Abstract
Background
Treatment of hyperphosphataemia is the primary goal of chronic kidney disease–mineral and bone disorder (CKD-MBD) management. This post hoc analysis of a randomized, Phase 3 study evaluated the effects of 1-year treatment with the phosphate binders sucroferric oxyhydroxide or sevelamer carbonate (‘sevelamer’) on CKD-MBD indices among dialysis patients with hyperphosphataemia.
Methods
After a 2- to 4-week washout from previous phosphate binders, 1059 patients were randomized 2:1 to sucroferric oxyhydroxide 1.0–3.0 g/day (n = 710) or sevelamer 2.4–14.4 g/day (n = 349) for up to 24 weeks. Eligible patients enrolled in a 28-week extension. This post hoc analysis was performed for patients who completed ≥1 year of continuous treatment (n = 549). As the treatment groups showed similar CKD-MBD outcomes, the data were pooled for this analysis.
Results
Phosphate-binder therapy was associated with significant and sustained 30% reductions in serum phosphorus (P < 0.001). Median intact fibroblast growth factor-23 (FGF-23) also significantly decreased (P < 0.001) by 64% over 1 year. Intact parathyroid hormone decreased significantly after 24 weeks (P < 0.001), but levels returned to near baseline values by Week 52; minimal changes in serum calcium were observed. Of the bone resorption markers evaluated, tartrate-resistant acid phosphatase 5b (TRAP5b) decreased significantly (P < 0.001), whereas CTx increased transiently but returned to baseline levels by Week 52. The bone formation markers bone-specific alkaline phosphatase and osteocalcin both increased over 1 year of treatment.
Conclusions
Overall, 1 year of sucroferric oxyhydroxide or sevelamer treatment significantly reduced serum FGF-23, which has been associated with clinical benefit in patients with CKD. The trend towards increased bone formation marker levels indicates a beneficial effect on bone metabolism.
Keywords: bone markers, dialysis, hyperphosphataemia, phosphate binder, sucroferric oxyhydroxide
ADDITIONAL CONTENT
An author video to accompany this article is available at: https://academic.oup.com/ndt/pages/author_videos.
INTRODUCTION
Hyperphosphataemia is a serious consequence of late-stage chronic kidney disease (CKD) and a major contributor to CKD–mineral and bone disorder (CKD-MBD) [1, 2].
As well as increases in parathyroid hormone (PTH) levels, hyperphosphataemia is typically accompanied by increases in serum fibroblast growth factor-23 (FGF-23). Increases in serum phosphorus, FGF-23 and PTH have each been independently associated with a greater risk of morbidity and mortality in CKD patients undergoing dialysis in epidemiological studies [3–5]. Elevated FGF-23 is also associated with progression of CKD [6], left ventricular hypertrophy [7], infections [8] and cardiovascular events [9] regardless of the presence of hyperphosphataemia.
Initial studies have shown that the treatment of hyperphosphataemia is a primary goal of CKD-MBD management [1, 10], and the majority of patients on dialysis are prescribed oral phosphate binders to achieve control of serum phosphorus levels [11]. These agents have also been shown to reduce FGF-23 levels [12–14]. Elevated FGF-23 has independently been associated with increased rates of cardiovascular events and mortality in dialysis patients [3], although more investigation is needed to establish whether interventions that lower FGF-23 levels lead to improved cardiovascular outcomes.
Sucroferric oxyhydroxide is non-calcium, iron-based phosphate binder approved for the treatment of hyperphosphataemia in dialysis patients. In an open-label, randomized 24-week Phase 3 study and its 28-week extension, sucroferric oxyhydroxide demonstrated similar phosphate-lowering efficacy to sevelamer carbonate (‘sevelamer’), but with a lower pill burden [15, 16].
There has, however, been a lack of large-scale clinical studies examining the impact of non-calcium-based phosphate binder therapy on CKD-MBD parameters.
Here we present a post hoc analysis of data from the initial Phase 3 study and its extension to assess the effects of serum phosphate control and FGF-23 levels with sucroferric oxyhydroxide and sevelamer on CKD-MBD parameters among dialysis patients who completed 1 year of treatment with sucroferric oxyhydroxide or sevelamer following a 2- to 4-week washout phase with cessation of all phosphate binders. Both phosphate binders had a comparable efficacy in the original 52-week treatment period, with no reason to expect different results in terms of CKD-MBD parameters. For this reason, the two treatment groups were pooled for this post hoc analysis.
MATERIALS AND METHODS
Design
This was a two-stage, randomized, active-controlled, parallel-group, multicentre, open-label and Phase 3 study (NCT01324128) [15] investigating the efficacy and safety of sucroferric oxyhydroxide versus sevelamer, followed by an extension study (NCT01464190) [16], in dialysis patients with hyperphosphataemia. Full details of the study designs have been described previously [15, 16].
The protocol was reviewed by Independent Ethics Committees or Institutional Review Boards, and the study was conducted in accordance with the Declaration of Helsinki Principles and the International Conference on Harmonisation E6 Guideline for Good Clinical Practice, Committee for Proprietary Medicinal Products guideline (CPMP/ICH/135/95), and was compliant with the European Union Clinical Trial Directive (Directive 2001/20/EC) and the Code of Federal Regulations for informed consent and protection of patient rights.
Participants
The full inclusion and exclusion criteria for the study participants have been described elsewhere [15]. In brief, eligible study patients were aged ≥18 years, had a history of hyperphosphataemia and prescription of stable doses of phosphate binders for ≥1 month before screening, required maintenance haemodialysis three times per week or peritoneal dialysis ≥3 months before screening, and had serum phosphate levels ≥1.94 mmol/L during washout. Patients were ineligible if, at screening, they had intact PTH (iPTH) levels >88 pmol/L or serum ferritin >4494 pmol/L.
Study treatment
Following a 2- to 4-week washout period, patients with serum phosphorus concentrations of ≥1.94 mmol/L were randomized 2:1 to receive sucroferric oxyhydroxide 1.0–3.0 g/day [2–6 tablets/day; starting dose 1.0 g/day (2 tablets/day)] or sevelamer 2.4–14.4 g/day [3–18 tablets/day; starting dose 4.8 g/day (6 tablets/day)] for 12 weeks’ dose titration (Weeks 1–12) followed by 12 weeks’ maintenance (Weeks 12–24). No other phosphate binders, or calcium-based antacids, were permitted during the study [16].
Eligible patients who had completed treatment in the initial 24-week efficacy and safety study were able to enter a 28-week extension study, continuing the same randomized treatment and dosage they were receiving at the end of the initial study [16].
Assessments, analysis sets and statistics
The analyses reported in this article were performed on the completers set, that is, all patients who completed at least 52 weeks of continuous treatment in the initial Phase 3 study and its extension study. The sucroferric oxyhydroxide and sevelamer groups were pooled for the purposes of the main post hoc analysis. A separate analysis examined any treatment differences between groups.
Serum concentrations of the following biochemical parameters were assessed at baseline, predefined time points over 1 year and end of study (Week 52): serum phosphorus, FGF-23, iPTH and total calcium (i.e. corrected for albumin); markers of bone resorption [tartrate-resistant acid phosphatase 5b (TRAP-5b) and carboxy-terminal cross-linking telopeptide of type 1 collagen (CTx)]; and markers of bone formation [osteocalcin (OST) and bone-specific alkaline phosphatase (BSAP)] [16]. Week 52 is defined as the last post-baseline non-missing value across both the Phase 3 and extension studies (i.e. last observation carried forward). For all analyses of the change from Week 24 to Week 52 in any endpoint, Week 24 refers to the baseline value for the extension study and Week 52 to the last post-baseline non-missing value across the extension study.
Analysis of serum samples derived from the completers set was performed at one of two central laboratories using standard validated methods. Serum FGF-23 was assessed using Immutopics Inc.: Human Intact FGF-23 enzyme-linked immunosorbent assay Kit.
Statistical tests were performed using two-sided tests at the 5% significance level. Wilcoxon signed-rank test is used for the change from baseline and Wilcoxon rank-sum test for the comparison of the changes from baseline between treatments. The analyses were conducted using SAS® version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Patient disposition and baseline demographics
In the initial Phase 3 study, 1059 patients were randomized to treatment: 710 to sucroferric oxyhydroxide and 349 to sevelamer (Supplementary data, Figure S1). A total of 1055 patients received one or more doses of study medication (the safety set: sucroferric oxyhydroxide, n = 707; sevelamer, n = 348). The completers set comprised 549 patients who received at least 52 weeks of continuous treatment (sucroferric oxyhydroxide, n = 322; sevelamer, n = 227).
There were no marked differences in baseline characteristics between patients in the sucroferric oxyhydroxide and sevelamer treatment groups (Supplementary data, Table S1). In the pooled population, the majority of patients were male, white and received haemodialysis (Table 1). Patients had been receiving dialysis for an average of 4 years prior to study entry. Approximately two-thirds (66.3%) of patients had previously received calcium-based phosphate binders, and over one-third (33.2%) had previously received sevelamer [15, 16].
Table 1.
Baseline patient demographics and clinical characteristics (completer set, n = 549)
| Total | |
|---|---|
| (n = 549) | |
| Mean (SD) age, years | 56 (14) |
| Sex, n (%) | |
| Male | 322 (58.7) |
| Ethnicity, n (%) | |
| White | 430 (78.3) |
| Black/African American | 100 (18.2) |
| Other | 19 (3.5) |
| Dialysis modality, n (%) | |
| Haemodialysis | 497 (90.5) |
| Peritoneal dialysis | 52 (9.5) |
| Reason for end-stage renal disease, n (%) | |
| Hypertension | 135 (24.6) |
| Glomerulonephritis | 137 (25.0) |
| Diabetic nephropathy | 133 (24.2) |
| Other | 144 (26.2) |
| Mean (SD) time from first dialysis, months | 52 (53) |
| Prior phosphate binders, n (%) | |
| Calcium-based | 364 (66.3) |
| Aluminium-based | 22 (4.0) |
| Lanthanum | 29 (5.3) |
| Sevelamer | 182 (33.2) |
| Other | 3 (0.6) |
| Prior use of sevelamer any time in previous 12 months, n (%) | 195 (35.5) |
Markers of serum phosphorus control
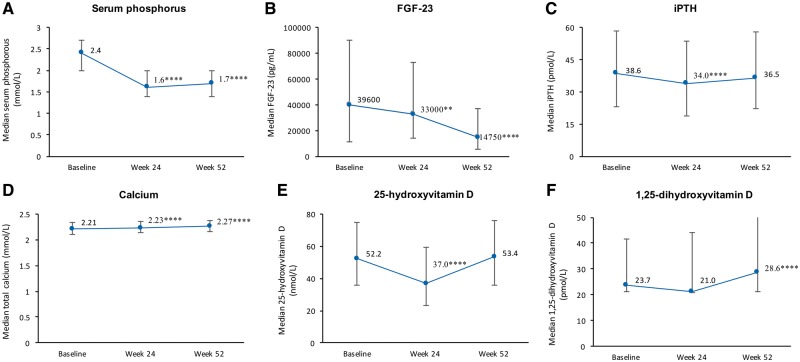
Phosphate binder treatment with sucroferric oxyhydroxide or sevelamer was associated with significant 30% reductions from baseline in serum phosphorus concentrations that were maintained for the duration of the 52-week treatment period (P < 0.0001) (Figure 1A). Serum FGF-23 concentrations progressively declined by 64% over the 1-year treatment period, with significant reductions from baseline to Week 24 (P = 0.0018) and to Week 52 (P < 0.0001). The potential effect of concomitant vitamin D receptor agonists (VDRAs) and calcimimetics on FGF-23 levels during study was evaluated in an exploratory subgroup analysis. No effect was apparent, with reductions in FGF-23 observed regardless of concomitant VDRAs or calcimimetics use (data not shown). However, the patient numbers were too small to draw meaningful conclusions from this analysis.
FIGURE 1.
Median serum concentration for serum phosphorus (A), FGF-23 (B), iPTH (C), calcium (D), 25-hydroxyvitamin D (E) and 1, 25-dihydroxyvitamin D (F) (completer set, n = 549). Error bars represent 25th and 75th percentiles. P-values are versus baseline: **P < 0.01, ****P < 0.0001.
There was a significant decrease in serum iPTH concentrations from baseline to Week 24 (P < 0.0001), but levels increased significantly from Week 24 to 52 (P < 0.0001), returning to near baseline values by Week 52 (Figure 1C). Following the initial washout period for other phosphate binders prior to the study baseline, 20.6% of patients had serum iPTH >66 pmol/L (>600 pg/mL), whereas 9.7% had levels >88 pmol/L (>800 pg/mL). By Week 24, these proportions were reduced to 16.1% and 6.2%, respectively, before climbing back to 20.6% and 12.2%, in line with rising iPTH levels at Week 52.
There were small but statistically significant (P < 0.0001) increases from baseline in serum total calcium concentrations during the 1-year study period (Figure 1D). Levels of 25-hydroxyvitamin D decreased significantly from baseline to Week 24, before returning to baseline levels by Week 52 (Figure 1E). Serum 1, 25-dihydroxyvitamin D decreased slightly until Week 24, but had increased significantly compared with baseline by Week 52 (P < 0.0001).
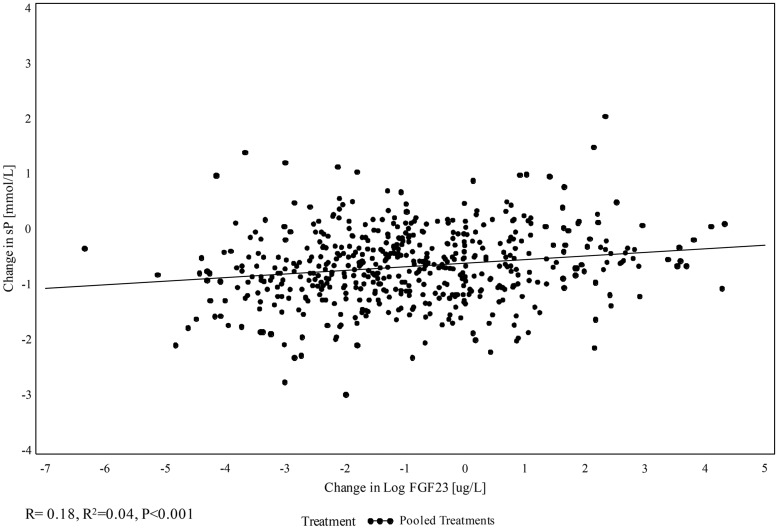
An exploratory analysis was performed to evaluate the relationship between changes in serum phosphorus and FGF-23 over the 52-week treatment period. This showed a weak, but statistically significant, correlation between log change in serum FGF-23 concentrations and change in serum phosphorus levels at Week 52 (R = 0.18; R2=0.035; P < 0.001) (Figure 2). The majority of patients experienced concomitant increases or decreases from baseline in both serum phosphorus and FGF-23. However, of patients who completed treatment and had available change from baseline values in both serum phosphorus and FGF-23, 31% (163/518) experienced an increase in one marker and a decrease in the other. Of these, 74% (121/163) experienced decreased serum phosphorus but increased FGF-23. There were no clear differences in baseline demographic and clinical characteristics for these subgroups.
FIGURE 2.
Change in log serum FGF-23 versus change in serum phosphorus (sP) at Week 52 (n = 518).
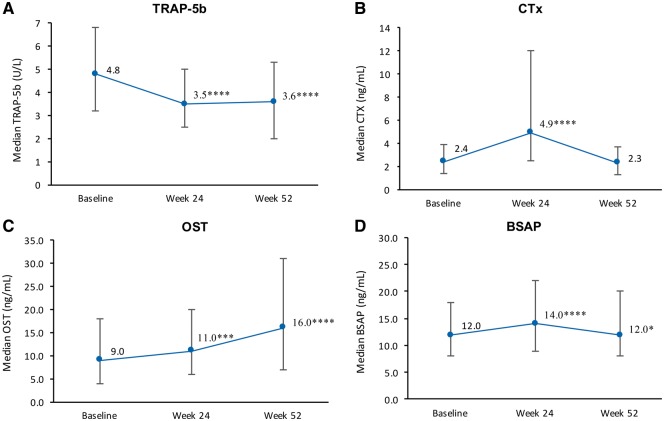
Serum markers of bone resorption
Serum TRAP-5b concentrations decreased significantly from baseline to Week 24 (P < 0.0001), and this initial reduction was maintained through to Week 52 (Figure 3A). By contrast, serum CTx concentrations increased from baseline to Week 24 (P < 0.0001), before decreasing from Weeks 24 to 52 (P < 0.0001) back to baseline levels (Figure 3B).
FIGURE 3.
Median serum concentration of markers of bone resorption, TRAP-5b (A) and CTx (B), and of bone formation, OST (C) and BSAP (D) (completer set, n = 549). Error bars represent 25th and 75th percentiles. P-values are versus baseline: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Serum markers of bone formation
Serum OST concentrations steadily increased over 1 year of treatment, with significant changes observed from baseline to Week 24 (P = 0.0005) and to Week 52 (P < 0.0001) (Figure 3C). Serum BSAP concentrations initially increased from baseline to Week 24 (P < 0.0001), but decreased from Weeks 24 to 52 (P < 0.0001), returning to around baseline levels (Figure 3D).
Differences between sucroferric oxyhydroxide and sevelamer
A separate analysis of the completer set revealed no statistically significant differences between the sucroferric oxyhydroxide and sevelamer treatment groups with respect to changes from baseline in serum phosphorus, FGF-23, iPTH, total calcium, 25-hydroxyvitamin D, 1, 25-dihydroxyvitamin D or OST (Supplementary data, Table S2). Over the 1-year treatment period (baseline to Week 52), there were small, but significantly greater, reductions in TRAP-5b and CTx in the sucroferric oxyhydroxide group, compared with the sevelamer group (median TRAP-5b: –1.3 versus –0.6 U/L, P = 0.023; median CTx: –0.3 versus 0.2 ng/mL; P = 0.006) (Supplementary data, Table S2). In addition, BSAP levels remained significantly above baseline at Week 52 for sevelamer but not sucroferric oxyhydroxide-treated patients. Changes in BSAP were statistically significant with sucroferric oxyhydroxide versus sevelamer (median 0.0 versus 2.0 ng/mL; P = 0.014).
DISCUSSION
This post hoc analysis evaluated the long-term effects of the non-calcium-based phosphate binders, sucroferric oxyhydroxide and sevelamer, on CKD-MBD parameters and markers of bone turnover in a large population of dialysis patients (n = 549). The two phosphate binders each had comparable effects on most of the CKD-MBD parameters, with the exception of small differences in long-term changes in markers of bone resorption (TRAP-5b and CTx) and bone formation (BSAP) (Supplementary data, Table S2). This confirmed the validity of pooling the data from the two groups for this analysis.
As previously reported, serum phosphorus control with sucroferric oxyhydroxide and sevelamer was maintained for the duration of the 52-week period in patients who completed both the initial Phase 3 study and subsequent extension study [15, 16].
The current analysis found that median serum FGF-23 levels declined throughout the course of the study, and were reduced by nearly two-third by the end of the 1-year treatment period. This exceeds the median three-fifth reduction seen with the calcimimetic cinacalcet in the EValuation Of Cinacalcet Hydrochloride Therapy to Lower CardioVascular Events (EVOLVE) trial [17], showing that similar reductions can be achieved by reducing serum phosphorus levels with non-calcium-based phosphate binders. The maximum FGF-23 reduction with cinacalcet was achieved by Week 20 before plateauing for the remaining 80 weeks of the treatment period. By contrast, FGF-23 continued to decrease throughout the 52 weeks of treatment with the non-calcium-based phosphate binders in this trial.
The results agree with previous non-clinical and clinical studies which suggested that both sucroferric oxyhydroxide and sevelamer may be more effective than calcium-based phosphate binders for the management of CKD-MBD. For example, a study conducted in adenine-induced CKD rats found that sucroferric oxyhydroxide reduced serum FGF-23 levels to a greater extent than calcium carbonate [18]. This study also showed that sucroferric oxyhydroxide was superior to calcium carbonate for preventing the development of vascular calcifications. In the clinical setting, studies in dialysis-dependent and non-dialysis CKD patients demonstrated that treatment with sevelamer was associated with a reduction in FGF-23 levels, whereas such decreases were generally not observed with the calcium-based comparators [19, 20]. The CALcium acetate MAGnesium carbonate study did show that calcium acetate/magnesium carbonate (Ca/Mg) decreased FGF-23 to a similar extent as sevelamer [21]. The reason for this result was unclear, but the authors indicated that the increase in magnesium and an interaction with the Ca-sensing receptor could provide a possible explanation, although the physiological mechanism remains to be confirmed [21]. A number of small clinical studies conducted in pre-dialysis and dialysis-dependent CKD patients with hyperphosphataemia have also reported that treatment with non-calcium-based phosphate binders (sevelamer and ferric citrate) were associated with a reduction in FGF-23 levels [13, 22–24].
The reduction in FGF-23 may be, in part, due to reductions of serum phosphorus concentrations provided by phosphate binder therapy. An exploratory analysis showed a statistically significant, but weak, correlation between changes in serum FGF-23 and phosphorus that occurred from baseline to Week 52. Despite the statistical significance, the correlation coefficient was low and the coefficient of determination (R2) was small (Figure 2), indicating that only a negligible proportion of the variance in the FGF-23 change from baseline was explained by the change from baseline in serum phosphorus. Therefore, while the relationship exists, it is not necessarily usable for predicting an expected change in FGF-23 based on a change in serum phosphorus.
Although the direction of change from baseline for serum phosphorus and FGF-23 (either increase or decrease) was concordant for the majority of patients, approximately one-third of patients with available changes from baseline for serum phosphorus and FGF-23 had discordant changes in these two parameters. However, the basis of these differences is unclear. As the majority of patients with discordant changes experienced increased FGF-23 despite decreased serum phosphorus, other factors influencing FGF-23 concentrations could potentially have been active in these patients, overcoming the effect of serum phosphorus.
It is interesting to note that FGF-23 levels continued to decrease during the extension study (Weeks 24 to 52), long after the initial serum phosphorus levels had stabilized. The reason for this phenomenon is unclear, and this study was not designed to investigate the mechanism of FGF-23 change following phosphate binder therapy. However, this observation has been reported in previous 12-week studies in non-dialysis and dialysis patients [25–28]. It may be speculated that the effect on FGF-23, as a bone-related biomarker downstream of serum phosphorus, may not be as immediately apparent as that on serum phosphorus. Alternatively, it is possible that mechanisms other than a change in serum phosphorus were affecting FGF-23 concentrations, or that biological adaptation to stable serum phosphorus control could in turn contribute to a further decrease in FGF-23.
Another possible explanation for the sustained decrease in FGF-23, taking into account that the majority of patients enrolled in the study were receiving calcium-based phosphate binders prior to study entry, is that the continued avoidance of calcium-loading with sucroferric oxyhydroxide and sevelamer treatment may have contributed towards this reduction of FGF-23 levels. Experiments using mouse models have shown that dietary calcium supplementation (leading to increased serum calcium levels) results in significant increases in serum levels of FGF-23 and increased FGF-23 expression in bone [29, 30]. There is also evidence from a clinical study comparing sevelamer versus calcium acetate in Stage 4 CKD patients with hyperphosphataemia, in which sevelamer was found to significantly reduce serum FGF-23 levels, whereas the calcium-based binder did not, despite both binders achieving similar levels of serum phosphorus reduction [23].
The precise role of FGF-23 in the pathogenesis of CKD-MBD remains to be fully elucidated. FGF-23 levels increase early in the pathogenesis of CKD (prior to detectable elevations in serum iPTH or phosphorus) as a physiological adaptation to maintain normal phosphorus balance by increasing urinary phosphate excretion in the kidney [31]. However, FGF-23 levels continue to rise as the disease advances, and by the time CKD progresses to end-stage renal disease these compensatory increases become maladaptive [31]. Studies have implicated elevated FGF-23 as a causative factor for left ventricular hypertrophy [7, 32], vascular calcification [33], impaired immune response [34], inflammation [35], infection [8] and a contributor towards increased mortality in dialysis patients [3]. The EVOLVE study suggests that treatment-induced reductions of FGF-23 levels have the potential to reduce the risk of cardiovascular death and major cardiovascular events in haemodialysis patients, although further evidence from prospective clinical studies is needed to support this association [17]. Therefore, a reduction of FGF-23 levels may be of clinical benefit in this patient population.
Small but significant reductions in serum iPTH concentrations were also observed during the initial Phase 3 study (Weeks 0 to 24); these may have occurred, at least in part, as the result of the initial phosphorus-lowering effect of phosphate binder therapy in patients enrolled in the study. Serum iPTH levels then increased back towards baseline levels during the extension study, which may reflect the natural course of secondary hyperparathyroidism in this dialysis population, although the overall changes were small.
Although transient changes in 25-hydroxyvitamin D and an increase in 1, 25-dihydroxyvitamin D by Week 52 were observed, these were not sufficient to draw clinically relevant conclusions.
Levels of serum total calcium were stable over 1 year of treatment. Although the changes observed were statistically significant, they were unlikely to have been clinically meaningful. These findings were not unexpected as patients’ serum calcium levels were regularly monitored during the course of the studies, with potential amends to therapy and dialysis prescription. Furthermore, serum calcium levels have been shown to be an unreliable indicator of calcium balance because such levels are usually maintained within a narrow range in this patient population [36, 37].
Conflicting results were obtained in terms of changes in markers of bone resorption: TRAP-5b levels decreased during the first 24 weeks of the study and then remained stable for the remainder of extension study, whereas CTx levels increased from baseline to Week 24 before returning to near baseline values by Week 52. However, changes in markers of bone formation were more consistent with significant increases being observed for both OST and BSAP during the initial 24-week study. Two-thirds of patients had been receiving calcium-based phosphate binders before entering the study; therefore, it is possible that treatment with non-calcium-based phosphate binders in this study may have led to an initial activation of bone formation, resulting in the observed temporary increases in OST and BSAP. This could also explain the unexpected discrepancy in the temporary downward trend of iPTH levels versus activated biomarkers. There were some small, but statistically significant, differences between the sucroferric oxyhydroxide and sevelamer treatment groups with respect to long-term changes in markers of bone resorption (TRAP-5b and CTx) and formation (BSAP) over the 1-year treatment period (Weeks 0–52). The specific reasons for these small differences between treatment groups are unclear and we consider them unlikely to be clinically relevant.
With respect to these bone marker findings, it is important to note that measurement and interpretation of bone turnover using these serum markers is challenging, mostly because normal reference ranges in the CKD population have not been established. In addition, some biomarkers are cleared via the kidney (e.g. CTx and OST); therefore, serum concentrations increase as the result of renal dysfunction [38]. In clinical practice, the most commonly utilized biochemical markers of bone turnover in CKD are BSAP and iPTH, whereas CTx, OST and TRAP-5b are still regarded as experimental and their routine measurement is not recommended by the guidelines [1]. In summary, the exploratory use of these markers of bone turnover may indicate an early increase in bone formation, but further research into their use and validation of reference ranges is required.
This analysis had several limitations. The decision to pool the two treatment groups meant that that the analysis was overpowered and therefore not all statistically significant changes detected were likely to have been clinically significant. Although there was no evidence of an impact of concomitant medications and changes in dialysis prescription on CKD-MBD parameters (e.g. VDRAs and calcimimetics), this could not be concluded statistically, as the numbers of patients involved were very small. In addition, a previous sub-analysis did show that sevelamer treatment interacted with oral VDRA to affect iPTH levels, while sucroferric oxyhydroxide did not [39].
In summary, this post hoc analysis showed that 52 weeks of treatment with the non-calcium-based phosphate binders sucroferric oxyhydroxide or sevelamer was associated with potentially beneficial changes in CKD-MBD parameters, notably FGF-23, suggesting potentially reduced risk of cardiovascular morbidity and mortality. Serum iPTH was well controlled for 52 weeks. The observed trend towards increased levels of bone formation markers, particularly during the first 24 weeks of the study, may indicate a beneficial effect of non-calcium-based phosphate-binder therapy for the activation of bone metabolism.
Supplementary Material
ACKNOWLEDGEMENTS
This study was sponsored by Vifor Pharma, Glattbrugg, Switzerland. All authors were involved in the preparation and approval of the manuscript in collaboration with Vifor Pharma. The authors would like to thank Amandine Perrin (Vifor Pharma) for statistics support. Editorial assistance was provided by AXON Communications, London, UK. The results presented in this paper have not been published previously in whole or part, except in the following scientific congress abstracts: Ketteler M et al. Effect of non-calcium phosphate binders on CKD-MBD indices in dialysis patients: a post hoc analysis of a Phase 3 study. Presented at the International Society of Nephrology-World Congress of Nephrology 2015.
FUNDING
This study, and editorial support for the publication of this article, was funded by Vifor (International) Inc., Switzerland.
AUTHORS’ CONTRIBUTIONS
Study design was performed by J.F. Study conduct was done by J.F., M.K., A.C.C., A.R., S.M.S. and B.S. Data collection was carried out by J.F., M.K., A.C.C., A.R., S.M.S. and B.S.. Data interpretation was done by M.K., S.M.S., A.C.C., A.R., B.S., V.R., S.W. and J.F. S.W. was responsible for the integrity of the data analysis. All authors contributed to data analysis, drafting manuscript, revising manuscript content and approving final version of manuscript.
CONFLICT OF INTEREST STATEMENT
M.K. has received lecture and consultancy honoraria by Amgen, Medice, Sanofi, Shire and Vifor Fresenius Medical Care Renal Pharma. S.M.S. has received consultancy fees from OPKO Health, Vifor Pharma, Amgen, Fresenius Medical Care, Litholink Corp. and NPS Pharma, and research funding from Abbott, Amgen, Fresenius Medical Care, Shire, OPKO Health and Vifor Pharma. A.C.C. has received consultancy fees or lecture fees from Vifor Pharma, Fresenius Medical Care and Amgen. A.R. has received consultancy fees from Fresenius Medical Care and Vifor Pharma, and lecture and consultancy fees from Sanofi. J.F. has received consulting fees or lecture fees from Amgen, Chugai, Fresenius Medical Care, Sanofi, Shire and Vifor Pharma Ltd. B.S. has received research support from Amgen, Vifor Pharma, Fresenius, Relypsa, ZS Pharma, Bayer, Hospira, Akebia and Rockwell. V.R. and S.W. are both employees of Vifor Pharma Ltd.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009; S1–S130 [DOI] [PubMed] [Google Scholar]
- 2. Hruska KA, Seifert M, Sugatani T.. Pathophysiology of the chronic kidney disease-mineral bone disorder. Curr Opin Nephrol Hypertens 2015; 24: 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gutiérrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 5. Tentori F, Blayney MJ, Albert JM. et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 6. Isakova T, Xie H, Yang W. et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chonchol M, Greene T, Zhang Y. et al. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 2016; 27: 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakano C, Hamano T, Fujii N. et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 2012; 50: 1266–1274 [DOI] [PubMed] [Google Scholar]
- 10. Ketteler M, Block GA, Evenepoel P. et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 11. Hutchison AJ, Smith CP, Brenchley PE.. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol 2011; 7: 578–589 [DOI] [PubMed] [Google Scholar]
- 12. Soriano S, Ojeda R, Rodríguez M. et al. The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol 2013; 80: 17–22 [DOI] [PubMed] [Google Scholar]
- 13. Koiwa F, Kazama JJ, Tokumoto A. et al. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Therapher Dial 2005; 9: 336–339 [DOI] [PubMed] [Google Scholar]
- 14. Block GA, Pergola PE, Uhlig K. et al. Ferric citrate reduced FGF23 in patients with non-dialysis dependent chronic kidney disease (NDD-CKD) and iron deficiency anemia (IDA) irrespective of the change in serum phosphate (P). Oral presentation at Kidney Week 2017 in New Orleans (Oct. 31–Nov. 5). Abstract TH-OR038
- 15. Floege J, Covic AC, Ketteler M. et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Floege J, Covic A, Ketteler M. et al. Long-term effects of iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 2015; 30: 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moe SM, Chertow GM, Parfrey PS. et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 2015; 132: 27–39 [DOI] [PubMed] [Google Scholar]
- 18. Phan O, Maillard M, Peregaux C. et al. PA21, a new iron-based noncalcium phosphate binder, prevents vascular calcification in chronic renal failure rats. J Pharmacol Exp Ther 2013; 346: 281–289 [DOI] [PubMed] [Google Scholar]
- 19. Lin HH, Liou HH, Wu MS. et al. Long-term sevelamer treatment lowers serum fibroblast growth factor 23 accompanied with increasing serum Klotho levels in chronic haemodialysis patients. Nephrology 2014; 19: 672–678 [DOI] [PubMed] [Google Scholar]
- 20. Oliveira RB, Cancela AL, Graciolli FG. et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol 2010; 5: 286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Covic A, Passlick-Deetjen J, Kroczak M. et al. A comparison of calcium acetate/magnesium carbonate and sevelamer-hydrochloride effects on fibroblast growth factor-23 and bone markers: post hoc evaluation from a controlled, randomized study. Nephrol Dial Transplant 2013; 28: 2383–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Block GA, Fishbane S, Rodriguez M. et al. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3-5. Am J Kidney Dis 2015; 65: 728–736 [DOI] [PubMed] [Google Scholar]
- 23. Yilmaz MI, Sonmez A, Saglam M. et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59: 177–185 [DOI] [PubMed] [Google Scholar]
- 24. Yokoyama K, Hirakata H, Akiba T. et al. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 2014; 9: 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liabeuf S, Ryckelynck JP, El Esper N. et al. Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am Soc Nephrol 2017; 12: 1930–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iguchi A, Yamamoto S, Yamazaki M. et al. Effect of ferric citrate hydrate on FGF23 and PTH levels in patients with non-dialysis-dependent chronic kidney disease with normophosphatemia and iron deficiency. Clin Exp Nephrol 2017. doi:10.1007/s10157-017-1510-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27. Spatz C, Roe K, Lehman E. et al. Effect of a non-calcium-based phosphate binder on fibroblast growth factor 23 in chronic kidney disease. Nephron Clin Pract 2013; 123: 61–66 [DOI] [PubMed] [Google Scholar]
- 28. Iguchi A, Kazama JJ, Yamamoto S. et al. Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron 2015; 131: 161–166 [DOI] [PubMed] [Google Scholar]
- 29. Shimada T, Yamazaki Y, Takahashi M. et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289: F1088–F1095 [DOI] [PubMed] [Google Scholar]
- 30. David V, Dai B, Martin A. et al. Calcium regulates FGF-23 expression in bone. Endocrinology 2013; 154: 4469–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wahl P, Wolf M.. FGF23 in chronic kidney disease. Adv Exp Med Biol 2012; 728: 107–125 [DOI] [PubMed] [Google Scholar]
- 32. Gutiérrez OM, Januzzi JL, Isakova T. et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turan MN, Kircelli F, Yaprak M. et al. FGF-23 levels are associated with vascular calcification, but not with atherosclerosis, in hemodialysis patients. Int Urol Nephrol 2016; 48: 609–617 [DOI] [PubMed] [Google Scholar]
- 34. Rossaint J, Oehmichen J, Van Aken H. et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 2016; 126: 962–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh S, Grabner A, Yanucil C. et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 2016; 90: 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kalantar-Zadeh K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer Adherence 2013; 7: 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spiegel DM, Brady K.. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int 2012; 81: 1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Babayev R, Nickolas TL.. Can one evaluate bone disease in chronic kidney disease without a biopsy? Curr Opin Nephrol Hypertens 2014; 23: 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sprague SM, Covic AC, Floege J. et al. Pharmacodynamic effects of sucroferric oxyhydroxide and sevelamer carbonate on VDRA bioactivity in dialysis patients. Am J Nephrol 2016; 44: 104–112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.