Abstract
Background: Gastroenteropancreatic neuroendocrine carcinoma (GEP-NEC) is a heterogeneous disease in terms of embryonic origin, aggressiveness, prognosis, and genomic profiling. Data regarding the efficacy of etoposide and cisplatin (EP) as a standard treatment of the primary tumor site in GEP-NEC are limited.
Materials and Methods: We analyzed 64 patients with histopathologically confirmed metastatic GEP-NEC who received EP at Samsung Medical Center, Seoul, Korea, between January 2010 and January 2018. Based on primary tumor site, outcome of treatment with EP was evaluated.
Results: Primary sites included 22 foregut-derived GEP-NECs (stomach, n = 6; duodenum, n = 4; pancreas, n = 12), 4 midgut-derived GEP-NECs, 5 hindgut-derived GEP-NECs of the rectum, 25 GEP-NECs originating from the hepatobiliary (HB) tract, and 12 GEP-NECs involving only intra-abdominal lymph nodes. No patient had a complete response (CR) and 17 had a partial response (PR), resulting in a 27.9% response rate (RR). When evaluating the efficacy of EP based on primary tumor site, the RR was most favorable in GEP-NECs involving only intra-abdominal lymph nodes, followed by GEP-NECs originating from foregut, midgut, HB, and hindgut. However, no statistically significant difference was observed for RR based on primary tumor site (P = 0.821). Similarly, no significant differences were found for progression-free survival (PFS) among patients with GEP-NECs arising from various primary tumor sites.
Conclusion: Results from this study showed that RR and PFS associated with EP treatment were not different based on the primary tumor site in patients with advanced or metastatic GEP-NEC.
Keywords: Gastroenteropancreatic neuroendocrine carcinoma, GEP-NEC, primary tumor site, etoposide, cisplatin
Introduction
Neuroendocrine tumors (NETs) are malignant growths originating from neuroendocrine cells. and their incidence has increased over the past 30 years 1. The majority of NETs are of gastroenteropancreatic (GEP) origin, arising in the foregut, midgut, or hindgut 2. Prognosis is different based on anatomic site, stage, and differentiation 3. The 5-year survival rate in patients with advanced stage NETs was poorer then in patients with early stage NET. In addition, patients with poorly differentiated neuroendocrine carcinomas (NECs) showed worse survival rate than those with well or moderately differentiated NETs 1.
Most patients with NECs have metastatic disease at diagnosis, with regional or distant metastasis 4. Consensus regarding the standardized cytotoxic chemotherapy regimen for metastatic GEP-NECs is lacking 5. Because the clinical behavior of GEP-NECs is similar to that of small-cell lung cancer (SCLC), which is responsive to etoposide and cisplatin (EP), EP has been the most widely used combination therapy in patients with extra-pulmonary NEC including GEP-NEC. In subgroup analyses by Moertel et al. and Mitry et al., EP showed favorable efficacy in NECs including GEP-NECs. Based on these studies, EP has been considered as the reference treatment for GEP-NECs 6, 7.
Data regarding the efficacy of EP based on primary tumor site in patients with GEP-NECs are lacking. Pancreatic NEC patients treated with cytotoxic chemotherapy showed a progression-free survival (PFS) of 8.9 months and an overall survival (OS) of 15 months 8. Gastric NEC patients that received EP chemotherapy showed a PFS of 7 months and an OS of 22 months 9. Moreover, hepatobiliary (HB) and pancreatic NEC patients receiving EP showed a 12% response rate (RR) and median OS of 6.9 months. Accordingly, EP showed a heterogeneous effect based on primary tumor site even if the GEP-NEC disease entity was the same.
Although EP has been considered the standard treatment for GEP-NECs, data for evaluating the efficacy of EP based on the primary tumor location of GEP-NEC are limited. Herein, we evaluated the impact of primary tumor site on the outcome of treatment with EP in patients with metastatic GEP-NEC.
Materials and methods
Patients
We analyzed 64 patients with histopathologically confirmed metastatic GEP-NEC who received EP at Samsung Medical Center, Seoul, Korea, between January 2010 and January 2018. The definition of GEP-NEC in this study was NEC arising in the GEP or HB system. The NECs were confirmed according to the 2010 WHO classification, additionally our study included poorly differentiated NECs with 20 or more mitoses/10 high power fields (HPF) and/or 20% Ki-67% index validated by experienced pathologists 10.
The clinicopathological characteristics collected from the 64 patients were age, gender, Eastern Cooperative Oncology Group Performance Status (ECOG PS), primary site, site of metastasis, number of metastatic sites, and outcome of treatment with EP.
Classification
NETs arise in a variety of anatomic locations, including the stomach, small intestine, colon, rectum, pancreas, liver, gall bladder, and multiple lymph nodes. In this study, based on embryological origin, primary tumor sites were classified as foregut (esophagus, stomach, duodenum, pancreas), midgut (appendix, ileum, cecum, ascending colon), and hindgut (distal large bowel, rectum) 11. To the above listed classification, HB (liver and biliary tract) and intra-abdominal lymph nodes origins were added.
Treatment
All patients received EP as the first line of palliative chemotherapy. Cisplatin (80 mg/m2) was administered intravenously (IV) over 1 h on the first day with adequate hydration. Etoposide (100 mg/m2/day) was administered IV over 90 min on days 1 - 3. This treatment was repeated every 3 weeks until progression, severe adverse reaction, or death. Tumor assessments based on computed tomography (CT) scans of the chest or abdomen were performed at baseline and every 2 cycles according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) 12.
Statistical Analyses
Descriptive statistics were reported as proportions and medians. Treatment outcomes were RR, PFS, and OS. The response to treatment was defined as complete response (CR), partial response (PR), stable disease, (SD) and progressive disease (PD), according to the RECIST 1.1 criteria. PFS was measured from the date of initial treatment to the date of death or the date of confirmed progression. OS was measured from the date of initial treatment to the date of death. PFS and OS were determined based on the Kaplan-Meier method, and survival curves were compared using log rank test. The 95% confidence interval (CI) was also computed for the median time to event.
Results
Patient characteristics
In this study, 64 patients that received EP were poorly differentiated and metastatic GEP-NEC at diagnosis. Patient demographics are presented in Table 1. The median age was 57 years (23 - 87 years). The proportion of male (n=46, 71.9%) was higher than female (n=18, 28.1%). The primary tumor sites included 22 foregut-derived GEP-NECs (stomach, n = 6; duodenum, n = 4; pancreas, n = 12), 4 midgut-derived GEP-NECs, 5 hindgut-derived GEP-NECs of the rectum, 25 GEP-NECs originating from the HB tract, and 12 GEP-NECs involving only intra-abdominal lymph nodes. Thirty-five patients had 1 or 2 metastatic sites, and 29 patients had 3 or more metastatic sites. The median number of EP cycles was 3 (range, 1 - 22 cycles).
Table 1.
Patient characteristics (n = 64)
| Parameter | n (%) |
|---|---|
| SEX | |
| Male | 46 (71.9%) |
| Female | 18 (28.1%) |
| Age, years | |
| Median, range | 57 (23 - 87) |
| ECOG | |
| 0 - 1 | 61 (95.3%) |
| >2 | 3 (4.9%) |
| Primary tumor site | |
| Foregut | 22 (34.4%) |
| Midgut | 4 (6.3%) |
| Hindgut | 5 (7.8%) |
| Hepatobiliary | 25 (39.1%) |
| Intra-abdominal lymph node | 8 (12.5%) |
| Metastatic site number | |
| 1 - 2 | 35 (54.7%) |
| ≥3 | 29 (45.4%) |
| Number of cycles, median | 3 (1 - 22) |
EP efficacy
Treatment responses are shown in Table 2. Among the 64 patients, none had CR and 17 had PR, resulting in a 27.9% RR. When evaluating the efficacy of EP based on primary tumor site, the RR was the most favorable in GEP-NEC patients with only intra-abdominal lymph node involvement, followed by GEP-NECs of foregut, midgut, HB, and hindgut origin. However, the RR based on the primary tumor site was not statistically significantly different (P = 0.821).
Table 2.
Treatment response based on primary tumor site of GEP-NEC
| Total | Foregut | Midgut | Hindgut | Hepatobiliary | Intra-Ab LNs | P value | |
|---|---|---|---|---|---|---|---|
| PR | 17 (27.9) | 7 (35.0) | 1 (25.0) | 1 (20.0) | 5 (20.8) | 3 (37.5) | |
| SD | 17 (27.9) | 3 (15.0) | 1 (25.0) | 1 (20.0) | 9 (37.5) | 3 (37.5) | |
| PD | 27 (44.3) | 10 (50.0) | 2 (50.0) | 3 (60.0) | 10 (41.7) | 2 (25.0) | |
| RR | 27.9 | 35.0 | 25.0 | 20.0 | 20.8 | 37.5 | 0.821 |
PFS
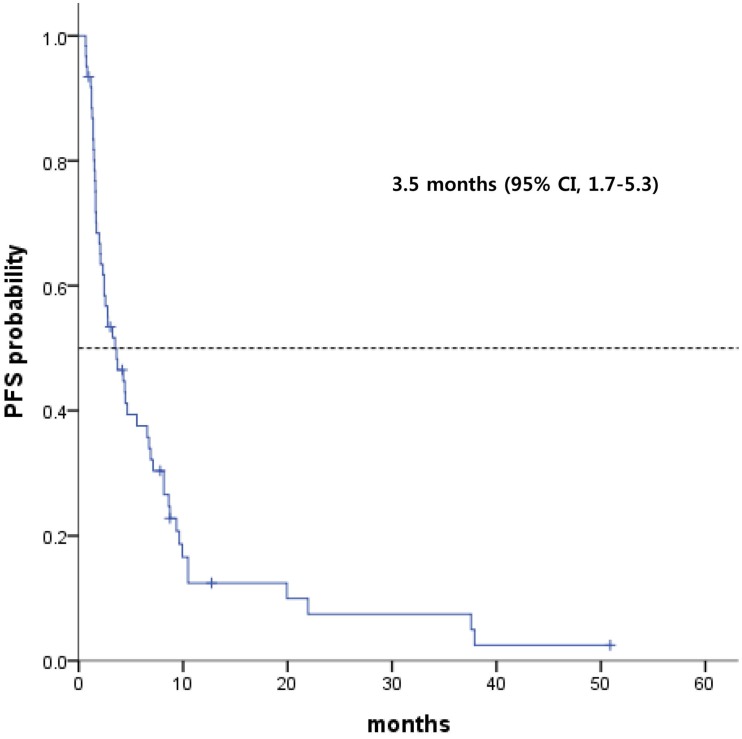
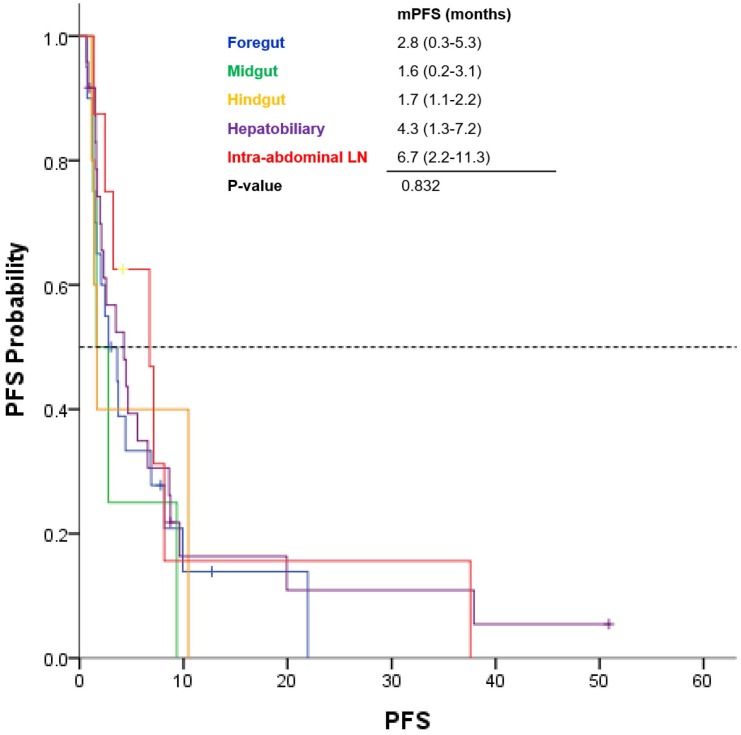
Among the 64 patients, the median PFS was 3.5 months (range, 1.7 - 5.3 months; Fig 1). GEP-NEC patients with only intra-abdominal lymph node involvement had a median PFS of 6.7 months, and those with HB tract involvement had a median PFS of 4.3 months (Fig 2). However, patients with midgut- and hindgut-derived GEP-NECs had a median PFS of 6.7 months and 4.3 months, respectively. Although a more favorable trend for PFS was observed in GEP-NEC patients with only intra-abdominal lymph node or with HB tract involvement than in those with midgut and hindgut-derived tumors, the PFS among patients with GEP-NECs arising from various primary tumor sites was not significantly different.
Fig 1.
Kaplan-Meier curves of progression-free survival (PFS) from etoposide plus cisplatin (n = 64)
Fig 2.
Kaplan-Meier curves of progression-free survival (PFS) from etoposide plus cisplatin based on primary tumor site of GEP-NEC
Discussion
NETs account for about 0.5% of all newly diagnosed malignancies and the most frequent primary site are the gastrointestinal tract (62-67%) and 12~20% patients already have metastasis at diagnosis 13. GEP-NECs are heterogeneous diseases in terms of embryonic origin, aggressiveness, prognosis, and genomic profiling 1. Platinum-based therapy with etoposide has been standard treatment for advanced or metastatic GEP-NECs 7, 14. In current study, the treatment outcomes according to different primary tumor sites were not statistically different in the poorly differentiated and metastatic NETs.
In clinic practice, NETs are generally sorted by histology and proliferation rate and tumor origins. Low to intermediate NETs have more treatment options than poorly differentiated NETs such as ablative therapy, trans-arterial embolization, selective radiation, surgery, somatostatin analogues and cytotoxic chemotherapy 15,16. The most NETs regardless of grade and primary tumor site are presented metastatic stage at diagnosis, so it is important to decrease tumor burden and control symptoms, if possible. However, cytotoxic chemotherapy is the most important treatment to high-grade or massive metastatic NETs. In the previous studies, a comparison of efficacy against cytotoxic EP chemotherapy is less reported for consideration of respective of the heterogeneity and primary tumor sites of GEP-NECs 6, 17, 18.
In this study, we evaluated the outcomes of treatment with EP as the standard chemotherapy based on primary tumor sites of foregut, midgut, hindgut, HB tract, and isolated intra-abdominal lymph nodes, together with entire NETs with poorly differentiated or high-grade and one more metastatic lesion. In previous study, it is likely that poorly differentiated and metastatic NETs itself is a worse prognostic factor than the primary tumor site as a prognostic factor.
Outcomes of chemotherapy treatment for GEP-NECs originating from different primary tumor sites were inconsistently reported among studies. In several studies, the HB and pancreatic NECs were less sensitive to EP, with a RR of 14% and median survival of 5.8 months 17. In another study, midgut and hindgut NETs had a lower RR to chemotherapies that included streptozotocin, 5-fluorouracil, doxorubicin, and cyclophosphamide regimens 19. However, data on outcomes to the EP chemotherapy based on various primary tumor sites in advanced or metastatic GEP-NECs are lacking. Although in several studies, the efficacy of chemotherapy in GEP-NECs with different primary tumor sites was investigated, those studies consisted of a small sample size and included various chemotherapy regimens 20,21.
In the present study, GEP-NEC patients with isolated intraperitoneal lymph nodes showed a favorable outcome trend to EP treatment compared to patients with GEP-NECs involving other primary tumor sites. This finding might be caused by other factors in addition to the characteristics of the primary tumor site. These patients are likely to receive additional anti-cancer treatments, especially local treatments, such as radiotherapy and surgery 22-24. These additional treatments combined with EP may contribute to favorable outcomes. In particular, among 8 GEP-NEC patients with isolated intraperitoneal lymph nodes, 5 patients simultaneously received radiotherapy, and 1 had a debulking operation.
However, the present study had several limitations such as a retrospective nature, small sample size, and heterogeneous patient population. The validation for findings of the present study was not conducted due to the rarity of the disease. Thus, the results in this study should be interpreted with caution. Nevertheless, this study is valuable because we investigated the outcomes of treatment with EP based on the primary tumor site in GEP-NEC patients. GEP-NEC is very heterogeneous disease entity and GEP-NEC patients with different primary tumor origin have known to have the different prognosis. The present study supported the evidence that EP treatment might be applied to GEP-NEC patients irrespective of the primary tumor sites. GPE-NEC is an orphan disease and difficult to study; thus, further collaborative research among nations and institutions is needed to validate this finding and to establish the new guidance in GEP-NECs.
Acknowledgments
This work was supported by funding from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2750, HI14C3418).
References
- 1.Yao JC, Hassan M, Phan A. et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Williams ED, Sandler M. The classification of carcinoid tum ours. Lancet. 1963;1:238–239. doi: 10.1016/s0140-6736(63)90951-6. [DOI] [PubMed] [Google Scholar]
- 3.Pape UF, Berndt U, Muller-Nordhorn J. et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 4.Oberg KE. Gastrointestinal neuroendocrine tumors. Ann Oncol. 2010;21(Suppl 7):vii72–80. doi: 10.1093/annonc/mdq290. [DOI] [PubMed] [Google Scholar]
- 5.Uri I, Grozinsky-Glasberg S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs) Clin Diabetes Endocrinol. 2018;4:16. doi: 10.1186/s40842-018-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moertel CG, Kvols LK, O'Connell MJ. et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Mitry E, Baudin E, Ducreux M. et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351–1355. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda M, Okuyama H, Takahashi H. et al. Chemotherapy for advanced poorly differentiated pancreatic neuroendocrine carcinoma. J Hepatobiliary Pancreat Sci. 2015;22:623–627. doi: 10.1002/jhbp.228. [DOI] [PubMed] [Google Scholar]
- 9.Okita NT, Kato K, Takahari D. et al. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14:161–165. doi: 10.1007/s10120-011-0025-5. [DOI] [PubMed] [Google Scholar]
- 10.International Classification of Diseases for Oncology. http://codes.iarc.fr/usingicdo.php. Accessed September 14; 2018. [Google Scholar]
- 11.Williams E, Sandler M. The classification of carcinoid tumours. Lancet. 1963;1(7275):238–239. doi: 10.1016/s0140-6736(63)90951-6. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3–7. doi: 10.1159/000080731. [DOI] [PubMed] [Google Scholar]
- 14.Kulke MH. Neuroendocrine tumors: is there a standard treatment? Gastrointest Cancer Res. 2008;2:152–153. [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson B, Kloppel G, Krenning E. et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors—well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology. 2008;87(1):8–19. doi: 10.1159/000111034. [DOI] [PubMed] [Google Scholar]
- 16.Faggiano A, Lo Calzo F, Pizza G. et al. The safety of available treatments options for neuroendocrine tumors. Expert Opin Drug Saf. 2017;20:1–13. doi: 10.1080/14740338.2017.1354984. [DOI] [PubMed] [Google Scholar]
- 17.Iwasa S, Morizane C, Okusaka T. et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 2010;40:313–318. doi: 10.1093/jjco/hyp173. [DOI] [PubMed] [Google Scholar]
- 18.Patta A, Fakih M. First-line cisplatin plus etoposide in high-grade metastatic neuroendocrine tumors of colon and rectum (MCRC NET): review of 8 cases. Anticancer Res. 2011;31:975–978. [PubMed] [Google Scholar]
- 19.O'Toole D, Hentic O, Corcos O. et al. Chemotherapy for gastro-enteropancreatic endocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):79–84. doi: 10.1159/000080747. [DOI] [PubMed] [Google Scholar]
- 20.Ekeblad S, Sundin A, Janson ET. et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 21.Oronsky B, Ma PC, Morgensztern D. et al. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991–1002. doi: 10.1016/j.neo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunz PL, Reidy-Lagunes D, Anthony LB. et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schurr PG, Strate T, Rese K. et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273–281. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhry H, Ellison TA, Maidment BW. et al. Radiation Therapy in Pancreatic Neuroendocrine Tumors: Favorable Outcomes and Low Toxicity in a Multi-institutional Experience. Int J Radiat Oncol. 2013;87:S308. [Google Scholar]