Abstract
Background
Acute necrotizing encephalopathy (ANE), known as influenza‐associated encephalitis, typically affects children.
Case presentation
A 70‐year‐old woman was admitted to the hospital with altered consciousness, a high temperature, and severe hypotension. Computed tomography (CT) of the head showed no abnormalities; thus, a diagnosis of suspected severe heat stroke was made. On day 2, repeated head CT revealed bilateral symmetrical lesions to the thalamus, and a rapid influenza antigen test was positive. Based on the CT findings and the medical history of influenza, a differential diagnosis of ANE was made. Subsequently, brain edema spread across the whole brain, and the patient died on day 21.
Conclusion
In elderly patients, differentiating ANE from severe heat stroke in a high‐temperature environment is difficult because of the similarities in clinical symptoms due to multiple organ failure.
Keywords: Bilateral symmetrical thalamic lesions, hyperthermia, influenza‐associated encephalopathy
Introduction
Acute necrotizing encephalopathy (ANE), known as influenza‐associated encephalitis, is characterized by progressive, diffuse swelling of the thalamus that can progress to life‐threatening brain edema. Acute necrotizing encephalopathy can progress to multiple organ failure, causing acute kidney injury or hepatic dysfunction, which accounts for the high mortality rate of 27%.1, 2 This condition typically occurs among children in East Asia but rarely in adults. Early treatment of affected patients is important,3 but it is difficult to distinguish ANE from other severe infectious diseases, Reye syndrome, mitochondrial encephalopathy, acute disseminated encephalomyelitis, hypoxic encephalopathy, Wernicke's encephalopathy, and heat stroke (HS).2
Severe HS can cause altered consciousness and multiple organ failure and is often fatal. In Japan, bath‐related fatal events have repeatedly occurred in elderly people. The major symptoms observed in these events were consciousness disturbance and exhaustion with high body temperature. A previous study reported an incidence of bath‐related events during the winter season of 10.09 cases per 100,000, and HS can cause such accidents.4 Here, we report a case of ANE with deep coma and multiple organ failure showing symptoms similar to severe HS. We also review published works and evaluate the appropriate diagnostic approach regarding the differential diagnosis of adult‐onset ANE.
Case report
In winter, a 70‐year‐old woman had taken a bath alone. When her family checked on her after 1 h, she was lying unconscious in a bathtub filled with hot water. The patient was initially transferred to another hospital. Given her comatose state (Glasgow Coma Scale [GCS] 3), severe hypotension, and multiple organ failure, she was immediately intubated and referred to our hospital for further treatment. On arrival to our hospital, her vital signs were as follows: blood pressure, 135/71 mmHg; heart rate, 105 b.p.m.; respiratory rate, 30 breaths/min; SpO2 100% using 10 L/min O2; and body temperature, 40°C. She had no stiffness in the neck and a normal Babinski reflex. Laboratory data revealed a normal white blood cell count (8,500/μL), hemoglobin, platelet, and electrolyte levels. By contrast, laboratory data showed mild elevation of C‐reactive protein (5.37 mg/dL), hepatic dysfunction (aspartate transaminase, 1,305 U/L; alanine transaminase, 894 U/L), acute kidney injury (blood urea nitrogen, 24.6 mg/dL; creatinine, 1.62 mg/dL), and coagulopathy (prothrombin time – international normalized ratio, 1.14; D‐dimer, 36.2 μg/dL). A computed tomography (CT) scan of the head was normal (Fig. 1A). A whole‐body CT scan showed no abnormalities in the lungs, kidneys, or liver; therefore, infectious disease was not suspected. A rapid influenza antigen test was not carried out. The patient had no history of endocrine disease, and she used no medications internally. Based on all these factors, we suspected this deep coma and multiple organ failure with high fever indicated severe HS by exclusion. We carried out fluid resuscitation with iced saline and cooling until her body temperature reached 39°C, and her consciousness gradually improved to GCS 7T (E2VTM5). The patient was admitted to the intensive care unit and received mechanical ventilation therapy under sedation with propofol (40 mg/h). We kept her body temperature at 36°C using adhesive surface cooling pads (Arctic Sun 5000; Bard Medical, Covington, GA, USA).
Figure 1.
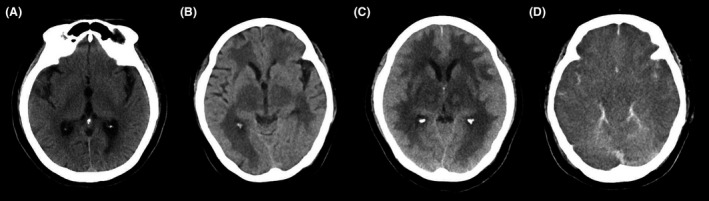
Computed tomography (CT) of a 70‐year‐old woman with suspected acute necrotizing encephalopathy on days 1, 2, 3, and 5 after admission. A, Computed tomography (CT) images of the head at admission showed no abnormalities. B, C, Head CT on (B) day 2 and (C) day 3 revealed progressive bilateral and symmetrical thalamic lesions. D, Head CT on day 5 showed diffuse swelling of the whole brain.
On day 2, the patient remained in a deep comatose state despite withdrawal of sedative drugs. A repeat head CT revealed bilateral and symmetrical low‐density areas and diffuse bilateral swelling of the thalamus. In addition, in another detailed discussion of the patient's medical history with her family, we learned she had suffered from a severe cough with high fever 2 days before hospital admission. A rapid influenza antigen test, undertaken after culturing scrapings taken from her nose, was positive. As the patient was hemodynamically unstable, we did not take a cerebrospinal fluid sample at admission. Additionally, as the head CT on day 2 also revealed diffuse brain swelling, we did not carry out a lumbar puncture. Based on the characteristic CT findings and medical history of influenza, we made a differential diagnosis of influenza A‐associated ANE. Intravenous peramivir (300 mg/day), steroid pulse therapy (1,000 mg/day), and D‐mannitol infusion (135 g/day) were added to her treatment protocol. Continuous renal replacement therapy and plasma exchange were also initiated. Surface cooling with Arctic Sun 5000 (Bard Medical) and mechanical ventilation therapy with a target PaCO2 of 30–40 mmHg were continued. Although acute kidney injury, acute hepatic failure, and coagulopathy improved, brain edema gradually affected the whole brain (Fig. 1B–D). The patient remained in a deep coma state, and spontaneous breathing ceased on day 4. Pupils were dilated and fixed bilaterally, and the pupillary light reflex had disappeared. An electroencephalogram taken at bedside on day 6 revealed electrocerebral inactivity. Treatment was withdrawn on day 7, and the patient died of ANE on day 21.
Discussion
Our case describes an elderly patient with ANE in whom the differential diagnosis of severe HS was first made. The clinical course of our case highlights two important issues: (i) ANE can have a similar presentation to severe HS in terms of clinical course during the acute phase but shows different radiological imaging findings, (ii) imaging findings characteristic of ANE might not be detectable in the ultra‐acute phase of the disease.
We also investigated previous reports of adult‐onset ANE and evaluated diagnostic characteristics. We searched the major Japanese databanks of the Igaku Chuou Zasshi using the key words “kyuseieshiseinousho” (ANE in Japanese) and “seijin” (adult in Japanese) and the electronic PubMed database using the key words (“acute necrotizing encephalopathy” OR “acute necrotizing encephalitis”) AND “adult”. We excluded cases involving patients younger than 20 years of age, cases of herpes encephalitis, and cases without a description of radiological findings. In total, we retrieved 19 cases of adult ANE, including our case.1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 All articles were reviewed for age, gender, GCS, presence of seizure episodes, elevated proteins in the spinal fluid, and aspartate transaminase/alanine transaminase elevations according to the diagnostic criteria for ANE in children proposed by Mizuguchi et al.2 Most patients affected by ANE were relatively young (median age, 39 years) and typically female (63.2%; Table 1). Acute necrotizing encephalopathy rarely occurred in elderly patients as in our case. The majority of patients with ANE (89.5%) presented with altered consciousness. Laboratory data and clinical course were similar for ANE and severe HS (Table 2). In our review, both ANE and severe HS presented with altered consciousness, hyperthermia, and dehydration, as in our case. In sum, these diseases show similar clinical presentation except for characteristic imaging findings and presence of prodromal symptoms. Elevated protein levels in the cerebrospinal fluid were found in nine of 12 patients with ANE in previous reports. Also, rapid influenza antigen tests could be helpful for early diagnosis and introduction of treatment.
Table 1.
Summary of cases of acute necrotizing encephalopathy in adults (n = 19)
| Number (%) | |
|---|---|
| Age, median (IQR) | 39 (25.5–61) |
| Female | 12/19 (63.2) |
| Consciousness disorder, GCS 3–12 | 17/19 (89.5) |
| Presence of seizure episodes | 7/19 (36.8) |
| Elevated protein in cerebrospinal fluid | 13/15 (86.7) |
| Elevated transaminase | 11/18 (61.1) |
| Bilateral symmetrical lesions | 19/19 (100) |
| No abnormal findings on initial CT | 8/12 (66.7) |
| Thalamic lesions on initial MRI | 9/11 (81.8) |
| Alive | 13/19 (68.4) |
CT, computed tomography; GCS, Glasgow Coma Scale; IQR, interquartile range; MRI, magnetic resonance imaging.
Table 2.
Comparison between acute necrotizing encephalopathy (ANE) and severe heat stroke (HS)
| ANE | Severe HS | |
|---|---|---|
| Prodromal symptoms | + | − |
| Consciousness disorder | + | + |
| Dehydration | ± | + |
| Hyperthermia | + | + |
| MOF | ± | ± |
| Abnormal findings on head CT/MRI | + | ± |
| Location of lesion(s) | Thalamus | Cerebellum hippocampus |
| Symmetry | + | + |
| Features | Progressive, diffuse swelling | Localized, small lesions |
CT, computed tomography; MOF, multiple organ failure; MRI, magnetic resonance imaging.
Our review showed that bilateral and symmetrical thalamic lesions are also detected in adult patients with ANE as they are in children, which marks a distinction from severe HS. Although the pathogenesis of ANE remains unclear, the associated radiological findings could reflect the pathology of the central thalamus with acute, drastic swelling due to cerebral vascular impairment.22 By contrast, radiologic imaging in patients with HS has revealed lesions in various locations, such as the cerebellum, caudate nucleus, hippocampus, external capsule, thalamus, splenium of the corpus callosum, subcortical white matter, and cortex.23 The cerebellum is particularly susceptible, which is explained by the vulnerability of the Purkinje cells within the cerebellum to heat‐induced injury. We speculate that the location of radiological findings could help in establishing a differential diagnosis.
Data extracted from our review also suggest that the abnormal CT findings characteristic of ANE are not often clearly detectable during the ultra‐acute phase. Similar to our case, ANE could not be diagnosed on admission in several previous cases without the characteristic lesions on the initial CT scan. The differential diagnosis in these previous cases was septic shock, anaphylactic shock, or viral encephalitis.10 A previous study of 41 child cases of ANE found that abnormal findings characteristic of ANE appeared on CT within 12 h after the onset of coma.24 In our review, only four of 12 patients with ANE showed bilateral thalamic lesions on the initial CT.7, 9, 19, 21 Conversely, lesions were detected by magnetic resonance imaging (MRI) in nine of 11 patients on admission.5, 6, 7, 9, 12, 13, 16, 19, 21 As diffusion‐weighted imaging is superior for detecting tissue damage in the cerebral parenchyma in the hyper‐acute phase, it could be more accurate in revealing cytotoxic edema in ANE. We, therefore, propose that MRI or a repeat CT scan within 12 h be undertaken to diagnose ANE in these patients.
Although ANE in adult patients is a fairly rare disease, our experience suggests that ANE should be considered as a differential diagnosis in comatose patients with hyperthermia and multiple organ failure. We also believe that our case is educational and valuable in the management of severe consciousness disturbance. In the case of severe consciousness disturbance, if there are no significant findings in the laboratory data, physicians tend to focus on radiological findings when searching for the cause. Even in our case, although diagnosis of ANE was obtained by characteristic radiological findings, therapeutic intervention was delayed. We emphasize that it is also necessary to consider cases such as ours from various perspectives, such as from cerebrospinal fluid findings and virus examinations. In the absence of contraindications and hemodynamic problems, cerebrospinal fluid findings could be helpful for diagnosis. We also reaffirmed the importance of screening for influenza during the differential diagnosis of high body temperature in winter in any situation.
Conclusion
We describe the difficulty encountered in differentiating ANE from severe HS in an elderly comatose patient in a high‐temperature environment due to similarities in laboratory data and clinical presentation. We propose that attention be paid to the characteristic imaging findings associated with ANE to make a differential diagnosis as soon as possible. As CT findings characteristic of ANE might not be apparent in the ultra‐acute phase, physicians should consider MRI or a repeat CT scan within 12 h of admission.
Disclosure
Approval of the research protocol: N/A.
Informed consent: Informed consent for publication of this case report was obtained from the patient's family.
Registry and registration no. of the study/trial: N/A.
Animal studies (if applicable): N/A.
Conflict of interest: None declared.
Funding information
No funding information provided.
References
- 1. Ishii N, Mochizuki H, Moriguchi‐Goto S et al An autopsy case of elderly‐onset acute necrotizing encephalopathy secondary to influenza. J. Neurol. Sci. 2015; 354: 129–30. [DOI] [PubMed] [Google Scholar]
- 2. Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997; 19: 81–92. [DOI] [PubMed] [Google Scholar]
- 3. Okumura A, Mizuguchi M, Kidokoro H et al Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009; 31: 221–7. [DOI] [PubMed] [Google Scholar]
- 4. Suzuki M, Shimbo T, Ikaga T, Hori S. Incidence and characteristics of bath‐related accidents. Intern. Med. 2019; 58: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alsolami A, Shiley K. Successful treatment of influenza‐associated acute necrotizing encephalitis in an adult using high‐dose oseltamivir and methylprednisolone: case report and literature review. Open Forum Infect. Dis. 2017; 4: oxf145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fasano A, Natoli GF, Cianfoni A et al Acute necrotizing encephalopathy: a relapsing case in a European adult. J. Neurol. Neurosurg. Psychiatry 2008; 79: 227–8. [DOI] [PubMed] [Google Scholar]
- 7. Iijima H, Wakasugi K, Ayabe M et al A case of adult influenza A virus‐associated encephalitis: magnetic resonance imaging findings. J. Neuroimaging 2002; 12: 273–5. [PubMed] [Google Scholar]
- 8. Katayama W, Watanabe S, Onuma K, Fujita K, Kamezaki T. An adult case of acute necrotizing encephalopathy with influenza virus infection. J. Ibaraki Soc. Rural Med. 2010; 23: 8–11. (Article in Japanese.) [Google Scholar]
- 9. Kato H, Hasegawa H, Iijima M, Uchigata M, Terada T, Okada Y. Brain magnetic resonance imaging of an adult case of acute necrotizing encephalopathy. J. Neurol. 2007; 254: 1135–7. [DOI] [PubMed] [Google Scholar]
- 10. Kojima T, Ishii M, Abe S, Kamimura R, Shimizu H. An adult case of acute necrotizing encephalopathy. J. Jpn Soc. Intensive Care Med. 2012; 19: 661–5. (Article in Japanese.) [Google Scholar]
- 11. Lee YJ, Smith DS, Rao VA et al Fatal H1N1‐related acute necrotizing encephalopathy in an adult. Case Rep. Crit. Care 2011; 2011: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang W, Shao Y, Cui Y et al Teaching NeuroImages: radiographic evolution in an adult case of acute necrotizing encephalopathy. Neurology 2018; 91: e490–1. [DOI] [PubMed] [Google Scholar]
- 13. Miyata E, Mizuno M, Mori K, Yasuno Y. An adult case of acute necrotizing encephalopathy. No To Shinkei 2002; 54: 354–5. (Article in Japanese.) [PubMed] [Google Scholar]
- 14. Mizuguchi M, Tomonaga M, Fukusato T, Asano M. Acute necrotizing encephalopathy with widespread edematous lesions of symmetrical distribution. Acta Neuropathol. 1989; 78: 108–11. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura Y, Miura K, Yamada I, Ino H, Mizobata T. A novel adult case of acute necrotizing encephalopathy of childhood with bilateral symmetric thalamic lesions. Rinsho Shinkeigaku (Clin. Neurol.) 2000; 40: 827–31. (Article in Japanese.) [PubMed] [Google Scholar]
- 16. Narra R, Mandapalli A, Kamaraju SK. Acute necrotizing encephalopathy in an adult. J. Clin. Imaging Sci. 2015; 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ochi N, Takahashi K, Yamane H, Takigawa N. Acute necrotizing encephalopathy in an adult with influenza A infection. Ther. Clin. Risk Manag. 2018; 14: 753–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saji N, Yamamoto N, Yoda J et al Adult case of acute encephalopathy associated with bilateral thalamic lesions and peripheral neuropathy. No To Shinkei 2006; 58: 1009–14. (Article in Japanese.) [PubMed] [Google Scholar]
- 19. Shirai T, Fujii H, Ono M et al A novel autoantibody against ephrin type B receptor 2 in acute necrotizing encephalopathy. J. Neuroinflammation 2013; 10: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolf K, Schmitt MT, Kollias S, Curt A. Acute necrotizing encephalopathy (ANE1): rare autosomal‐dominant disorder presenting as acute transverse myelitis. J. Neurol. 2013; 260: 1545–53. [DOI] [PubMed] [Google Scholar]
- 21. Zhou C, Wu L, Wu J, Zhang H. Acute necrotizing encephalopathy secondary to sepsis. Ann. Saudi Med. 2014; 34: 451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mizuguchi M, Hayashi M, Nakano I et al Concentric structure of thalamic lesions in acute necrotizing encephalopathy. Neuroradiology 2002; 44: 489–93. [DOI] [PubMed] [Google Scholar]
- 23. Fuse A, Yamashiro K, Oji Y et al Reversible focal cerebral cortical lesions in a patient with heat stroke. Intern. Med. 2013; 52: 377–80. [DOI] [PubMed] [Google Scholar]
- 24. Mizuguchi M, Abe J, Mikkaichi K et al Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J. Neurol. Neurosurg. Psychiatry 1995; 58: 555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


