Abstract
Background
Indications for using veno‐arterial extracorporeal membrane oxygenation (V‐A ECMO) in sepsis cases remain unclear.
Case Presentation
A 66‐year‐old man with pre‐existing chronic heart failure developed severe pneumonia resulting in refractory septic shock. He was diagnosed with septic cardiomyopathy based on depressed left ventricular ejection fraction and a dilated left ventricle based on a transthoracic echocardiogram. We initiated V‐A ECMO on day 3 because the shock did not respond to conventional therapy. The patient's hemodynamics improved, and his infection was reduced. He recovered fully and was discharged on day 107 with his cardiac function restored to its baseline.
Conclusion
Septic cardiomyopathy is a form of reversible myocardial dysfunction. Veno‐arterial extracorporeal membrane oxygenation should be considered for septic cardiomyopathy with intractable circulatory failure. Pre‐existing chronic heart failure is not a contraindication for VA‐ECMO.
Keywords: Cardiomyopathy, extracorporeal membrane oxygenation, heart failure, sepsis, shock
Introduction
Septic shock has a high mortality rate1 and was formerly categorized as a distributive shock, characterized by a hyperdynamic state, decreased systemic vascular resistance, and increased microvascular permeability. However, some septic shock patients develop septic cardiomyopathy, causing cardiac dysfunction, left ventricular dilation, and decreased ejection fraction.2 Septic cardiomyopathy can be fatal, but cardiac function often recovers 7–10 days after onset.2
Veno‐arterial extracorporeal membrane oxygenation (V‐A ECMO) provides cardiopulmonary support to patients with refractory cardiovascular failure of various etiologies.3 Its use has recently increased due to technical advances and accumulated experience. Some studies report successful V‐A ECMO use for septic shock.4
However, V‐A ECMO indications for sepsis are unclear. Veno‐arterial extracorporeal membrane oxygenation is contraindicated in chronic/irreversible organ dysfunction patients.5 We report the successful use of V‐A ECMO for severe septic cardiomyopathy in a patient with pre‐existing chronic heart failure.
Case
A 66‐year‐old man was transferred to our hospital for multiple organ failure caused by severe pneumonia. He had pre‐existing chronic heart failure (New York Heart Association class II), with 40% left ventricular ejection fraction (LVEF) caused by atrial fibrillation.
On examination, his vital signs were as follows: Glasgow Coma Scale score, 15 (E4V5M6); blood pressure, 139/86 mmHg with 0.15 μg/kg/min norepinephrine infusion; heart rate, 192 b.p.m.; respiratory rate, 24 breaths/min; body temperature, 38°C; and oxygen saturation, 92% with 10 L/min oxygen. Arterial blood gas analysis revealed hypoxia and metabolic acidosis with hyperlactatemia. Blood examinations revealed elevated inflammatory markers and impaired renal function (Table 1). A transthoracic echocardiogram (TTE) showed diffuse left ventricular hypokinesis with 30% LVEF and no findings suggesting infective endocarditis. Computed tomography revealed consolidation of the lower left lung lobe. He was diagnosed with pneumonia, septic shock, acute kidney injury, and acute exacerbation of chronic heart failure.
Table 1.
Laboratory data of a 66‐year‐old man on admission for multiple organ failure caused by severe pneumonia
| Hematology | Biochemistry | Hemostasis | |||||
| WBC | 11,900/μL | CRP | 43.7 mg/dL | PT | 19.2 sec | ||
| RBC | 390 × 104/μL | Procalcitonin | 176 ng/mL | PT‐INR | 1.54 | ||
| Ht | 35.1% | ALT | 19 IU/L | APTT | 38.7 sec | ||
| Plt | 15.3 × 104/μL | LDH | 221 IU/L | Fibrinogen | >800 mg/dL | ||
| ALP | 149 IU/L | FDP | 6.2 μg/mL | ||||
| Blood gas analysis | T. Bil | 0.5 mg/dL | D‐dimer | 1.4 μg/mL | |||
| pH | 7.429 | BUN | 149 mg/dL | ATIII | 73% | ||
| PaCO2 | 31.2 mmHg | Cre | 4.56 mg/dL | ||||
| PaO2 | 62.4 mmHg | Na | 125 mEq/L | ||||
|
|
19.2 mmol/L | K | 4.8 mEq/L | ||||
| BE | −10.4 mmol/L | Cl | 88 mEq/L | ||||
| Lactate | 3.1 mmol/L | Ca | 8.3 mEq/L | ||||
| SaO2 | 90.6% | CPK | 394 IU/L | ||||
| Mb | 2,288 ng/mL | ||||||
| TP | 6.9 g/dL | ||||||
| Alb | 1.9 g/dL | ||||||
| NTproBNP | 28,397 pg/dL | ||||||
Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; ATIII, antithrombin III; BE, base excess; BUN, blood urea nitrogen; Ca, calcium; CPK, creatine kinase; Cl, chloride; Cre, creatinine; CRP, C‐reactive protein; FDP, fibrinogen degradation products; Ht, hematocrit; K, potassium; LDH, lactate dehydrogenase; Mb, myoglobin; Na, sodium; NTproBNP, N terminal pro brain natriuretic peptide; Plt, platelets; PT, prothrombin time; PT‐INR, prothrombin time – international normalized ratio; T. Bil, total bilirubin; TP, total protein; RBC, red blood cells; WBC, white blood cells.
After admission, antibiotics (meropenem, linezolid, clindamycin, and minomycin), hydrocortisone, vasopressors (norepinephrine and vasopressin), and landiolol were initiated. Continuous renal replacement therapy and mechanical ventilation therapy were also initiated. Before introducing ECMO, the ventilator was set to the airway pressure release mode (high airway pressure, 16 cm H2O; fraction of inspired oxygen, 0.6). Blood gas analysis showed pCO2 46.1 mmHg and pO2 116 mmHg. However, hyperlactatemia and tachycardia persisted, and purpura appeared over the limbs and trunk. The TTE findings on the second hospitalization day revealed refractory cardiac failure with 10% LVEF regardless of the improving oxygenation; thus, septic cardiomyopathy was suspected. His hemodynamics remained unstable; metabolic acidosis progressed despite intra‐aortic balloon pump (IABP) placement on day 2. Streptococcus pneumoniae was found in the blood and sputum cultures. Because of his intractable shock, V‐A ECMO was implemented as a bridging therapy on day 3. The pre‐ECMO implantation Sequential Organ Failure Assessment (SOFA) score was 15 points, and Acute Physiology and Chronic Health Evaluation II score was 26 points.
Once V‐A ECMO was initiated, the patient gradually recovered from the infection and circulatory failure and showed decreased serum lactate levels and cardiac index and LVEF recovery. He was successfully weaned from V‐A ECMO on day 12 and IABP on day 13 (Fig. 1). Abscessed left lower pulmonary lobe resection was carried out on day 16. He was taken off mechanical ventilation and continuous renal replacement therapy on day 19 and discharged home in an ambulatory condition on day 107. The TTE at discharge showed that his cardiac function had returned to baseline.
Figure 1.
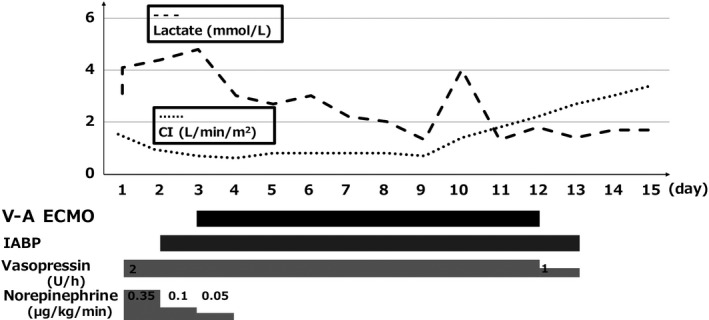
Shifts in serum lactate levels and the cardiac index (CI) over the clinical course in a 66‐year‐old man with refractory septic shock caused by pneumonia. Circulatory failure was prolonged despite conventional treatment. Veno‐arterial extracorporeal membrane oxygenation (V‐A ECMO) was initiated on day 3 and he recovered from circulatory failure with decreasing serum lactate levels and increasing CI during V‐A ECMO support. IABP, intra‐aortic balloon pump.
Discussion
Septic cardiomyopathy is reversible at 7–10 days after onset of sepsis.2 It occurs in 18%‐65% sepsis patients. Unfortunately, there are no formal diagnostic criteria for septic cardiomyopathy. Repeated TTE could enable better systolic and diastolic function assessment over time. There is no significant difference in mortality between patients with or without septic cardiomyopathy;6 however, the differences observed could be due to variations in fluid loading condition. Sepsis‐induced cardiogenic shock can cause septic cardiomyopathy, which is sometimes intractable and fatal.7
An important indication for V‐A ECMO is reversible cardiac dysfunction. The 2016 Surviving Sepsis Campaign Guidelines state that ECMO can be considered as rescue therapy in refractory hypoxia patients.8 Extracorporeal membrane oxygenation, especially V‐A ECMO, indications for sepsis remain unclear.
Circulatory failure in sepsis comprises distributive, hypovolemic, and cardiogenic shock.9 If the main causes of septic shock are vascular permeability and vasodilation, draining sufficient volumes of venous blood to generate blood flow on ECMO is difficult. Conversely, sepsis‐induced cardiogenic shock, presumed to be septic cardiomyopathy, is considered a better indicator for V‐A ECMO because septic cardiomyopathy can be reversed in 7–10 days. A report describing septic shock patients receiving V‐A ECMO found that myocardial dysfunction was associated with a better prognosis.4 Our patient was diagnosed with septic cardiomyopathy based on depressed LVEF and dilated left ventricle on repeated TTE. His circulatory failure was mainly caused by septic cardiomyopathy with little vascular permeability. His cardiac function returned to baseline within 10 days, and we could successfully wean him from V‐A ECMO, which was instrumental in saving him. Although V‐A ECMO increases afterload and decreases stroke volume, it plays a key role in providing systemic perfusion in place of the injured native heart. Because we speculated that his cardiac function would recover, we made his systemic perfusion dependent on V‐A ECMO until it improved, at the cost of his own cardiac function. The effectiveness of IABP with V‐A ECMO for septic cardiomyopathy remains unknown. However, IABP helped wean our patient off V‐A ECMO and might have contributed to improving his outcome.
In our patient, pre‐existing chronic heart failure could have been a contraindication to ECMO. The Extracorporeal Life Support Organization states that contraindications to V‐A ECMO include chronic organ dysfunction.5 Veno‐arterial ECMO is indicated for decompensated chronic heart failure patients, but their mortality is high.10 Few reports of V‐A ECMO for septic shock patients with chronic heart failure exist. Even if patients with chronic heart failure develop further deterioration of cardiac function due to septic cardiomyopathy, which is reversible, they could recover to their baseline cardiac function. Therefore, pre‐existing chronic heart failure is not a contraindication for VA‐ECMO in septic cardiomyopathy patients. A pre‐ECMO SOFA score >11, idiopathic cardiomyopathy as the cause of pre‐existing chronic heart failure, cardiac disease duration >2 years pre‐ECMO, and pre‐ECMO blood lactate >4 mmol/L are independent predictors of 1‐year mortality in patients receiving V‐A ECMO for acute decompensated heart failure.10 Regarding septic cardiomyopathy in chronic heart failure, the SOFA score and blood lactate level might not be mortality predictors because both are affected by sepsis itself, in addition to cardiogenic shock. However, cardiac disease duration and the cause of pre‐existing chronic heart failure would influence prognosis, even in septic cardiomyopathy patients. In this case, the pre‐ECMO cardiac disease duration was 18 months. Shorter pre‐ECMO cardiac disease duration might be a favorable predictive factor in patients with intractable septic cardiomyopathy superimposed on chronic heart failure that requires V‐A ECMO.
Conclusion
When managing intractable septic cardiomyopathy, V‐A ECMO should be considered. Pre‐existing chronic heart failure is not a contraindication for introducing V‐A ECMO in septic cardiomyopathy patients. Cardiac disease duration might influence prognosis in these patients.
Disclosure
Approval of the research protocol: N/A.
Informed consent: Written consent for the publication of this case report was obtained from the patient.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding information
No funding information provided.
References
- 1. Freund Y, Lemachatti N, Krastinova E et al Prognostic accuracy of sepsis‐3 criteria for in‐hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017; 317: 301–8. [DOI] [PubMed] [Google Scholar]
- 2. Parker MM, Shelhamer JH, Bacharach SL et al Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 1984; 100: 483–90. [DOI] [PubMed] [Google Scholar]
- 3. Kilic A, Shukrallah BN, Kilic A, Whitson BA. Initiation and management of adult veno‐arterial extracorporeal life support. Ann. Transl. Med. 2017; 5: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brechot N, Luyt CE, Schmidt M et al Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit. Care Med. 2013; 41: 1616–26. [DOI] [PubMed] [Google Scholar]
- 5. Extracorporeal Life Support Organization (ELSO): Guidelines.[homepage on the internet]. Available from: https://www.elso.org/Resources/Guidelines.aspx. Accessed 25 July 2018.
- 6. Sevilla Berrios RA, O'Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30‐day mortality in patients with severe sepsis and septic shock: a systematic review and meta‐analysis. J. Crit. Care 2014; 29: 495–9. [DOI] [PubMed] [Google Scholar]
- 7. Weil MH, Nishijima H. Cardiac output in bacterial shock. Am. J. Med. 1978; 64: 920–2. [DOI] [PubMed] [Google Scholar]
- 8. Rhodes A, Evans LE, Alhazzani W et al Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017; 43: 304–77. [DOI] [PubMed] [Google Scholar]
- 9. Vincent JL, De Backer D. Circulatory shock. N. Engl. J. Med. 2013; 369: 1726–34. [DOI] [PubMed] [Google Scholar]
- 10. Dangers L, Brechot N, Schmidt M et al Extracorporeal membrane oxygenation for acute decompensated heart failure. Crit. Care Med. 2017; 45: 1359–66. [DOI] [PubMed] [Google Scholar]


