Abstract
Cardiovascular mortality is very high in chronic and end-stage kidney disease (ESKD). However, risk stratification data are lacking. Sudden cardiac deaths are among the most common cardiovascular causes of death in these populations. As a result, many studies have assessed the prognostic potential of various electrocardiographic parameters in the renal population. Recent data from studies of implantable loop recordings in haemodialysis patients from five different countries have shed light on a pre-eminent bradyarrhythmic risk of mortality. Importantly, heart block addressed by permanent pacing system was detected in a proportion of patients during the prolonged recording periods. Standard electrocardiogram is inexpensive, non-invasive and easily accessible. Hence, risk prediction models using this simple investigation tool could easily translate into clinical practice. We believe that electrocardiographic assessment is currently under-valued in renal populations. For this review, we identified studies from the preceding 10 years that assessed the use of conventional and novel electrocardiographic biomarkers as risk predictors in chronic and ESKD. The review indicates that conventional electrocardiographic markers are not reliable for risk stratification in the renal populations. Novel parameters have shown promising results in smaller studies, but further validation in larger populations is required.
Keywords: cardiovascular, CKD, ECG, ESRD
INTRODUCTION
Non-dialysis chronic kidney disease (CKD) is characterized by much higher cardiovascular mortality and morbidity when compared with the general population. This risk increases exponentially in end-stage kidney disease (ESKD) [1]. US Renal registry data indicate that sudden death and/or fatal arrhythmia is the documented cause of death in ∼26% of ESKD patients [2].
Although atherosclerotic disease is common in CKD and ESKD, evidence indicates that it accounts for only a small proportion of cardiovascular deaths in this population [3]. Furthermore, extrapolating evidence from the general population for cardiac risk modification has proven to be of limited benefit in dialysis patients. Statin therapy for primary prevention does not reduce cardiac risk in dialysis patients [4] and coronary revascularization [3], or use of implantable cardioverter defibrillators [5] based on the current guidelines (i.e. guidelines developed based on studies in cardiac patients), does not reduce arrhythmic mortality in CKD and ESKD patients. In the general population, most fatal arrhythmic events are triggered by underlying myocardial ischaemia, usually in the presence of coronary artery disease [6], and are frequently tachyarrhythmias although bradyarrhythmic sudden deaths also occur. In advanced CKD and ESKD, the mechanism, timeline and specific rhythm of such events are not fully understood. Non-conventional cardiovascular risk factors such as electrolyte imbalances, volume shifts and blood pressure changes have been implicated in extremely high sudden death rates after the long interdialytic interval of the typical three session of haemodialysis (HD) a week [7]. Recent studies of prolonged implantable loop recording in five different HD cohorts have suggested that bradyarrhythmic events may be more common than ventricular arrhythmia in causing sudden cardiac deaths (SCDs) [8]. Although the underlying mechanisms are far from clear, ∼10% of the patients in these cohorts were noted to have heart block or other bradyarrhythmia that could be treated with permanent pacing systems, and this itself should make the case for more frequent use of standard electrocardiogram (ECG) in dialysis populations. In recent years, data from experimental and population-based studies have led to advances in our understanding of the underlying cardiovascular disease mechanisms. This led to focussing on the dynamic interplay between myocardial structural changes, vascular changes, autonomic imbalance, inflammation, and fluid and electrolyte shifts that can lead to arrhythmias [9].
The presumed high burden of arrhythmic deaths in dialysis patients has led to a renewed interest in the evaluation of electrocardiographic parameters as potential risk predictors. The standard 12-lead ECG is an easily accessible and inexpensive bedside test. Moreover, the implementation of advanced software in most modern electrocardiographic machines means that vectorcardiographic indices can be derived with accuracy from standard 12-lead ECGs.
AIMS OF THE REVIEW
This review aims at providing an overview of studies that assessed the use of selected electrocardiographic and vectorcardiographic parameters taken from standard 12-lead and continuous Holter electrocardiography for the purpose of cardiac risk stratification in the CKD and ESKD populations.
REVIEW METHODOLOGY
Data sources and search strategy
MEDLINE through PubMed, Google Scholar and Cochrane Library were searched to identify potentially relevant articles and abstracts. Furthermore, we reviewed the bibliographies of the selected articles for additional relevant studies. The search terms are presented in Table 1.
Table 1.
Keywords used as Boolean operators or search terms
| Renal disease | Outcomes | Parameters |
|---|---|---|
| CKD | Survival | LVH |
| HD | Death | QTc |
| PD | Mortality | QT |
| Chronic kidney disease | Cardiovascular outcomes | PR |
| Renal disease | Cardiac outcomes | QRS–T angle |
| Haemodialysis | TCRT | |
| Haemodialysis | HRV | |
| Peritoneal dialysis | Left ventricular hypertrophy | |
| Dialysis | Heart rate variability | |
| ECG | ||
| Electrocardiogram | ||
| Electrocardiographic |
Eligibility of studies
Studies in any of the CKD, HD and peritoneal dialysis (PD) populations were considered for inclusion if they met the following criteria: published between January 2007 and December 2016; investigated at least 50 participants in the initial cohort; had a mean follow-up time of at least 1 year; any external, non-invasive ECG methodology (standard 12-lead, Holter, etc.); assessed death and/or cardiac outcomes as an endpoint; studied the association of left ventricular hypertrophy (LVH), QTc interval, QRS complex, PR interval, QRS–T angle and/or heart rate variability (HRV) with these endpoints.
Figure 1 shows a schematic representation of the different components of a standard ECG in sinus rhythm. Figure 2 shows a representation of QRS–T angle from vectorcardiograms.
FIGURE 1.
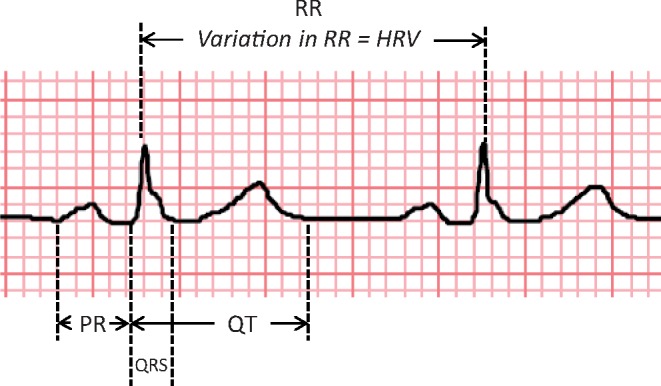
Schematic diagram of ECG (sinus rhythm).
FIGURE 2.
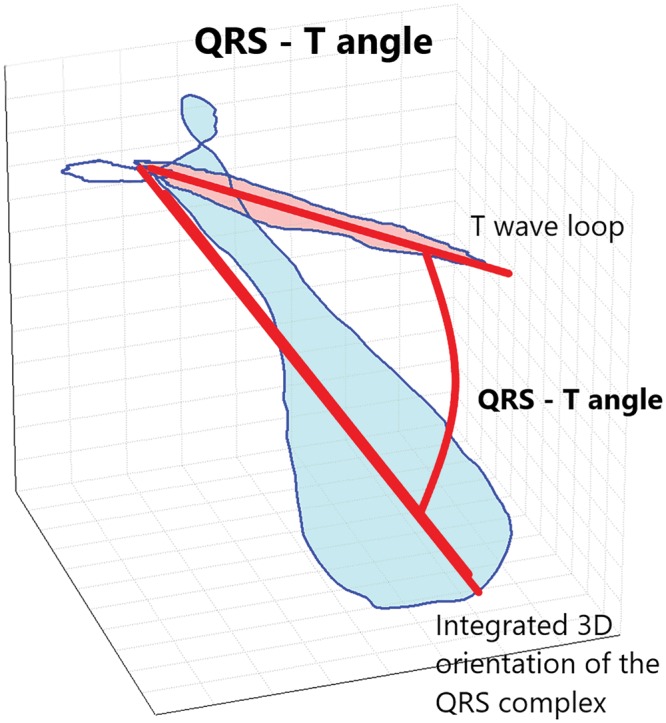
A representation of QRS–T angle from vectorcardiograms.
Cardiac outcomes included coronary events, arrhythmic events, cardiac failure or a combination of these. Death included all-cause mortality and, where available, sudden death as defined by the authors.
Studies are presented in two categories, one for dialysis and the other for CKD. Due to the paucity of studies including PD patients, studies in PD and HD are not listed separately.
ECG PROGNOSTIC INDICES
Electrocardiographic LVH
LVH is a common finding in advanced CKD and ESKD [10]. Up to 75% of patients have LVH upon initiation of maintenance dialysis [10], and increased echocardiographic left ventricular mass index (LVMI) is associated with adverse cardiovascular outcomes and SCD [11]. A summary of studies detailed below is found in Table 2.
Table 2.
Studies evaluating the association of electrocardiographic LVH with clinical outcomes in chronic renal disease
| References | Population | Sample size | Follow-up | Results | Comments |
|---|---|---|---|---|---|
| Covic et al. [12] | Prevalent HD and PD | 418 | 67 months ( mean) | LVH by Novacode predictive of cardiovascular mortality (HR = 3.04, 95% CI 1.11–8.28; P < 0.05) | 11 other methods not predictive |
| Kim et al. [13] | Incident HD | 317 | 27.4 months (mean) | LVH by Sokolow–Lyon voltage duration product (HR = 3.43, 95% CI 1.32–892; P = 0.011) and Cornell voltage duration product (HR = 3.07, 95% CI 1.16–8.11; P = 0.024) predictive of cardiovascular mortality | 50% discordance between ECG and echocardiographic diagnosis of LVH |
| Cice et al. [15] | Prevalent HD | 407 | 46 months (mean) | LVH with strain predictive of cardiovascular deaths (P < 0.05) and sudden deaths (P < 0.01) | Univariate analysis |
| Krane et al. [14] | HD with diabetes | 1253 | 48 months (mean) | LVH with Sokolow–Lyon criteria was predictive of sudden death (HR = 1.60, 95% CI 1.05–2.44; P = 0.027) | A trend towards higher risk for cardiovascular endpoints was detected |
| Agarwal and Light [16] | CKD, excluding ESRD | 387 | 90 months (median) | LVH with Sokolow–Lyon criteria prognostic for all-cause mortality (HR = 2.84, 95% CI 1.50–5.37; P < 0.001) | Multivariate analysis including adjustment for blood pressure |
Dialysis
Covic et al. [12] evaluated the prognostic value of estimating LVH by 12 different sets of commonly used electrocardiographic criteria in a retrospective, observational, single-centre study, which included both prevalent HD and PD patients [12]. Novacode, a method that does not use voltage criteria but incorporates repolarization indices into an algorithm, was found to be predictive of cardiovascular mortality, while 11 other methods, including the widely used Sokolow–Lyon and Cornell criteria, were not.
A Korean prospective observational study of incident HD patients [13] compared the prognostic value for cardiovascular mortality of commonly used ECG criteria for LVH, namely Sokolow–Lyon and Cornell, with the voltage duration product method that encompasses the QRS duration. The diagnosis of LVH using voltage duration product methods was an independent risk factor for cardiovascular outcomes, but LVH defined by fixed voltage Sokolow–Lyon and Cornell was not. Approximately half of the individuals with an echocardiographic diagnosis of LVH did not have a matching electrocardiographic one.
Krane et al. [14], in a study of 1253 maintenance HD patients with diabetes, identified that ECG LVH with Sokolow–Lyon criteria was predictive of sudden death and stroke [hazard ratio (HR) = 1.60, 95% confidence interval (CI) 1.05–2.44; P = 0.027], but not of all-cause mortality, cardiac deaths and myocardial infarction, although a trend towards statistical significance for cardiovascular endpoints was observed.
Cice et al. [15], in a prospective study of normotensive maintenance HD patients without coronary artery disease, found that the strain pattern on the ECG was associated with cardiovascular and sudden death.
CKD
There is a paucity of studies assessing the association between ECG diagnosis of LVH and mortality or cardiovascular outcomes. Agarwal and Light, in a cross-sectional study of 387 patients that included 243 patients with various degrees of CKD, found a statistically significant association between diagnosis of LVH with Sokolow–Lyon criteria and all-cause mortality. The LVH group had perhaps unsurprisingly higher baseline blood pressure readings, but the association between LVH and mortality still persisted even after adjustment for blood pressure [16].
Comment
The electrocardiographic detection of LVH in CKD patients correlates poorly with LVH diagnosis using echocardiography. This observation is in line with the findings in the general population [17], suggesting that changes in electrical remodelling depicted by ECG LVH do not reflect anatomical structural changes established by echocardiogram and that they carry additional independent prognostic information. On the other hand, the predictive value of ECG LVH with fixed voltage criteria is variable in dialysis patients and this may be the result of a variable and fluctuant impact of fluid and electrolyte status on the ECG waveform. Timing of the ECG is important as fluid removal immediately after dialysis leads to an increase in ECG voltage due to impedance changes, which is gradually attenuated as fluid accumulates until the next dialysis session [18]. As a result, an inter-dialytic ECG may obfuscate the presence of LVH in a patient with large inter-dialytic fluid gains, which itself is in turn an independent mortality risk factor [19].
QT interval
The electrocardiographic QT interval represents the time from the onset of ventricular depolarization to the completion of repolarization. QTc is the value of QT after correction for heart rate. The Bazett formula is the most commonly used method for QT correction in clinical studies [20]. Other formulae (Fridericia, Framingham, Hodges, etc.) tend to provide similar estimates when resting heart rates are close to 60 b.p.m. [21]. Clinically meaningful prolongation of QTc is often defined as QTc >460 ms in women and QTc >450 ms in men [22].
Electrocardiographic QT duration reflects both cardiac conduction and repolarization and is influenced by electrolyte shifts, myocardial ischaemia and structural heart disease. QTc prolongation increases the risk of ventricular tachyarrhythmia. A summary of studies detailed below is found in Table 3.
Table 3.
Studies evaluating the association of QTc with clinical outcomes in chronic renal disease
| References | Population | Sample size | Follow up | Results | Comments |
|---|---|---|---|---|---|
| Hage et al. [23] | HD and PD evaluated for transplantation | 280 | 40 months (mean) | QTc independent predictor of survival (HR = 1.008, 95% CI 1.001–1.014; P = 0.016) | |
| Flueckiger et al. [24] | CKD 5 and ESRD evaluated for renal transplantation | 930 | 37.2 months (median) | QTc >450 ms associated with risk of death in adjusted analysis (HR = 1.71, 95% CI 1.11–2.63; P = 0.0158) | |
| Deo et al. [27] | CKD | 3939 | 90 months (median) | Prolonged QTc associated with all cause (HR = 1.46, 95% CI 1.16–1.84) and cardiovascular mortality (HR = 1.72, 95% CI 1.19–2.49) | Association with cardiovascular death ceased to exist in subgroup adjusted analysis that included LVMI and LVEF |
| Dobre et al. [26] | CKD 3–5 | 1165 | 123.6 months (mean) | Prolonged QT was associated with 61% higher risk for cardiovascular events (HR = 1.61, 95% CI 1.16–2.23) | Predominantly CKD 3 (95.6% of study population) |
| Genovesi et al. [25] | HD | 122 | 46.8 months (median) | Prolonged QTc independently associated with all cause mortality (HR = 2.16, 95% CI 1.20–3.91; P = 0.011) and sudden death (HR = 8.33, 95% CI 1.71–40.48; P = 0.009) | |
| Krane et al. [14] Malik et al. [71] | HD with diabetes CKD | 1253 6565 | 48 months (mean) 159.6 months | QT interval not associated with outcomes QTc improved the risk prediction of traditional models (P < 0.00001 for all-cause mortality and P < 0.00001 for cardiovascular mortality) |
Dialysis
Hage et al. [23] found that QT prolongation was an independent predictor of all-cause mortality in a prospective cohort of both HD and PD patients evaluated for renal transplantation (HR = 1.008, 95% CI 1.001–1.014; P = 0.016). This study did not show any difference in the proportion of patients with QT prolongation between HD and PD.
In another prospective study of both incident and prevalent dialysis patients evaluated for renal transplantation, Flueckiger et al. [24] showed similar associations between the prolongation of the QT interval and all-cause mortality in 930 patients (HR = 1.71, 95% CI 1.11–2.63; P = 0.0158).
Genovesi et al. [25] used 24-h Holter electrocardiography in a cohort of 122 prevalent HD patients. The mean QTc was estimated in three periods: during dialysis treatment for 4 h, 4 h after dialysis treatment and the remaining 16 h after dialysis treatment [25]. After a median follow-up of 3.9 years, QTc prolongation was found to be independently associated with SCD (HR = 8.33, 95% CI 1.71–40.48; P = 0.009). Interestingly, the mean QTc interval did not change significantly during or after dialysis.
In contrast to the previous observational studies, a large multicentre randomized controlled trial of statin therapy in diabetic HD patients, the German Diabetes and Dialysis study (4D, Die Deutsche Diabetes Dialyse Studie), did not find any association between the duration of the QTc interval and cardiovascular outcomes [14].
CKD
In the CKD population, several observational studies have identified a link between QTc duration and cardiovascular outcomes [24, 26, 27]. Deo et al., in a prospective study of almost 4000 CKD patients, found that prolongation of the QTc interval was associated with all-cause and cardiovascular mortality. This association, however, ceased to exist in sub-group analysis adjusted for LVMI and left ventricular ejection fraction (LVEF) [27].
Similarly, Dobre et al. [26] in a study of mainly CKD 3 patients demonstrated that QTc was associated with cardiovascular events.
In the National Health and Nutrition Examination Survey III, the addition of QTc in the adjusted model that included traditional risk factors for cardiovascular mortality improved risk prediction for all-cause and cardiovascular mortality. The main strengths of this study were the large sample size (6565 individuals) and the long follow-up period (median follow-up 13.3 years).
Comment
Fluid and electrolyte shifts may affect QT interval; fluid and potassium removal both contribute to QTc prolongation at the end of the dialysis, whereas calcium changes are less consistent and can have a variable effect on the QTc [18]. Genovesi et al. [28] have previously reported that low potassium and calcium dialysate are associated with prolongation of QTc interval towards the end of HD. Also, Bazett’s correction, which has been used in many of the studies, is known to lead to artificially prolonged QTc values in the presence of increased heart rate. Although this is of little concern when dealing with singular QTc measurements in any given patient, it might represent a potential source of bias in statistical studies linking outcomes to QTc duration.
QRS complex—amplitude and duration
The electrocardiographic QRS complex represents the electrical activation of the ventricular myocardium, spreading from septal activation to the depolarization of the base of the ventricular free walls.
A broad QRS complex (>120 ms) has been used as a marker of cardiac dyssynchrony in studies evaluating the incidence of SCD in patients with heart failure [29], and is one of the criteria for resynchronization therapy in congestive heart failure [30].
Dialysis
A Spanish prospective study of 285 incident HD and PD patients with generally well-preserved left ventricular function did not show any independent association between QRS duration and SCD incidence [31].
CKD
The use of the QRS complex for cardiovascular risk prediction is poorly investigated and appears to be unreliable as an independent marker based on the currently available evidence. Research suggests that the QRS interval duration increases with the progression of CKD [32].
In a prospective study of 3587 individuals with mainly early to moderate CKD [mean estimated glomerular filtration rate (eGFR) 50–60 mL/min/1.73 m2, median follow-up 7.5 years), Deo et al. identified prolongation of the QRS interval as an independent risk predictor for cardiovascular death, even after adjustment for LVMI and ejection fraction. For QRS duration of 100–119 ms, the HR was 1.64 (95% CI 1.20–2.25) and for QRS >120 ms, the HR was 1.75 (95% CI 1.17–2.62) [27].
Comment
The amplitude of the QRS complex increases after HD [33, 34]. The latter is thought to be a result of the changes in body fluid volume. Fluid removal also leads to a decrease in tissue conductivity which, as a result, affects the surface voltage of the electrocardiographic complexes [35]. Therefore, the change of the QRS amplitude with different fluid status is a result of different thorax impedance and not of electrophysiological cardiac changes.
LBBB versus RBBB QRS morphology
There are few data available in comparing left bundle branch block (LBBB) versus right bundle branch block (RBBB). In the study of diabetic patients on HD by Krane et al. [14], neither RBBB nor LBBB showed any association with mortality or cardiovascular outcomes in multivariate analysis adjusting for comorbidities and demographics. The presence of LBBB may obscure the electrocardiographic diagnosis of LVH as they both cause conduction delays and as a result the inclusion of LBBB as a separate variable in a model that includes electrocardiographic LVH is not without problems [36]. Covic et al. [12], in a study of HD patients that compared different electrocardiographic methods of LVH estimation and their association with outcomes, also noted that LBBB was associated with all-cause mortality in univariate analysis. However, they suggested caution while using LBBB and ECG LVH in the same model.
PR interval
The electrocardiographic PR interval represents the propagation of the myocardial electrical impulse between atrial depolarization and the onset of ventricular depolarization, and is normally between 120 and 200 ms. The PR interval is also affected by fluid and electrolyte shifts. In the general population, prolongation of the PR interval has been associated with increased risk of developing atrial fibrillation, of requiring pacemaker implantation and of overall mortality [37]. A summary of the studies detailed below is found in Table 4.
Table 4.
Studies evaluating the association of PR interval with clinical outcomes in CKD
| References | Population | Sample size | Follow-up | Results | Comments |
|---|---|---|---|---|---|
| Flueckiger et al. [24] | CKD 5 and ESRD evaluated for renal transplantation | 930 | 37.2 months (median) | PR interval was associated with all-cause mortality (HR = 1.97, 95% CI 1.18–3.29; P = 0.090) | |
| Deo et al. [27] | CKD | 3939 | 90 months (median) | PR >200 ms is associated with cardiovascular mortality (HR = 1.62, 95% CI 1.19–2.19) | |
| Green et al. [11] | HD and PD | 323 | 43.2 months (mean) | No independent association between PR interval and cardiovascular outcomes in multivariate analysis | |
| Kestenbaum et al. [40] | CKD | 600 | 110.4 months (median) | No independent association between PR prolongation and incident cardiovascular events | |
| Badarau et al. [38] | HD | 116 | 17.5 months (median) | Log pre- and post-dialysis difference in PR interval predicts cardiovascular events (HR = 0.387, 95% CI 0.251–0.597; P < 0.001) | |
| Silva et al. [39] | HD | 100 | 14 months (mean) | The duration of the PR interval was independently associated with bradyarrhythmias (odds ratio = 1.05, 95% CI 1.02–1.08; P < 0.001) | Candidates for renal transplantation |
Dialysis
Flueckiger et al. [24], in their study of 930 transplant candidates undergoing HD, demonstrated that prolonged PR interval was associated with all-cause mortality in multivariate analysis (HR = 1.97, 95% CI 1.18–3.29; P = 0.090).
Green et al. [11] undertook a prospective observational study of 211 HD and 112 PD patients and identified a significant association between prolongation of the PR interval and cardiovascular outcomes in univariate, but not in multivariate, analysis (mean follow-up 3.6 years).
Another prospective study of 116 HD patients by Badarau et al. evaluated that the PR interval derived from standard ECGs were acquired 5 min before and 30 min after a HD session. In this study, for the majority of patients, the PR interval decreased after dialysis and in multivariate Cox regression analysis, the difference between the pre- and post-dialysis PR interval duration was identified as an independent predictor of cardiovascular outcomes with longer PR having a lower risk (HR for log of change in PR = 0.387, 95% CI 0.251–0.597; P < 0.001), but not of all-cause mortality [38].
A Brazilian prospective observational study aimed to evaluate the incidence of arrhythmias and their associations with ECG findings in a cohort of 100 HD patients using implantable loop recorders. During a follow-up period of 424 ± 124 days, prolongation of the PR interval was found to be independently associated with the development of bradyarrhythmias [39].
CKD
In a prospective study of 3587 patients with different stages of pre-dialysis CKD, a prolonged PR interval was identified as an independent predictor of cardiovascular mortality (HR = 1.62, 95% CI 1.19–2.19) [27].
In contrast, Kestenbaum et al. [40] prospectively studied 600 individuals with a moderate degree of CKD (median eGFR 53 mL/min/1.73 m2) and did not observe any independent association between PR prolongation and incident cardiovascular events [40].
Comment
In conclusion, the PR interval demonstrates variable associations with mortality in CKD and ESKD that may be explained by fluid and electrolyte influences on PR interval. The link between prolonged PR interval and mortality is unclear, but it may be related to mortality associated with bradyarrhythmias or atrial fibrillation.
QRS–T angle
In the last decade, there has been increasing interest in the spatial QRS–T angle that is defined as the angular difference between the orientation of the three-dimensional (3D) QRS and T vectorcardiographic loops that are either directly captured or calculated from the standard 12-lead recordings. This is because the angle has emerged as a novel marker for cardiac risk stratification [41]. A number of studies in different populations have demonstrated an association between a wide spatial QRS–T angle and cardiovascular and all-cause mortality [42].
The spatial QRS–T angle can easily be measured either on vectorcardiograms recorded using the Frank electrode positions [43] or by following orthogonal transformation from a digital 12-lead ECG using conversion systems such as Kors or inverse Dower matrices [44, 45]. In these methods, the spatial orientation of the orthogonal XYZ leads is defined anatomically and is subject independent. A novel descriptor uses singular value decomposition to construct a mathematically derived subject-dependent 3D space optimizing the orthogonal leads in order to capture most of the ECG energy in each individual, and calculates the difference between the global direction of depolarization and repolarization expressed as an average cosine of the angles between the QRS and T vectors [total cosine R-to-T (TCRT)] [46]. Figure 2 depicts the TCRT.
The definition and range of normal and abnormal QRS–T angles in healthy individuals depend on the method of estimation as well as on gender, age and underlying heart rate [47–50]. The spatial QRS–T angle may be calculated by several methods including using the peak angular difference between the QRS and T-vectors, their mean angular difference [51], the angle between the spatial mean QRS vector and spatial peak T-vector [52] and by using the average cosine of the angles between the QRS and T-vectors [53]. Therefore, ‘absolute’ values of the QRS–T angle should only be referenced in relation to the individual studies and methods they derive from. A summary of the studies detailed below is found in Table 5.
Table 5.
Studies evaluating the association of spatial QRS–T angle and outcomes in ESRD
| References | Population | Sample size | Follow-up | Results | Comments |
|---|---|---|---|---|---|
| de Bie et al [54] | HD and PD | 277 | 25.2 months (mean) | QRS–T angle independent predictor of all cause mortality (HR = 2.33, 95% CI 1.46–3.70; P < 0.01) and SCD (HR = 2.99, 95% CI 1.04–8.60; P < 0.05) | Single surface ECG |
| Poulikakos et al. 2014 [55] | HD | 81 | 18 months | Extremely high TCRTs in patients who experienced arrhythmic events | Holter |
| Couderc et al. [57] | HD patients above the age of 40 with history of diabetes or hypertension | 50 | 13 months | Statistically significant increase of the QRS–T angle after the dialysis session in the non-survivor group (P < 0.05) | Holter |
| Tereshchenko et al. [52] | Incident HD | 358 | 864.6 person years | Spatial QRS–T angle >75° was independently associated with all-cause (HR = 2.38, 95% CI 1.41–4.04; P = 0.001) and cardiovascular mortality (HR = 2.99, 95% CI 1.31–6.82; P = 0.01) | 5 min SA ECG |
Dialysis
Several studies evaluated the prognostic value of spatial QRS–T angle for all-cause and cardiovascular mortality in dialysis patients. In a retrospective study of 277 incident HD and PD patients, de Bie et al. [54] identified abnormal spatial QRS–T angle as an independent predictor of all-cause mortality (HR = 2.33, 95% CI: 1.46–3.70; P < 0.01) and SCD (HR = 2.99, 95% CI 1.04–8.60; P < 0.05) after multivariate analysis [54]. An abnormal spatial QRS–T angle was defined as >130° in men and >116° in women in that study, and the length of follow-up was 2.1 ± 1.7 years.
In a pilot study of 81 prevalent HD patients, which used continuous Holter electrocardiographic recordings, Poulikakos et al. [55] reported higher TCRT values (expressed in degrees) in individuals who suffered major arrhythmic events (TCRT) [56].
Couderc et al. calculated the QRS–T angle from ECG Holter recordings in a study of 50 prevalent HD patients. They demonstrated a statistically significant greater average QRS–T angle in the first 6 h after initiation of the dialysis session compared with pre-dialysis that correlated with all-cause mortality [57].
A large prospective study of incident HD patients by Tereshchenko et al. evaluated the spatial QRS–T angle for risk stratification in a cohort of patients of predominantly African origin with overall normal LVEFs. The authors calculated the QRS–T angle as the angle between spatial mean QRS vector and spatial peak T-vector in averaged XYZ ECG from 5 min signal-averaged ECGs. In multivariate adjusted analysis, a spatial QRS–T angle >75° was independently associated with all-cause (HR = 2.38, 95% CI 1.41–4.04; P = 0.001) and cardiovascular mortality (HR = 2.99, 95% CI 1.31–6.82; P = 0.01) [52].
CKD
There were no suitable studies at the time of this review.
Comment
The QRS–T angle has showed promising results for risk prediction in dialysis patients. However, there is a need for standardization of the measurement [53] so that normal limits and clinically relevant risk stratification dichotomies can be established.
HRV
HRV gained popularity, among other ECG parameters, because of its importance for cardiovascular risk prediction [58, 59]. HRV measurement is based on different assessments of the oscillations of the intervals between consecutive cardiac beats. It has been used as a surrogate method of assessing the sympathetic and parasympathetic cardiac autonomic modulation [60]. Reduced HRV has been associated with increased mortality in different populations including healthy individuals and patients post-myocardial infarction [61, 62]. HRV can be measured using time- and frequency domain methods as well as employing nonlinear dynamics analyses. Standards of HRV assessment are available [63, 64] and are followed in most of the risk assessment studies. A summary of studies detailed below is found in Table 6.
Table 6.
Studies evaluating the association of HRV and outcomes in CKD
| References | Population | Sample size | Follow-up | Results | Comments |
|---|---|---|---|---|---|
| Oikawa et al. [65] | HD | 383 | 2110 ± 903 days | Independent association between reduced SDNN and all-cause (HR = 2.181, 95% CI 1.530–3.108; P < 0.001) and cardiovascular mortality (HR = 2.114, 95% CI 1.200–3.725; P = 0.01) | |
| Chandra et al. [68] | CKD 3–5 | 305 | 2.7 years (median) | A LF/HF ratio below the median was associated with a significantly increased risk of cardiac events (HR = 2.52; P = 0.002) | |
| Pei et al. [66] | PD | 81 | 43.78 ± 14.77 months | LF/HF below the median significantly associated with all-cause mortality (P = 0.012) | |
| Suzuki et al. [67] | HD | 281 | 87 months (median) | The scaling component α1 was independently associated with all-cause mortality (HR = 1.46, 95% CI 1.16–1.85; P = 0.001) | None of the traditional HRV parameters was associated with mortality |
| Badarau et al. [38] | HD | 116 | 17.5 months (mean) | Association between VLF component and all-cause mortality (log VLF, HR = 1.741, 95% CI 1.047–2.895; P = 0.033) |
SDNN = standard deviation of normal to normal R–R intervals; VLF = very low frequency; LF = low frequency; HF = high frequency.
Dialysis
In a study of 383 incident and prevalent HD patients, Oikawa et al. [65] reported an independent association between reduced overall HRV and all-cause (HR = 2.181, 95% CI 1.530–3.108; P < 0.001) and cardiovascular mortality (HR = 2.114, 95% CI 1.200–3.725; P = 0.01) [65].
A study of 81 PD patients also reported on the prognostic value of spectral HRV assessment for all-cause mortality during 4 years of follow-up [66]. In a prospective study of 281 prevalent HD patients, Suzuki et al. evaluated different HRV measures. Time and spectral assessment of short-term HRV indices predicted mortality but after adjusting for age, LVEF, serum albumin, C-reactive protein and calcium × phosphate product, only one of the nonlinear dynamics parameters was an independent mortality predictor (HR = 1.46, 95% CI 1.16–1.85; P = 0.001) [67].
Badarau et al. [38] reported an association between very low frequency HRV and all-cause mortality in a study of 116 HD patients (HR = 1.741, 95% CI 1.047–2.895; P = 0.033), but did not find such an association with the other spectral HRV components.
In the latter two studies, the 24-h Holter ECG was recorded during interdialytic interval, whereas in other studies, it took place on a dialysis day that included the dialysis session.
CKD
A multicentre prospective study of 305 patients with CKD stages 3–5 demonstrated a strong association between decreased spectral HRV parameters and the cumulative probability of adverse cardiovascular events [68].
Comment
The variable results of studies using out-of-hospital 24-h Holter ECGs can be explained by the difficulty in standardizing the environmental factors that influence HRV assessment, including HD [69], during the recording. Indeed, total 24-h R–R interval variability analysis of recordings in truly ambulating out-of-hospital patients is of little prognostic value because of the differences in the environmental challenges to which the autonomic system responds, and is no longer recommended as a favoured approach for autonomic nervous system assessment [70, 71].
It has also been reported that high phosphate and parathyroid hormone levels [72] and fluid overload [73] are associated with reduced HRV in HD patients, whereas daily HD [74] and haemofiltration [75] are associated with less pronounced reductions in HRV compared with standard HD.
CONCLUSION
A number of electrocardiographic parameters have been used as potential risk predictors in advanced renal disease and dialysis with variable results. The use of conventional ECG parameters is severely limited by the influence of fluid and electrolyte shifts on their measurements. Inconsistency and lack of reproducibility make them unreliable as independent biomarkers.
In the case of the PR interval prolongation, in particular the link between abnormal PR and mortality might reflect the mortality risk associated with bradyarrhythmias or atrial fibrillation. In the determination of electrocardiographic LVH, the use of Novacode has shown promising results. Novacode has the advantage of not relying on voltage criteria, but requires computer processing of EGC waveform. Hence, unlike conventional methods such as Sokolow–Lyon, LVH cannot be determined by manual observation.
We elected to omit QTc dispersion from the review in order to keep the presented results more relevant. Previous research has indicated that QT dispersion as a metric is problematic as it has very poor reproducibility and cannot be used consistently for risk stratification. There is also some controversy regarding the meaning of QT dispersion as some previous research has questioned whether it truly represents repolarization heterogeneity [76, 77].
Novel markers, such as the QRS–T angle, have shown promising results in HD cohorts. However, the definitions of abnormal QRS–T angle vary significantly depending on the method of calculation used. Further standardization is therefore required. Moreover, the prognostic value of the QRS–T angle needs to be evaluated in larger prospective studies. In general, there is a paucity of studies assessing electrocardiographic markers as risk prediction tools in PD when compared with HD.
In summary, larger and more comprehensive studies are required, including those assessing the evolution of electrocardiographic changes from CKD to HD and PD and the relation of these changes to cardiac mortality. In addition, every opportunity should be taken to include serial ECG recordings in all larger randomized controlled trials examining cardiovascular and mortality outcomes. Risk stratification models that incorporate echocardiographic, electrocardiographic and laboratory parameters together will likely lead to more sensitive and specific risk prediction. Finally, the serious and potentially treatable bradyarrhythmias being detected by implantable loop recording in dialysis patients would itself justify a more regular and perhaps protocolled use of ECG in these populations.
FUNDING
M.M. reports grants from British Heart Foundation (NH/16/2/32499) during the conduct of the study.
REFERENCES
- 1. Caskey FCC, Dawnay A, Farrington K. et al. 18th Annual Report of the Renal Association, UK Renal Registry. Nephron 2016; 132: 927100468 [Google Scholar]
- 2.US Renal Data System. 2016Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2017; 69: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herzog CA, Strief JW, Collins AJ. et al. Cause-specific mortality of dialysis patients after coronary revascularization: why don't dialysis patients have better survival after coronary intervention? Nephrol Dial Transplant 2008; 23: 2629–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barylski M, Nikfar S, Mikhailidis DP. et al. Statins decrease all-cause mortality only in CKD patients not requiring dialysis therapy—a meta-analysis of 11 randomized controlled trials involving 21, 295 participants. Pharmacol Res 2013; 72: 35–44 [DOI] [PubMed] [Google Scholar]
- 5. Chonchol M, Goldenberg I, Moss AJ. et al. Risk factors for sudden cardiac death in patients with chronic renal insufficiency and left ventricular dysfunction. Am J Nephrol 2007; 27: 7–14 [DOI] [PubMed] [Google Scholar]
- 6. Spaulding CM, Joly L-M, Rosenberg A. et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336: 1629–1633 [DOI] [PubMed] [Google Scholar]
- 7. Foley RN, Gilbertson DT, Murray T. et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107 [DOI] [PubMed] [Google Scholar]
- 8. Kalra PA, Green D, Poulikakos D.. Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int 2018; 93: 781–783 [DOI] [PubMed] [Google Scholar]
- 9. Di Lullo L, Rivera R, Barbera V. et al. Sudden cardiac death and chronic kidney disease: From pathophysiology to treatment strategies. Int J Cardiol 2016; 217: 16–27 [DOI] [PubMed] [Google Scholar]
- 10. Foley RN, Parfrey PS, Harnett JD. et al. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 1995; 5: 2024–2031 [DOI] [PubMed] [Google Scholar]
- 11. Green D, Ritchie JP, Abidin N. et al. The association of ECG and echocardiographic abnormalities with sudden cardiac death in a dialysis patient cohort. J Nephrol 2014; 27: 81–86 [DOI] [PubMed] [Google Scholar]
- 12. Covic AC, Buimistriuc LD, Green D. et al. The prognostic value of electrocardiographic estimation of left ventricular hypertrophy in dialysis patients. Ann Noninvasive Electrocardiol 2013; 18: 188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim SJ, Oh HJ, Yoo DE. et al. Electrocardiographic left ventricular hypertrophy and outcome in hemodialysis patients. PLoS One 2012; 7: e35534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krane V, Heinrich F, Meesmann M. et al. Electrocardiography and outcome in patients with diabetes mellitus on maintenance hemodialysis. Clin J Am Soc Nephrol 2009; 4: 394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cice G, Benedetto AD, Andrea AD. et al. Heart rate as independent prognostic factor for mortality in normotensive hemodialysed patients. J Nephrol 2008; 21: 704–712 [PubMed] [Google Scholar]
- 16. Agarwal R, Light RP.. Determinants and prognostic significance of electrocardiographic left ventricular hypertrophy criteria in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bacharova L, Estes HE, Schocken DD. et al. The 4th Report of the Working Group on ECG diagnosis of left ventricular hypertrophy. J Electrocardiol 2017; 50: 11–15 [DOI] [PubMed] [Google Scholar]
- 18. Poulikakos D, Malik M.. Challenges of ECG monitoring and ECG interpretation in dialysis units. J Electrocardiol 2016; 49: 855–859 [DOI] [PubMed] [Google Scholar]
- 19. Flythe JE, Curhan GC, Brunelli SM.. Disentangling the ultrafiltration rate–mortality association: The respective roles of session length and weight gain. Clin J Am Soc Nephrol 2013; 8: 1066–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920; 7: 353–370 [Google Scholar]
- 21. Goldenberg I, Moss AJ, Zareba W.. QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol 2006; 17: 333–336 [DOI] [PubMed] [Google Scholar]
- 22. Zhang Y, Post WS, Blasco-Colmenares E. et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology 2011; 22: 660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hage FG, de Mattos AM, Khamash H. et al. QT prolongation is an independent predictor of mortality in end-stage renal disease. Clin Cardiol 2010; 33: 361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flueckiger P, Pastan S, Goyal A. et al. Associations of ECG interval prolongations with mortality among ESRD patients evaluated for renal transplantation. Ann Transplant 2013; 19: 257–268 [DOI] [PubMed] [Google Scholar]
- 25. Genovesi S, Rossi E, Nava M. et al. A case series of chronic haemodialysis patients: mortality, sudden death, and QT interval. Europace 2013; 15: 1025–1033 [DOI] [PubMed] [Google Scholar]
- 26. Dobre M, Brateanu A, Rashidi A. et al. Electrocardiogram abnormalities and cardiovascular mortality in elderly patients with CKD. Clin J Am Soc Nephrol 2012; 7: 949–956 [DOI] [PubMed] [Google Scholar]
- 27. Deo R, Shou H, Soliman EZ. et al. Electrocardiographic measures and prediction of cardiovascular and noncardiovascular death in CKD. J Am Soc Nephrol 2016; 27: 559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Genovesi S, Dossi C, Viganò MR. et al. Electrolyte concentration during haemodialysis and QT interval prolongation in uraemic patients. Europace 2008; 10: 771–777 [DOI] [PubMed] [Google Scholar]
- 29. Linde C, Leclercq C, Rex S. et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40: 111–118 [DOI] [PubMed] [Google Scholar]
- 30.NICE. Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for Arrhythmias and Heart Failure National Institute for Health and Care Excellence 2014; Technology appraisal guidance [TA314]
- 31. Vazquez E, Sanchez-Perales C, Garcia-Garcia F. et al. Sudden death in incident dialysis patients. Am J Nephrol 2014; 39: 331–336 [DOI] [PubMed] [Google Scholar]
- 32. Majima S, Tanaka M, Okada H. et al. The PR interval and QRS duration could be predictors of renal function decline. Atherosclerosis 2015; 240: 105–109 [DOI] [PubMed] [Google Scholar]
- 33. Fuenmayor AJ, Vasquez CJ, Fuenmayor AM. et al. Hemodialysis changes the QRS amplitude in the electrocardiogram. Int J Cardiol 1993; 41: 141–145 [DOI] [PubMed] [Google Scholar]
- 34. Drighil A, Madias JE, Yazidi A. et al. P-wave and QRS complex measurements in patients undergoing hemodialysis. J Electrocardiol 2008; 41: 60.e1–67 [DOI] [PubMed] [Google Scholar]
- 35. Madias JE, Narayan V.. Augmentation of the amplitude of electrocardiographic QRS complexes immediately after hemodialysis: a study of 26 hemodialysis sessions of a single patient, aided by measurements of resistance, reactance, and impedance. J Electrocardiol 2003; 36: 263–271 [DOI] [PubMed] [Google Scholar]
- 36. Surawicz B, Childers R, Deal BJ. et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. J Am College Cardiol 2009; 53: 976–981 [DOI] [PubMed] [Google Scholar]
- 37. Cheng S, Keyes MJ, Larson MG. et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA 2009; 301: 2571–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Badarau S, Siriopol D, Drugus D. et al. Electrocardiogram abnormalities and heart rate variability in predicting mortality and cardiovascular events among hemodialyzed patients. Int Urol Nephrol 2015; 47: 1703–1708 [DOI] [PubMed] [Google Scholar]
- 39. Silva RT, Martinelli Filho M, Peixoto Gde L. et al. Predictors of arrhythmic events detected by implantable loop recorders in renal transplant candidates. Arq Bras Cardiol 2015; 105: 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kestenbaum B, Rudser KD, Shlipak MG. et al. Kidney function, electrocardiographic findings, and cardiovascular events among older adults. Clin J Am Soc Nephrol 2007; 2: 501–508 [DOI] [PubMed] [Google Scholar]
- 41. Strauss DG, Mewton N, Verrier RL. et al. Screening entire health system ECG databases to identify patients at increased risk of death. Circ Arrhythm Electrophysiol 2013; 6: 1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, Zhu Q, Zhu L. et al. Spatial/frontal QRS-T angle predicts all-cause mortality and cardiac mortality: a meta-analysis. PLoS One 2015; 10: e0136174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frank E. An accurate, clinically practical system for spatial vectorcardiography. Circulation 1956; 13: 737–749 [DOI] [PubMed] [Google Scholar]
- 44. Schreurs CA, Algra AM, Man SC. et al. The spatial QRS-T angle in the Frank vectorcardiogram: accuracy of estimates derived from the 12-lead electrocardiogram. J Electrocardiol 2010; 43: 294–301 [DOI] [PubMed] [Google Scholar]
- 45. Kors J, Van Herpen G, Sittig A. et al. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J 1990; 11: 1083–1092 [DOI] [PubMed] [Google Scholar]
- 46. Zabel M, Malik M.. Morphological assessment of T wave patterns (chapter 35). In: Malik M, Camm AJ (eds). Dynamic electrocardiography Wiley-Blackwell, 2004; 305–357 [Google Scholar]
- 47. Scherptong RWC, Henkens IR, Man SC. et al. Normal limits of the spatial QRS-T angle and ventricular gradient in 12-lead electrocardiograms of young adults: dependence on sex and heart rate. J Electrocardiol 2008; 41: 648–655 [DOI] [PubMed] [Google Scholar]
- 48. Kardys I, Kors JA, van der Meer IM. et al. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J 2003; 24: 1357–1364 [DOI] [PubMed] [Google Scholar]
- 49. Smetana P, Batchvarov VN, Hnatkova K. et al. Sex differences in repolarization homogeneity and its circadian pattern. Am J Physiol Heart Circ Physiol 2002; 282: H1889–H1H97 [DOI] [PubMed] [Google Scholar]
- 50. Smetana P, Batchvarov VN, Hnatkova K. et al. Ventricular gradient and nondipolar repolarization components increase at higher heart rate. Am J Physiol Heart Circ Physiol 2004; 286: H131–H136 [DOI] [PubMed] [Google Scholar]
- 51. Cortez DL, Schlegel TT.. When deriving the spatial QRS-T angle from the 12-lead electrocardiogram, which transform is more Frank: regression or inverse Dower? J Electrocardiol 2010; 43: 302–309 [DOI] [PubMed] [Google Scholar]
- 52. Tereshchenko LG, Kim ED, Oehler A. et al. Electrophysiologic substrate and risk of mortality in incident hemodialysis. J Am Soc Nephrol 2016; 27: 3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hnatkova K, Seegers J, Barthel P. et al. Clinical value of different QRS-T angle expressions. Europace 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Bie MK, Koopman MG, Gaasbeek A. et al. Incremental prognostic value of an abnormal baseline spatial QRS-T angle in chronic dialysis patients. Europace 2013; 15: 290–296 [DOI] [PubMed] [Google Scholar]
- 55. Poulikakos D, Banerjee D, Malik M.. Major arrhythmic events and T wave morphology descriptors in hemodialyzed patients. J Electrocardiol 2014; 47: 240–243 [DOI] [PubMed] [Google Scholar]
- 56. Acar B, Yi G, Hnatkova K. et al. Spatial, temporal and wavefront direction characteristics of 12-lead T-wave morphology. Med Biol Eng Comput 1999; 37: 574–584 [DOI] [PubMed] [Google Scholar]
- 57. Couderc J, Xia J, McGrath M. et al. Increased repolarization heterogeneity is associated with increased mortality in hemodialysis patients. Comput Cardiol 2011; 2011: 845–848 [Google Scholar]
- 58. La Rovere MT, Bigger JT, Marcus FI. et al. ; ATRAMI Investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998; 351: 478–484 [DOI] [PubMed] [Google Scholar]
- 59. Tsuji H, Venditti FJ, Manders ES. et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994; 90: 878–883 [DOI] [PubMed] [Google Scholar]
- 60. Pomeranz B, Macaulay R, Caudill MA. et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol 1985; 248: H151–H153 [DOI] [PubMed] [Google Scholar]
- 61. Kleiger RE, Miller JP, Bigger JT. et al. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987; 59: 256–262 [DOI] [PubMed] [Google Scholar]
- 62. Kleiger RE, Stein PK, Bigger JT.. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 2005; 10: 88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malik M. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 1996; 17: 354–381 [PubMed] [Google Scholar]
- 64. Sassi R, Cerutti S, Lombardi F. et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015; 17: 1341–1353 [DOI] [PubMed] [Google Scholar]
- 65. Oikawa K, Ishihara R, Maeda T. et al. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol 2009; 131: 370–377 [DOI] [PubMed] [Google Scholar]
- 66. Pei J, Tang W, Li L-X. et al. Heart rate variability predicts mortality in peritoneal dialysis patients. Ren Fail 2015; 37: 1132–1137 [DOI] [PubMed] [Google Scholar]
- 67. Suzuki M, Hiroshi T, Aoyama T. et al. Nonlinear measures of heart rate variability and mortality risk in hemodialysis patients. Clin J Am Soc Nephrol 2012; 7: 1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chandra P, Sands RL, Gillespie BW. et al. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant 2011; 27: 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tong Y-Q, Hou H-M.. Alteration of heart rate variability parameters in nondiabetic hemodialysis patients. Am J Nephrol 2007; 27: 63–69 [DOI] [PubMed] [Google Scholar]
- 70. Wellens HJ, Schwartz PJ, Lindemans FW. et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014; 35: 1642–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Malik M, Huikuri H, Lombardi F. et al. ; on behalf of the e-Health/Digital Rhythm Study Group of the European Heart Rhythm Association. The purpose of heart rate variability measurements. Clin Auton Res 2017; 27: 139–140 [DOI] [PubMed] [Google Scholar]
- 72. Poulikakos D, Malik M, Banerjee D.. Parathyroid hormone and heart rate variability in haemodialysis patients. Nephron Clin Pract 2014; 126: 110–115 [DOI] [PubMed] [Google Scholar]
- 73. Ferrario M, Moissl U, Garzotto F. et al. Effects of fluid overload on heart rate variability in chronic kidney disease patients on hemodialysis. BMC Nephrol 2014; 15: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chan CT, Chertow GM, Daugirdas JT. et al. Effects of daily hemodialysis on heart rate variability: results from the Frequent Hemodialysis Network (FHN) Daily Trial. Nephrol Dial Transplant 2014; 29: 168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Genovesi S, Bracchi O, Fabbrini P. et al. Differences in heart rate variability during haemodialysis and haemofiltration. Nephrol Dial Transplant 2007; 22: 2256–2262 [DOI] [PubMed] [Google Scholar]
- 76. Rautaharju PM. QT and dispersion of ventricular repolarization: the greatest fallacy in electrocardiography in the 1990s. Circulation 1999; 99: 2476c-2479. [PubMed] [Google Scholar]
- 77. Malik M, Acar B, Gang Y. et al. QT dispersion does not represent electrocardiographic interlead heterogeneity of ventricular repolarization. J Cardiovasc Electrophysiol 2000; 11: 835–843 [DOI] [PubMed] [Google Scholar]


