indicate subjects with systolic pulmonary artery pressure (sPap) > 36 mmHg. The dashed lines indicate the LLN5 (z‐score < −1.645). The absolute values indicate the number of the total 96 test results for DMCO that fall into each quadrant (Q1–Q4).
In 30 subjects with analyzable CT scans, the median extent of fibrosis was 15% (IQR25–75%, 2 to 26%), while ground glass attenuation was 1% (IQR25–75%, 0 to 9%). As the between‐observer agreement of CT scans was very good for both fibrosis (weighted K, 0.92; 95% confidence interval, 0.84 to 0.99) and ground glass (weighted K, 0.82; 95% confidence interval, 0.70 to 0.94), the mean of two readings was retained for analysis. Both FVC and TLC z‐scores correlated significantly with mean lung density and fibrosis extent (Fig. 3A and B) but were above the LLN5 not only in subjects with < 5% fibrosis, but also in a consistent number (11 and 8, respectively) of those with ≥ 5% fibrosis. DLCO and DLNO also correlated significantly with mean lung density and fibrosis extent (Fig. 4A and B). However, DLNO but not DLCO was below the LLN5 in all subjects with ≥ 5% fibrosis. Moreover, DLNO was below the LLN5 in four out of nine subjects with ≤ 5% fibrosis. There were no significant correlations between ground glass extent and any measure of lung function.
Figure 3.
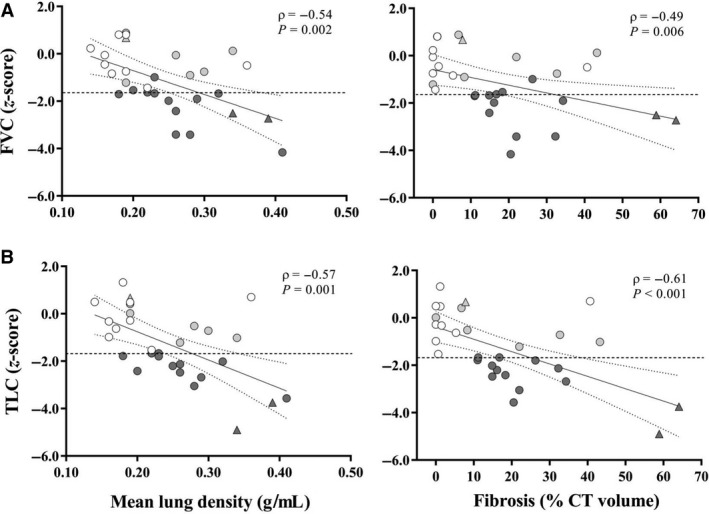
Correlations between mean lung density (g·mL−1) or fibrosis extent (% CT volume) and z‐scores of (A) forced vital capacity (FVC) and (B) TLC. Symbols indicate SSc subjects with spirometry and TLC > LLN5 (white), DLCO < LLN5 (light grey), and TLC < LLN5 (dark grey) with (n = 11) or without (n = 3) DLCO < LLN5. Triangles indicate subjects with sPap > 36 mmHg. CI95% of the best‐fit regression line is marked by dotted lines whereas horizontal dashed line indicates the 5th percentile of reference values (−1.645 z‐score).
Figure 4.
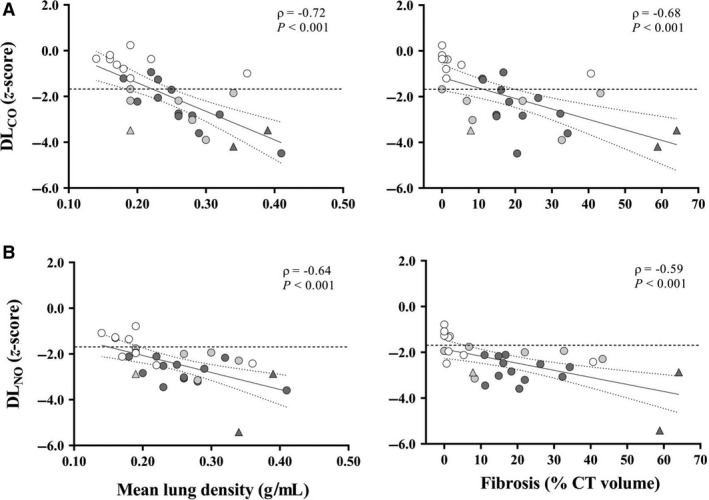
Correlations between mean lung density (g·mL−1) or fibrosis extent (% CT volume) and z‐scores of (A) standard DLCO and (B) DLNO. Symbols indicate SSc subjects with spirometry and TLC > LLN5 (white), DLCO < LLN5 (light grey), and TLC < LLN5 (dark grey) with (n = 11) or without (n = 3) DLCO < LLN5. Triangles indicate subjects with sPap > 36 mmHg. CI95% of the best‐fit regression line is marked by dotted lines whereas horizontal dashed line indicates the 5th percentile of reference values (−1.645 z‐score).
Discussion
The main findings of the present study can be summarized as follows: (1) DLNO was reduced in 56% of subjects with SSc and in 22% of those with normal lung volumes and standard DLCO, (2) standard DLCO was normal in 30% of subjects with reduced DLNO, (3) DMCO and VC partitioning did not provide consistent explanations for individual differences in DLNO and DLCO, and (4) both DLNO and DLCO were significantly correlated with CT measurements of ILD but only the former was consistently reduced in all subjects with fibrosis extent ≥ 5%.
Comments on methodology
To account for the possible bias due to an increased FIO2 (Munkholm et al. 2018) by an automatic flushing procedure with 100% O2 before each test in the commercially available system used in the present study, we determined DMCO and VC using DLCO measured either in combination with DLNO or separately by standard technique (Figs. 5 and 6). These required different breath‐hold times, that is, 4–6 and 9–11 sec, respectively, but this seems to have a negligible effect on final DLCO values (Graham et al. 1985).
Figure 5.
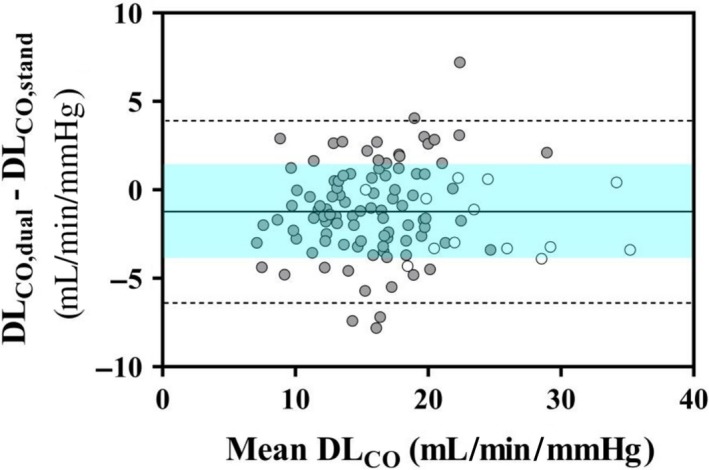
Bland‐Altman plot of the difference between absolute values of lung diffusing capacity for CO measured in mild hyperoxia (DLCO,dual) and normoxia (DLCO,stand) (y‐axis) vs. mean DLCO value (x‐axis) in healthy controls (white circles) and SSc subjects (grey circles). The standard deviation (SD) of mean difference is bounded by the shaded area included between the horizontal dashed lines indicating 95% confidence interval (CI95%). It is noteworthy the scattered fluctuations of data around the mean value and the limits of agreement exceeding CI95% in four cases.
Figure 6.
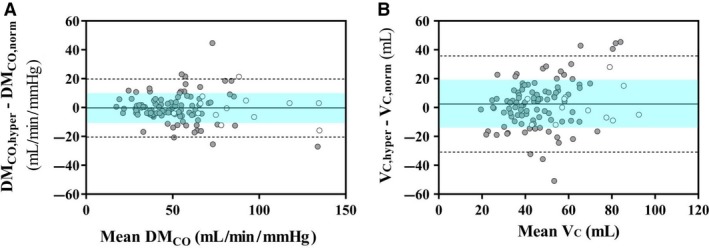
Bland‐Altman plots of the difference between absolute values of alveolar membrane diffusive conductance for CO (DMCO) (left panel) and pulmonary capillary blood volume (VC) (right panel) both derived in mild hyperoxia and normoxia versus their respective mean value (x‐axis) in healthy controls (white circles) and SSc subjects (grey circles). The SD of mean differences is bounded by the shaded areas included between the horizontal dashed lines indicating CI95%. It is noteworthy the scattered fluctuations of data around the mean value of the relevant parameter and the limits of agreement exceeding CI95% in six and seven cases for DMCO and VC, respectively.
In contrast to previous studies (Overbeek et al. 2008; Pernot et al. 2012; Sivova et al. 2013; Guarnieri et al. 2015), lung function data were expressed as z‐scores, instead of absolute or percentage values, to avoid age‐, sex‐, and height‐biases. Although the 2.5th percentile (z‐score of −1.96) has been suggested as a lower limit of normal for DLNO measurement (Zavorsky et al. 2017), we used the 5th percentile (z‐score −1.645) in order to make the results comparable with standard DLCO and avoid false negatives in subjects with established clinical diagnosis (Quanjer et al. 1993). On the other hand, no subject of the control group had DLNO, DMCO, or VC z‐scores below −1.645, thus making the possibility of false positives unlikely.
Study limitations
First, the whole group of our control subjects showed a DLNO mean z‐score of ‐0.69 ± 0.53. However, we used the reference equation (Zavorsky et al. 2017) that provided the lowest SD (0.58) of z‐scores of our local dataset of healthy subjects. Moreover, all individual z‐scores were definitely above the quoted LLN5 (−1.645) for DLNO. Second, only 30 CT scans taken within 3 months from lung function studies were considered to reduce temporal variability and were analyzed at six axial levels. The CT images of 13 subjects were also suited for advanced quantitative analysis using all CT slices, and mean lung density was strongly correlated with that of the six axial levels (r = 0.99; P < 0.001). Thus, it seems reasonable to assume that the latter was representative of the whole lungs. Third, sPap was derived from TRV, which may not be detectable by transthoracic Doppler echocardiograpy in about half of subjects with invasively determined PAH (O'Leary et al. 2018). Although an estimated sPap > 50 mmHg has been arbitrarily recommended by previous guidelines to make PAH likely (Galiè et al. 2009), in a large recent study values ≥ 36 mmHg have been shown to provide a > 90% positive predictive value (Greiner et al. 2014). Nevertheless, PAH cannot be ruled out in subjects with undetected TRV. Fourth, while lung function tests were obtained in a sitting position, CT scans were acquired in supine posture, which might have altered VC (Cotton et al. 1990) and possibly mean lung density. It seems, however, unlikely that changes in body position might have altered the extent of fibrotic changes. Finally, 40 ppm of NO in the inspired gas could decrease hypoxic pulmonary vasoconstriction (Asadi et al. 2015), but this was found for PAO2 values < 60 mmHg (Glenny and Robertson 2011), thus well below the values of 125.4 ± 8.5 and 99.1 ± 3.3 mmHg calculated for the hyperoxic and normoxic conditions of this study, respectively.
Comments on results
The value of DLCO partitioning into DMCO and VC subcomponents was investigated in five previous studies, two using the multistep PAO2 method (Overbeek et al. 2008; Pernot et al. 2012) and three using the DLNO‐DLCO method (Sivova et al. 2013; Guarnieri et al. 2015; Degano et al. 2017). Altogether, these studies showed that DMCO could be reduced in subjects with ILD, irrespective of the presence of PAH (Overbeek et al. 2008; Pernot et al. 2012; Sivova et al. 2013; Guarnieri et al. 2015; Degano et al. 2017), but also in subjects with isolated PAH without ILD (Guarnieri et al. 2015). By contrast, VC was reduced in subjects with PAH without ILD (Guarnieri et al. 2015; Degano et al. 2017) but highly variable in subjects with ILD, whether associated with PAH (Overbeek et al. 2008; Pernot et al. 2012; Sivova et al. 2013; Guarnieri et al. 2015; Degano et al. 2017), or not (Overbeek et al. 2008; Pernot et al. 2012; Guarnieri et al. 2015; Degano et al. 2017). Although it has been suggested that DLNO may be more sensitive than standard DLCO in subjects without ILD or PAH (Guarnieri et al. 2015), none of the above studies reported direct comparisons between these two measures in individual subjects.
Consistent with previous investigations (Abramson et al. 1991; Steen et al. 1992; Jacobsen et al. 1997; Guarnieri et al. 2015; Degano et al. 2017), a substantial number of SSc subjects of the present study had standard lung function measurements, including DLCO, within their respective normal ranges, that is, the 90% confidence interval. New findings of this study are that DLNO was reduced not only in all subjects with restriction, but also in 95% of those with reduced DLCO but no restriction, and about 22% of those with otherwise normal lung function. Consistent with these findings is the reduction in DLNO also observed in subjects with minimal or no fibrosis on CT scan. By converse, DLCO was normal in 30% of subjects with reduced DLNO, including individuals with lung restriction or likely PAH. In keeping with this finding is the normal DLCO also observed in some subjects with 10–40% fibrosis on CT scan. As 70–80% of the total resistance to CO uptake is deemed to be in the blood red cells (Guénard et al. 1987; Borland and Higenbottam 1989), a reduction in DLCO without lung restriction has been considered as a sign of PAH (Wilson et al. 1964; Steen et al. 1992). Moreover, as the red cell resistance to NO uptake is only 40% of total (Borland et al. 2014), DLCO should be expected to be reduced in subjects without restriction or ILD more than DLNO and this difference be associated with VC rather than DMCO decrement. Neither of these predictions is supported by the present findings.
A possible reason for DLNO being more sensitive than DLCO even in the absence of restriction or fibrosis may be that DMCO is not simply related to membrane thickness and alveolar surface but to total gas exchange area, including vessel and red cell surfaces, whereas VC is related to pulmonary blood volume only (Kang and Sapoval 2016). Disproportionate reductions in gas exchange area may be the consequence of geometric heterogeneity of vascular bed, due to capillary remodeling or obliteration with blood volume being redistributed to unaffected lung regions, thus reducing DMCO but not total VC (Pande et al. 1975; Oppenheimer et al. 2006). DMCO impairment might be also the consequence of uneven blood red cell distribution within the alveolar capillaries with enhanced erythrocyte clustering and deformation (Hsia et al. 1997) due to increased plasma viscosity (Spengler et al. 2004), thus reducing their surface but not volume (Oppenheimer et al. 2006). These mechanisms are in keeping with previous data from Farha et al. (2013) showing that decreased gas transfer in idiopathic PAH may be due to loss of either DMCO or VC and may explain the variability in results of the present study in subjects with sPap > 36 mmHg. Another reason for decreased DMCO without VC changes could be a thickening of alveolar‐to‐capillary membrane by the presence of interstitial edema. However, there was a strict correlation between mean lung density and % fibrosis extent (r = 0.80; P < 0.001, data not shown) and there was no significant ground glass attenuation in most of subjects, which makes this mechanism unlikely. On the other hand, owing to the strict dependence of DLCO on specific blood θ CO (Roughton and Forster 1957), a preserved VC may attenuate the effects of alveolar‐to‐capillary membrane thickening by expanding the surface area available for gas exchange, which may explain the preserved DLCO in subjects with lung restriction or fibrosis.
Conclusions
Collectively, the results of the present study suggest that the measurement of DLNO may be of clinical value in the diagnostic workup of SSc, because it is more sensitive than DLCO, either in the presence or absence of lung restriction or fibrosis. However, DMCO and VC partitioning does not seem to be useful to tell whether different results of DLNO and DLCO are primarily due to vascular or interstitial lung disease in individual subjects. Finally, decreased DLCO in the absence of lung restriction does not allow to suspect PAH without ILD.
Conflict of Interest
G.B., A.G., M.B. and M.P. have no financial/nonfinancial interests to disclose; M.O. received personal fees from Imbio LLC for consultancies and a grant for Ph.D. course from Menarini Foundation; V.B. received personal fees and nonfinancial support for consultancy, given lecture, and travel reimbursement from ndd Medizintechnik.
Barisione G., Garlaschi A., Occhipinti M., Baroffio M., Pistolesi M., Brusasco V.. Value of lung diffusing capacity for nitric oxide in systemic sclerosis. Physiol Rep, 7 (13), 2019, e14149, 10.14814/phy2.14149
Funding Information
None declared.
Trial registry: ClinicalTrials.gov PRS: No.: NCT03601520 Unique Protocol ID: IRCCS‐SSc‐2018.
References
- Aaron, S. D. , Dales R. E., and Cardinal P.. 1999. How accurate is spirometry at predicting restrictive impairment. Chest 115:869–873. [DOI] [PubMed] [Google Scholar]
- Abramson, M. J. , Barnett A. J., Littlejohn G. O., Smith M. M., and Hall S.. 1991. Lung function abnormalities and decline of spirometry in scleroderma: an overrated danger? Postgrad. Med. J. 67:632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi, A. K. , Sá R. C., Kim N. H., Theilmann R. J., Hopkins S. R., Buxton R. B., et al. 2015. Inhaled nitric oxide alters the distribution of blood flow in the healthy human lung, suggesting active hypoxic pulmonary vasoconstriction in normoxia. J. Appl. Physiol. 118:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano, Y. , and Sato S.. 2015. Vasculopathy in scleroderma. Semin. Immunopathol. 37:489–500. [DOI] [PubMed] [Google Scholar]
- Baldwin Ede, F. , Cournand A., and Richards D. W. Jr. 1949. Pulmonary insufficiency; a study of 39 cases of pulmonary fibrosis. Medicine (Baltimore) 28:1–25. [PubMed] [Google Scholar]
- Barisione, G. , Bacigalupo A., Brusasco C., Scanarotti C., Penco S., Bassi A. M., et al. 2014. Mechanisms for reduced pulmonary diffusing capacity in haematopoietic stem cell transplantation recipients. Respir. Physiol. Neurobiol. 194:54–61. [DOI] [PubMed] [Google Scholar]
- Barisione, G. , Brusasco C., Garlaschi A., Baroffio M., and Brusasco V.. 2016. Lung diffusing capacity for nitric oxide as a marker of fibrotic changes in idiopathic interstitial pneumonias. J. Appl. Physiol. 120:1029–1038. [DOI] [PubMed] [Google Scholar]
- Bohr, C. 1909. Über die spezifische Tätigkeit der Lungen bei der respiratorischen Gasaufnahme und ihr Verhalten zu der durch die Alveolarwand stattfindenden Gasdiffusion. Skandinavisches Archiv. für Physiologie 22:221–280. [Google Scholar]
- Borland, C. D. , and Cox Y.. 1991. Effect of varying alveolar oxygen partial pressure on diffusing capacity for nitric oxide and carbon monoxide, membrane diffusing capacity and lung capillary blood volume. Clin. Sci. 81:759–765. [DOI] [PubMed] [Google Scholar]
- Borland, C. D. , and Higenbottam T. W.. 1989. A simultaneous single breath measurement of pulmonary diffusing capacity with nitric oxide and carbon monoxide. Eur. Respir. J. 2:56–63. [PubMed] [Google Scholar]
- Borland, C. , Bottrill F., Jones A., Sparkes C., and Vuylsteke A.. 2014. The significant blood resistance to lung nitric oxide transfer lies within the red cell. J. Appl. Physiol. 116:32–41. [DOI] [PubMed] [Google Scholar]
- Carlsen, E. , and Comroe J. H. Jr. 1958. The rate of uptake of carbon monoxide and of nitric oxide by normal human erythrocytes and experimentally produced spherocytes. J. Gen. Physiol. 42:83–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombi, D. , Dinkel J., Weinheimer O., Obermayer B., Buzan T., Nabers D., et al. 2015. Visual vs fully automatic histogram‐based assessment of idiopathic pulmonary fibrosis (IPF) progression using sequential multidetector computed tomography (MDCT). PLoSOne 10:e0130653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotes, J. E. , Dabbs J. M., Elwood P. C., Hall A. M., McDonald A., and Saunders M. J.. 1972. Iron‐deficiency anaemia: its effect on transfer factor for the lung (diffusing capacity) and ventilation and cardiac frequency during sub‐maximal exercise. Clin. Sci. 42:325–335. [DOI] [PubMed] [Google Scholar]
- Cotton, D. J. , Graham B. L., and Mink J. T.. 1990. Pulmonary diffusing capacity in adult cystic fibrosis: reduced positional changes are partially reversed by hyperoxia. Clin. Invest. Med. 13:82–91. [PubMed] [Google Scholar]
- Crapo, R. O. , Kanner R. E., Jensen R. L., and Elliott C. G.. 1988. Variability of the single‐breath carbon monoxide transfer factor as a function of inspired oxygen pressure. Eur. Respir. J. 1:573–574. [PubMed] [Google Scholar]
- Degano, B. , Soumagne T., Delaye T., Berger P., Perez T., Guillien A., et al. 2017. Combined measurement of carbon monoxide and nitric oxide lung transfer does not improve the identification of pulmonary hypertension in systemic sclerosis. Eur. Respir. J. 50:1701008 10.1183/13993003.01008-2017. [DOI] [PubMed] [Google Scholar]
- Farha, S. , Laskowski D., George D., Park M. M., Tang W. H., Dweik R. A., et al. 2013. Loss of alveolar membrane diffusing capacity and pulmonary capillary blood volume in pulmonary arterial hypertension. Respir. Res. 14:6 10.1186/1465-9921-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster, R. E . 1987. Diffusion of gases across the alveolar membrane. In: Handbook of Physiology. The Respiratory System. Gas exchange. Bethesda, MD: Am. Physiol. Soc., 3, 71‐88.
- Galiè, N. , Hoeper M. M., Humbert M., Torbicki A., Vachiery J. L., Barbera J. A., et al. 2009. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 30:2493–2537. [DOI] [PubMed] [Google Scholar]
- Gattinoni, L. , Chiumello D., and Cressoni M.. 2005. Pulmonary computed tomography and adult respiratory distress syndrome. Swiss Med. Wkly 35:169–174. [DOI] [PubMed] [Google Scholar]
- Gibson, Q. H. , and Roughton F. J.. 1957. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J. Physiol. 136:507–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny, R. W. , and Robertson H. T.. 2011. Spatial distribution of ventilation and perfusion: mechanisms and regulation. Compr. Physiol. 1:375–395. [DOI] [PubMed] [Google Scholar]
- Graham, B. L. , Mink J. T., and Cotton D. J.. 1985. Effect of breath‐hold time on DLCO(SB) in patients with airway obstruction. J. Appl. Physiol. 58:1319–1325. [DOI] [PubMed] [Google Scholar]
- Graham, B. L. , Mink J. T., and Cotton D. J.. 2002. Effects of increasing carboxyhemoglobin on the single breath carbon monoxide diffusing capacity. Am. J. Respir. Crit. Care Med. 165:1504–1510. [DOI] [PubMed] [Google Scholar]
- Greiner, S. , Jud A., Aurich M., Hess A., Hilbel T., Hardt S., et al. 2014. Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J. Am. Heart Assoc. 3:e001103 10.1161/jaha.114.001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri, G. , Zanatta E., Mason P., Scarpa M. C., Pigatto E., Maestrelli P., et al. 2015. Determinants of impairment in lung diffusing capacity in patients with systemic sclerosis. Clin. Exp. Rheumatol. 33(Suppl 91):S80–S86. [PubMed] [Google Scholar]
- Guénard, H. , Varène N., and Vaida P.. 1987. Determination of lung capillary blood volume and membrane diffusing capacity by measurement of NO and CO transfer. Respir. Physiol. 70:113–120. [DOI] [PubMed] [Google Scholar]
- Guénard, H. J. , Martinot J.‐B., Martin S., Maury B., Lalande S., and Kays C.. 2016. In vivo estimates of NO and CO conductance for haemoglobin and for lung transfer in humans. Respir. Physiol. Neurobiol. 228:1–8. [DOI] [PubMed] [Google Scholar]
- Hatle, L. , Angelsen B. A., and Tromsdal A.. 1981. Non‐invasive estimation of pulmonary artery systolic pressure with Doppler ultrasound. Br. Heart. J. 45:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen, F. , Khanna D., Fransen J., Johnson S. R., Baron M., Tyndall A., et al. 2013. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 65:2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia, C. C. , Chuong C. J., and Johnson R. L. Jr. 1997. Red cell distortion and conceptual basis of diffusing capacity estimates: finite element analysis. J. Appl. Physiol. 83:1397–1404. [DOI] [PubMed] [Google Scholar]
- Hsia, C. C. , Wagner P. D., Dane D. M., Wagner H. E., and Johnson R. L. Jr. 2008. Predicting diffusive alveolar oxygen transfer from carbon monoxide‐diffusing capacity in exercising foxhounds. J. Appl. Physiol. 105:1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, S. , Halberg P., Ullman S., Høier‐Madsen M., Petersen J., Mortensen J. A., et al. 1997. A longitudinal study of pulmonary function in Danish patients with systemic sclerosis. Clin. Rheumatol. 16:384–390. [DOI] [PubMed] [Google Scholar]
- Jones, R. S. , and Meade F.. 1961. A theoretical and experimental analysis of anomalies in the estimation of pulmonary diffusing capacity by the single breath method. Q. J. Exp. Physiol. Cogn. Med. Sci. 46:131–143. [DOI] [PubMed] [Google Scholar]
- Kang, M. Y. , and Sapoval B.. 2016. Time‐based understanding of DLCO and DLNO. Respir. Physiol. Neurobiol. 225:48–59. [DOI] [PubMed] [Google Scholar]
- Khanna, D. , Gladue H., Channick R., Chung L., Distler O., Furst D. E., et al. 2013. Recommendations for screening and detection of connective tissue disease‐associated pulmonary arterial hypertension. Arthritis Rheum. 65:3194–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee, I. , Zanen P., Biesma D. H., and van den Bosch J. M.. 2005. The effect of red cell transfusion on nitric oxide diffusing capacity. Respiration 72:512–516. [DOI] [PubMed] [Google Scholar]
- Macintyre, N. , Crapo R. O., Viegi G., Johnson D. C., van der Grinten C. P., Brusasco V., et al. 2005. Standardisation of the single breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 26:720–735. [DOI] [PubMed] [Google Scholar]
- Miller, M. R. , Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., et al. 2005. Standardisation of spirometry. Eur. Respir. J. 26:319–338. [DOI] [PubMed] [Google Scholar]
- Mukerjee, D. , St George D., Knight C., Davar J., Wells A. U., Du Bois R. M., et al. 2004. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford) 43:461–466. [DOI] [PubMed] [Google Scholar]
- Munkholm, M. , Marott J. L., Bjerre‐Kristensen L., Madsen F., Pedersen O. F., Lange P., et al. 2018. Reference equations for pulmonary diffusing capacity of carbon monoxide and nitric oxide in adult Caucasians. Eur. Respir. J. 19:1500677 10.1183/13993003.00677-2015 [DOI] [PubMed] [Google Scholar]
- O'Leary, J. M. , Assad T. R., Xu M., Farber‐Eger E., Wells Q. S., Hemnes A. R., et al. 2018. Lack of a tricuspid regurgitation Doppler signal and pulmonary hypertension by invasive measurement. J. Am. Heart Assoc. 7:e009362 10.1161/jaha.118.009362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer, B. W. , Berger K. I., Hadjiangelis N. P., Norman R. G., Rapoport D. M., and Goldring R. M.. 2006. Membrane diffusion in diseases of the pulmonary vasculature. Respir. Med. 100:1247–1253. [DOI] [PubMed] [Google Scholar]
- Overbeek, M. J. , Groepenhoff H., Voskuyl A. E., Smit E. F., Peeters J. W., Vonk‐Noordegraaf A., et al. 2008. Membrane diffusion‐ and capillary blood volume measurements are not useful as screening tools for pulmonary arterial hypertension in systemic sclerosis: a case control study. Respir. Res. 9:68 10.1186/1465-9921-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande, J. N. , Gupta S. P., and Guleria J. S.. 1975. Clinical significance of the measurement of membrane diffusing capacity and pulmonary capillary blood volume. Respiration 32:317–324. [DOI] [PubMed] [Google Scholar]
- Pellegrino, R. , Viegi G., Brusasco V., Crapo R. O., Burgos F., Casaburi R., et al. 2005. Interpretative strategies for lung function tests. Eur. Respir. J. 26:948–968. [DOI] [PubMed] [Google Scholar]
- Pernot, J. , Puzenat E., Magy‐Bertrand N., Manzoni P., Gondouin A., Bourdin H., et al. 2012. Detection of interstitial lung disease in systemic sclerosis through partitioning of lung transfer for carbon monoxide. Respiration 84:461–468. [DOI] [PubMed] [Google Scholar]
- Phansalkar, A. R. , Hanson C. M., Shakir A. R., Johnson R. L. Jr, and Hsia C. C.. 2004. Nitric oxide diffusing capacity and alveolar microvascular recruitment in sarcoidosis. Am. J. Respir. Crit. Care Med. 169:1034–1040. [DOI] [PubMed] [Google Scholar]
- Quanjer, P. H. , Tammeling G. J., Cotes J. E., Pedersen O. F., Peslin R., and Yernault J. C.. 1993. Lung volumes and forced ventilatory flows. Report Working Party, Standardization of Lung Function Tests, European Community for Steel and Coal and European Respiratory Society. Eur. Respir. J. 6(Suppl 16):5–40. [PubMed] [Google Scholar]
- Quanjer, P. H. , Stanojevic S., Cole T. J., Baur X., Hall G. L., Culver B. H., et al. 2012. Multi‐ethnic reference values for spirometry for the 3‐95‐yr age range: the global lung function 2012 equations. Eur. Respir. J. 40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton, F. J. , and Forster R. E.. 1957. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J. Appl. Physiol. 11:290–302. [DOI] [PubMed] [Google Scholar]
- Schneider, P. D. , Wise R. A., Hochberg M. C., and Wigley F. M.. 1982. Serial pulmonary function in systemic sclerosis. Am. J. Med. 73:385–394. [DOI] [PubMed] [Google Scholar]
- Schoenfeld, S. R. , and Castelino F. V.. 2015. Interstitial lung disease in scleroderma. Rheum. Dis. Clin. North Am. 41:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivova, N. , Launay D., Wémeau‐Stervinou L., De Groote P., Remy‐Jardin M., Denis G., et al. 2013. Relevance of partitioning DLCO to detect pulmonary hypertension in systemic sclerosis. PLoS ONE 8:e78001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler, M. I. , Svetaz M. J., Leroux M. B., Leiva M. L., and Bottai H. M.. 2004. Association between capillaroscopy, haemorheological variables and plasma proteins in patients bearing Raynaud's phenomenon. Clin. Hemorheol. Microcirc. 30:17–24. [PubMed] [Google Scholar]
- Stanojevic, S. , Graham B. L., Cooper B. G., Thompson B. R., Carter K. W., Francis R. W., et al. 2017. Official ERS technical standards: global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur. Respir. J. 50:1700010 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- Steen, V. D. , Graham G., Conte C., Owens G., and Medsger T. A. Jr. 1992. Isolated diffusing capacity reduction in systemic sclerosis. Arth. Rheumat. 35:765–770. [DOI] [PubMed] [Google Scholar]
- Wanger, J. , Clausen J. L., Coates A., Pedersen O. F., Brusasco V., Burgos F., et al. 2005. Standardisation of the measurement of lung volumes. Eur. Respir. J. 26:511–522. [DOI] [PubMed] [Google Scholar]
- Wilhelm, E. , Battino R., and Wilcock R. J.. 1977. Low‐pressure solubility of gases in liquid water. Chem. Rev. 77:219–262. [Google Scholar]
- Wilson, R. J. , Rodnan G. P., and Robin E. D.. 1964. An early pulmonary physiologic abnormality in progressive systemic sclerosis (diffuse scleroderma). Am. J. Med. 36:361–369. [DOI] [PubMed] [Google Scholar]
- Yushkevich, P. A. , Piven J., Hazlett H. C., Smith R. G., Ho S., Gee J. C., et al. 2006. User‐guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31:1116–1128. [DOI] [PubMed] [Google Scholar]
- Zavorsky, G. S. , and Murias J. M.. 2006. A small amount of inhaled nitric oxide does not increase lung diffusing capacity. Eur. Respir. J. 27:1251–1257. [DOI] [PubMed] [Google Scholar]
- Zavorsky, G. S. , Hsia C. C., Hughes J. M., Borland C. D., Guénard H., van der Lee I., et al. 2017. Standardisation and application of the single‐breath determination of nitric oxide uptake in the lung. Eur. Respir. J. 49:1600962 10.1183/13993003.00962-2016 [DOI] [PubMed] [Google Scholar]


