Abstract
Background
Kidney allograft half-life has not improved despite excellent short-term survival. Recent long-term surveillance biopsy studies identify accumulating glomerulosclerosis (GS) to be associated with late allograft loss. While podocyte depletion is well known to drive proteinuria and GS in animal models and human glomerular diseases, its role in renal allograft loss of function is generally not recognized.
Methods
To address these questions, we collected urine from 125 kidney allograft recipients in the first posttransplant year for urine pellet messenger RNA (mRNA) and protein analysis, with a median follow up of 4.5 years.
Results
Using multivariable linear models adjusted for proteinuria, transplant, recipient and donor factors, we observed that the average urine pellet podocin mRNA normalized to urine creatinine (UPodCR) in the first posttransplant year was significantly associated with an estimated glomerular filtration rate (eGFR) decline (P = 0.001). The relationship between UPodCR and eGFR decline persisted even among recipients who were nonproteinuric and who had no recurrent or de novo glomerular disease identified on 1-year protocol biopsy. Finally, we identified recipient, donor and recipient:donor body surface area mismatch ratio to be independently associated with UPodCR early after transplantation. A larger donor was protective, while a larger recipient and increased recipient:donor size mismatch ratio were associated with increased UPodCR.
Conclusions
These findings support the concept that in kidney allografts, accelerated podocyte loss precedes proteinuria and is associated with inferior long-term allograft outcomes as measured by eGFR decline and may be initiated by recipient:donor size mismatch. Modulating factors driving early podocyte detachment after kidney transplantation may help improve long-term outcomes.
Keywords: glomerulosclerosis, graft failure, kidney transplantation, podocytes, proteinuria
INTRODUCTION
With the remarkable advances in improving short-term allograft survival, the focus has now shifted toward extending long-term allograft half-life. Two surveillance biopsy studies point to the high prevalence of glomerular disease (GD) in long-term stable allografts [1, 2]. In the tacrolimus era, Stegall et al. [2] noted that by 10 years posttransplant, glomerulosclerosis (GS) was pervasive in allografts that were losing function, while transplant glomerulopathy (TG) and rejection were uncommon. These authors concluded that ‘while almost all renal allografts sustain major histologic injury by 10 years after transplantation, much damage appears nonimmunologic, suggesting that new approaches are needed to decrease late injury’. At the same time, proteinuria is well known to be associated with worse long-term allograft outcomes [3, 4]. The cause of increased GS prevalence and its contribution to long-term outcome remains unclear. Mitigating mechanisms of post-transplant GD could lead to improved outcomes in a setting where even a small improvement of allograft half-life would significantly impact societal costs and quality and quantity of life and help reduce the demand–supply gap in kidney transplantation.
There is now substantial support for the concept that hyperfiltration occurs after unilateral nephrectomy in man and in allografts [5–9]. Both model systems and human studies are compatible with progressive podocyte depletion from glomeruli serving as a mechanism by which hyperfiltration-associated events are transduced to GS [10–13]. Importantly, podocyte detachment from glomeruli becomes increased under conditions of hypertrophic stress, where they can be measured in the urine pellet as a noninvasive biomarker [11, 14]. Compatible with these concepts, we previously reported that podocyte detachment after kidney transplantation was increased on average 6-fold above that of controls, with lower levels (2-fold above control) being present in long-lived stable allografts and higher levels (10- to 20-fold above control) present in allografts with TG or recurrent GD [15]. In a second simulation study, we showed that this degree of accelerated podocyte detachment in association with the decrease in podocyte number per glomerulus associated with age would predict an allograft half-life close to the observed value at ∼15 years, as well as the well-known relationship of older donor age to worse allograft outcomes [16–18]. These data suggest that not only is accelerated podocyte detachment associated with worse allograft outcome, but there must be a rather strong relationship between them.
If the above observations are correct, the urine pellet podocin messenger RNA (mRNA) that is normalized to urine creatinine (UPodCR) would be predicted to be strongly associated with allograft function decline and graft loss. To test this hypothesis, we performed an observational study in which urine samples from allograft recipients were used to assess the relationship between UPodCR in the first year after transplantation compared with other risk factors known to be associated with worse allograft survival, with the slope of estimated glomerular filtration rate (eGFR) decline measured for >4.5 years of follow-up. In addition to the two glomerular podocyte mRNA markers (podocin and nephrin), we also measured urine pellet mRNAs for a distal tubular/collecting duct marker (aquaporin2) and a profibrotic marker [transforming growth factor β1 (TGF-β1)] to better understand the relationships between podocyte detachment in relation to tubular and profibrotic events measured using the same method in the same urine samples.
MATERIALS AND METHODS
Study design, participants and setting
We conducted an observational study of consenting adult patients presenting either for a kidney transplant or to the outpatient kidney transplant clinic at the University of Michigan from February 2012 to May 2015. All patients were approached and written and informed consent was obtained (IRB-HUM00055525). Patients were followed through 30 June 2017, the initiation of dialysis or death, whichever was earlier. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Declaration of Helsinki. Patients received induction per University of Michigan protocol at that time and the majority were on standard triple immunosuppression with tacrolimus, mycophenolate mofetil and prednisone at discharge.
Outcomes
Our primary outcome of interest was the rate of allograft function decline using the annualized slope of eGFR. The eGFR values were estimated according to the Modification of Diet in Renal Disease (MDRD) equation [19]. The MDRD equation has previously been validated in kidney transplantation as a measure of renal function [20, 21]. In addition, allograft function decline assessed as the eGFR slope has been validated in kidney transplantation as a surrogate of long-term outcomes [22]. As a secondary analysis, we evaluated two additional outcomes. First, we assessed for factors associated with death-censored graft loss, then for factors associated with increased UPodCR in the first 3 months posttransplant. We used the 3-month time point to be consistent with our previous study, which showed that maximal glomerular hypertrophy occurs by 3-months posttransplant [15].
Predictor variables
Recipient, donor demographic and clinical data as well as transplant characteristics are presented in Table 1. The ratio of recipient body surface area (BSA) to donor BSA (RD mismatch) was used as a surrogate of recipient-to-donor size mismatch [23]. BSA was calculated using the DuBois equations [24]. A ratio ≥1 would imply that the recipient’s BSA is larger than or equal to that of the donor and <1 would imply that the recipient is smaller than the donor.
Table 1.
Predictors of eGFR slope over a median 4.5 years of follow-up: univariable and multivariable analysis
| Univariable analysis |
Multivariable analysis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β coef. | SE | P-value | LCL | UCL | β coef. | SE | P-value | LCL | UCL | |
| Urine markers | ||||||||||
| Log UPodCR | −2.58 | 0.56 | <0.001 | −3.7 | −1.46 | −2.17 | 0.62 | 0.001 | −3.41 | −0.93 |
| Log UNephCR | −1.83 | 0.64 | 0.005 | −3.09 | −0.56 | |||||
| Log UAqp2CR | 0.09 | 0.53 | 0.86 | −0.96 | 1.15 | |||||
| Log UTGFβ1CR | −0.82 | 0.32 | 0.01 | −1.46 | −0.18 | −0.14 | 0.35 | 0.69 | −0.83 | 0.55 |
| Log UProtCR | −1.44 | 0.56 | 0.01 | −2.55 | −0.34 | 0.15 | 0.59 | 0.81 | −1.03 | 1.33 |
| Recipient | ||||||||||
| Age | −0.02 | 0.04 | 0.69 | −0.10 | 0.07 | |||||
| Black race | −4.80 | 1.61 | 0.004 | −7.98 | −1.60 | −1.91 | 1.48 | 0.20 | −4.85 | 1.00 |
| Gender, male | 3.02 | 1.23 | 0.02 | 0.59 | 5.47 | 1.41 | 1.21 | 0.25 | −0.98 | 3.80 |
| BSA | 2.15 | 2.33 | 0.36 | −2.48 | 6.77 | |||||
| cPRA | −0.05 | 0.02 | 0.02 | −0.10 | −0.01 | −0.02 | 0.02 | 0.26 | −0.06 | 0.02 |
| Previous transplant | −1.47 | 2.72 | 0.59 | −6.87 | 3.92 | |||||
| Dialysis vintage (ref: 0–2 years) | ||||||||||
| Preemptive | 2.23 | 1.56 | 0.16 | −0.86 | 5.33 | |||||
| Dialysis >2 years | 0.71 | 1.44 | 0.62 | −2.15 | 3.57 | |||||
| Cause of renal disease (ref: PKD) | ||||||||||
| Congenital/familial | 1.22 | 3.15 | 0.70 | −5.01 | 7.46 | |||||
| Glomerular | −0.35 | 2.36 | 0.88 | −5.04 | 4.33 | |||||
| Hypertension | 1.40 | 2.57 | 0.59 | −3.70 | 6.51 | |||||
| Diabetes | −2.67 | 2.30 | 0.25 | −7.22 | 1.88 | |||||
| Other | −3.16 | 2.54 | 0.22 | −8.21 | 1.88 | |||||
| Donor | ||||||||||
| Age | −0.00 | 0.05 | 0.94 | −0.10 | 0.09 | |||||
| AA (ref: non-AA) | −2.81 | 1.97 | 0.16 | −6.71 | 1.09 | |||||
| Male sex | −1.62 | 1.26 | 0.20 | −4.12 | 0.89 | |||||
| BSA | 1.71 | 2.29 | 0.46 | −2.83 | 6.25 | |||||
| Transplant | ||||||||||
| Cadaveric transplant | −1.92 | 1.31 | 0.15 | −4.52 | 0.68 | |||||
| Cold ischemia time | −0.18 | 0.08 | 0.03 | −0.33 | −0.02 | −0.10 | 0.07 | 0.16 | −0.25 | 0.04 |
| HLA mismatch | ||||||||||
| 3–4 | 0.88 | 1.59 | 0.58 | −2.27 | 4.02 | |||||
| 5–6 | 0.64 | 1.63 | 0.70 | −2.60 | 3.87 | |||||
| First-year events | ||||||||||
| DSA in first year | −1.50 | 1.57 | 0.34 | −4.62 | 1.62 | |||||
| Rejection in first year | −0.19 | 1.22 | 0.88 | −6.37 | 5.43 | |||||
| First-year biopsies | ||||||||||
| GGS >10% (ref: GGS <10%) | −5.67 | 1.78 | 0.002 | −9.19 | −2.15 | −4.20 | 1.65 | 0.01 | −7.50 | −0.94 |
| GD (TG or recurrent disease) | −8.66 | 2.22 | <0.001 | −13.1 | −4.26 | −5.77 | 2.04 | 0.006 | −9.83 | −1.70 |
| IFTA (ref: No IFTA) | ||||||||||
| Mild | 0.77 | 1.33 | 0.57 | −1.87 | 3.40 | 0.63 | 1.15 | 0.59 | −1.65 | 2.90 |
| Moderate–severe | −3.88 | 1.84 | 0.04 | −7.54 | −0.23 | −1.24 | 1.76 | 0.48 | −4.72 | 2.25 |
Coefficient values of urine markers represent the coefficients of their log-transformed values. The slope of eGFR can be obtained by multiplying the β-coefficient with the log of the fold increase desired. For example, a 3-fold increase in UPodCR would be β × log2(3) = −2.17 × 1.58 = −3.4 mL/min/1.73 m2/year. The β-coefficient for other values represents the change in eGFR in mL/min/1.73 m2/year. Urine nephrin was excluded from multivariable analysis due to collinearity with podocin mRNA. IFTA was defined as mild (<25%), moderate (25–50%) and severe as (>50% of the total area).
Coef., coefficient; SE, standard error; UPodCR, urine podocin mRNA:urine creatinine ratio; UNephCR, urine nephrin mRNA:creatinine ratio, UAqp2CR, urine aquaporin2 mRNA:urine creatinine ratio; UTGFβ1CR, urine transforming growth factor β mRNA:urine creatinine ratio; UProtCR, urine protein:urine creatinine ratio; AA, African American; cPRA, calculated panel reactive antibody; PKD, polycystic kidney disease, HLA, human leukocyte antigen; TG, transplant glomerulopathy; LCL, lower confidence limit; UCL, upper confidence limit.
Urine sample processing and mRNA assay
Urine pellet mRNA was assayed as previously described [14]. See the Supplementary data section entitled ‘Urine mRNA methodology, interpretation and limitations’ for details. All urine mRNA markers (podocin, nephrin, aquaporin2 and TGF-β1) were first normalized to urinary creatinine (to account for volume and dilution), log transformed (base 2) and then averaged. When only one sample was collected in the first year (13 patients), the individual values represented the ‘average’. A similar strategy was applied for assessment of proteinuria. These averaged log-transformed urine values were then used as independent predictors of allograft function in our primary analysis. A detectable mRNA signal was the only criterion used for inclusion or exclusion of urine samples.
Biopsy data
Biopsy data were obtained from implantation, 3-, 6- and 12-month surveillance biopsies or other indication biopsies performed in the first year and detailed results were abstracted from the patient’s chart. We used rejection in the first year as a binary variable. If the patient had two or more rejections in the first year, then the higher grade of rejection was used for analysis. Borderline rejection was excluded. For assessment of the relationship of histology with the average of urine mRNA markers, we utilized 1-year surveillance biopsies. If 1-year biopsies were not performed, we utilized the last performed biopsy within the first year. Banff 2007 classification was utilized at the University of Michigan at that time [25]. Donor-specific antibody data were obtained at the time of surveillance or indication biopsies.
Measurements
Assessment of the relationship between allograft loss of function and average UPodCR in the first year
The first eGFR data point used for this analysis coincided with the first urine collection for measurement of urine mRNA markers and proteinuria. Collection of eGFR data continued until the end of the follow-up period. For each subject we first performed a linear regression with eGFR (in mL/min/1.73 m2) as the dependent variable and time (from first collection) as the independent variable. The resulting estimated slope (in mL/min/1.73 m2/year) was used to derive the annualized eGFR decline for every patient. As a second step, in a linear regression we used the slope of eGFR as the dependent variable and donor, recipient, transplant factors and average of urine mRNA markers as well as proteinuria over the first year as independent variables.
Assessment of the relationship between average UPodCR in the first year and death-censored graft loss
As a secondary analysis, we assessed predictors of death-censored graft loss, using Cox proportional hazards model.
Assessment of the relationship between recipient and donor BSA mismatch and UPodCR
To analyze the determinants of UPodCR early after transplantation, we restricted analysis to grafts with one or more urine collections in the first 3 months posttransplantation. Due to the variability in the number of urine collections per patient, we used a linear mixed effects model with UPodCR as the dependent variable and an a priori set of independent predictors that was hypothesis and literature driven. These included donor age, type of kidney (living versus cadaveric), cold ischemia time, donor BSA, recipient BSA and the RD mismatch ratio (as a measure of the workload). Nonsignificant variables (P < 0.1) were removed sequentially, leaving only the significant variables in the final model (backward selection).
Statistical analysis
For normally distributed data, means of continuous variables were reported with their standard error. For nonnormal data, we reported medians with interquartile ranges (IQRs). Correlational studies were done using Spearman’s correlation. Student’s t-test was used to compare means of continuous variables across two groups while analysis of variance was used for two or more groups. Stata 13 I/C (StataCorp, College Station, TX, USA) was used for all analyses.
RESULTS
Demographic recipient, transplantation and donor characteristics are shown in Table 2. For the 125 allograft recipients, 10 327 eGFR observations were used to estimate the slope over the median 4.5-year follow-up with an average of 76 observations per allograft (range 9–345). In the first year, 534 urine samples were collected [mean 4/patient (range 1–15)] with a median time to collection after transplantation of 132 days (IQR 55–216). Only 13 of the 125 patients had a single urine sample collection in the first year (10.4%).
Table 2.
Recipient, donor and transplant characteristics
| Recipient | |
| Age (years), mean ± SD | 48.2 ± 14.8 (range 18–75) |
| Race | |
| Non-AA | 106 (84.8) |
| AA | 19 (14.6) |
| Males | 79 (63.2) |
| BMI (kg/m2), mean ± SD | 29.0 ± 5.4 |
| BSA (kg/m2), mean ± SD | 1.98 ± 0.26 |
| Cause of renal disease | |
| Congenital/familial | 8 (6.4) |
| PKD | 14 (11.2) |
| Glomerular | 28 (22.4) |
| Hypertension | 17 (13.6) |
| Diabetes | 39 (31.2) |
| Other | 19 (15.2) |
| Dialysis vintage | |
| Preemptive | 28 (22.4) |
| Dialysis 0–2 years | 60 (48.0) |
| Dialysis >2 years | 37 (29.6) |
| Calculated panel reactive antibody >20 | 22 (17.6) |
| Previous transplantation | 6 (4.8) |
| Induction (thymoglobulin) | 106 (85) |
| No induction | 19 (15) |
| Maintenance immunosuppression | |
| Tacrolimus, mycophenolate mofetil, steroid | 121 (96.8) |
| Tacrolimus, mycophenolate mofetil | 1(0.8) |
| Tacrolimus, everolimus, steroid | 2(1.6) |
| Cyclosporine, mycophenolate, steroid | 1(0.8) |
| Transplantation | |
| Living donor | 84 (67.2) |
| Cold ischemia time, median (IQR) | 3.0 (1.7–10.0) |
| HLA mismatch | |
| 0–2 | 30 (24.0) |
| 3–4 | 53 (42.0) |
| 5–6 | 42 (33.6) |
| Donor | |
| Age (years), mean ± SD | 40.2 ± 12.4 (range 7–64) |
| Race | |
| Non-AA | 111 (88.8) |
| AA | 14 (11.2) |
| Males | 53 (42.4) |
| BMI (kg/m2), mean ± SD | 26.9 ± 5.4 |
| BSA (kg/m2), mean ± SD | 1.86 ± 0.28 |
Values presented as n (%) unless stated otherwise. Diseases included congenital/familial (prune belly, renal hypoplasia, medullary cystic disease, renal agenesis, congenital obstructive uropathy), glomerular (IgA, FSGS, membranous, SLE, Wegner’s granulomatosis, Alport’s, chronic glomerulonephritis, Henoch–Schöenlein purpura) and other (lithium toxicity, pyelonephritis, obstructive uropathy, interstitial nephropathy, unknown).
AA, African American; BMI, body mass index; PKD, polycystic kidney disease; HLA, human leukocyte antigen; FSGS, focal segmental glomerulosclerosis; SLE, systemic lupus erythematosus.
Transplant outcomes
In total, 124 of 125 (>99%) recipients had at least one allograft biopsy (surveillance or indication) in the first year. Of these, 93 patients had a surveillance biopsy at 1 year ± 2 months. Biopsy-proven acute rejection [T-cell-mediated rejection (TCMR) Grade ≥1A or antibody-mediated rejection (AMR)] in the first year was diagnosed in 14 patients (11.2%). Of these, 12 had TCMR and 2 had mixed rejection [25]. The majority, 117/125 (93.6%), had donor-specific antibody (DSA) measured per protocol in the first year, of which 11 (9.4%) developed de novo DSA. Twenty allografts were lost by the end of the follow-up period (30 June 2017), of which 10 were due to death with function and 10 returned to dialysis/retransplantation [associated with rejection in 5 (2 with AMR, 2 with mixed and 1 with severe TCMR), 1 with primary nonfunction, 1 with interstitial fibrosis and tubular atrophy (IFTA), 2 with chronic thrombotic microangiopathy and 1 with TG complicated by recurrent immunoglobulin A nephropathy]. Eight allografts had glomerular disease (GD), including recurrent disease (n = 4) or de novo TG (n = 4). Mild IFTA was present in 51.2% of biopsies, with 13.2% having moderate–severe IFTA. Twenty allografts had >10% global GS (GGS). BK viremia (>5000 copies/mL) was present in 13 patients (10.5%), of which 4 had biopsy-proven BK virus nephropathy. Confirmed cytomegalovirus (CMV) infection was present in 5 patients (4.0%), while 9 of 83 nondiabetic recipients developed posttransplant diabetes mellitus (PTDM).
Long-term eGFR decline is associated with increased UPodCR occurring in the first year after transplantation
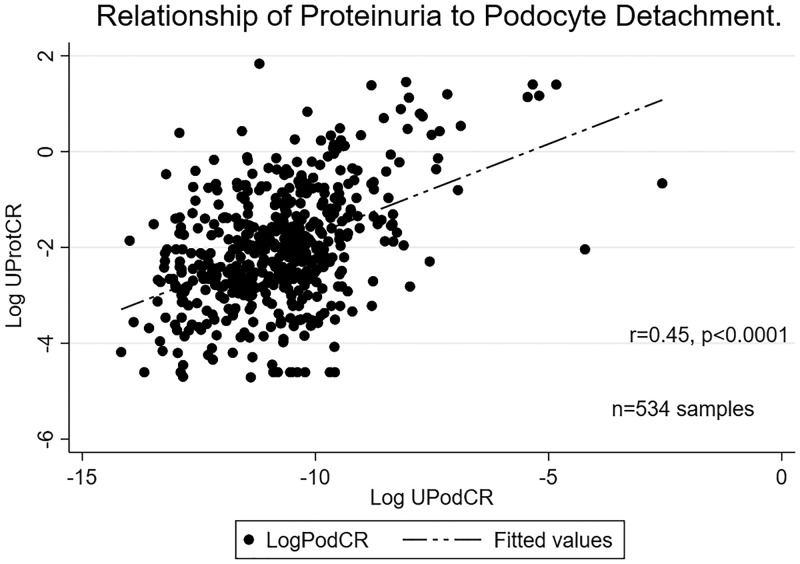
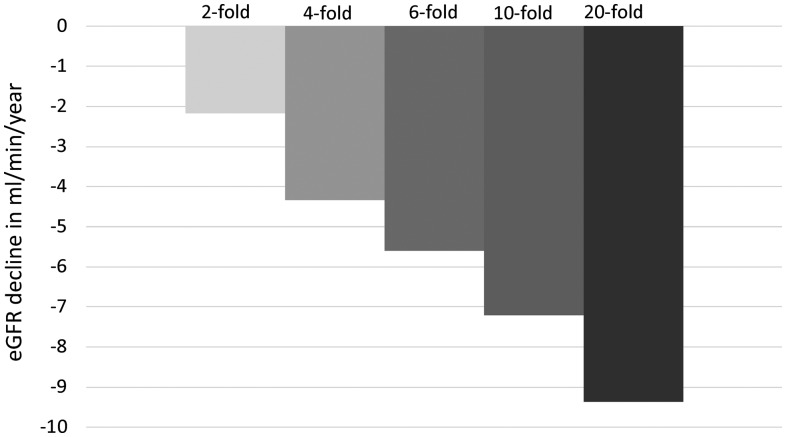
Table 1 demonstrates the multivariable analysis where only UPodCR, GGS > 10% and recurrent and de novo GD remained as significant predictors. Figure 1 demonstrates the moderate correlation of UPodCR with proteinuria (r = 0.45, p < 0.0001) among all the samples analyzed in the first year. Proteinuria, while being identified as a significant predictor of allograft function decline in a univariable analysis, was no longer significant in multivariable analysis. Removal of recipients with single urine values (n = 13) did not change the strength or direction of any association in the multivariable model. Figure 2 demonstrates the relationship between the fold increase in average podocyte loss and slope of eGFR.
FIGURE 1.
Relationship of urinary podocin mRNA normalized to urinary creatinine (UPodCR) and urine protein normalized to urinary creatinine (UProtCR). The figure demonstrates a moderate correlation with a Spearman’s rho of 0.45 (P < 0.0001) among samples obtained in the first year posttransplantation.
FIGURE 2.
Relationship of average urine pellet podocin mRNA in the first year and long-term renal function decline. The figure demonstrates that an increase in the average urine pellet podocin mRNA (UPodCR) in the first year is associated with eGFR decline. As an example, based on the coefficients obtained from the multivariable linear regression model, if the average eGFR of a kidney transplant by the end of the first year is 45 mL/min, then a 10-fold increased UPodCR, such as seen in TG, will be associated with a 7.2 mL/min/year loss in eGFR and thus the graft would be expected to reach end stage in another 4–5 years (eGFR <15 mL/min).
Donor and recipient BSA mismatch is associated with increased UPodCR in the first 3 months posttransplant
A larger donor was protective, while a larger recipient or a larger RD BSA mismatch ratio was associated with increased UPodCR in the first 3 months posttransplant (Table 3). When proteinuria (UProtCR) in the first 3 months after transplantation was used as the dependent variable there was no significant association with recipient BSA, donor BSA or recipient:donor mismatch ratio. In addition, to check for residual confounding by native kidney proteinuria or podocyturia, an independent univariable analysis did not reveal an association of 3-month or 12-month average UPodCR or UProtCR with preemptive versus nonpreemptive status, similar to a previous study [4]. Further, there was no association between rejection by 3 months and UPodCR at 3 months (data not shown).
Table 3.
Predictors of increased urine pellet podocin mRNA (UPodCR) in the first 3 months posttransplant (multivariable model)
| β coef. | SE | P-value | LCL | UCL | |
|---|---|---|---|---|---|
| Recipient:donor BSA mismatch | 10.2 | 5.12 | 0.05 | 0.14 | 20.2 |
| Recipient BSA | 5.0 | 2.58 | 0.05 | 0.00 | 10.1 |
| Donor BSA | −5.7 | 2.63 | 0.03 | −10.85 | −0.52 |
Initial hypothesis-driven multivariable model was adjusted for donor age, type of kidney (living versus cadaveric), cold ischemia time, donor BSA, recipient BSA and the RD BSA mismatch ratio (as a measure of the workload). In the next steps, nonsignificant variables were removed one at a time, leaving only the significant variables in the mode (backward selection) [38].
Based on β coefficients, a 0.1 kg/m2 increase in the donor BSA was associated with a 33% lower UPodCR. In contrast, a 0.1 kg/m2 increase in recipient BSA was associated with a 1.4-fold increase in average UPodCR. Similarly, a 0.1 increase in the recipient:donor BSA mismatch ratio was associated with a 2.0. Could write out just as 2-fold increase in UPodCR. Thus, for example, if the recipient BSA was 20% larger than the donor BSA, then one would expect an almost 4-fold increased UPodCR compared with a donor and recipient pair with a similar BSA.
BSA, body surface area (DuBois equation); Coef., coefficient (see text); SE, standard error; LCL, lower confidence limit; UCL, upper confidence limit.
Increased UPodCR in the first year was associated with death-censored graft loss
In a secondary analysis (limited by only 10 graft failures), we identified UPodCR to be a significant predictor of graft loss (Table 4). Due to the low death-censored graft failure rate, a multivariable analysis was not performed.
Table 4.
Predictors of death-censored graft failure for a median 4.5 years of follow-up: univariable analysis
| HR | SE | P-value | LCL | UCL | |
|---|---|---|---|---|---|
| Urine markers | |||||
| Log UPodCR | 2.26 | 0.65 | 0.004 | 1.29 | 3.97 |
| Log UNephCR | 1.88 | 0.66 | 0.07 | 0.95 | 3.76 |
| Log UAqp2CR | 0.81 | 0.27 | 0.53 | 0.43 | 1.55 |
| Log UTGFβ1CR | 1.31 | 0.23 | 0.13 | 0.93 | 1.85 |
| Log UProtCR | 1.90 | 0.55 | 0.03 | 1.08 | 3.35 |
| Recipient | |||||
| Age | 1.00 | 0.02 | 0.99 | 0.96 | 1.05 |
| Black race | 4.56 | 3.06 | 0.02 | 1.22 | 17.0 |
| Gender, male | 0.65 | 0.43 | 0.52 | 0.17 | 2.41 |
| BSA | 2.89 | 3.61 | 0.39 | 0.25 | 33.3 |
| cPRA | 1.01 | 0.01 | 0.11 | 0.99 | 1.03 |
| Dialysis vintage (ref: no dialysis or <2 years) | |||||
| Dialysis >2 years | 2.28 | 0.68 | 0.006 | 1.27 | 4.10 |
| Previous transplant | 4.47 | 3.63 | 0.07 | 0.91 | 22.0 |
| Donor | |||||
| Age | 1.01 | 0.03 | 0.61 | 0.96 | 1.07 |
| Black race | 4.20 | 2.98 | 0.04 | 1.05 | 16.9 |
| Male sex | 1.22 | 0.82 | 0.77 | 0.33 | 4.53 |
| BSA | 0.29 | 0.33 | 0.28 | 0.03 | 2.74 |
| Transplant | |||||
| Cadaveric transplant | 2.66 | 1.79 | 0.14 | 0.71 | 9.95 |
| Cold ischemia time | 1.08 | 0.04 | 0.03 | 1.00 | 1.15 |
| HLA mismatch | |||||
| 3–4 | 0.15 | 0.17 | 0.1 | 0.02 | 140 |
| 5–6 | 0.79 | 0.56 | 0.74 | 0.20 | 3.19 |
| First year | |||||
| DSA in first year | 1.86 | 1.50 | 0.44 | 0.39 | 9.02 |
| Rejection in first year | 0.84 | 0.58 | 0.80 | 0.22 | 3.23 |
| 1-year serum creatinine | 3.81 | 2.05 | 0.01 | 1.33 | 10.96 |
| First-year biopsies | |||||
| (ref: GGS <10%) | |||||
| GGS >10% | 5.39 | 3.63 | 0.01 | 1.44 | 20.2 |
| Recurrent or de novo GD | 13.0 | 8.80 | 0.0001 | 3.47 | 49.0 |
| IFTA (ref: No IFTA) | |||||
| Mild | 1.21 | 1.06 | 0.82 | 0.22 | 6.70 |
| Moderate–severe | 3.10 | 2.84 | 0.22 | 0.52 | 18.7 |
Due to nonconvergence of the model, preemptive and dialysis time <2 years were combined into one group. Given that only 10 graft losses (events) occurred in the absence of death, we did not perform multivariable analysis. Cause of ESRD was not shown in the tables due to the nonconvergence of models. IFTA was defined as mild (<25%), moderate (25–50%) and severe (>50% of the total area).
UNephCR, urine nephrin mRNA:creatinine ratio, UAqp2CR, urine aquaporin 2 mRNA:urine creatinine ratio; UTGFβ1CR, urine transforming growth factor β mRNA:urine creatinine ratio; UProtCR, urine protein:urine creatinine ratio; AA, African American; cPRA, calculated panel reactive antibody; HLA, human leukocyte antigen; DSA, donor-specific antibody; GGS, global glomerulosclerosis; LCL, lower confidence limit; UCL, upper confidence limit.
Increased UPodCR is also associated with eGFR decline in the subset of allografts without observable recurrent or de novo GDs and without proteinuria
For this subgroup analysis, allografts with an average UProtCR ≥0.3 g/g (n = 22) or observable recurrent and de novo GD were excluded. In the remainder (n = 98), we observed that UPodCR remained significantly associated with eGFR decline (log UPodCR β coefficient −1.19, P = 0.048).
Recurrent or de novo GD and UPodCR
The small subset of allografts with recurrent or de novo GD (n = 8) had quantitatively higher levels of first-year-averaged podocyturia than grafts without recurrent or de novo GD (P = 0.07). Similarly, grafts with recurrent or de novo GD had quantitatively higher proteinuria (P = 0.1) compared with those without GD.
Relationship of proteinuria to graft loss
Similar to a previous study by Amer et al. [4], we found that proteinuria was related to death-censored allograft loss in a univariable analysis {hazard ratio [HR] 1.90 [95% confidence interval (CI) 1.08–3.35], P = 0.03} (Table 4).
There was no relationship of BK viremia and nephropathy, CMV infection and development of PTDM status or pretransplant diabetes with UPodCR. Only 32.8% of our patients received angiotensin II blockers in the first year and there was no relationship between their use and average 1-year UPodCR (P = 0.14).
DISCUSSION
In this analysis of 125 kidney allograft recipients whose urine pellets were analyzed in the first posttransplant year, we observed several key findings. First, a higher average UPodCR in the first year was independently associated with a steeper slope of eGFR decline by 4.5 years of follow-up. Second, even among nonproteinuric recipients and those with no observable recurrent or de novo GD by the first year, increased UPodCR was significantly associated with long-term allograft function decline. Lastly, we found that a smaller donor BSA (surrogate for smaller kidney size) relative to the recipients BSA was associated with increased UPodCR in the first 3 months post-transplant. Given the absolute specificity of urine podocin mRNA to the podocyte, these findings are compatible with the concept that ‘subclinical’ glomerular injury begins very early after transplantation and is associated with inferior long-term allograft outcomes.
Consistent with prior animal and human data demonstrating a relationship between podocyte depletion, progressive GS and loss of renal function [15, 26, 27], we noted that the average UPodCR in the first year was independently associated with the rate of GFR decline, a novel finding in the human transplant setting. In this analysis of urine markers, proteinuria (in the first year) did not predict the slope of eGFR either with or without adjustment for UPodCR. This finding is compatible with the concept that early after transplant, podocyte loss is occurring but is not yet sufficient to cause clinically significant proteinuria, as has been recently documented in Alport’s syndrome (a podocyte depletion disease) [26]. Consistent with this hypothesis, on a subgroup analysis in which recipients with clinically significant proteinuria (≥0.3 g/g) and those with observable recurrent and de novo GD identified on 1-year protocol biopsies were excluded, UPodCR remained a predictor of allograft function decline. This suggests that accelerated podocyte loss begins very early after transplantation, precedes proteinuria and has started to have an observable impact on allograft function. Consistent with this concept is a prior report demonstrating that microalbuminuria predicts long-term allograft loss even in the absence of overt proteinuria [28]. However, unlike podocin mRNA, microalbuminuria lacks the specificity of a noninvasive biomarker of subclinical podocyte injury [29].
Similar to prior observations, we found that the presence of moderate–severe IFTA when assessed alone was significantly associated with allograft loss, however, on adjustment for other histological factors such as GGS and recurrent and de novo GD, this association was lost. In parallel, the observed relationship of the urine pellet profibrotic marker (TGF-β1) to allograft loss of function was lost after multivariable adjustment. Although the cause for these findings remains unclear, we speculate that IFTA and urine TGF-β1 mRNA may in part be secondary to upstream glomerular injury. Indeed, in most GDs, interstitial fibrosis is significantly associated with renal function decline [30, 31]. This is also reported in the transplantation setting, where IFTA is more common in biopsies with glomerulopathy than in those without [32]. Furthermore, globally sclerosed glomeruli involute over time and become difficult to detect, thereby leaving downstream tubule–interstitial scarring as the major histologic finding.
Several reports have demonstrated the impact of donor and recipient size mismatch on long-term allograft outcome [7, 9, 33]. Giral et al. [7] reported that transplantation of a smaller kidney into a larger recipient was associated with biopsy-proven progressive GS, proteinuria and faster loss of function. While the hyperfiltration hypothesis has been postulated to help explain these findings, the mechanism by which glomerular hypertrophy, hyperfiltration and hypertension leads to progressive glomerular injury has not been elucidated. We have previously shown that there is an average 20–25% increase in glomerular volume (glomerular hypertrophy associated with compensatory kidney hypertrophy) that occurs by 3 months posttransplant (accompanied by a proportional reduction in podocyte density) and this is associated with an average 6-fold increased rate of podocyte detachment [15]. Two other studies have also noted increased glomerular volume in protocol biopsies [34, 35]. In one study, glomerular volume on protocol biopsies correlated with recipient body mass index, suggesting that the glomerular hypertrophic process was exacerbated by recipient and donor size mismatch. In addition, indirect data supporting increased allograft hyperfiltration come from the observation that the glomerular volume increase correlated positively with creatinine clearance and obese kidney transplant recipients experience a larger increase in filtration fraction [36]. In this report we demonstrate that in the first 3 months post-transplant, a donor and recipient size mismatch was associated with accelerated loss of podocytes in urine. This finding is also consistent with the hypothesis proposed by Kriz and Lemley [37] that the triad of glomerular hypertension, hypertrophy and hyperfiltration leads to an increased mechanical detachment of podocytes. This increase in UPodCR along with the observed 20–25% reduction in podocyte density from the glomerular volume increase would itself be predicted to poise allografts for glomerular destabilization, podocyte depletion and eventual progressive GS, a finding now observed in long-term surveillance biopsies [1, 2, 27].
While alloimmune and inflammatory mechanisms leading to IFTA are all well known to be capable of causing allograft loss, we were not able to find a statistically significant relationship between these variables (DSA, rejection) and eGFR slope or graft outcome. We speculate that the study may have been underpowered to see these effects (the cumulative rate of rejection and de novo DSA formation in this study were 11.4% and 9.2%, respectively). It is also possible that the relationship between immune mechanisms and allograft function decline might be mediated by podocyte loss, although we did not note any increased podocyte loss in those with and without rejection by the end of the first year (data not shown). It may also be that the remarkable success of the transplant community in mitigating these forces using current immunosuppression has reduced their dominating impact on long-term graft survival, leaving posttransplant GD to be a leading cause of allograft failure.
Our study has the limitations and biases of any single-center retrospective study. Besides having recipients who were mostly male (63%) and received living donor kidneys, major limitations of our study were that the timing of the initial and subsequent urine collections in patients were quite variable (IQR 55–216 days, ∼2–7 months), although we tried to minimize its impact by using linear mixed models while determining factors associated with podocyte loss. Second, the urine collections were not always timed with the biopsies and thus a direct relationship between histology, rejection and urine markers could not be ascertained. Third, we did not perform detailed glomerular morphometric and podometric analysis on serial biopsies in the patients whose urine was collected. Finally, due to the low rate of recurrent and de novo GD as well as rejection in the first year, we could not establish a direct relationship between these events and UPodCR. Regardless, our findings support the idea that irrespective of the cause, podocyte loss in the first year predicts long-term outcomes and does so even before significant proteinuria and GD are evident. This would be compatible with the idea that urinary podocyte loss may be an excellent noninvasive biomarker of subclinical glomerular injury. To test their utility as biomarkers of subclinical glomerular injury, further validation studies need to be performed. In addition, carefully conducted prospective studies where timed urine mRNA markers outlined in this study are directly compared with detailed serial biopsy information and allograft function are required.
In summary, our data support the concept that subclinical glomerular injury manifested by accelerated podocyte loss begins very early after transplantation and, even in the absence of proteinuria and recurrent and de novo GD, predicts long-term allograft function. Further, we note that hypertrophic kidney stress incurred at the time of kidney transplantation per se may also have a role in initiating and driving subsequent long-term allograft function decline. While the current study focused on early podocyte loss, further studies are needed to identify additional immune and nonimmune accelerators of podocyte loss that are active beyond the first posttransplant year. Understanding the biology of early and late podocyte loss may help formulate strategies to reduce the burden of progressive GS and thereby extend allograft half-life.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of the University of Michigan transplant study coordinators and the transplant patients who consented for their urine samples to be used for study.
FUNDING
This work was supported by the National Institutes of Health. A.S.N. acknowledges support from the Michigan Nutrition and Obesity Research Center (5 P30 DK089503-08) as well as the University of Michigan O’Brien Kidney Translational Core Center (4 P30 DK081943-09). R.C.W. acknowledges the support of the National Institutes of Health (grants R01 DK 46073 and R01 DK 102643) and the University of Michigan O’Brien Kidney Translational Core Center (P30 DK081943).
CONFLICT OF INTEREST STATEMENT
L.W. reports a consultancy agreement with Novartis outside of the current work. All other authors declare no conflicts of interest. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Nankivell BJ, Borrows RJ, Fung CL. et al. The natural history of chronic allograft nephropathy. N Engl J Med 2003; 349: 2326–2333 [DOI] [PubMed] [Google Scholar]
- 2. Stegall MD, Cornell LD, Park WD. et al. Renal allograft histology at 10 years after transplantation in the tacrolimus era: evidence of pervasive chronic injury. Am J Transplant 2018; 18: 180–188 [DOI] [PubMed] [Google Scholar]
- 3. Amer H, Cosio FG.. Significance and management of proteinuria in kidney transplant recipients. J Am Soc Nephrol 2009; 20: 2490–2492 [DOI] [PubMed] [Google Scholar]
- 4. Amer H, Fidler M, Myslak M. et al. Proteinuria after kidney transplantation, relationship to allograft histology and survival. Am J Transplant 2007; 7: 2748–2756 [DOI] [PubMed] [Google Scholar]
- 5. Gutierrez-Millet V, Nieto J, Praga M. et al. Focal glomerulosclerosis and proteinuria in patients with solitary kidneys. Arch Intern Med 1986; 146: 705–709 [PubMed] [Google Scholar]
- 6. Praga M, Hernandez E, Herrero JC. et al. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int 2000; 58: 2111–2118 [DOI] [PubMed] [Google Scholar]
- 7. Giral M, Foucher Y, Karam G. et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J Am Soc Nephrol 2010; 21: 1022–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lenihan CR, Busque S, Derby G. et al. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest 2015; 125: 1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller AJ, Kiberd BA, Alwayn IP. et al. Donor–recipient weight and sex mismatch and the risk of graft loss in renal transplantation. Clin J Am Soc Nephrol 2017; 12: 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 2007; 71: 1205–1214 [DOI] [PubMed] [Google Scholar]
- 11. Fukuda A, Chowdhury MA, Venkatareddy MP. et al. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 2012; 23: 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukuda A, Wickman LT, Venkatareddy MP. et al. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 2012; 81: 40–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishizono R, Kikuchi M, Wang SQ. et al. FSGS as an adaptive response to growth-induced podocyte stress. J Am Soc Nephrol 2017; 28: 2931–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wickman L, Afshinnia F, Wang SQ. et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol 2013; 24: 2081–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y, Hodgin JB, Afshinnia F. et al. The two kidney to one kidney transition and transplant glomerulopathy: a podocyte perspective. J Am Soc Nephrol 2015; 26: 1450–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naik AS, Afshinnia F, Cibrik D. et al. Quantitative podocyte parameters predict human native kidney and allograft half-lives. JCI Insight 2016; 1: e86943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodgin JB, Bitzer M, Wickman L. et al. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol 2015; 26: 3162–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander JW, Bennett LE, Breen TJ.. Effect of donor age on outcome of kidney transplantation. A two-year analysis of transplants reported to the United Network for Organ Sharing Registry. Transplantation 1994; 57: 871–876 [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masson I, Flamant M, Maillard N. et al. MDRD versus CKD-EPI equation to estimate glomerular filtration rate in kidney transplant recipients. Transplantation 2013; 95: 1211–1217 [DOI] [PubMed] [Google Scholar]
- 21. Pöge U, Gerhardt T, Palmedo H. et al. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant 2005; 5: 1306–1311 [DOI] [PubMed] [Google Scholar]
- 22. Clayton PA, Lim WH, Wong G, Chadban SJ.. Relationship between eGFR decline and hard outcomes after kidney transplants. J Am Soc Nephrol 2016; 27: 3440–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan JC, Paik J, Chertow GM. et al. Validity of surrogate measures for functional nephron mass. Transplantation 2011; 92: 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du Bois D, Du Bois E.. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5: 303–311 [PubMed] [Google Scholar]
- 25. Solez K, Colvin R, Racusen L. et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8: 753–760 [DOI] [PubMed] [Google Scholar]
- 26. Ding F, Wickman L, Wang SQ. et al. Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport syndrome. Kidney Int 2017; 92: 1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wharram BL, Goyal M, Wiggins JE. et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 2005; 16: 2941–2952 [DOI] [PubMed] [Google Scholar]
- 28. Halimi JM, Buchler M, Al-Najjar A. et al. Urinary albumin excretion and the risk of graft loss and death in proteinuric and non‐proteinuric renal transplant recipients. Am J Transplant 2007; 7: 618–625 [DOI] [PubMed] [Google Scholar]
- 29. Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12: 364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Risdon R, Sloper J, De Wardener H.. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 1968; 292: 363–366 [DOI] [PubMed] [Google Scholar]
- 31. Bohle A, Mackensen-Haen S, Gise H.. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol 1987; 7: 421–433 [DOI] [PubMed] [Google Scholar]
- 32. Gloor JM, Sethi S, Stegall MD. et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant 2007; 7: 2124–2132 [DOI] [PubMed] [Google Scholar]
- 33. Kasiske BL, Snyder JJ, Gilbertson D.. Inadequate donor size in cadaver kidney transplantation. J Am Soc Nephrol 2002; 13: 2152–2159 [DOI] [PubMed] [Google Scholar]
- 34. Alperovich G, Maldonado R, Moreso F. et al. Glomerular enlargement assessed by paired donor and early protocol renal allograft biopsies. Am J Transplant 2004; 4: 650–654 [DOI] [PubMed] [Google Scholar]
- 35. Azevedo F, Alperovich G, Moreso F. et al. Glomerular size in early protocol biopsies is associated with graft outcome. Am J Transplant 2005; 5: 2877–2882 [DOI] [PubMed] [Google Scholar]
- 36. Bosma RJ, Kwakernaak AJ, van der Heide JJ. et al. Body mass index and glomerular hyperfiltration in renal transplant recipients: cross-sectional analysis and long-term impact. Am J Transplant 2007; 7: 645–652 [DOI] [PubMed] [Google Scholar]
- 37. Kriz W, Lemley KV.. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 2015; 26: 258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989; 79: 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.