Abstract
Background
Cognitive outcomes in preterm (PT) children have been associated with microstructural properties of white matter. PT children who experienced neonatal inflammatory conditions have poorer cognitive outcomes than those who did not. The goal of this study was to contrast white matter microstructure and cognitive outcomes after preterm birth in relation to the presence or absence of severe inflammatory conditions in the neonatal period.
Methods
PT children (n = 35), born at gestational age 22–32 weeks, were classified as either PT+ (n = 12) based on a neonatal history of inflammatory conditions, including bronchopulmonary dysplasia, necrotizing enterocolitis or culture positive sepsis, or PT- (n = 23) based on the absence of the three inflammatory conditions. Full term (FT) children (n = 43) served as controls. Participants underwent diffusion MRI and cognitive testing (intelligence, reading, and executive function) at age 6 years. The corpus callosum was segmented into 7 regions using deterministic tractography and based on the cortical projection zones of the callosal fibers. Mean fractional anisotropy (FA) and mean diffusivity (MD) were calculated for each segment. General linear models with planned contrasts assessed group differences in FA, MD and cognitive outcomes. Pearson correlations assessed associations of white matter metrics and cognitive outcome measures.
Results
FA was significantly lower and MD was significantly higher in PT+ compared to PT- or FT groups in multiple callosal segments, even after adjusting for gestational age. Executive function scores, but not intelligence or reading scores, were less favorable in PT+ than in PT- groups. Among the entire sample, occipital FA was significantly correlated with IQ (r = 0.25, p < 0.05), reading (r = 0.32, p < 0.01), and executive function (r = −0.28, p < 0.05) measures. Anterior frontal FA and superior parietal FA were significantly correlated with executive function (r = −0.25, r = 0.23, respectively, p < 0.05).
Conclusions
We observed differences in the white matter microstructure of the corpus callosum and in the cognitive skills of 6-year-old PT children based on their history of neonatal inflammation. Neonatal inflammation is one medical factor that may contribute to variation in long-term neurobiological and neuropsychological outcomes in PT samples.
Keywords: Diffusion MRI, White matter, Tractography, Prematurity, Corpus callosum, Inflammation, Neurodevelopmental outcomes, Executive function
Abbreviations: PT, preterm; PT, preterm without inflammation; PT+, preterm with inflammation; FT, full term; GA, gestational age; BW, birthweight; dMRI, diffusion magnetic resonance imaging; DTI, diffusion tensor imaging; FA, Fractional Anisotropy; MD, Mean Diffusivity; AFQ, Automated Fiber Quantification; WASI-II, Wechsler Abbreviated Scale of Intelligence, Second Edition; WRMT-III, Woodcock Reading Mastery Tests, Third Edition; BRIEF, Behavior Rating Inventory of Executive Function; GEC, Global Executive Composite; FDR, false discovery rate; SD, standard deviation; CI, confidence interval; ANOVA, analysis of covariance
Highlights
-
•
Preterm neonatal inflammation related to 6 yr corpus callosum microstructure.
-
•
Corpus callosum microstructure was similar in healthy preterms and full terms.
-
•
Preterm neonatal inflammation related to 6 yr executive function.
-
•
Corpus callosum microstructure correlated with cognitive function.
1. Introduction
Children born very or extremely preterm (PT), at gestational ages <32 weeks, are at high risk for poor neurodevelopmental outcomes compared to children born full term (FT). However, within the PT population, outcomes are highly variable (Anderson, Doyle, and Victorian Infant Collaborative Study, 2003; Bright et al., 2017). Many studies have documented that children who experience serious inflammatory complications during the neonatal period are at higher risk for poor cognitive outcomes than children spared these conditions (Mitha et al., 2013; Schlapbach et al., 2012; Stoll et al., 2004; Leviton et al., 2018). Adverse outcomes in PT children have been attributed, at least in part, to injury to the white matter of the brain (Back and Miller, 2014; Volpe et al., 2011). Diffusion neuroimaging studies have identified white matter microstructure differences between PT and FT samples throughout childhood and into adulthood (Kontis et al., 2009; Mullen et al., 2011; Nagy et al., 2003; Thompson et al., 2011; Vangberg et al., 2006). These microstructural differences can be detected in the absence of gross white matter abnormalities and possibly reflect white matter injury and subsequent dysmaturity, shown in animal models of preterm birth (Volpe et al., 2011; Back, 2015). Alternatively, or in addition, microstructural differences observed in older preterm children may also reflect white matter changes from recovery, compensation, and on-going development. Studies have yet to examine whether a history of neonatal inflammation relates to variability in later white matter microstructure at school age and whether such variation in white matter properties mediates neurodevelopmental outcomes at those ages. The goal of this study was to contrast white matter microstructure and cognitive outcomes in three groups of 6-year-old children: PT children who experienced major inflammatory conditions during the neonatal period (PT+), PT children who did not experience such inflammatory conditions (PT-), and FT children.
The white matter of the brain is highly susceptible to injury from complications of preterm birth, including hypoxia, ischemia, and inflammation. PT infants have immature immune systems and limited regulation of cerebral blood flow. PT infants are, thus, vulnerable to infections and illnesses that can create a diffuse inflammatory response throughout the body and trigger oxidative stress in the brain. Between 24 and 32 weeks gestational age, immature progenitor cells and pre-oligodendrocytes predominate in the oligodendrocyte cell line, which is ultimately responsible for myelinating axons. These immature cells are particularly susceptible to destruction by oxidative stress. Although capable of regenerating after destruction, the oligodendrocyte cell line after injury often shows failure to fully mature or myelinate (Back and Miller, 2014). Such injuries and subsequent dysmaturity of the oligodendrocyte cell line are thought to contribute to long-term white matter abnormalities observed in children born preterm (Volpe, 2009).
Three major inflammatory complications that are prevalent in PT infants are likely to set up this unfortunate cascade. Bronchopulmonary dysplasia, also known as chronic lung disease, results from subacute or chronic inflammation affecting the immature respiratory system. Necrotizing enterocolitis is a bacterial infection of the gastrointestinal tract. Sepsis is a bacterial infection in the blood. All three conditions are capable of inducing hypoxia, ischemia, and, in particular, inflammation affecting the brain (Behrman, Butler, and Institute of Medicine (U. S.). Committee on Understanding Premature Birth and Assuring Healthy Outcomes, 2007). Brain regions most vulnerable to inflammatory-related injuries are periventricular white matter regions (Volpe, 2009).
Diffusion MRI (dMRI) has become a preferred method for assessing and characterizing the properties of white matter pathways in the brain after PT birth (Basser and Pierpaoli, 1996; Feldman et al., 2010). dMRI studies of preterm neonates, performed at or near term, have observed variation in microstructural properties. In a systematic review of 22 at- or near-term dMRI studies of preterm neonates, fractional anisotropy (FA) and mean diffusivity (MD) were most commonly reported, with 20 and 16 studies, respectively. Axial diffusivity (AD) and radial diffusivity (RD) were assessed in 12 of the reviewed studies (Pannek et al., 2014). Lower FA, higher MD, and/or higher RD in preterm neonates compared to term infants was reported in multiple white matter regions and pathways (Thompson et al., 2011; Ball et al., 2013). Lower FA, higher MD, and higher RD in PT neonates is consistent with reductions in myelin and loss of axonal fibers from oxidative damage (Back and Miller, 2014; Volpe, 2009). Beyond the newborn period, in childhood and adolescence, differences in white matter microstructure can be found comparing PT and FT children, though the pattern of findings varies as a function of age, white matter pathway, and analytic strategies (Groeschel et al., 2014; Li et al., 2015; Travis et al., 2015).
Emerging evidence from dMRI studies suggests that neonatal inflammatory conditions may contribute to variability in white matter microstructure seen in PT children. One dMRI study found that the apparent effects of gestational age on a composite measure of brain FA, measured at near term, could be explained by necrotizing enterocolitis and mechanical ventilation (Bonifacio et al., 2010). In another dMRI study, FA of the corpus callosum measured at term-equivalent age was associated with incidence of chronic lung disease and infection (Shim et al., 2012). However, studies have yet to determine whether variability in white matter microstructure observed in school-aged PT children relates to prior history of neonatal inflammation and whether such variations relate to neurodevelopmental outcomes.
To address these questions, the present study sought to determine if inflammatory conditions in the neonatal period would be associated with corpus callosum microstructure at age 6. We chose to limit our analyses to the corpus callosum for several reasons. Abnormalities in corpus callosum microstructure are known sequelae of PT birth. PT children consistently demonstrate lower FA and higher MD in the corpus callosum compared to FT children (Dodson et al., 2017; Hasegawa et al., 2011; Jo et al., 2012; Li et al., 2015; Mullen et al., 2011; Skranes et al., 2007). In addition, the corpus callosum contains fiber tracts that traverse through periventricular white matter regions that demonstrate susceptibility to preterm injuries induced by hypoxia, ischemia, and inflammation (Back et al., 2007). The corpus callosum has also been implicated in a range of cognitive functions. We chose to focus on intelligence, reading, and executive function because previous studies have found associations of white matter metrics of the corpus callosum and these domains in FT and PT children. In PT children, MD in the genu, body, and splenium has been negatively correlated, and FA positively correlated with IQ (Kontis et al., 2009; Eikenes et al., 2011). Both positive and negative associations have been reported between reading abilities and FA measured from medial and posterior regions of the corpus callosum in samples of preterm and full term children (Andrews et al., 2010). FA across the corpus callosum has been positively associated with executive function tasks such as orienting (Murray et al., 2016) and spatial working memory/strategy (Loe et al., 2019). In the corpus callosum and in other white matter regions, associations between white matter and cognitive functions often differ between FT and PT children (Loe et al., 2019; Dodson et al., 2018; Feldman et al., 2012; Travis et al., 2016). These findings suggest that the neurobiology underlying structure-function relationships may differ between FT and PT children.
In the present study, we divided a sample of PT children into those who did and did not experience the three previously described inflammatory conditions—bronchopulmonary dysplasia, necrotizing enterocolitis, and sepsis. We then compared white matter microstructure of the corpus callosum and results of cognitive assessments in the PT children with and without neonatal inflammatory conditions and both groups to a sample of FT controls. Two specific aims guided the analyses: 1. To describe FA and MD of the corpus callosum in the 3 groups using dMRI. We hypothesized that the PT group with neonatal inflammatory conditions would have lower FA and higher MD than the PT group without inflammation and the FT comparison group. 2. To describe long-term neurocognitive outcomes in the 3 groups. We hypothesized that the PT group with neonatal inflammatory conditions would have lower IQ, weaker reading skills, and relatively poorer executive function than PT children without inflammation and FT controls. To be complete, we performed secondary analyses to explore whether white matter metrics in the corpus callosum would be correlated with cognitive measures. If the PT group who experienced inflammation had both altered white matter microstructure and adverse neurodevelopmental outcomes, we anticipated a strong correlation between brain structure and cognitive functions.
2. Methods
2.1. Design
This study was exploratory because children were enrolled on the basis of premature birth, irrespective of neonatal complications.
2.2. Participants
Participants were enrolled in a longitudinal study investigating the neural basis of reading in children born preterm. Children were recruited from the San Francisco Bay Area from 2012 to 2015. For the analyses presented here, PT birth was restricted to children with gestational age (GA) ≤ 32 weeks because of the susceptibility of white matter to injury is high at these gestational ages. FT birth was defined as GA ≥ 37 weeks or birth weight ≥ 2, 500 g. Children who completed a 30 direction dMRI acquisition protocol (see below) at age 6 years and had neonatal clinical information available were included in the current study. The sample included 35 PT children and 43 FT children. Full inclusion and exclusion criteria have been previously described (Dodson et al., 2017). Briefly, exclusion criteria for all participants included presence of a seizure disorder, hydrocephalus, sensorineural hearing loss, or genetic disorders. The experimental protocol was approved by the Stanford University Institutional Review Board #IRB-22233. A parent or legal guardian provided informed written consent and participants were compensated for participation. Parent reports were used to collect demographic characteristics. We obtained measures for socioeconomic status using a modified Hollingshead Index (Hollingshead, 1975).
Participants were divided into 3 groups based on medical chart review of the PT participants: the PT inflammation positive group (PT+, n = 12) experienced culture positive sepsis, medical or surgical necrotizing enterocolitis, and/or bronchopulmonary dysplasia characterized by >28 days of ventilation and/or supplemental oxygen; 7 participants had one inflammatory condition, 3 participants had 2 inflammatory conditions, and 2 participants had 3 inflammatory conditions. The PT inflammation negative group (PT-, n = 23) experienced none of these events. The FT (n = 43) group served as controls. Medical chart review was also used to compare the PT groups on other complications of PT birth: small for gestational age (≤ 3rd percentile birth weight for gestational age), retinopathy of prematurity or immature retinae, patent ductus arteriosus, presence of hyperbilirubinemia, and abnormal near-term T1-weighted MRI scans.
2.3. Procedures
2.3.1. Diffusion MRI measures, acquisition, and analyses
MRI data were acquired on a 3 T Discovery MR750 scanner (General Electric Healthcare, Milwaukee, WI, USA) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA) at the Center for Cognitive and Neurobiological Imaging at Stanford University (www.cni.stanford.edu). All subjects were scanned without the use of sedation. We collected a high-resolution T1-weighted anatomical image using a 5-min inversion recovery (IR)-prep 3D fast-spoiled gradient (FSPGR) sequence collected in the sagittal plane (0.9-mm3 voxel size) and a diffusion-weighted sequence consisting of a 30-direction diffusion-weighted scan (b = 1000 s/mm2) with 3 b = 0 scans. Tensor fitting was implemented using the RESTORE algorithm (Robust Estimation of Tensors by Outlier Rejection) (Chang et al., 2005). This algorithm excludes outlier data points automatically, and thus takes care of noisy volumes, including ones caused by subject motion. Imaging parameters, data preprocessing steps, including procedures for motion correction and diffusion tensor estimation are described in the Supplementary Material and in Dodson et al. (Dodson et al., 2018). We chose to analyze 2 diffusion tensor metrics: Fractional anisotropy (FA), a sensitive index of the directionality of water diffusion within the voxels of the brain, and mean diffusivity (MD), an index of the magnitude of water diffusion within the voxels. Deterministic tractography, fiber tract identification, segmentation and quantification were implemented using the Automated Fiber Quantification (AFQ; https://github.jyeatman/AFQ) software package (Yeatman et al., 2012) and MATLAB (MATLAB and Statistics Toolbox Release R2014a, The MathWorks, Inc., Natick, Massachusetts, United States). Details of these procedures are described in the Supplementary Material and have been reported in previous publications (Dodson et al., 2017, Dodson et al., 2018; Travis et al., 2017).
We used AFQ to segment the corpus callosum into 7 discrete and non-overlapping regions based on the cortical zone where fiber projections terminated (anterior frontal, superior frontal, motor, superior parietal, posterior parietal, temporal, and occipital). This method allows for anatomically specific and functionally relevant divisions of the corpus callosum (Fig. 1) (Dougherty et al., 2007; Huang et al., 2005). Tract profiles of each tissue parameter (FA and MD) were calculated at 30 equidistant locations along the central portion of each fiber tract bounded by the same two regions of interest used for tract segmentation. This procedure generated an FA or MD tract profile that described the variations in either FA or MD along the central portion of the tract. Tract profiles were then averaged to produce a single mean value for each of the 7 segments. Tracking was successful in all segments except for the motor segment in one PT+ participant and the posterior parietal segment in one FT participant.
Fig. 1.
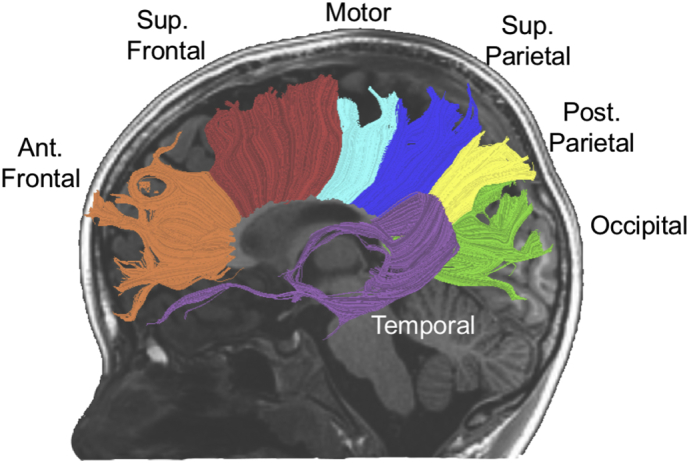
Tractography of 7 segments of callosal white matter tracts displayed on a mid-sagittal T1-weighted image from a representative preterm subject: occipital = green, temporal = purple, posterior parietal (Post. Parietal) = yellow, superior parietal (Sup. Parietal) = blue, motor = aqua, superior frontal (Sup. Frontal) = red, anterior frontal (Ant. Frontal) = orange. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3.2. Cognitive assessments
Participants completed a battery of standardized measures of cognitive skills. Intellectual abilities (IQ) were assessed using the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) (Wechsler and Hsiao-pin, 2011); we used full scale IQ, a composite score (mean = 100 and standard deviation = 15) based on four subtest T-scores (Vocabulary, Similarities, Block Design, and Matrix Reasoning). Reading ability was measured by the Woodcock Reading Mastery Tests, Third Edition (WRMT-III) (Woodcock, 2011). We used Basic Skills standard scores (mean = 100, standard deviation = 15), which assess children's ability to decode words. Executive Function was assessed using a parent-report measure, the Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al., 2000). We used the Global Executive Composite (GEC) T-score (mean 50, standard deviation = 10), which is an overall score based on ratings of 8 clinical scales encompassing behavioral regulation (Inhibit, Shift, and Emotional Control) and metacognition (Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor). Higher GEC scores indicate increasing problems in the domain of executive function. Scores at or above 65 are considered clinically significant. Cognitive assessments were obtained in all participants except for a missing reading measure in one FT participant.
2.4. Statistical analyses
2.4.1. Demographic variables
We computed separate one-way analyses of variance (ANOVAs) to compare continuous variable demographics, including gestational age, birthweight, age at scan, and socioeconomic status, between groups with Tukey-Kramer or Games-Howell post-hoc tests to follow-up on significant differences, depending on homogeneity of variances. Fisher's Exact tests were calculated to compare sex, race, and ethnicity across the three groups (PT+, PT-, FT).
2.4.2. Group comparisons of white matter microstructural properties and cognitive measures
2.4.2.1. Primary analyses: FA, MD, IQ, reading, and executive function
Two series of general linear models with planned contrasts were computed to examine the contribution of group (PT+, PT-, FT) to (1) FA or MD of each corpus callosum segment, and (2) cognitive outcomes. Two additional series of general linear models, including only PT children with gestational age as a covariate, were conducted to examine the contribution of group (PT+, PT-) to (1) FA or MD of each corpus callosum segment and (2) cognitive outcomes, independent of gestational age. Group differences were considered to be significant at p < 0.05. To control for multiple comparisons across segments and cognitive measures, the significance of omnibus tests was determined using a false discovery rate (FDR) of 5% (Benjamini and Hochberg, 1995).
2.4.2.2. Secondary analyses: AD, and RD
To investigate the contributions of AD and RD to group differences in FA, we conducted a one-way multivariate analysis of variance (MANOVA) (Travis et al., 2015; Dodson et al., 2017). For the MANOVA, group (FT, PT-, PT+) served as the between-subjects variable and mean AD and mean RD within the significant segment served as the dependent variables. We chose to enter AD and RD into the same model to reduce the number of comparisons and increase power for detecting group differences. To identify whether significant group effects identified in the MANOVA were the result of group differences in AD, RD or both, we then performed post hoc univariate general linear models with planned contrasts.
2.4.3. Correlations between white matter microstructural and cognitive measures
Pearson correlations were used to assess the strength of the association between FA or MD and cognitive measures in the combined sample of FT and PT participants. Moderation analysis based on the method of Hayes (Hayes, 2017) was used to assess whether correlations differed between groups. Correlations were considered to be significant at p < 0.05. To control for multiple comparisons across segments and cognitive measures, significance using a FDR of 5% was also computed (Benjamini and Hochberg, 1995).
3. Results
3.1. Participant characteristics
Characteristics of the FT, PT+ and PT- participants are shown in Table 1. PT+ had lower GA and lower birthweight (BW) than the PT- group. GA and BW were highly correlated r = 0.815, p < 0.0005. Because of collinearity, we controlled only for GA in analyses of the PT groups. Children in the 3 groups did not differ in age at dMRI scan, race, or socioeconomic status (all p > 0.05).
Table 1.
Participant characteristics by group with subgroup comparisons.
| FT (n = 43) |
PT- (n = 23) |
PT+ (n = 12) |
Comparison PT- to FT |
Comparison PT+ to FT |
Comparison PT+ to PT- |
|
|---|---|---|---|---|---|---|
| Mean (SD) | Mean difference (95% CI) | Mean difference (95% CI) | Mean difference (95% CI) | |||
| Gestational Age, weeks | 40 (1) | 30 (1) | 27 (3) | 9 (8, 10)⁎⁎ |
12 (10, 14)⁎⁎ |
3 (1, 5)⁎ |
| Birthweight, grams | 3320 (412) | 1463 (293) | 1002 (552) | 1858 (1647, 2069)⁎⁎ |
2318 (1871, 2765)⁎⁎ |
460 (14, 906)⁎ |
| Socioeconomic Statusa | 59 (10) | 50 (16) | 52 (14) | N.S. | N.S. | N.S. |
| Age at dMRI, years | 6.2 (0.2) | 6.2 (0.2) | 6.2 (0.1) | N.S. | N.S. | N.S. |
| Number (%) | ||||||
| Male | 16 (37) | 16 (70) | 6 (50) | ⁎ | N.S. | N.S. |
| Non-white | 12 (28) | 8 (35) | 4 (33) | N.S. | N.S. | N.S. |
FT = Full Term, PT- = Preterm without Inflammation, PT+ = Preterm with inflammation.
N.S. = Non-significant, p ≥ 0.05; CI = confidence interval.
Socioeconomic status measured by a modified Hollingshead Index.
p < 0.05.
p < 0.0005.
Medical information for the PT participants is presented in Table 2. We found no differences between PT+ and PT- in the percentage of children born small for gestational age, who had patent ductus arteriosus, or hyperbilirubinemia (all p > 0.05). Abnormal clinical MRI findings were documented in 9 participants (PT- = 4, PT+ = 5, p = 0.1). White matter abnormalities were documented as mildly abnormal in 7 and severity was not rated in 2 participants. In the PT- group, abnormal MRI findings were one each of the following: bilateral immature white matter, increased signal intensity in T2, cerebellar malformation, and not specified. In the PT+ group, abnormal MRI findings were one each of the following: grade 1 IVH, small periventricular lesions, multiple small hemorrhages, multifocal hemorrhage, remaining bleed. The PT- group had a lower incidence of intraventricular hemorrhage greater than or equal to grade 2 and retinopathy of prematurity than the PT+ group. Gestational age was significantly lower in children who experienced retinopathy of prematurity (t = 7.56 p < 0.0005) or who experienced intraventricular hemorrhage ≥ 2 (t = 2.72, p = 0.01). Gestational age was not significant different in children who were or were not small for gestational age (t = 0.51, p = 0.6) or those with and without hyperbilirubinemia (t = 0.97, p = 0.3).
Table 2.
Medical risk factors for preterm infants.
| PT- n (%) | PT+ n (%) | |
|---|---|---|
| Culture Positive Sepsis | N/A | 6 (50) |
| Bronchopulmonary Dysplasia | N/A | 7 (58) |
| Necrotizing Enterocolitis | N/A | 6 (50) |
| Small for Gestational Age | 1 (5) | 2 (17) |
| Patent Ductus Arteriosus | 8 (35) | 7 (58) |
| Hyperbilirubinemia | 11 (52) | 9 (82) |
| Retinopathy of Prematurity⁎⁎ | 0 (0) | 8 (67) |
| Intraventricular Hemorrhage, ≥ Grade 2⁎ | 0 (0) | 3 (27) |
| Abnormal Near-Term MRI | 4 (18) | 5 (45) |
PT- = preterm without inflammation, PT+ = preterm with inflammation.
p < 0.05.
p < 0.0005.
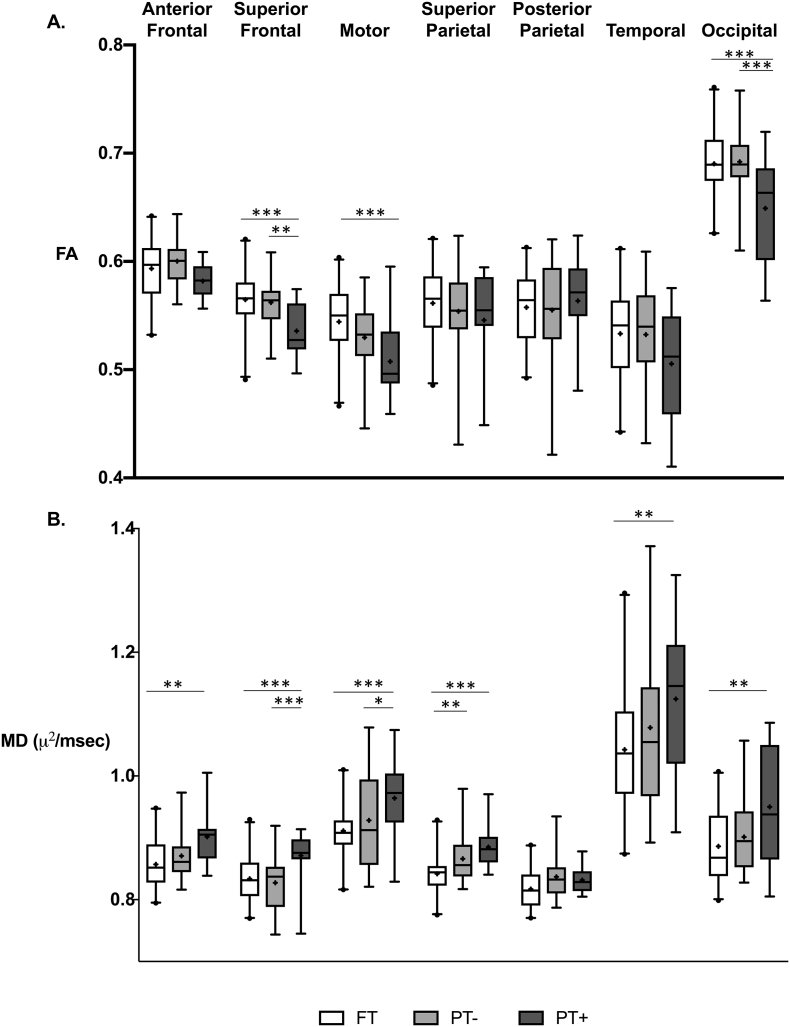
3.2. FA of white matter in the FT, PT-, and PT+ groups
Mean values and the general linear model for FA are shown in Table 3 and Fig. 2A. In the analysis of the three groups, the general linear model revealed significant main effects of group in the superior frontal, motor, and occipital segments. Planned comparisons showed significantly lower FA in PT+ compared to FT and compared to PT-. Differences in mean FA between PT- and FT were not statistically significant in any segment. After covarying for gestational age, a main effect of group remained significant only for the superior frontal segment, indicating that inflammation had an independent effect on mean FA (Table S1).
Table 3.
General linear model and planned contrasts of mean FA of 7 segments of the corpus callosum for the three participant groups.
| Main Effects |
Comparison PT- to FT |
Comparison PT+ to FT |
Comparison PT+ to PT- |
|||||
|---|---|---|---|---|---|---|---|---|
| FT (n = 43) |
PT- (n = 23) |
PT+ (n = 12) |
F (2,75) | η2 | Mean Difference (95% CI) |
Mean Difference (95% CI) |
Mean Difference (95% CI) |
|
| Anterior Frontal | 0.59 (0.03) | 0.60 (0.02) | 0.58 (0.02) | 2.27 | 0.06 | |||
| Superior Frontal | 0.56 (0.03) | 0.56 (0.02) | 0.54 (0.02) | 6.94⁎ | 0.16 | 0.00 (-0.02,0.01) |
-0.03 (-0.05,-0.01)⁎⁎ |
-0.03 (-0.04,-0.01)⁎ |
| Motora | 0.54 (0.03) | 0.53 (0.03) | 0.51 (0.04) | 6.17⁎ | 0.14 | -0.02 (-.03,0.0) |
-0.04 (-0.06,-0.02)⁎ |
-0.02 (-0.06,-0.00) |
| Superior Parietal | 0.56 (0.03) | 0.55 (0.04) | 0.55 (0.05) | 0.89 | 0.02 | |||
| Posterior Parietala | 0.56 (0.03) | 0.56 (0.05) | 0.56 (0.04) | 0.20 | 0.01 | |||
| Temporal | 0.53 (0.04) | 0.53 (0.05) | 0.51 (0.05) | 1.81 | 0.05 | |||
| Occipital | 0.69 (0.03) | 0.69 (0.03) | 0.65 (0.05) | 8.18⁎ | 0.18 | 0.00 (-0.02,0.02) |
-0.04 (-0.06,-0.02)⁎⁎ |
-0.04 (-0.07,-0.02)⁎⁎ |
FT = Full Term, PT- = Preterm without Inflammation, PT+ = Preterm with inflammation.
df = 2, 74.
p < 0.01.
p < 0.001.
Fig. 2.
White matter microstructure box plots by segment and group. Box plots illustrate median (─), mean (+), 1st and 3rd quartiles (━), 2.5–97.5%ile (┴), and outliers (•). General linear models for the three groups with planned contrasts as shown in Table 3, Table 4. A. FA = Fractional Anisotropy, B. MD = Mean Diffusivity. FT = Full Term, PT- = Preterm without inflammation, PT+ = Preterm with inflammation. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. Secondary analyses of AD and RD in the FT, PT-, and PT+ groups
In tract segments in which we found significant group differences in FA, MANOVAs revealed significant multivariate effects of Group (FT, PT-, or PT+) in the Superior Frontal (F = 6.4, p < 0.0005), Motor (F = 3.5, p = 0.009), and Occipital (F = 4.0, p = 0.004) segments. The subsequent post-hoc general linear models and planned contrasts are presented in Table S4. In the superior frontal segment, the PT+ group showed significantly increased AD and significantly increased RD compared to FT and PT- groups. In the motor and occipital segments, the PT+ group showed significantly increased RD compared to FT and PT- groups.
3.4. MD of white matter in the FT, PT-, and PT+ groups
Mean values and the general linear model for MD are shown in Table 4 and Fig. 2B. In the analysis of the three groups, the general linear model revealed significant main effects of group in all segments. Planned comparisons showed significantly higher MD in PT+ compared to FT in 6 of 7 segments and compared to PT- MD in 5 of 7 segments. PT- MD was higher than FT MD in one segment. After covarying for gestational age, a main effect of group remained significant only in the superior frontal segment, indicating that inflammation had an independent effect on mean MD (Table S2).
Table 4.
General linear model and planned contrasts of mean MD (μ2/msec) of 7 segments of the corpus callosum for the three participant groups.
| Main effects |
Comparison PT- to FT |
Comparison PT+ to FT |
Comparison PT+ to PT- |
|||||
|---|---|---|---|---|---|---|---|---|
| FT (n = 43) |
PT- (n = 23) |
PT+ (n = 12) |
F (2,75) |
η2 | Mean Difference (95% CI) |
Mean Difference (95% CI) |
Mean Difference (95% CI) |
|
| Anterior Frontal | 0.86 (0.04) | 0.87 (0.04) | 0.90 (0.04) | 5.60⁎⁎ | 0.13 | 0.02 (−0.01, 0.04) |
0.04 (0.02, 0.07)⁎⁎ |
0.03 (0.00, 0.08) |
| Superior Frontal | 0.83 (0.03) | 0.82 (0.04) | 0.88 (0.02) | 11.69⁎⁎⁎ | 0.24 | −0.01 (−0.03, 0.01) |
0.05 (0.03, 0.07)⁎⁎⁎ |
0.06 (0.03, 0.08)⁎⁎⁎ |
| Motora | 0.91 (0.05) | 0.92 (0.08) | 0.98 (0.05) | 5.74⁎⁎ | 0.13 | 0.01 (−0.02, 0.04) |
0.07 (0.03, 0.10)⁎⁎⁎ |
0.05 (0.01, 0.09)⁎ |
| Superior Parietal | 0.84 (0.03) | 0.87 (0.04) | 0.88 (0.04) | 8.66⁎⁎⁎ | 0.19 | 0.03 (0.01, 0.04)⁎⁎ |
0.04 (0.02, 0.06)⁎⁎⁎ |
0.02 (−0.01, 0.04) |
| Posterior Parietala, b | 0.82 (0.03) | 0.84 (0.03) | 0.83 (0.02) | 3.23 | 0.08 | |||
| Temporal | 1.04 (0.10) | 1.07 (0.13) | 1.14 (0.11) | 4.02⁎ | 0.10 | 0.03 (−0.03, 0.08) |
0.10 (0.03, 0.17)⁎⁎ |
0.07 (−0.01, 0.15) |
| Occipital | 0.89 (0.06) | 0.90 (0.06) | 0.95 (0.10) | 4.46⁎ | 0.11 | 0.02 (−0.01, 0.05) |
0.06 (0.02, 0.10)⁎⁎ |
0.04 (0.00, 0.09) |
FT = Full Term, PT- = Preterm without Inflammation, PT+ = Preterm with inflammation. MD = mean diffusivity.
df = 2, 74.
Omnibus test does not survive False Discovery Rate correction for multiple comparisons.
p < 0.05.
p < 0.01.
p < 0.001.
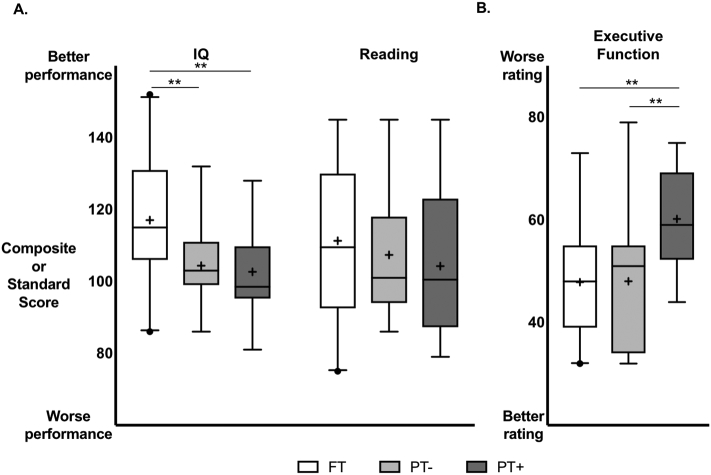
3.5. Cognitive outcomes of the FT, PT-, and PT+ groups
Mean values and general linear models for cognitive measures are shown in Table 5 and Fig. 3. In the analysis of the three groups, the general linear model revealed a significant main effect of group in IQ. Planned comparisons showed significantly lower IQ in PT+ and in PT- compared to FT. Differences in IQ between PT+ and PT- were not statistically significant, either with or without covarying for gestational age (Table S3). Thus, both PT groups had lower IQ scores than the FT group.
Table 5.
General linear model and planned contrasts of the three cognitive outcome measures for the three participant groups.
| Main Effects |
Comparison PT- to FT |
Comparison PT+ to FT |
Comparison PT+ to PT- |
|||||
|---|---|---|---|---|---|---|---|---|
| FT (n = 43) |
PT- (n = 23) |
PT+ (n = 12) |
F (2,75) |
η2 | Mean difference (95% CI) |
Mean difference (95% CI) |
Mean difference (95% CI) |
|
| IQ | 117.1 (16.4) | 101.4 (9.1) | 102.3 (10.0) | 7.95⁎⁎ | 0.18 | −12.7 (−20.2, −5.1)⁎⁎ |
−14.4 (−23.9, −4.9)⁎⁎ |
−1.7 (−12.1, 8.7) |
| Readinga | 111.3 (21.0) | 107.4 (18.3) | 104.3 (20.7) | 0.68 | 0.02 | |||
| Executive Function | 47.9 (10.7) | 47.8 (10.1) | 60.8 (10.8) | 6.11⁎⁎ | 0.14 | −0.2 (−5.6, 5.9) |
12.3 (5.0, 19.6)⁎⁎ |
12.1 (4.2, 20.0)⁎⁎ |
FT = Full Term, PT- = Preterm without Inflammation, PT+ = Preterm with inflammation. IQ is measured by the WASI-II full scale IQ composite score. Reading is measured by the WRMT-III Basic Skills standard score. Executive function is measured by the BRIEF Global Executive Composite T-score.
df = 2, 74.
p < 0.01.
Fig. 3.
Cognitive measure box plots by assessment and group. Box plots illustrate median (─), mean (+), 1st and 3rd quartiles (━), 2.5–97.5%ile (┴), and outliers(•) for A. IQ and Reading and B. Executive Function. General linear models for the three groups with planned contrasts as shown in Table 5. IQ is measured by the WASI-II full scale IQ composite score. Reading is measured by the WRMT-III Basic Skills standard score. Executive function is measured by the BRIEF Global Executive Composite T-score. FT = Full Term, PT- = Preterm without inflammation, PT+ = Preterm with inflammation. ** p < 0.01.
There was no significant main effect of group in reading.
In the analysis of the three groups, the general linear model revealed a significant main effect of group in executive function. Planned comparisons showed significantly higher executive function scores in PT+ compared to FT and compared to PT-, indicating poorer parent ratings of executive function in PT+ compared to the other two groups. Differences in executive function scores between PT- and FT were not statistically significant. After covarying for gestational age, a main effect of group remained significant, indicating that inflammation had an independent effect on parent ratings of executive function beyond the degree of prematurity (Table S3).
3.6. Correlations between white matter metrics and cognitive outcomes
Correlations between FA and between MD and IQ, reading, and executive function are shown in Table 6. IQ and reading were significantly positively correlated with occipital FA. Executive function was negatively correlated with occipital FA. These associations remained significant after correcting for multiple comparisons. Executive function was additionally significantly negatively correlated with anterior frontal FA and significantly positively correlated with superior parietal FA. These associations did not survive corrections for multiple comparisons. We had anticipated stronger correlations between IQ, reading, and executive function and white matter microstructure. Upon visual inspection of scatter plots of FA in relation to outcomes, the direction of the association appeared to be different for the FT and PT+ children in the anterior frontal and occipital segments as shown in Fig. S1. Therefore, we investigated whether group (FT, PT-, PT+) moderated the significant structure-function correlations. The interactions between group and mean FA fell short of statistical significance for all cognitive outcomes (all p > 0.05), most likely due to the fact that our sample size was not sufficiently large to detect significant interactions of this magnitude in structure-function correlations.
Table 6.
Pearson correlations between white matter metrics and cognitive outcomes in the combined sample of FT and PT participants.
| IQ |
Reading |
Executive function |
|
|---|---|---|---|
| Subdivision | r | ||
| FA | |||
| Anterior Frontal | 0.06 | 0.02 | −0.25⁎, a |
| Superior Frontal | 0.08 | 0.13 | 0.02 |
| Motor | 0.13 | 0.12 | 0.04 |
| Superior Parietal | 0.04 | 0.06 | 0.23⁎, a |
| Posterior Parietal | 0.07 | −0.03 | −0.07 |
| Temporal | −0.11 | 0.13 | −0.12 |
| Occipital | 0.25⁎ | 0.32⁎⁎ | −0.29⁎ |
| MD | |||
| Anterior Frontal | −0.19 | 0.00 | 0.05 |
| Superior Frontal | −0.10 | −0.12 | 0.14 |
| Motor | −0.07 | −0.14 | 0.10 |
| Superior Parietal | −0.22 | −0.22 | 0.03 |
| Posterior Parietal | −0.22 | −0.08 | 0.11 |
| Temporal | −0.03 | −0.20 | 0.15 |
| Occipital | −0.22 | −0.07 | 0.16 |
FA = Fractional Anisotropy, MD = Mean Diffusivity.
p < 0.05.
p < 0.01.
Does not survive False Discovery Rate Correction for multiple comparisons.
4. Discussion
4.1. Summary of results
In this exploratory investigation of 6-year-old children, we contrasted two white matter metrics from diffusion MRI and cognitive outcomes in three groups: PT+, PT children with a history of neonatal inflammatory conditions; PT-, those PT children without inflammation; and FT children. We found that the PT+ group had decreased FA and increased MD in multiple segments of the corpus callosum compared to the PT- and FT groups; we found minimal differences in white matter metrics between the PT- and FT groups. Secondary analyses of AD and RD demonstrated that decreased FA in the PT+ was driven by significantly decreased RD in three segments of the corpus callosum and also by significantly increased AD only in the superior frontal segment. We also found that the PT+ group had less favorable scores compared to the PT- group on a parent-report measure of executive function. Other cognitive outcomes were not different across the two PT groups. We found significant associations between FA and cognitive outcomes, particularly in the occipital segment. Thus, the results led to the intriguing suggestion that inflammatory events in the newborn period may be associated with long-term properties of white matter pathways in the brains of children born preterm.
4.2. White matter outcomes of PT+ and PT- groups
Consistent with our initial hypotheses, the major finding of this study was that at age 6 years, the group of children born preterm who experienced any of the major inflammatory events in the neonatal period had alterations in mean white matter microstructural metrics compared to children born preterm without major inflammatory events. The present study extends findings of previous studies that have observed white matter differences at near term in preterm groups defined by presence or absence of single inflammatory conditions including prolonged ventilation or development of chronic lung disease (Alexandrou et al., 2014; Anjari et al., 2009; Rose et al., 2014; Teli et al., 2018), sepsis (Chau et al., 2012; Krishnan et al., 2007), or necrotizing enterocolitis (Pogribna et al., 2013). Here, the pattern for group differences in FA was generally consistent with the results of a meta-analysis that showed reduced FA in PT compared to FT groups in the genu, body and splenium (Li et al., 2015). A novel finding was that children born preterm who did not experience the major inflammatory events in the neonatal period showed minimal differences in white matter metrics compared to the full term group. Thus, these findings were suggestive that inflammation in the neonatal period may be an important contributor to brain structure of children born preterm. Our analytic strategy was to include children with one or more of three serious inflammatory conditions into one group, PT+. This approach may lead to more consistent findings than consideration of each condition separately. Using a sensitive tractography method for subdividing the corpus callosum facilitated the identification of group differences that many not have been evident had we considered the corpus callosum as a single region of interest (Basser and Pierpaoli, 1996; Feldman et al., 2010).
The findings of lower FA, higher RD and higher MD in the PT+ group are consistent with decreased levels of myelination, as shown in animal models of preterm birth (Hagberg et al., 2002). However, diffusion metrics including FA and MD are known to index multiple tissue properties in addition to myelin (Basser and Pierpaoli, 1996; Jones and Cercignani, 2010). Thus, the observed white matter changes (low FA; high RD and MD) within the PT groups may also reflect variations in axonal properties, such as decreased fiber densities and fiber coherence or increased axonal diameter, that have been shown to occur in different proportions across regions of the corpus callosum (Aboitiz et al., 1992). Future studies employing MRI techniques more specific to myelin (Mezer et al., 2013) and to axonal properties, such as diameter (Assaf et al., 2008), fiber coherence, and density (Berman et al., 2018; Zhang et al., 2012), are required to clarify the impact of inflammatory events in the aftermath of preterm birth on tissue properties across regions of the corpus callosum.
4.3. Cognitive outcomes of PT+ and PT- groups
The cognitive outcome findings in this study were complex. We found group differences between PT+ and PT- only in parent reports of executive function skills. Executive function skills are a heterogeneous set of cognitive abilities required to manage complex tasks and include working memory, cognitive flexibility, and inhibitory control. Decrements in executive function skills are highly prevalent among children born preterm (Burnett et al., 2018; Murray et al., 2016; Scott et al., 2017; Stalnacke et al., 2018; Taylor and Clark, 2016). We used a parent-report composite measure of executive functioning. Parent reports may not be directly comparable to laboratory- or performance-based tasks (Loe et al., 2015). However, the parent-report measure may capture clinically meaningful outcomes at an age when commonly used performance based tasks can be at floor values. Thus, the results suggest that executive function skills may be sensitive to neonatal medical history.
We did not find PT+/PT- group differences in intelligence or reading measures despite differences in white matter metrics of the corpus callosum. While medical history may contribute to white matter microstructure, clinical outcomes likely depend on many additional factors. Environmental input, educational experiences, and other variables may be equally or more important than neurobiology in determining clinical outcomes in these domains. Further investigation in larger cohorts will be necessary to identify whether children born FT or PT without neonatal inflammation may utilize different skills and/or neural pathways than PT with neonatal inflammation to accomplish the same tasks.
4.4. Correlations between white matter and cognitive outcomes
Consistent with previous studies, we identified correlations between cognitive measures and FA in multiple segments of the corpus callosum (Eikenes et al., 2011; Kontis et al., 2009; Odegard et al., 2009; Vallesi et al., 2016). Specifically, we found that higher FA in the occipital segment correlated with better performance on measures of IQ, reading, and better parent ratings of executive function. We also observed negative and positive correlations between executive function and FA in an anterior and a medial segment, respectively. This pattern of findings in generally consistent with previous studies that have observed positive and negative associations between cognitive and motor outcomes and FA from the corpus callosum (Dougherty et al., 2007) and other cerebral tracts in preterm and full term children (Dodson et al., 2018; Feldman et al., 2012; Travis et al., 2016; Groeschel et al., 2014). Tissue properties known to contribute to diffusion metrics (eg., axonal density, axonal diameter, fiber coherence and myelin) have been shown to vary across regions of the corpus callosum (Aboitiz et al., 1992). Variability in FA was also recently shown to be highly correlated with quantitative T1 MRI metrics for assessing myelin content in posterior but not anterior segments of the corpus callosum in preterm and full term children (Travis et al., 2019). Based on such evidence, we speculate that positive structure-behavior associations observed within posterior callosal regions may reflect individual differences in myelin content whereas negative structure-behavior associations observed within anterior callosal segment may reflect individual differences in axonal properties, such as axonal diameter or fiber coherence. Similar to previous studies, we also found that structure-behavior associations did not always occur within the same callosal segments in which we observed group differences (Travis et al., 2016). Such findings suggest that individual differences in cognitive abilities may depend on multiple pathways and tissue properties beyond those found to differ between PT and FT groups. Clarifying the tissue properties that contribute to the observed pattern structure-behavior associations will benefit from future studies able to combine multiple neuroimaging techniques for assessing myelin content (eg., quantitative T1 MRI (Mezer et al., 2013), fiber, and axonal properties (Zhang et al., 2012; Assaf et al., 2008).
4.5. Limitations
In this exploratory analysis, the sample size was modest. We used a simplistic definition of “inflammation” to group study participants. We did not consider maternal inflammatory events. The three inflammatory conditions were most common among children born at earlier gestational ages. While necrotizing enterocolitis, bronchopulmonary dysplasia, and sepsis all cause a strong systemic inflammatory response, we do not know the exact mechanism by which these conditions would lead to altered white matter. In the future, prospective measures of systemic inflammation in relation to outcomes, particularly among infants born extremely prematurely, would propel this line of research. We recognize that systemic inflammation may affect white matter metrics and outcomes in a variety of ways. Systemic inflammation affects the permeability and function of the blood-brain barrier (Banks and Erickson, 2010) and can also incite a local inflammatory response in the brain (Brochu et al., 2011), both of which may lead to altered white matter maturation (Wang et al., 2006). However, these major inflammatory complications may also lead to or be a proxy for other disturbances, including acute or chronic hypoxia, ischemia, or prolonged poor nutritional status. Inflammatory complications may be a marker for other environmental influences associated with altered white matter microstructure, such as increased number of invasive or painful procedures (Vinall et al., 2014). These related endogenous or exogenous insults may be the ultimate culprits impairing normal white matter development.
5. Conclusions and future directions
Dividing children born preterm into those who did and did not experience major inflammatory complications was useful for identifying differences in white matter metrics of the corpus callosum and in executive function skills. Further in depth exploration of neonatal inflammatory experiences and white matter pathways beyond the corpus callosum will contribute to understanding brain-behavior associations in the aftermath of preterm birth. Clinical care continues to evolve since these children were born. Newer practices, such as the standardized use of hydrocortisone to prevent chronic lung disease (Doyle et al., 2017) may be specifically relevant to investigations of white matter and outcomes in children born preterm. Studies of interventions to improve neurodevelopmental outcomes may consider inflammatory events in assigning risk status or in providing early interventions for improving neurodevelopmental outcomes.
Funding
This work was supported by National Institutes of Health [NICHD grant RO1-HD069162], and Health Resources and Services Administration Maternal Child Health Bureau [T77MC09796] to Heidi M Feldman, PI. Dr. Dubner is the Stanford Maternal Child Health Research Institute Tashia and John Morgridge Endowed Postdoctoral Fellow, who supplied support. The work was also supported by the National Institutes of Health [5K99HD084749] and the 2014 Society for Developmental and Behavioral Pediatrics Young Investigator Award to Katherine E Travis, PI.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101832.
Appendix A. Supplementary data
Supplementary material 1.
Supplementary material 2.
References
- Aboitiz F., Scheibel A.B., Fisher R.S., Zaidel E. Fiber composition of the human Corpus callosum. Brain Res. 1992;598(1):143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Alexandrou G., Martensson G., Skiold B., Blennow M., Aden U., Vollmer B. White matter microstructure is influenced by extremely preterm birth and neonatal respiratory factors. Acta Paediatr. 2014;103(1):48–56. doi: 10.1111/apa.12445. [DOI] [PubMed] [Google Scholar]
- Anderson, P., L. W. Doyle, and Group Victorian Infant Collaborative Study Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Andrews J.S., Ben-Shachar M., Yeatman J.D., Flom L.L., Luna B., Feldman H.M. Reading performance correlates with white-matter properties in preterm and term children. Dev. Med. Child Neurol. 2010;52(6):e94–100. doi: 10.1111/j.1469-8749.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjari M., Counsell S.J., Srinivasan L., Allsop J.M., Hajnal J., Rutherford M.A., David E.A. The Association of Lung Disease with cerebral white matter abnormalities in preterm infants. Pediatrics. 2009;124(1):268. doi: 10.1542/peds.2008-1294. [DOI] [PubMed] [Google Scholar]
- Assaf Y., Blumenfeld-Katzir T., Yovel Y., Basser P.J. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn. Reson. Med. 2008;59(6):1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A. Brain injury in the preterm infant: new horizons for pathogenesis and prevention. Pediatr. Neurol. 2015;53(3):185–192. doi: 10.1016/j.pediatrneurol.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A., Miller S.P. Brain injury in premature neonates: a primary cerebral Dysmaturation disorder? Ann. Neurol. 2014;75(4):469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A., Riddle A., McClure M.M. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38(2 Suppl):724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Ball G., Boardman J.P., Aljabar P., Pandit A., Arichi T., Merchant N., Rueckert D., Edwards A.D., Counsell S.J. The influence of preterm birth on the developing Thalamocortical Connectome. Cortex. 2013;49(6):1711–1721. doi: 10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Banks W.A., Erickson M.A. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Behrman R.E., Butler A.S., Institute of Medicine (U. S.). Committee on Understanding Premature Birth and Assuring Healthy Outcomes . National Academies Press; Washington, D.C.: 2007. Preterm Birth: Causes, Consequences, and Prevention. [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57(1):289–300. [Google Scholar]
- Berman S., West K.L., Does M.D., Yeatman J.D., Mezer A.A. Evaluating G-ratio weighted changes in the Corpus callosum as a function of age and sex. Neuroimage. 2018;182:304–313. doi: 10.1016/j.neuroimage.2017.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacio S.L., Glass H.C., Chau V., Berman J.I., Xu D., Brant R., Barkovich A.J., Poskitt K.J., Miller S.P., Ferriero D.M. Extreme premature birth is not associated with impaired development of brain microstructure. J. Pediatr. 2010;157(5):726–732. doi: 10.1016/j.jpeds.2010.05.026. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright H.R., Babata K., Allred E.N., Erdei C., Kuban K.C.K., Joseph R.M., O'Shea T.M., Leviton A., Dammann O. Neurocognitive outcomes at 10 years of age in extremely preterm newborns with late-onset bacteremia. J. Pediatr. 2017;187:43–49. doi: 10.1016/j.jpeds.2017.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochu M.E., Girard S., Lavoie K., Sebire G. Developmental regulation of the Neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: an experimental study. J. Neuroinflammation. 2011;8(May):55. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett A.C., Anderson P.J., Lee K.J., Roberts G., Doyle L.W., Cheong J.L.Y. Trends in executive functioning in extremely preterm children across 3 birth eras. Pediatrics. 2018;141(1) doi: 10.1542/peds.2017-1958. [DOI] [PubMed] [Google Scholar]
- Chang L.C., Jones D.K., Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn. Reson. Med. 2005;53(5):1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Chau V., Brant R., Poskitt K.J., Tam E.W., Synnes A., Miller S.P. Postnatal infection is associated with widespread abnormalities of brain development in premature Newborns. Pediatr. Res. 2012;71(3):274–279. doi: 10.1038/pr.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson C.K., Travis K.E., Ben-Shachar M., Feldman H.M. White matter microstructure of 6-year old children born preterm and full term. Neuroimage Clin. 2017;16:268–275. doi: 10.1016/j.nicl.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson C.K., Travis K.E., Borchers L.R., Marchman V.A., Ben-Shachar M., Feldman H.M. White matter properties associated with pre-Reading skills in 6-year-old children born preterm and at term. Dev. Med. Child Neurol. 2018;60(7):695–702. doi: 10.1111/dmcn.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R.F., Ben-Shachar M., Deutsch G.K., Hernandez A., Fox G.R., Wandell B.A. Temporal-Callosal pathway diffusivity predicts phonological skills in children. Proc. Natl. Acad. Sci. U. S. A. 2007;104(20):8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L.W., Cheong J.L., Ehrenkranz R.A., Halliday H.L. Early (< 8 Days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017;10:Cd001146. doi: 10.1002/14651858.CD001146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenes L., Løhaugen G.C., Brubakk A.M., Skranes J., Håberg A.K. Young adults born preterm with very low birth weight demonstrate widespread white matter alterations on brain DTI. NeuroImage. 2011;54(3):1774–1785. doi: 10.1016/j.neuroimage.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Feldman H.M., Yeatman J.D., Lee E.S., Barde L.H., Gaman-Bean S. Diffusion tensor imaging: a review for Pediatric researchers and clinicians. J. Dev. Behav. Pediatr. 2010;31(4):346–356. doi: 10.1097/DBP.0b013e3181dcaa8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H.M., Lee E.S., Loe I.M., Yeom K.W., Grill-Spector K., Luna B. White matter microstructure on diffusion tensor imaging is associated with conventional magnetic resonance imaging findings and cognitive function in adolescents born preterm. Dev. Med. Child Neurol. 2012;54(9):809–814. doi: 10.1111/j.1469-8749.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G.A., Isquith P.K., Guy S.C., Kenworthy L. Psychological Assessment Resources; 2000. BRIEF: Behavior Rating Inventory of Executive Function. [Google Scholar]
- Groeschel S., Tournier J.D., Northam G.B., Baldeweg T., Wyatt J., Vollmer B., Connelly A. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. Neuroimage. 2014;87(February):209–219. doi: 10.1016/j.neuroimage.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Hagberg H., Peebles D., Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and Excitotoxic insults. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8(1):30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Yamada K., Morimoto M., Morioka S., Tozawa T., Isoda K., Murakami A. Development of Corpus callosum in preterm infants is affected by the prematurity: in vivo assessment of diffusion tensor imaging at term-equivalent age. Pediatr. Res. 2011;69(3):249–254. doi: 10.1203/PDR.0b013e3182084e54. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. 2nd ed. Guilford Press; 2017. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- Hollingshead A. Yale University Department of Psychology; New Haven, CT: 1975. Four Factor Index of Social Status. [Google Scholar]
- Huang H., Zhang J., Jiang H., Wakana S., Poetscher L., Miller M.I., van Zijl P.C., Hillis A.E., Wytik R., Mori S. DTI Tractography based Parcellation of white matter: application to the mid-sagittal morphology of Corpus callosum. Neuroimage. 2005;26(1):195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Jo H.M., Cho H.K., Jang S.H., Yeo S.S., Lee E., Kim H.S., Son S.M. A comparison of microstructural maturational changes of the Corpus callosum in preterm and full-term children: a diffusion tensor imaging study. Neuroradiology. 2012;54(9):997–1005. doi: 10.1007/s00234-012-1042-8. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Kontis D., Catani M., Cuddy M., Walshe M., Nosarti C., Jones D., Wyatt J., Rifkin L., Murray R., Allin M. Diffusion tensor MRI of the Corpus callosum and cognitive function in adults born preterm. Neuroreport. 2009;20(4):424–428. doi: 10.1097/WNR.0b013e328325a8f9. [DOI] [PubMed] [Google Scholar]
- Krishnan M.L., Dyet L.E., Boardman J.P., Kapellou O., Allsop J.M., Cowan F., Edwards A.D., Rutherford M.A., Counsell S.J. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120(3):e604–e609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- Leviton A., Joseph R.M., Fichorova R.N., Allred E.N., Gerry Taylor H., Michael O'Shea T., Dammann O. Executive dysfunction early postnatal biomarkers among children born extremely preterm. J. Neuroimmune Pharmacol. 2018 doi: 10.1007/s11481-018-9804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Sun Z., Han Y., Gao L., Yuan L., Zeng D. Fractional anisotropy alterations in individuals born preterm: a diffusion tensor imaging meta-analysis. Dev. Med. Child Neurol. 2015;57(4):328–338. doi: 10.1111/dmcn.12618. [DOI] [PubMed] [Google Scholar]
- Loe I.M., Chatav M., Alduncin N. Complementary assessments of executive function in preterm and full-term Preschoolers. Child Neuropsychol. 2015;21(3):331–353. doi: 10.1080/09297049.2014.906568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe I.M., Adams J.N., Feldman H.M. Executive function in relation to white matter in preterm and full term children. Front. Pediatr. 2019;6(418) doi: 10.3389/fped.2018.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezer A., Yeatman J.D., Stikov N., Kay K.N., Cho N.J., Dougherty R.F., Perry M.L. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat. Med. 2013;19(12):1667–1672. doi: 10.1038/nm.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitha A., Foix-L'Helias L., Arnaud C., Marret S., Vieux R., Aujard Y., Thiriez G. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2):e372–e380. doi: 10.1542/peds.2012-3979. [DOI] [PubMed] [Google Scholar]
- Mullen K.M., Vohr B.R., Katz K.H., Schneider K.C., Lacadie C., Hampson M., Makuch R.W., Reiss A.L., Constable R.T., Ment L.R. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54(4):2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.L., Thompson D.K., Pascoe L., Leemans A., Inder T.E., Doyle L.W., Anderson J.F.I., Anderson P.J. White matter abnormalities and impaired attention abilities in children born very preterm. Neuroimage. 2016;124(Pt A):75–84. doi: 10.1016/j.neuroimage.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Skare S., Andersson J.L., Lilja A., Flodmark O., Fernell E. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr. Res. 2003;54(5):672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Odegard T.N., Farris E.A., Ring J., McColl R., Black J. Brain connectivity in non-Reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47(8–9):1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Pannek K., Scheck S.M., Colditz P.B., Boyd R.N., Rose S.E. Magnetic resonance diffusion Tractography of the preterm infant brain: a systematic review. Dev. Med. Child Neurol. 2014;56(2):113–124. doi: 10.1111/dmcn.12250. [DOI] [PubMed] [Google Scholar]
- Pogribna U., Yu X., Burson K., Zhou Y., Lasky R.E., Narayana P.A., Parikh N.A. Perinatal clinical antecedents of white matter microstructural abnormalities on diffusion tensor imaging in extremely preterm infants. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J., Vassar R., Cahill-Rowley K., Stecher Guzman X., Hintz S.R., Stevenson D.K., Barnea-Goraly N. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. Neuroimage Clin. 2014;5:169–177. doi: 10.1016/j.nicl.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapbach L.J., Adams M., Proietti E., Aebischer M., Grunt S., Borradori-Tolsa C., Bickle-Graz M. Outcome at two years of age in a Swiss National Cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12(December):198. doi: 10.1186/1471-2431-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M.N., Hunter S.J., Joseph R.M., O'Shea T.M., Hooper S.R., Allred E.N., Leviton A., Kuban K. Neurocognitive correlates of attention-deficit hyperactivity disorder symptoms in children born at extremely low gestational age. J. Dev. Behav. Pediatr. 2017;38(4):249–259. doi: 10.1097/DBP.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S.Y., Jeong H.J., Son D.W., Jeong J.S., Oh S.H., Park S.Y., Ryu T.H., Kim Y.B., Cho Z.H. Altered microstructure of white matter except the Corpus callosum is independent of prematurity. Neonatology. 2012;102(4):309–315. doi: 10.1159/000341867. [DOI] [PubMed] [Google Scholar]
- Skranes J., Vangberg T.R., Kulseng S., Indredavik M.S., Evensen K., Martinussen M., Dale A.M., Haraldseth O., Brubakk A.-M. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain J. Neurol. 2007;130(Pt 3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Stalnacke J., Lundequist A., Bohm B., Forssberg H., Smedler A.C. A longitudinal model of executive function development from birth through adolescence in children born very or extremely preterm. Child Neuropsychol. 2018:1–18. doi: 10.1080/09297049.2018.1477928. [DOI] [PubMed] [Google Scholar]
- Stoll B.J., Hansen N.I., Adams-Chapman I. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- Taylor H.G., Clark C.A. Executive function in children born preterm: risk factors and implications for outcome. Semin. Perinatol. 2016;40(8):520–529. doi: 10.1053/j.semperi.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teli R., Hay M., Hershey A., Kumar M., Yin H., Parikh N.A. Postnatal microstructural developmental trajectory of Corpus callosum subregions and relationship to clinical factors in very preterm infants. Sci. Rep. 2018;8(1):7550. doi: 10.1038/s41598-018-25245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Inder T.E., Faggian N., Johnston L., Warfield S.K., Anderson P.J., Doyle L.W., Egan G.F. Characterization of the Corpus callosum in very preterm and full-term infants utilizing MRI. Neuroimage. 2011;55(2):479–490. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Adams J.N., Ben-Shachar M., Feldman H.M. Decreased and increased anisotropy along major cerebral white matter tracts in preterm children and adolescents. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Ben-Shachar M., Myall N.J., Feldman H.M. Variations in the neurobiology of Reading in children and adolescents born full term and preterm. Neuroimage Clin. 2016;11:555–565. doi: 10.1016/j.nicl.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Adams J.N., Kovachy V.N., Ben-Shachar M., Feldman H.M. White matter properties differ in 6-year old readers and pre-readers. Brain Struct. Funct. 2017;222(4):1685–1703. doi: 10.1007/s00429-016-1302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Castro M.R.H., Berman S., Dodson C.K., Mezer A.A., Ben-Shachar M., Feldman H.M. More than myelin: probing white matter differences in prematurity with quantitative T1 and diffusion MRI. NeuroImage Clin. 2019;22:101756. doi: 10.1016/j.nicl.2019.101756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallesi A., Mastrorilli E., Causin F., D'Avella D., Bertoldo A. White matter and task-switching in young adults: a diffusion tensor imaging study. Neuroscience. 2016;329:349–362. doi: 10.1016/j.neuroscience.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangberg T.R., Skranes J., Dale A.M., Martinussen M., Brubakk A.M., Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage. 2006;32(4):1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Vinall J., Miller S.P., Bjornson B.H., Fitzpatrick K.P.V., Poskitt K.J., Brant R., Synnes A.R., Cepeda I.L., Grunau R.E. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics. 2014;133(3):412–421. doi: 10.1542/peds.2013-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J., Kinney H.C., Jensen F.E., Rosenberg P.A. The developing Oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 2011;29(4):423–440. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Rousset C.I., Hagberg H., Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin. Fetal Neonatal Med. 2006;11(5):343–353. doi: 10.1016/j.siny.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wechsler D., Hsiao-pin C. 2nd Ed. Pearson; San Antonio, TX: 2011. WASI-II: Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Woodcock R.W. 3rd Ed. Pearson; San Antonio, TX: 2011. Woodcock Reading Mastery Tests: WRMT-III. [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating Fiber-tract quantification. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo Neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1.
Supplementary material 2.