Abstract
Previously, we succeeded in transplanting autologous nasal mucosal cell sheets in the middle ears of 5 patients, who underwent cholesteatoma resection, which prevents recurrence of cholesteatoma in clinical settings. Current good manufacturing practice (GMP) standards for human cell cultivation requires the establishment of cell processing centers (CPC) which act as germ-free facilities. However, due to practical difficulties involved in establishing and maintaining such facilities at each individual hospital, a functional transport system is felt to be needed for the continuation of effective regenerative therapy. In the current study, nasal mucosal tissue and autologous blood obtained from 3 human volunteers were transported for over 3 h. Disinfected nasal tissues were cultured using keratinocyte culture medium, which included autologous serum prepared from blood. After 24 d, cultured nasal mucosal cells were transported for over 3 h and subsequently assessed for cell number, viability and purity. Moreover, CK4, CK8, and CK18 were analyzed the suitability of these nasal mucosal cell sheets for middle ear regenerative therapy.
Overall, we confirmed that nasal mucosal cell sheets can be fabricated using transported nasal mucosal tissue and blood. This study would be contribute to establish a new regenerative therapy for clinical application, accompanied with transportation between companies and hospitals.
Keywords: Nasal mucosal cell sheet, Cell processing center (CPC), Transportation, Cytokeratin expression
Highlights
-
•
Transportation of nasal mucosal tissue and blood.
-
•
Characterizing of nasal mucosal cell sheet by immunohistology.
-
•
Transportation of nasal mucosal cell sheet as pre-clinical study.
1. Introduction
Cultured autologous mucosal cell sheets fabricated on temperature-responsive cell culture surfaces have been successfully used to treat body tissue injury [1], [2], [3]. Usually, cell sheet sources are selected from tissues similar to the host tissue. For example, while oral mucosal cells are selected as a cell source for esophageal tissue regeneration [4], skeletal muscle cells are selected for heart regeneration [5].
In otolaryngology, middle ear mucosal regeneration is important for post-operative outcomes following cholesteatoma or adhesive otitis media related surgery. Middle ear mucosa comprises simple epithelial cells, stratified columnar cells, ciliated cells, and secretory cells [6], which regulate gas exchange and middle ear aeration in addition to preventing recurrence of severe otitis media. As noted above, oral mucosal cell sheet has been proceeded by other group, however, stratified squamous epithelial cells is different from a classification point of view. Therefore, as otolaryngologists, we selected nasal cells, classified as respiratory epithelial tissue and pseudostratified ciliated epithelial tissue [7], as a cell source for middle ear mucosal regenerative therapy. We successfully transplanted these in the middle ears of 5 patients following tympanoplasty, whereby middle ear aeration and hearing acuity were improved [8].
These cell sheets were cultured in a sterile room, termed the cell-processing center (CPC), which met current good manufacturing practice (GMP) standards. Although a CPC is needed for cultivation of human cells, various factors associated with management and costs prevent the establishment of such CPCs at all hospitals. Therefore, development of a protocol for transportation of human tissue, blood, and cell sheets between hospitals and CPCs was felt to be important. Previous studies have indicated the possibility of fabricating cell sheets using oral mucosal tissue and autologous serum, transported approximately 1200 km for over 7 h by airplane [4], [9]. In such cases, both hospitals are equipped with CPCs and serum is prepared prior to transportation [4]. Following the transportation study, Jikei University planned new clinical study with transportation of nasal mucosal cell sheet with St. Marianna University. The distance is approximately 26 km and takes 1 h. For purposes of the current clinical study, we aimed to transport both nasal mucosal tissue and human blood specimens. Therefore, in this study, blood and nasal mucosal tissue samples of volunteers were transported for 3 h, following which serum was prepared and the nasal mucosal epithelial cells cultivated until cell sheet stage. Subsequently, we evaluated the quality of these nasal mucosal epithelial cell sheets based on properties such as cell number, viability, and purity, both before and after transportation. Moreover, in order to reemphasize the objectives of this study, we analyzed cytokeratin expression in the nasal mucosal and oral mucosal cell sheets, compared to that in middle ear mucosal tissue. We expect to expand our research by performing clinical trials involving transported tissue and blood as our next project.
2. Methods
2.1. Preparation of human serum from transported blood samples
This study was approved by the Institutional Review Board of Jikei University, Tokyo, Japan. Informed consent was obtained from all volunteers. Moreover, infections of human immunodeficiency virus, syphilis, and hepatitis B and C virus in the patients should be confirmed for all volunteers.
Human serum was prepared from blood and used for keratinocyte culture medium (KCM). 90 mL of blood was collected slowly using a 21-gauge needle, and added to a 50-mL centrifuge tube. Blood was transported for over 3 h, on foot or by car, to a CPC. A blood clot was produced by incubating blood at 37 °C for 30 min. Blood was centrifuged at 1830 g for 10 min at room temperature, and serum was collected as the supernatant. Centrifugation and collection of serum were performed twice. Serum was sterilized using 0.2-μm pore polyether sulfone filter.
2.2. Preparation of keratinocyte culture medium (KCM)
KCM was prepared according to previously described methods [10]. Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher, MA, USA) and DMEM with Ham's F-12 medium (Gibco) were mixed at a 1:1 ratio. The medium was supplemented with 5% autologous human serum, 0.3 μM saxizon (Takeda yakuhin kogyo, Osaka, Japan), 140.0 mU/mL humulin (Novo Nordisk, Bagsvaerd, Denmark), 2.0 nM triiodothyronine (MP Biomedicals, CA, USA), 0.2 μM epidermal growth factor (Higeta-Shoyu, Tokyo, Japan), 1.0 nM cholera toxin (Wako Pure Chemicals, Osaka, Japan), 100 U/mL penicillin, 69 μM streptomycin (Wako Pure Chemicals), and 0.4 μg/mL of amphotericin B (Bristol-Myers Squibb, NY, USA).
2.3. Fabrication of the nasal mucosal cell sheet
Nasal mucosal tissue was collected from inferior nasal turbinate mucosa via nasal endoscopy. Harvested nasal mucosa was disinfected using povidone-iodine, and then transferred into 30 mL of DMEM with 100 U/mL of penicillin, 69 μM streptomycin, and 1.0 μM of amphotericin B. The tissue was then transported in an ice box for over 3 h on foot or by car. Primary culture was started at CPC following transportation.
Nasal mucosal tissue was sterilized twice using povidone-iodine and washed using phosphate-buffered saline (PBS); (Wako Pure Chemicals). Then, mucosal tissue was cut into 1-mm cubes. A type I collagen-coated 60-mm culture dish (BD BioCoat, Franklin Lakes, NJ, USA) was completely covered by 750 μL of the KCM. Next, 4 mucosal tissue cubes were placed on the dish, and incubated at 37 °C in a humidified atmosphere containing 5% CO2. One day following incubation 1 mL of KCM was added gently to the dish and 1 h later another 2 mL were added. The medium was replaced several times during 2 weeks of primary culture. Following 2 weeks of cultivation, the cells were collected via trypsin digestion, and seeded on a 6-well temperature-responsive cell culture insert (CellSeed, Tokyo, Japan) at a density of 2.0 × 105 cells/cm2. The cell sheet was detached after 10 d by reducing the temperature to 20 °C for 30 min. A schematic of these procedures is shown in Fig. 1.
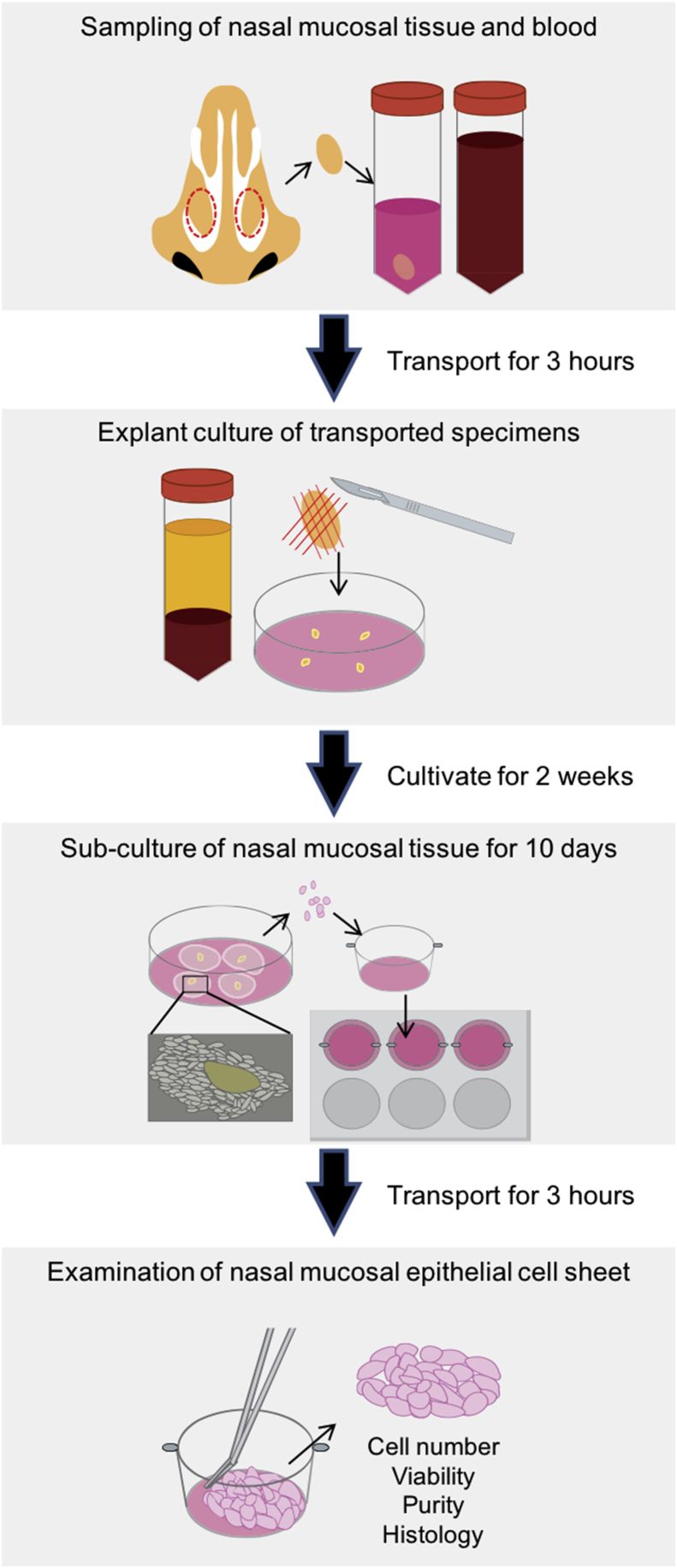
Fig. 1.
Schematic diagram of the outline of this study. Human blood and nasal mucosal tissue were maintained for 3 h at room temperature and 4 °C, respectively. Subsequently, serum was prepared and explant cultures were initiated. After 24 d, cultured cells were transported again for 3 h at 37 °C. Finally, the transported cell sheets we analyzed for quality and compared with non-transported cell sheets.
Moreover, for the purpose of comparison, oral mucosal cell sheets were fabricated in our laboratory using 5% fetal bovine serum according to methods described in our previous article [11].
2.4. Analysis of the fabricated cell sheet and conditioned medium
The cell sheets were subjected to various quality tests, such as sterility tests, mycoplasma tests, and Limulus colorimetric tests. These were performed in conditioned sub-cultivation media. Criteria for the conditioned medium were; < 1.0 EU/mL of endotoxin, sterility, absence of mycoplasma by loop-mediated isothermal amplification (LAMP), and cultivation according to previously described methods [12]. Moreover, fabricated cell sheets were evaluated for cell number, viability, and cell purity. Cell number and viability of one whole cell sheet were analyzed using trypan blue assay (Sigma–Aldrich, MO, USA) with a cytometer. To determine whether cultured cells developed epithelial characteristics, keratin-positive epithelial cell marker rate was analyzed via flow cytometry (MACSQuant® Analyzer, Miltenyi Biotec, Germany). In compliance with previous clinical studies, determinant criteria were selected as; cell count >1.0 × 105 cells, > 70% of viability, and >70% keratin positive cell rate.
2.5. Immunofluorescence analysis of explant cultured cells
Nasal mucosal tissue were cultured on a slide chamber (Thermo Fisher) for 6 days. Explant cultured nasal mucosal cells were fixed using 100% ethanol for 20 min. Following removal of ethanol, the samples were blocked for 1 h using blocking buffer (Nakarai tesque, Kyoto, Japan). Sections were then incubated with pan-cytokeratin (Abcam, Cambridge, UK) and Ki67 (Dako, CA, USA) antibodies diluted in PBS at 4 °C overnight. Following primary antibody reaction, sections were washed with shading. Samples were then incubated with FITC-conjugated secondary antibodies (Invitrogen, CA, USA) and the nuclei stained using 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) at room temperature for 1 h.
2.6. Immunohistochemistry (IHC)
Harvested nasal mucosal cell sheets were fixed using 4% paraformaldehyde (Muto Pure Chemicals, Tokyo, Japan). After dehydration, the samples were embedded in paraffin. Human native nasal mucosal tissue, middle ear mucosal tissue, and oral mucosal tissue were obtained via endoscopic sinus surgery, mastoidectomy and tonsillectomy, respectively. These specimens were also embedded in paraffin. Paraffin embedded samples were cut into 4-μm-thick sections and placed on glass slides. The sections were deparaffinized by xylene and ethanol, and then washed with pure water. The sections were stained with hematoxylin and eosin (HE staining). For immunohistochemistry, deparaffinized sections were heated with citrate (Dako) to uncover antigens. Next, the sections were incubated with a peroxidase blocking solution (Dako) and a protein blocking solution (Nacalai tesque) to prevent non-specific reactions. The sections were then incubated with cytokeratin 4 (Abcam), cytokeratin 8 (Santa Cruz, CA, USA) and cytokeratin 18 (Abcam), diluted in blocking buffer at 4 °C overnight. Following primary antibody reaction, sections were washed thrice with PBS, 5 min each turn. Samples were then allowed to react with secondary antibodies and HRP polymer (Dako) at room temperature for 1 h. The sections were washed again and covered with 3,3′-diaminobenzidine (Dako). Nuclei were stained using hematoxylin.
3. Results
3.1. Explant culturing of transported nasal mucosal cells
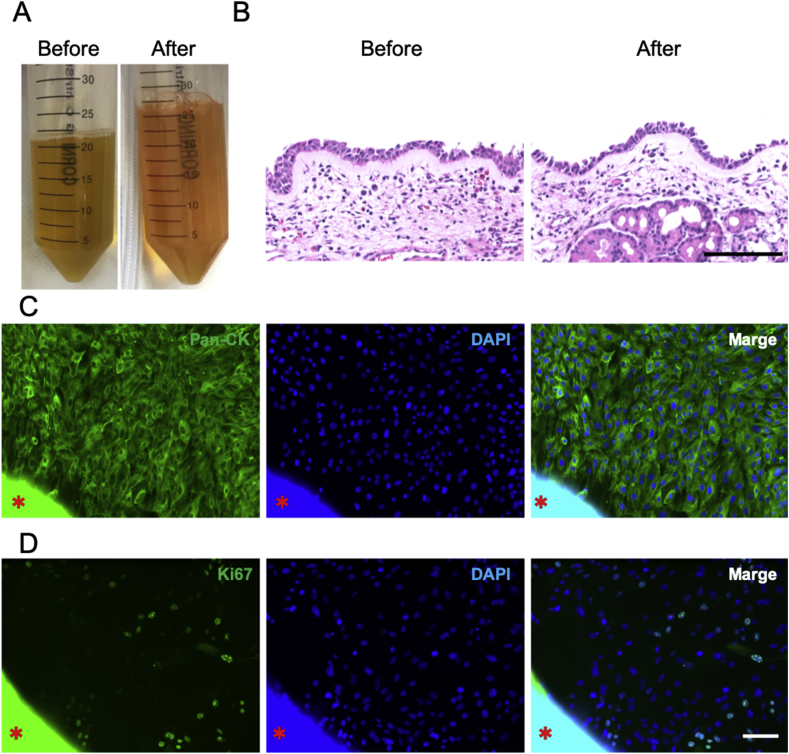
Nasal mucosal tissue and blood samples were collected during the intraoperative period of endoscopic sinus surgery (ESS) under general anesthesia. These specimens were transported for 3 h. After transportation, over 40 mL of human serum was extracted from the blood, both with and without transportation (Fig. 2A). There were no significant differences in color and viscosity changes between sera produced from blood with and without transportation. Moreover, no significant differences were observed in histological findings of nasal mucosal tissue before and after transportation (Fig. 2B). Then, explant culture was initiated with the prepared serum, and collective cellular outgrowth was observed at the periphery of adhered nasal mucosal tissue at 6 d following culture. The epithelial marker, pan-cytokeratin, and the proliferation marker, Ki67, were expressed in outgrowth cells (Fig. 2C and D). This indicated that transported nasal mucosal epithelial cells had proliferated. After 14 d of cultivation, a total of 1.0 × 106 nasal mucosal cells with >95% viability were collected.
Fig. 2.
Explant culture of nasal mucosal tissue using specimens following transportation. (A) Human serum fabricated from blood before and after transportation. (B) HE staining of nasal mucosal tissue before and after transportation. (C, D) Immunostaining results of pan-cytokeratin (C, Green), Ki67 (D, Green), and DAPI (Blue) for transported nasal mucosa 6 d following explant culture. The asterisks show the nasal mucosal tissue. Scale bar, 100 μm.
3.2. Examination of the nasal mucosal epithelial cell sheet
Epithelial cells collected from explant culture were seeded at a density of 2.0 × 105 viable cells/cm2 on a temperature-responsive cell culture insert. We observed that cells continued proliferating in the KCM and reached confluence 3–5 d following sub-cultivation. These cells were of a cuboidal shape and maintained confluence 10 d after sub-cultivation (Fig. 3A). We confirmed that the thermo-box could keep an inner temperature of over 35 °C for more than 3 h, even when the outside temperature was 4 °C (Fig. 3B). The conditioned medium was examined for bacteria, fungi, and M. pneumoniae using LAMP, Limulus colorimetric tests, and culture tests. Limulus colorimetric tests (n = 3) indicated that the endotoxin score was under 0.06 EU/mL. In addition, the medium tested negative for M. pneumoniae according to both LAMP and culture tests. Therefore, the conditioned medium had not been contaminated as a result of the GMP-grade culture method.
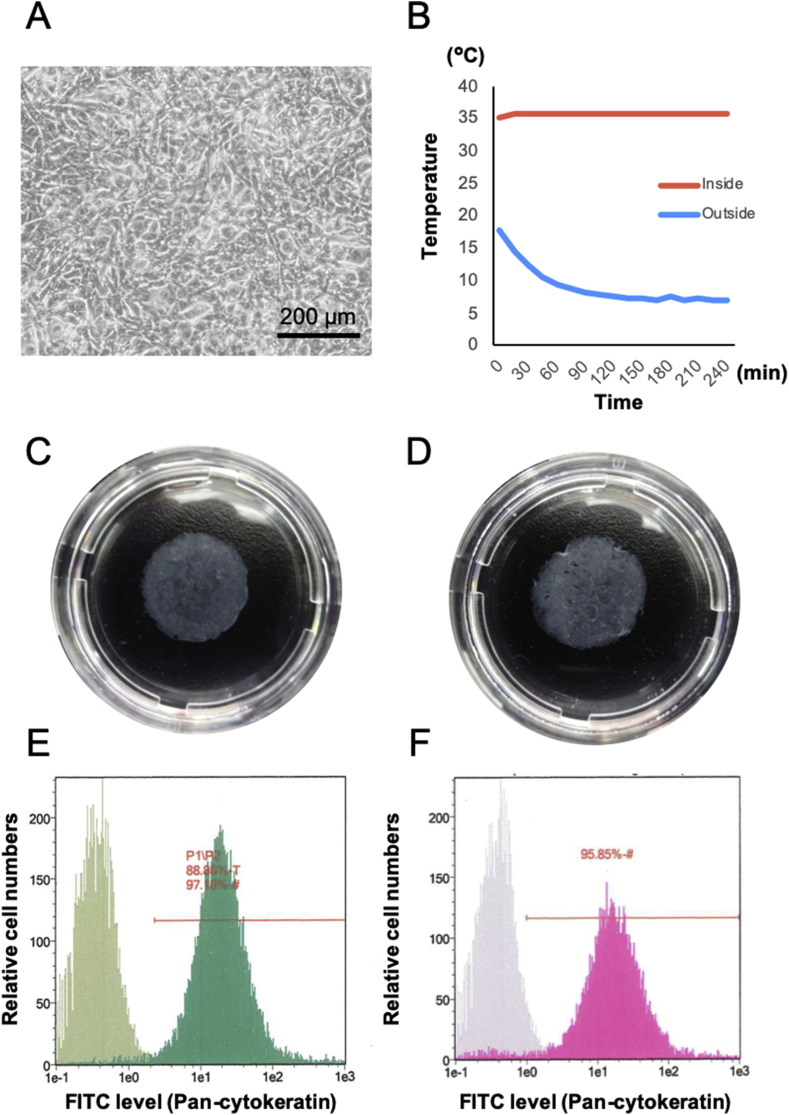
Fig. 3.
Characteristics of the nasal mucosal epithelial cell sheet with or without transportation. (A) Phase-contrast microscopy results of nasal epithelial cells at 10 d of sub-culture. (B) Temperature record of inside (red line) and outside (blue line) the thermo-box temperature during 3 h transportation. (C) Quality of the cell sheet before (C, E) and after (D, F) transportation. (C, D) Photographs of the harvested nasal mucosal cell sheet before and after transportation with 35 mm dish. (E, F) Flowcytometry results of pan-cytokeratin expression for the nasal mucosal epithelial cell sheet before and after transportation.
Confluent cells were detached as a cell sheet from the temperature-responsive surface at 20 °C (Fig. 3C and D). The fabricated cell sheet had over 1.0 × 105 cells with >90% viability. Moreover, flow cytometry result showed a high rate of keratin positive cells (Fig. 3E and F). These scores met the criteria for pre-transplant tests determined in our previous clinical study, which were determined using an oral mucosal epithelial cell sheet. Therefore, it was concluded that we had succeeded in fabricating transplantable human nasal mucosal epithelial cell sheets from transported blood and tissue samples.
3.3. Histological evaluation of the nasal mucosal cell sheet and native tissues
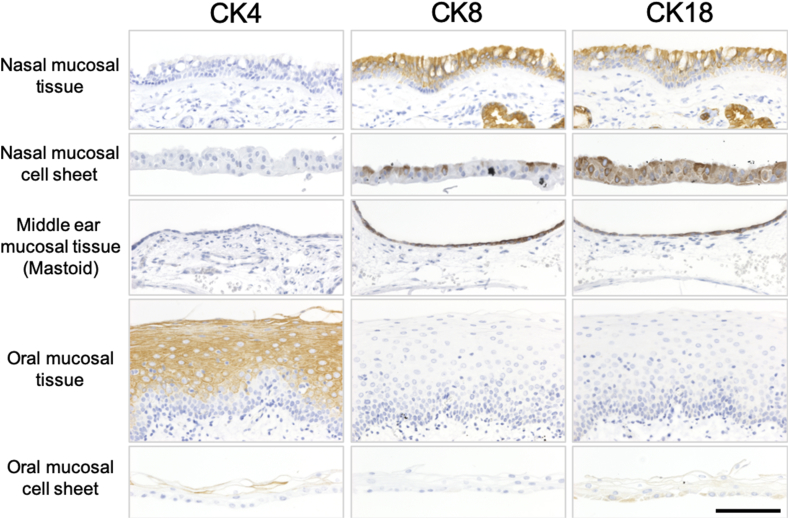
To reconfirm the characteristics of the transported nasal mucosal cell sheet, cytokeratin 4 (CK4), cytokeratin 8 (CK8), and cytokeratin 18 (CK18) were analyzed via immunohistology. The histological images showed similar thicknesses between the nasal mucosal epithelial layer and the middle ear mucosal epithelial layer. On the other hand, oral mucosal epithelial thickness was different from that of other tissues such as stratified squamous epithelial tissue. CK4, known as a differentiated mucosal stratified squamous epithelial marker [13], was hardly detected in the nasal epithelial tissue, the nasal epithelial cell sheet or the middle ear epithelia, but was detected in the oral mucosal epithelia (Fig. 4). CK8 and CK18, known as simple layer epithelial markers [13], were detected in the nasal epithelial tissue, the nasal epithelial cell sheet, and the middle ear epithelial tissue, whereas low levels of staining were detected in oral mucosal epithelial tissue and oral mucosal cell sheet (Fig. 4). These results suggested that the nasal mucosal epithelial cell sheets may be more suitable than oral mucosal cell sheets for middle ear regenerative medicine.
Fig. 4.
Cytokeratin expressions of native tissue and cell sheet. Immunohistological results of CK4 (left column), CK8 (center column), and CK18 (right column) for nasal mucosal tissue, nasal mucosal cell sheet, middle ear mucosal tissue, oral mucosal tissue, and oral mucosal cell sheet. Scale bar, 100 μm.
4. Discussion
Regenerative therapy using cell sheets shows much promise as an upcoming mode of cell therapy. Middle ear cholesteatoma is a middle ear disease which leads to hearing loss, dizziness, facial paralysis, and brain abscesses [14], [15]. Several techniques have been proposed to diagnose cholesteatoma, including keratinized stratified squamous epithelial markers [16]. Although regeneration of middle ear mucosa using cultured grafts is considered to be important, stratified squamous epithelial cells may potentially prevent aeration of the middle ear cavity by producing keratinized cholesteatoma like debris. Transplantation of non-stratified epithelial grafts for mucosal regeneration is considered to be suitable for regenerating thinner middle ear mucosal epithelial layers. Although nasal mucosal cell sheets have been characterized in our previous study [17], we reconfirmed that, in a manner similar to middle ear mucosa, nasal mucosal epithelial cell sheets also expressed CK8 and CK18 (Fig. 4). This is because cytokeratin expression is an important characteristic of epithelial tissues [13], [18]. Nasal mucosa, which is easily harvested by otolaryngologists, is considered to be the only thin epithelial cell source obtainable without resorting to anesthesia and risking post-operative complications [8]. Moreover, it allows both the patient and the hospital to manage a course of treatment within a single department. Reportedly, thin layer grafts support maximum total pressure in the middle ear following surgery [8], [19].
Despite several ongoing studies targeting the development of serum-free culture, serum remains an essential additive [20]. We aimed to develop human serum from transported blood. In order to maintain pH balance in the medium, serum should be prepared without hemolysis. Hemolysis observed in 1 d between 4 °C and 20 °C was minimal [21], and it was hardly observed in serum made from transported blood (Fig. 1A). Further, it functioned well in regard to cell proliferation (Fig. 2D).
Previous studies have indicated that untransported human oral mucosal epithelial cells do not encounter fungal contamination [2], [22]. However, transported conditioned medium was contaminated with Candida albicans [12]. Based on these findings, amphotericin B at a concentration of 1.0 μg/mL was added to prevent any fungal growth in the transported medium, in order to produce cell sheets sans contamination [12]. However, high concentrations of amphotericin B in media can inhibit not only Candida proliferation, but also that of mammalian cells [23]. In general, as growth oriented epithelial stem cells are located at the basal layer of stratified squamous epithelial tissue [24], they may be protected from pharmacological agents such as amphotericin B, by cell−cell adhesion in the upper layers of the oral mucosal epithelia [25]. On the other hand, nasal epithelial tissue is classified as pseudostratified ciliated epithelial tissue, which exhibits less cell−cell adhesion compared to stratified squamous epithelial tissue. Nevertheless, epithelial cells proliferating on the type I collagen dish grew into a nasal mucosal epithelial cell sheet even when a transportation medium with a high concentration of amphotericin B was used (Fig. 2D). Moreover, the cell sheet consisted of numerous cells with high viability and purity, thus satisfying criteria required for transplantation (Fig. 3E and F, Table 1). Therefore, our fabrication of transplantable human nasal mucosal epithelial cell sheets using blood and transported tissue may be considered a success. To be establish as a regenerative medicine product, both nasal tissue and nasal cell sheets should be tested for how long they maintain quality during transport, the optimum transport conditions, potential for tumorigenesis after transportation, and transport conditions at below 0 °C or over 37 °C, in further studies.
Table 1.
Quality check result of human nasal mucosal epithelial cell sheet.
| Transportaion | Cell number | Viability (%) | Keratin positive rate | |
|---|---|---|---|---|
| Donor 1 | Before | 2.5 × 105 cells/sheet | >99.9% | 92.5% |
| After | 1.4 × 105 cells/sheet | 98.6% | 98.2% | |
| Donor 2 | Before | 2.0 × 105 cells/sheet | 98.6% | 96.0% |
| After | 5.0 × 105 cells/sheet | 96.1% | 96.8% | |
| Donor 3 | Before | 5.4 × 105 cells/sheet | 95.6% | 97.2% |
| After | 2.5 × 105 cells/sheet | 95.1% | 95.9% |
In this study, we confirmed the suitability of the nasal mucosal cell sheets for middle ear mucosal regenerative therapy, based on cytokeratin expressions, and succeeded in carrying out pre-clinical study for nasal mucosal cell sheet with transportation.
Disclosure statement
Yamato Masayuki is a stakeholder of CellSeed Inc., and Tokyo Women's Medical University is currently receiving research funding from CellSeed Inc. Yamato Masayuki is also an advisor of Helios and Nippi.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 18K16907, AMED under Grant Number JP18bk0104051, and The Jikei University Strategic Prioritizing Research Fund. The authors thank three volunteer donors for donation of nasal mucosal tissue and blood. Furthermore, we also thank Kanako Taya and Eika Hayashi for their help in fabricating the cell sheet.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 2.Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588. doi: 10.1053/j.gastro.2012.04.050. e2. [DOI] [PubMed] [Google Scholar]
- 3.Iwata T., Yamato M., Washio K., Yoshida T., Tsumanuma Y., Yamada A. Periodontal regeneration with autologous periodontal ligament-derived cell sheets – a safety and efficacy study in ten patients. Regen Ther. 2018;9:38–44. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi N., Isomoto H., Kobayashi S., Kanai N., Kanetaka K., Sakai Y. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7:17460. doi: 10.1038/s41598-017-17663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawa Y., Miyagawa S., Sakaguchi T., Fujita T., Matsuyama A., Saito A. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2011;42:181–184. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 6.Albiin N., Hellstrom S., Stenfors L.E., Cerne A. Middle ear mucosa in rats and humans. Ann Otol Rhinol Laryngol Suppl. 1986;126:2–15. doi: 10.1177/00034894860950s501. [DOI] [PubMed] [Google Scholar]
- 7.Jafek B.W. Ultrastructure of human nasal mucosa. The Laryngoscope. 1983;93:1576–1599. doi: 10.1288/00005537-198312000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto K., Yamato M., Morino T., Sugiyama H., Takagi R., Yaguchi Y. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen Med. 2017;2:6. doi: 10.1038/s41536-017-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oie Y., Nozaki T., Takayanagi H., Hara S., Hayashi R., Takeda S. Development of a cell sheet transportation technique for regenerative medicine. Tissue Eng Part A. 2014;20:373–382. doi: 10.1089/ten.tec.2013.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takagi R., Yamato M., Murakami D., Kondo M., Ohki T., Sasaki R. Fabrication and validation of autologous human oral mucosal epithelial cell sheets to prevent stenosis after esophageal endoscopic submucosal dissection. Pathobiology. 2011;78:311–319. doi: 10.1159/000322575. [DOI] [PubMed] [Google Scholar]
- 11.Morino T., Takagi R., Yamamoto K., Kojima H., Yamato M. Explant culture of oral mucosal epithelial cells for fabricating transplantable epithelial cell sheet. Regen Ther. 2018;10:36–45. doi: 10.1016/j.reth.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takagi R., Kobayashi S., Yamato M., Owaki T., Kasai Y., Hosoi T. How to prevent contamination with Candida albicans during the fabrication of transplantable oral mucosal epithelial cell sheets. Regen Ther. 2015;1:1–4. doi: 10.1016/j.reth.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moll R., Divo M., Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo C.L., Shiao A.S., Yung M., Sakagami M., Sudhoff H., Wang C.H. Updates and knowledge gaps in cholesteatoma research. BioMed Res Int. 2015;2015:854024. doi: 10.1155/2015/854024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad S.C., Shin S.H., Russo A., Di Trapani G., Sanna M. Current trends in the management of the complications of chronic otitis media with cholesteatoma. Curr Opin Otolaryngol Head Neck Surg. 2013;21:446–454. doi: 10.1097/MOO.0b013e3283646467. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto-Fukuda T., Takahashi H., Koji T. Animal models of middle ear cholesteatoma. J Biomed Biotechnol. 2011;2011:394241. doi: 10.1155/2011/394241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hama T., Yamamoto K., Yaguchi Y., Murakami D., Sasaki H., Yamato M. Autologous human nasal epithelial cell sheet using temperature-responsive culture insert for transplantation after middle ear surgery. J Tissue Eng Regenerat Med. 2017;11:1089–1096. doi: 10.1002/term.2012. [DOI] [PubMed] [Google Scholar]
- 18.Kasai Y., Sugiyama H., Takagi R., Kondo M., Owaki T., Namiki H. Brush biopsy of human oral mucosal epithelial cells as a quality control of the cell source for fabrication of transplantable epithelial cell sheets for regenerative medicine. Regen Ther. 2016;4:71–77. doi: 10.1016/j.reth.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto K., Hama T., Yamato M., Uchimizu H., Sugiyama H., Takagi R. The effect of transplantation of nasal mucosal epithelial cell sheets after middle ear surgery in a rabbit model. Biomaterials. 2015;42:87–93. doi: 10.1016/j.biomaterials.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Kondo M., Yamato M., Takagi R., Namiki H., Okano T. Membrane-permeable calpain inhibitors promote rat oral mucosal epithelial cell proliferation by inhibiting IL-1 alpha signaling. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gershfeld N.L., Murayama M. Thermal instability of red blood cell membrane bilayers: temperature dependence of hemolysis. J Membr Biol. 1988;101:67–72. doi: 10.1007/BF01872821. [DOI] [PubMed] [Google Scholar]
- 22.Jonas E., Sjoqvist S., Elbe P., Kanai N., Enger J., Haas S.L. Transplantation of tissue-engineered cell sheets for stricture prevention after endoscopic submucosal dissection of the oesophagus. Unit Eur Gastroenterol J. 2016;4:741–753. doi: 10.1177/2050640616631205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uribe C.C., Dos Santos de Oliveira F., Grossmann B., Kretzmann N.A., Reverbel da Silveira T., Giugliani R. Cytotoxic effect of amphotericin B in a myofibroblast cell line. Toxicol Vitro. 2013;27:2105–2109. doi: 10.1016/j.tiv.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Camidge D.R., Pemberton M.N., Growcott J.W., Johnstone D., Laud P.J., Foster J.R. Assessing proliferation, cell-cycle arrest and apoptotic end points in human buccal punch biopsies for use as pharmacodynamic biomarkers in drug development. Br J Canc. 2005;93:208–215. doi: 10.1038/sj.bjc.6602686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Presland R.B., Dale B.A. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]