Abstract
Disseminated intravascular coagulation (DIC) is a frequent complication in sepsis. Once patients develop DIC, the mortality rate increases significantly. Moreover, recent studies have suggested that coagulation disorder plays a significant role in the development of organ dysfunction in sepsis. Thus, the early detection of DIC is vital in sepsis care, and the Japanese Association for Acute Medicine established a set of original diagnostic criteria in 2006 (JAAM DIC). Since then, the usefulness of the JAAM DIC has been repeatedly reported, and these criteria have been widely adopted in emergency and critical care settings in Japan. Different criteria have also been released by the International Society on Thrombosis and Haemostasis (ISTH overt‐DIC), and the latter criteria are presently considered to be the international standard. Compared with the JAAM DIC, the ISTH overt‐DIC criteria are stricter and the timing of diagnosis is later. This discrepancy is because of conceptual differences. As many physicians think sepsis‐associated DIC is the target of anticoagulant therapies in Japan, the JAAM DIC criteria were designed to allow the early initiation of treatment. As other countries do not provide DIC‐specific treatments, early diagnosis is not necessary, and this situation has led to a significant gap. However, as overt‐DIC is a late‐phase coagulation disorder, a need for early detection has been advocated, and members of the ISTH have recently proposed the category of sepsis‐induced coagulopathy. In this review, we introduce the strengths and weaknesses of the major criteria including JAAM‐DIC, ISTH overt‐DIC, sepsis‐induced coagulopathy, and Japanese Society on Thrombosis and Haemostasis‐DIC.
Keywords: Coagulopathy, diagnostic criteria, disseminated intravascular coagulation, sepsis/multiple organ failure
Introduction
The International Society on Thrombosis and Haemostasis (ISTH) has defined disseminated intravascular congestion (DIC) as “an acquired syndrome characterized by the intravascular activation of coagulation with loss of localization arising from different causes. It can originate from and cause damage to the microvasculature, which if sufficiently severe, can produce organ dysfunction”.1 Indeed, a wide variety of diseases can cause DIC, and the resulting clinical symptoms can differ considerably. However, a unique point of DIC is that, despite differences in the underlying diseases, a diagnosis can be made using the same diagnostic criteria, and the ISTH has released a set of overt‐DIC diagnostic criteria that consists of a combination of coagulation test results.1 The hallmark of DIC is systemic activation of coagulation resulting in (i) increased levels of fibrin‐related markers, (ii) the derangement of coagulation systems as expressed by the results of global coagulation tests, (iii) a decreased platelet count as a consequence of activation of platelets and thrombus formation. These outcomes form the essential components of the diagnostic criteria. The concept behind these criteria originated from the criteria of the Japanese Ministry of Health and Welfare (JMHW).2 Subsequently, although the ISTH overt‐DIC criteria have become the global standard,3, 4 other diagnostic criteria are also commonly used. For example, the Japanese Association for Acute Medicine (JAAM) released the JAAM DIC diagnostic criteria for acute DIC by eliminating a decreased fibrinogen level as a criterion,5 and the clinical usefulness of the JAAM DIC has been reported repeatedly.5, 6
Although the same diagnostic criteria have been utilized for the diagnosis of DIC arising from different backgrounds, recent studies have elucidated characteristic differences in DIC that are dependent on the underlying disease. For example, sepsis‐associated DIC is often complicated with organ dysfunction, whereas DIC associated with hematologic malignancy is predominantly complicated with bleeding.7 These differences arise from mechanistic differences, and DIC is divided into a thrombotic phenotype and a fibrinolytic phenotype. Thus, it would be rational to create different diagnostic criteria depending on the individual disease background. Following these concepts, the Japanese Society on Thrombosis and Hemostasis (JSTH) proposed new DIC diagnostic criteria.8 Moreover, dynamic changes in the coagulation status have also been recognized. For example, an initial excess of fibrinolysis is followed by the suppression of fibrinolysis in trauma‐induced DIC.9 Meanwhile, coagulation activation in combination with the excessive suppression of fibrinolysis and a disrupted anticoagulant system is observed in sepsis‐associated DIC.10 The clarification of these differences in pathogenesis has supported progress in the establishment of simpler diagnostic criteria. Active members of the ISTH DIC Scientific Standardization Committee (SSC) recently proposed the simplest version to date. Namely, a diagnosis of sepsis‐induced coagulopathy (SIC) consists of only three items: sepsis‐3 (infection with organ dysfunction), platelet count, and the prothrombin time ratio.11 The purpose of this review is to explain the significance of diagnosing DIC in sepsis, and to describe the history of developing popularly used diagnostic criteria (i.e., ISTH overt‐DIC and JAAM DIC), and strengths and weaknesses of those criteria. Finally, we introduce the details of the new criteria, that is, JSTH DIC and SIC. We think the understanding of the concepts and features of the individual criteria will help the proper management of sepsis‐associated DIC.
Importance of diagnosing DIC in sepsis
Recent knowledge supports the idea that DIC should not be recognized only as a coagulation disorder or hemostatic failure, but also as a delayed symptom of emerging systemic vascular inflammation caused by endothelial dysfunction.12, 13 As tissue malcirculation is the essential factor in the progression to septic organ failure, DIC and shock are the two crucial complications that can affect patient outcome.14, 15 A recent survey revealed that approximately one‐third of patients with sepsis who were treated in the intensive care unit (ICU) had shock as a complication, and more than half of them had DIC.16 The effectiveness of anticoagulant therapy for sepsis‐associated DIC has been intensively studied in Japan, and recent studies have repeatedly reported that anticoagulant therapy is only effective in patients with severe coagulopathy and DIC.6, 17, 18 Furthermore, subgroup analyses in large‐scale randomized controlled trials, including KyberSept and PROWESS, have examined the effect of anticoagulants in patients with sepsis and have reported trends toward a greater risk reduction in mortality among patients with DIC than among patients without DIC.19, 20 Therefore, the discrimination of patients with DIC from those without is vital.21 A clue to the proper understanding of DIC is that DIC is not a distinct disease category, but rather a continuous condition arising from coagulation disorder and coagulopathy. Therefore, it is not natural to create a border between those entities.22 However, as the benefit of anticoagulant therapy is reportedly correlated with the degree of the coagulation disorder, this condition needs to be categorized so that patients capable of benefiting from specific, appropriate therapy can receive such treatment.23
Accordingly, the primary objective of diagnosing DIC is to improve patient outcome through the initiation of specific treatments. In other words, the diagnostic criteria must be designed so as to categorize candidates who are most likely to benefit from a particular therapy.21 The validation of diagnostic criteria should also be undertaken from this point of view; however, no such prospective cohort studies have been made. In addition, one large‐scale randomized controlled trial exists that has evaluated the efficacy of recombinant thrombomodulin.24 In this trial, treatment was started after a diagnosis had been made using the JMHW DIC criteria, and a better DIC resolution was reported in the study group, compared with a group treated with unfractionated heparin. One must remember that the development of an adequate scoring system has facilitated the development of clinical trial protocols for DIC tremendously and has enabled the identification of likely candidates and risk stratification for new treatments.25 The DIC score can also be used as a surrogate outcome measure.26 More recently, Umemura et al.27 reported that ISTH overt‐DIC screening on the day of ICU admission was associated with a lower mortality, and the association became stronger if the screening was repeated 2 days later, suggesting that DIC screening by itself might be capable of reducing mortality. We agree that the ability of diagnostic criteria to discriminate survivors and non‐survivors at the timing of DIC diagnosis is important. Having a scoring system capable of reflecting the severity of a patient's condition would also be preferable.28 However, such characteristics are not mandatory for diagnostic criteria, and the DIC score should be used as a parameter that acts independently of other severity markers.
There exists a fundamental limitation in designing the diagnostic criteria. As long as conventional global clotting assays continue to be used, very few advances can be achieved. Platelet counts, prothrombin time (PT) test, and the levels of fibrin/fibrinogen degradation products (FDPs) are common components in the major sets of diagnostic criteria, and modifications of these cut‐offs and/or scores will only change the balance between sensitivity and specificity in anticipating mortality. Instead, a better understanding of the concept behind each diagnostic criterion should be emphasized. For example, the JAAM criteria are more sensitive than the ISTH overt‐DIC and JMHW DIC criteria and are presumably more suitable for early diagnosis. However, the ISTH overt‐DIC and JMHW DIC criteria are superior at excluding other conditions that should be differentiated from DIC. We think that there should be two different roles for diagnostic criteria: as a platform for DIC study, and for use in daily clinical practice. Preferably, a single criterion should contribute to both of these roles; if not, however, it might be better to have different criteria depending on the purpose (Fig. 1).
Figure 1.
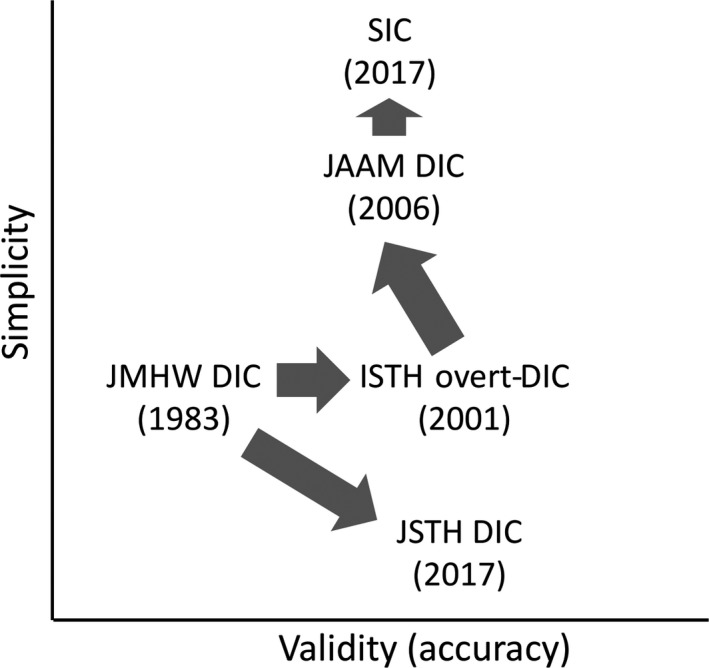
The intention for the diagnostic criteria for disseminated intravascular coagulation (DIC) and coagulopathy in sepsis in terms of validity versus simplicity. ISTH, International Society on Thrombosis and Haemostasis; JAAM, Japanese Association for Acute Medicine; JMHW, Japanese Ministry of Health and Welfare; JSTH, Japanese Society on Thrombosis and Hemostasis; SIC, sepsis‐induced coagulopathy.
International and Japanese diagnostic criteria
The release of the JMHW DIC criteria started a new epoch in the study of DIC. These criteria consisted of the presence of a primary cause, clinical symptoms, and four hemostatic laboratory tests.2 Since then, several sets of criteria have been proposed based on similar concepts. All of these sets of criteria have adopted a scoring system and include a combination of coagulation markers as there is no single test that is capable of defining or ruling out a diagnosis of DIC. Thus, this system is thought to be of utmost importance in assessing the whole clinical picture of the patient and the diagnosis.29 Currently, there are two sets of popular criteria: the ISTH overt‐DIC criteria, and the JAAM DIC criteria. Each of them has their own advantages and drawbacks. A significant difference is that the former criteria cover all the causes of DIC, whereas the latter were specially designed for the diagnosis of acute DIC.
International Society on Thrombosis and Haemostasis overt‐DIC
The progression of DIC causes reductions in hemostatic factors including platelets and clotting factors, and the patients finally reach a state of consumptive coagulopathy.30 In 1983, the JMHW created the first diagnostic criteria for DIC; these criteria consisted of the platelet count, PT, FDP, and fibrinogen level. The JMHW criteria adopted a scoring system that was suitable for evaluating continuous changes and detecting severity. However, some problems could not be ignored. First, although DIC is always a complication that occurs secondary to a basal disease, the presence of the basal disease was counted as a score. Clinicians also complained about the FDP criterion, as the measurement of this parameter was less common than the measurement of D‐dimer in many countries outside Japan. Thus, the SSC of the ISTH reviewed the JMHW criteria and released a new set of criteria for overt‐DIC in 2001 (Table 1).1 The JMHW DIC criteria and the ISTH overt‐DIC criteria were both established based on the definition that DIC consists of “systemic intravascular coagulation” and “consumptive coagulopathy”; however, the latter criteria were authorized by an international society and became the global standard. There are always strong and weak points for all sets of diagnostic criteria, and it is not always possible to decide which is superior. The JMHW criteria were the first DIC criteria and were established in Japan; however, the global standard for the diagnosis of DIC should probably be the ISTH overt‐DIC criteria.4 As DIC is a common and serious condition, various diagnostic criteria have been released other than the two above‐mentioned criteria, causing further confusion8, 31, 32 Thus, the establishment of an accepted standard is needed. One must remember that the establishment of different standards hinders progress in clinical practice and research on DIC by causing division, rather than a unified approach and it is just like yielding the condition after the collapse of the Tower of Babel.
Table 1.
International Society on Thrombosis and Haemostasis (ISTH) overt disseminated intravascular coagulation (DIC), Japanese Society on Acute Medicine (JAAM) DIC, and sepsis‐induced coagulopathy (SIC) scoring systems
| Item | Score | ISTH overt‐DIC range | JAAM DIC range | SIC range |
|---|---|---|---|---|
| Platelet count (×109/L) | 3 | – |
<80 ≥50% decrease within 24 h |
− |
| 2 | <50 | − | <100 | |
| 1 | ≥50, <100 |
≥80, <120 ≥30% decrease within 24 h |
≥100, <150 | |
| FDP (D‐dimer) | 3 | Strong increase |
≥25 μg/mL (use conversion chart) |
− |
| 2 | Moderate increase | − | − | |
| 1 | − |
≥10, <25 μg/mL (use conversion chart) |
− | |
| Prothrombin time (PT ratio) | 2 | ≥6 s | − | >1.4 |
| 1 | ≥3 s, <6 s | ≥1.2 (PT ratio) | >1.2, ≤1.4 (PT ratio) | |
| Fibrinogen (g/mL) | 1 | <100 | − | − |
| SIRS score | 1 | − | >3 | − |
| SOFA score | 2 | − | − | ≥2 |
| 1 | − | − | 1 | |
| Total score for DIC or SIC | ≥5 | ≥4 | ≥4 |
Total Sequential Organ Failure Assessment (SOFA) score is the sum of four items: respiratory SOFA, cardiovascular SOFA, hepatic SOFA, and renal SOFA.
–, not applicable; FDP, fibrin/fibrinogen degradation product; SIRS, systemic inflammatory response syndrome.
Which criteria are optimal for use has been a major interest of many physicians, and research on this topic is ongoing. However, validating the diagnostic accuracy of criteria can be difficult, as DIC is a conceptual diagnosis and cannot be confirmed pathologically or using other definitive methods.33, 34, 35 Some attempts have been made to compare diagnostic accuracy by comparing the predictive value for mortality.36, 37 However, this type of comparison might not be appropriate for evaluating diagnostic accuracy because the diagnostic criteria are not measures of severity like the Acute Physiology and Chronic Health Evaluation (APACHE) or Sequential Organ Failure Assessment (SOFA) scores. To begin with, the ability of criteria to discriminate candidates who are likely to benefit from a specific therapy is of foremost importance. For example, a retrospective analysis revealed that patients with ISTH overt‐DIC who were treated with recombinant activated protein C tended to have a greater relative risk reduction in mortality, compared with patients without treatment and such trend could not be seen in patients without ISTH overt‐DIC.20 This example supports the usefulness of the overt‐DIC criteria. In future studies, the performance of criteria should be confirmed plausibly in prospective controlled studies. Another critical performance of standard criteria is to determine the optimal timing for intervention, and the performance of such criteria should also be examined from this perspective. Finally, we must caution again that persisting with individual diagnostic criteria hampers progress in research and clinical practice in this field.
Japanese Association for Acute Medicine DIC
In the early 21st century, Japanese physicians complained about the delay in the diagnosis of infection‐based DIC. Although the ISTH overt‐DIC criteria had already been released as a global consensus, a general agreement could not be achieved in Japan because of some obstacles. First, the ISTH criteria did not enable any remarkable advances. Second, the cut‐off values for the fibrin‐related markers were not clearly shown. Finally, the timing of diagnosis was still later than that achievable using the JMHW criteria. Japanese physicians requested simpler and easier‐to‐use diagnostic criteria that could enable early diagnosis, especially for use in emergency and critical care fields. Under this background, JAAM started a project to create new criteria specifically designed for the diagnosis of acute diseases, including sepsis and trauma, and the JAAM DIC criteria were launched in 2006.5 In the JAAM DIC criteria, the fibrinogen level was omitted, and the selection of D‐dimer was allowed as another fibrin‐related marker. Dynamic changes in the platelet count were also included in the JAAM DIC. A unique feature of the JAAM criteria was the inclusion of systemic inflammatory response syndrome (SIRS). Although SIRS does not directly reflect coagulation disorders, it can help to identify the presence of sepsis. After several validation studies, the JAAM DIC criteria quickly spread and became the standard for diagnosis in Japan.38, 39, 40 In comparison to the ISTH overt‐DIC criteria, the JAAM DIC criteria can reportedly detect twice as many cases at an earlier timing.41, 42 Following the dissemination of the JAAM DIC criteria, the identification of septic DIC patients who could benefit from anticoagulant therapy has become part of routine clinical practice in Japan.
Despite the high incidence of DIC and its prognostic implications, the treatment of this detrimental condition has not been very successful in most countries since the withdrawal of recombinant activated protein C.43, 44 In contrast, anticoagulant therapy using antithrombin and recombinant thrombomodulin has been extensively accepted and is being used in daily practice in Japan.17, 45, 46, 47, 48 The latest Japanese Clinical Practice Guidelines for the Management of Sepsis and Septic Shock (J‐SSCG 2016) were created based on clinical evidence recommending the use of antithrombin and they turned out to fit the reality.49 In J‐SSCG 2016, the use of the JAAM DIC criteria was recommended to determine the timing of intervention. Other studies have revealed that the survival benefits of anticoagulant therapy were only seen in DIC patients, and not in non‐DIC patients.6, 23
As mentioned above, the JAAM DIC diagnostic criteria are useful and are still considered to be the standard of practice in Japan. The JAAM DIC criteria have gained positive evaluations and have an established reputation in some other geographical areas.50, 51 However, almost 14 years have passed, and the SIRS category adopted by the JAAM DIC is no longer used in Sepsis‐3;52 thus, new criteria that fit the new sepsis definition are expected.53
Future perspectives
There are two approaches to designing diagnostic criteria: creating practical criteria or creating precise criteria. As DIC is a common complication of sepsis, patients are treated not only in the ICU but also in general wards; therefore, simple and easy to use criteria are appreciated.4 However, the development of more precise criteria is also needed for scientific purposes. For the latter case, the inclusion of molecular markers is a rational approach.54 As long as access to new biomarkers is restricted and we continue to use only global hemostatic markers, that is, platelet count, PT test, FDP/D‐dimer, and fibrinogen, we will only be able to modulate the sensitivity and specificity of diagnostic criteria. Recently, two opposing types of criteria have been introduced. The ISTH have released practical diagnostic criteria for SIC,11 while the JSTH have proposed specific criteria that include antithrombin activity and molecular markers.8, 55
Sepsis‐induced coagulopathy
As already mentioned, the ISTH has defined DIC as being characterized by the systemic intravascular activation of coagulation arising from different causes.1 This definition implies that DIC can develop from various causes yet manifests similar changes in coagulation markers. Thus, the same diagnostic criteria should be applied regardless of the underlying diseases. However, this concept has recently begun to change with advances in understanding of the pathophysiology of DIC. For example, DIC secondary to infection is characterized by the excessive suppression of fibrinolysis arising from the overproduction of plasminogen activator inhibitor‐1.56, 57 This response is recognized as part of the host defense mechanisms, and coagulation progresses with fibrinolysis shutdown.58, 59 In contrast, such suppression is not seen in DIC arising as a complication of hematologic malignancies.60 As a result, the thrombotic phenotype of DIC, which is associated with infection, often develops in cases with organ dysfunction, whereas systemic bleeding is a feature of the fibrinolytic phenotype of DIC that often accompanies hematopoietic malignancy.60 Consequently, although the activation of coagulation is a universal hallmark of all types of DIC, the elevation in fibrin‐related markers is not associated with the severity of sepsis in a linear manner.61 By contrast, increased PT prolongation is correlated with the 28‐day mortality rate.61, 62
It would be more efficient to develop individual diagnostic criteria reflecting the pathophysiology of individual types of DIC.4, 8 The SIC criteria were constructed by the members of the DIC SSC of ISTH and were proposed in 2017 to categorize patients with “sepsis and coagulation disorders”; these criteria were designed to fit the new definition of sepsis.11 Similar ideas have been offered previously, and a simple diagnostic system composed of the platelet count and PT test has been examined.63, 64, 65 The SIC diagnostic criteria are simple and are composed of three items: platelet count, PT‐international normalized ratio, and the SOFA score. The SOFA score was included to confirm the presence of sepsis but not to reflect its severity; therefore, the score was regarded as 2 even when the SOFA score was 2 or more. If the SIC criteria could identify a similar category of patients as the JAAM DIC criteria, it would be preferable for Japanese physicians.
The utility of the SIC score has been repeatedly validated. First, the DIC SSC members of ISTH examined the diagnostic rate and mortality in sepsis patients with coagulopathy. As a result, almost all the patients with overt‐DIC had SIC, and SIC had preceded overt‐DIC in every case. The mortality rate for overt‐DIC was significantly higher than that for SIC (32.5% versus 23.1%).66 A comparison of the SIC and JAAM DIC criteria was also carried out, and the prevalence of patients with SIC was comparable to that of patients with JAAM DIC. The SIC criteria also showed similar performance for predicting the 28‐day mortality as the JAAM DIC criteria.67 Thus, the SIC criteria were suggested to identify a category similar to that identified by the JAAM DIC criteria. In addition, Ding et al.68 reported a strong correlation between the SIC and the ISTH overt‐DIC categories. They also reported a similar area under the receiver operating characteristic curve value for the two categories (SIC 0.658 versus overt‐DIC 0.684). The latest validation study reported the usefulness of the SIC criteria based on data from a Japanese cohort of septic patients. This study reported that the positive rate for ISTH overt‐DIC was approximately half of that for SIC, while the mortality rates for the two sets of criteria were comparable.69 Furthermore, the beneficial effects of anticoagulant therapy were observed in patients with coagulopathy as defined using both sets of criteria.69 The ISTH SSC is currently planning to propose a simplified “two‐step” scoring system for the early detection of DIC consisting of screening according to the SIC score as the first step; patients meet the criteria for SIC, then the overt‐DIC score would be calculated as the second step.
Japanese Society on Thrombosis and Hemostasis DIC
In 2016, a working group of the JSTH proposed new diagnostic criteria, and these criteria were authorized in 2017 (Table 2).8, 55 The main feature of the new criteria was the design of individual scoring methods that depended on the basal disease. The disease types were classified as hematopoietic disorder type, infectious type, and basic type based on the underlying conditions. The scoring for the platelet count was omitted in the hematopoietic type, whereas the scoring for the fibrinogen level was eliminated from the infectious type as fibrinogen is an acute phase protein and a reduction in its level is rarely seen in cases with infection. Other than the above, the antithrombin activity and molecular markers were added as new items. Antithrombin activity has been thought to be a sensitive marker for DIC, especially in cases with sepsis, and its utility for the prediction of mortality has been repeatedly reported.70, 71 As for molecular markers, thrombin–antithrombin complex (TAT), soluble fibrin (SF), and prothrombin fragment 1+2 (F1+2) have been adopted to increase the sensitivity.72 Both TAT and SF were used for the exclusion of other diseases that mimic DIC. In cases with infectious‐type DIC, the score was calculated based on the sum of the scores for the platelet count, FDP, PT ratio, antithrombin activity, and molecular markers (TAT, SF, or F1+2). Regarding the score for the platelet count, the score covered a range of points, 0–3, which was the same as that used in the JMHW, with the addition of another 1 point if a decrease of 30% or more occurs within 24 h. The ranges and points were 0–3 for FDP, and 0–2 for the PT ratio, and the ranges and scoring methods were the same as those used by the JMHW criteria. The antithrombin activity was not included as a test item in the original JMHW criteria, but it was adopted as a new JSTH criterion. A score of 1 point was assigned for antithrombin activity of 70% or less. For the molecular markers, 1 point was given if these values increase to 2‐fold or more of the respective upper limits of the standard range. The threshold for the diagnosis of infectious‐type DIC was set to more than 5 points.
Table 2.
Japanese Society on Thrombosis and Hemostasis disseminated intravascular coagulation (DIC) scoring systems for infection
| Item | Score | ISTH overt‐DIC range |
|---|---|---|
| Platelet count (×109/L) | 3 | ≤50 |
| 2 | >50, ≤80 | |
| 1 | >80, ≤120 | |
| +1 | ≥30% decrease within 24 h | |
| FDP (μg/mL) | 3 | ≥40 |
| 2 | <40, ≥20 | |
| 1 | <20, ≥10 | |
| Prothrombin time ratio | 2 | ≥1.67 |
| 1 | >1.25, <1.67 | |
| Antithrombin (%) | 1 | ≤70 |
| TAT, SF, F1+2 | 1 | ≥2‐fold of normal upper limit |
| Liver failurea | −3 | Yes |
| Total score for DIC | ≥5 |
For institutions that measure only D‐dimer, 1 point will be added if D‐dimer increases ≥2‐fold the normal upper limit.
Corresponds to “a prothrombin time activity of ≤40% or an international normalized ratio value of ≥1.5 due to severe liver dysfunction seen within 8 weeks of onset of initial symptoms following liver impairment that develops in a normal liver or a liver that is thought to exhibit normal liver function” (acute liver failure) or “cirrhosis with a Child–Pugh classification of B or C (≥7 points)” (chronic liver failure).
F1+2, prothrombin fragment 1 + 2; FDP, fibrin/fibrinogen degradation product; ISTH, International Society on Thrombosis and Haemostasis; SF, soluble fibrin; TAT, thrombin–antithrombin complex.
Although the advantages of including these molecular markers are understandable,73, 74 the drawbacks of these measures are a greater complexity and cost. Also, measurements of these markers are still not routinely carried out in many hospitals in Japan, at present. Therefore, Iba et al. eliminated the molecular markers, reduced the FDP score, and adjusted the total score for the diagnosis, then examined the performance of this simplified JSTH DIC scoring system. As a result, the simplified JSTH DIC scoring system was shown to have a performance similar to that of the original JSTH DIC scoring system in terms of mortality prediction.61 The JSTH DIC diagnostic criteria are probably suitable for research purposes but might require modification for clinical application. As evidence of the validation of the new JSTH criteria remains scarce, further studies are warranted to confirm the usefulness of these criteria.
Summary
The ISTH overt‐DIC criteria were constructed in conjunction with the DIC definition established by the ISTH and are considered to be an international standard, whereas the JAAM DIC criteria were specifically designed for the diagnosis of acute DIC including sepsis‐associated DIC. The JAAM DIC criteria are more sensitive and can detect DIC at an earlier timing. Moreover, they are the only diagnostic criteria that can determine the optimal timing of anticoagulant therapy. As the ISTH overt‐DIC are not suitable for early diagnosis, the ISTH created another category that defines a pre‐DIC status, namely SIC. A two‐step diagnostic strategy using SIC and overt‐DIC has been proposed. After all, the characteristic differences among sets of diagnostic criteria must be understood to select the optimal criteria according to the purpose.
Disclosure
Ethical consideration in this guideline: N/A.
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: TI is an advisory for JIMRO, Japan Blood Products Organization, and Asahi Kasei Pharma. YU received lecture fees from Asahi Kasei Pharma, Japan Blood Products Organization, CSL Behring. EW received grants from Asahi Kasei Pharma, Japanese Association for Complement Research, and personal fees from Alexion Pharma. SK received lecture fees from Asahi Kasei Pharma, CSL Behring, Nihon Pharmaceutical, and Japan Blood Products Organization. The other authors have no conflict of interest.
Funding Information
No funding information provided.
References
- 1. Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001; 86: 1327–30. [PubMed] [Google Scholar]
- 2. Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl. Haematol. 1983; 49: 265–75. [DOI] [PubMed] [Google Scholar]
- 3. Toh CH, Hoots WK. The scoring system of the scientific and standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5‐year overview. J. Thromb. Haemost. 2007; 5: 604–6. [DOI] [PubMed] [Google Scholar]
- 4. Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat. Rev. Dis. Primers 2016; 2: 16037. [DOI] [PubMed] [Google Scholar]
- 5. Gando S, Iba T, Eguchi Y et al A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit. Care Med. 2006; 34: 625–31. [DOI] [PubMed] [Google Scholar]
- 6. Yamakawa K, Umemura Y, Hayakawa M et al Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit. Care 2016; 20: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Semeraro N, Ammollo CT, Semeraro F, Colucci M. Coagulopathy of acute sepsis. Semin. Thromb. Hemost. 2015; 41: 650–8. [DOI] [PubMed] [Google Scholar]
- 8. Asakura H, Takahashi H, Uchiyama T et al Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb. J. 2016; 14: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shakur H, Roberts I, Bautista R et al Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant hemorrhage (CRASH‐2): a randomized, placebo‐controlled trial. Lancet 2010; 376: 23–32. [DOI] [PubMed] [Google Scholar]
- 10. Levi M, Sivapalaratnam S. Disseminated intravascular coagulation: an update on pathogenesis and diagnosis. Expert Rev. Hematol. 2018; 11: 663–72. [DOI] [PubMed] [Google Scholar]
- 11. Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis‐induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open 2017; 7: e017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delabranche X, Boisramé‐Helms J, Asfar P et al Microparticles are new biomarkers of septic shock‐induced disseminated intravascular coagulopathy. Intensive Care Med. 2013; 39: 1695–703. [DOI] [PubMed] [Google Scholar]
- 13. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis . J. Thromb. Haemost. 2018; 16: 231–41. [DOI] [PubMed] [Google Scholar]
- 14. Ogura H, Gando S, Saitoh D et al Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J. Infect. Chemother. 2014; 20: 157–62. [DOI] [PubMed] [Google Scholar]
- 15. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016; 2: 16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayakawa M, Saito S, Uchino S et al Characteristics, treatments, and outcomes of severe sepsis of 3195 ICU‐treated adult patients throughout Japan during 2011‐2013. J. Intensive Care 2016; 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis‐associated disseminated intravascular coagulation: the view from Japan. J. Thromb. Haemost. 2014; 12: 1010–9. [DOI] [PubMed] [Google Scholar]
- 18. Umemura Y, Yamakawa K. Optimal patient selection for anticoagulant therapy in sepsis: an evidence‐based proposal from Japan. J. Thromb. Haemost. 2018; 16: 462–4. [DOI] [PubMed] [Google Scholar]
- 19. Kienast J, Juers M, Wiedermann CJ et al Treatment effects of high‐dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J. Thromb. Haemost. 2006; 4: 90–7. [DOI] [PubMed] [Google Scholar]
- 20. Dhainaut JF, Yan SB, Joyce DE et al Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J. Thromb. Haemost. 2004; 2: 1924–33. [DOI] [PubMed] [Google Scholar]
- 21. Gando S, Meziani F, Levi M. What's new in the diagnostic criteria of disseminated intravascular coagulation? Intensive Care Med. 2016; 42: 1062–4. [DOI] [PubMed] [Google Scholar]
- 22. Simmons J, Pittet JF. The coagulopathy of acute sepsis. Curr. Opin. Anaesthesiol. 2015; 28: 227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta‐analysis of randomized controlled trials. J. Thromb. Haemost. 2015; 14: 518–30. [DOI] [PubMed] [Google Scholar]
- 24. Saito H, Maruyama I, Shimazaki S et al Efficacy and safety of recombinant human soluble thrombomodulin (ART‐123) in disseminated intravascular coagulation: results of a phase III, randomized, double‐blind clinical trial. J. Thromb. Haemost. 2007; 5: 31–41. [DOI] [PubMed] [Google Scholar]
- 25. Wada H, Thachil J, Di Nisio M et al Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines. J. Thromb. Haemost. 2013; 11: 761–7. [DOI] [PubMed] [Google Scholar]
- 26. Dempfle CE. Comparing DIC scores: not an easy task indeed. Thromb. Res. 2009; 124: 651–2. [DOI] [PubMed] [Google Scholar]
- 27. Umemura Y, Yamakawa K, Hayakawa M, Hamasaki T, Fujimi S. Screening itself for disseminated intravascular coagulation may reduce mortality in sepsis: a nationwide multicenter registry in Japan. Thromb. Res. 2018; 161: 60–6. [DOI] [PubMed] [Google Scholar]
- 28. Levi M, Schultz MJ. What do sepsis‐induced coagulation test result abnormalities mean to intensivists? Intensive Care Med. 2017; 43: 581–3. [DOI] [PubMed] [Google Scholar]
- 29. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br. J. Haematol. 2009; 145: 24–33. [DOI] [PubMed] [Google Scholar]
- 30. Levi M, Ten Cate H. Disseminated intravascular coagulation. N. Engl. J. Med. 1999; 341: 586–92. [DOI] [PubMed] [Google Scholar]
- 31. Lee JH, Song JW, Song KS. Diagnosis of overt disseminated intravascular coagulation: a comparative study using criteria from the International Society versus the Korean Society on Thrombosis and Hemostasis. Yonsei Med. J. 2007; 48: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang M, Kou H, Deng J et al Retrospective evaluation of new Chinese diagnostic scoring system for disseminated intravascular coagulation. PLoS ONE 2015; 10: e0129170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toh CH, Alhamdi Y. Current consideration and management of disseminated intravascular coagulation. Hematology Am. Soc. Hematol. Educ. Program 2013; 2013: 286–91. [DOI] [PubMed] [Google Scholar]
- 34. Iba T. The meanings of DIC diagnostic criteria. Thromb. Res. 2012; 129: e269–70. [DOI] [PubMed] [Google Scholar]
- 35. Thachil J. The elusive diagnosis of disseminated intravascular coagulation: does a diagnosis of DIC exist anymore? Semin. Thromb. Hemost. 2019; 45: 100–7. [DOI] [PubMed] [Google Scholar]
- 36. Takemitsu T, Wada H, Hatada T et al Prospective evaluation of three different diagnostic criteria for disseminated intravascular coagulation. Thromb. Haemost. 2011; 105: 40–4. [DOI] [PubMed] [Google Scholar]
- 37. Singh RK, Baronia AK, Sahoo JN et al Prospective comparison of new Japanese Association for Acute Medicine (JAAM) DIC and International Society of Thrombosis and Hemostasis (ISTH) DIC score in critically ill septic patients. Thromb. Res. 2012; 129: e119–25. [DOI] [PubMed] [Google Scholar]
- 38. Gando S, Saitoh D, Ogura H et al Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit. Care Med. 2008; 36: 145–50. [DOI] [PubMed] [Google Scholar]
- 39. Kushimoto S, Gando S, Saitoh D et al Clinical course and outcome of disseminated intravascular coagulation diagnosed by Japanese Association for Acute Medicine criteria. Comparison between sepsis and trauma. Thromb. Haemost. 2008; 100: 1099–105. [PubMed] [Google Scholar]
- 40. Gando S, Saitoh S, Ogura H et al A multicenter prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit. Care 2013; 17: R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saito S, Uchino S, Hayakawa M et al Epidemiology of disseminated intravascular coagulation in sepsis and validation of scoring systems. J. Crit. Care 2018; 50: 23–30. [DOI] [PubMed] [Google Scholar]
- 42. Gando S, Saitoh D, Ogura H et al Disseminated intravascular coagulation (DIC) diagnosed based on the Japanese Association for Acute Medicine criteria is a dependent continuum to overt DIC in patients with sepsis. Thromb. Res. 2009; 123: 715–8. [DOI] [PubMed] [Google Scholar]
- 43. Ranieri VM, Thompson BT, Barie PS et al Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012; 366: 2055–64. [DOI] [PubMed] [Google Scholar]
- 44. Rhodes A, Evans LE, Alhazzani W et al Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017; 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 45. Hayakawa M, Yamakawa K, Saito S et al Recombinant human soluble thrombomodulin and mortality in sepsis‐induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb. Haemost. 2016; 115: 1157–66. [DOI] [PubMed] [Google Scholar]
- 46. Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis‐associated disseminated intravascular coagulation: an observational nationwide study. J. Thromb. Haemost. 2014; 12: 1470–9. [DOI] [PubMed] [Google Scholar]
- 47. Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis‐associated disseminated intravascular coagulation: a perspective from Japan. Int. J. Hematol. 2016; 103: 253–61. [DOI] [PubMed] [Google Scholar]
- 48. Hayakawa M, Kudo D, Saito S et al Antithrombin supplementation and mortality in sepsis‐induced disseminated intravascular coagulation: a multicenter retrospective observational study. Shock 2016; 46: 623–31. [DOI] [PubMed] [Google Scholar]
- 49. Nishida O, Ogura H, Egi M et al The Japanese clinical practice guidelines for management of sepsis and septic shock 2016 (J‐SSCG 2016). Acute Med. Surg. 2018; 5: 3–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jhang WK, Ha E, Park SJ. Evaluation of disseminated intravascular coagulation scores in critically ill pediatric patients with septic shock. J. Crit. Care 2018; 47: 104–8. [DOI] [PubMed] [Google Scholar]
- 51. Di Nisio M, Baudo F, Cosmi B et al Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb. Res. 2012; 129: e177–84. [DOI] [PubMed] [Google Scholar]
- 52. Singer M, Deutschman CS, Seymour CW et al The third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iba T, Di Nisio M, Thachil J et al Revision of the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) diagnostic criteria using antithrombin activity. Crit. Care 2016; 20: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wada H, Sakuragawa N, Mori Y et al Hemostatic molecular markers before the onset of disseminated intravascular coagulation. Am. J. Hematol. 1999; 60: 273–8. [DOI] [PubMed] [Google Scholar]
- 55. Wada H, Takahashi H, Uchiyama T et al The approval of revised diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb. J. 2017; 15: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koyama K, Madoiwa S, Nunomiya S et al Combination of thrombin‐antithrombin complex, plasminogen activator inhibitor‐1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit. Care 2014; 18: R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hack CE. Fibrinolysis in disseminated intravascular coagulation. Semin. Thromb. Hemost. 2001; 27: 633–8. [DOI] [PubMed] [Google Scholar]
- 58. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013; 13: 34–45. [DOI] [PubMed] [Google Scholar]
- 59. Schmitt FCF, Manolov V, Morgenstern J et al Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann. Intensive Care. 2019; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J. Intensive Care 2014; 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iba T, Di Nisio M, Thachil J et al A proposal of the modification of Japanese Society on Thrombosis and Hemostasis (JSTH) Disseminated Intravascular Coagulation (DIC) diagnostic criteria for sepsis‐associated DIC. Clin. Appl. Thromb. Hemost. 2018; 24: 439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iba T, Arakawa M, Ohchi Y et al Prediction of early death in patients with sepsis‐associated coagulation disorder treated with antithrombin supplementation. Clin. Appl. Thromb. Hemost. 2018; 1076029618797474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kinasewitz GT, Zein JG, Lee GL, Nazir SA, Taylor FB Jr. Prognostic value of a simple evolving disseminated intravascular coagulation score in patients with severe sepsis. Crit. Care Med. 2005; 33: 2214–21. [DOI] [PubMed] [Google Scholar]
- 64. Taylor FB Jr, Kosanke S, Randolph M et al Retrospective description and experimental reconstitution of three different responses of the baboon to lethal E. coli . Circ. Shock 1994; 42: 92–103. [PubMed] [Google Scholar]
- 65. Lyons PG, Micek ST, Hampton N, Kollef MH. Sepsis‐associated coagulopathy severity predicts hospital mortality. Crit. Care Med. 2018; 46: 736–42. [DOI] [PubMed] [Google Scholar]
- 66. Iba T, Arakawa M, Di Nisio M et al Newly proposed sepsis‐induced coagulopathy precedes international society on thrombosis and haemostasis overt‐disseminated intravascular coagulation and predicts high mortality. J. Intensive Care Med. 2018; 885066618773679. [DOI] [PubMed] [Google Scholar]
- 67. Iba T, Arakawa M, Levy JH et al Sepsis‐induced coagulopathy and japanese association for acute medicine DIC in coagulopathic patients with decreased antithrombin and treated by antithrombin. Clin. Appl. Thromb. Hemost. 2018; 24: 1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ding R, Wang Z, Lin Y, Liu B, Zhang Z, Ma X. Comparison of a new criteria for sepsis‐induced coagulopathy and International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul. Fibrinolysis 2018; 29: 551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamakawa K, Yoshimura J, Ito T, Hayakawa M, Hamasaki T, Fujimi S. External validation of the two newly proposed criteria for assessing coagulopathy in sepsis. Thromb. Haemost. 2018; 119: 203–12. [DOI] [PubMed] [Google Scholar]
- 70. Fourrier F, Chopin C, Goudemand J et al Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest 1992; 101: 816–23. [DOI] [PubMed] [Google Scholar]
- 71. Iba T, Gando S, Murata A et al Predicting the severity of systemic inflammatory response syndrome (SIRS)‐associated coagulopathy with hemostatic molecular markers and vascular endothelial injury markers. J. Trauma 2007; 63: 1093–8. [DOI] [PubMed] [Google Scholar]
- 72. Aota T, Wada H, Fujimoto N et al The valuable diagnosis of DIC and pre‐DIC and prediction of a poor outcome by the evaluation of diagnostic criteria for DIC in patients with hematopoietic injury established by the Japanese Society of Thrombosis and Hemostasis. Thromb. Res. 2016; 147: 80–4. [DOI] [PubMed] [Google Scholar]
- 73. Iba T, Ito T, Maruyama I et al Potential diagnostic markers for disseminated intravascular coagulation of sepsis. Blood Rev. 2016; 30: 149–55. [DOI] [PubMed] [Google Scholar]
- 74. Aota T, Wada H, Fujimoto N et al Evaluation of the diagnostic criteria for the basic type of DIC established by the Japanese Society of Thrombosis and Hemostasis. Clin. Appl. Thromb. Hemost. 2017; 23: 838–43. [DOI] [PubMed] [Google Scholar]


