Abstract
Background
Liver transplantation is an effective therapy for end‐stage liver diseases and acute liver failure. After the operation, however, recipients may suffer grafts loss induced by alloimmune reaction, which is termed as acute allograft rejection. The interaction between costimulatory molecules, CD276, and its ligand, TREML2, promotes T cell‐mediated immune response, as well as acute or chronic allograft rejection. Our research aimed at correlating genetic polymorphisms of CD276/TREML2 with acute rejection, and evaluating its prognostic value of acute rejection after liver transplantation.
Methods
The study enrolled a total of 388 recipients. Among them, acute allograft rejection was observed in 54 cases. We performed single nucleotide polymorphism genotyping of CD276, including rs11072431, rs11574495, rs12593558, rs12594627, rs2127015, rs3816661 and rs7176654, and TREML2, including rs4714431, rs6915083, rs7754593, and rs9394767 from preoperative peripheral blood genome DNA.
Results
We found rs2127015 of CD276, rs6915083 and rs7754593 of TREML2, and HBV infection as well were associated with acute rejection. And, rs2127015 influences CD276 expression. Moreover, we established a risk assessment model, composited by statistically proved risk factors.
Conclusion
By integrating both clinical and genetic variables, liver transplant recipients can be categorized into different risk groups, and might benefit from individualized therapies.
Keywords: acute rejection, CD276, costimulatory molecule, liver transplantation, SNP
1. INTRODUCTION
Liver transplantation is an effective therapy for end‐stage liver diseases and liver failure. Since immunosuppressive agents have been introduced for clinical use, the incidence of allograft rejection decreases dramatically. However, acute rejection episode, normally resulted from inadequate immunosuppression, would still occur among 15%–45% recipients within months (Farges et al., 1996; Hubscher, 2009; Ingulli, 2010). And, it might lead to graft loss, increased risk of chronic organ dysfunction, and suboptimal long‐term outcomes with decreased allograft half‐life by 34% (Ingulli, 2010).
Allogeneic grafts induce fierce T cell‐mediated immune responses in recipients, and the response is the main cause of rejection and graft dysfunction (Sanchez‐Fueyo & Strom, 2011). T cells from both innate and adaptive immune system play central roles in regulating immune reactions during rejection episode (Clarkson & Sayegh, 2005; Ingulli, 2010; Rothstein & Sayegh, 2003). First, host T cells allorecognize donor‐derived antigens, and are activated by costimulatory signals (Hubscher, 2009; Ingulli, 2010). During this step, antigens are recognized by the interaction between major histocompatibility complexes (MHC) on antigen‐presenting cells (APC) and T‐cell receptors (TCR) on T cells. Then the recognition stimulates T cells and alters the intracellular transcriptional profiles. Once activated, host T cells would undergo clonal expansion, differentiate into effector T cells, migrate into allograft, and accelerate the destruction of donor organ (Hubscher, 2009; Ingulli, 2010). It leads to mixed inflammatory cells infiltration, usually mononuclear, in portal tracts, and is the most common histology characteristic of acute rejection (Hubscher, 2009).
Recent studies indicated that costimulatory molecules might serve as important therapeutic targets for preventing allograft rejection. For instance, B7 proteins, belonging to IgG superfamily, normally express on membrane of APCs. The best‐studied B7 proteins are CD80 (OMIM #112203) and CD86 (OMIM #601020). Depending on the counterparts engaged on T cells surface, they could provide either positive or negative costimulatory signal. T cells could be positively stimulated by APCs when CD80 or CD86 interacts with CD28 (OMIM #186760), or negatively stimulated by CTLA4 (OMIM #123890) (Clarkson & Sayegh, 2005). Commercial recombinant CTLA4 protein has provided extended graft survival for renal recipients (Dell‐Olio & Kelly, 2010; Post, Douglas, & Mulligan, 2005; Snanoudj, Zuber, & Legendre, 2010).
Initial works on CD276 (OMIM #605715) suggested a positive costimulatory effect on T‐cells activation. In conjunction with anti‐CD3 monoclonal antibody, CD276 positively stimulated T‐cell proliferation, with enhanced IFN‐γ production and CD8 + cytotoxic activity. Although in a cardiac transplantation model, graft rejection developed rapidly in both CD276‐/‐ mice and control mice equally, brief treatment of immunosuppressive regimens, to CD276‐/‐ mice, led to prolonged survival time and decreased incidence of rejections (L. Wang et al., 2005). CD276 also showed the effect of negative costimulation, such as inhibiting T‐cell activation and effector cytokine production (Clarkson & Sayegh, 2005; Rothstein & Sayegh, 2003). TREML2 (Triggering receptor expressed on myeloid cell‐like transcript 2, OMIM #609715) has been identified as a ligand of CD276 for positive costimulation (Hashiguchi et al., 2008; Kobori et al., 2010). Since CD276 can positively activate T cells via TREML2, we speculated a participation of both molecules in graft intolerance.
Nowadays, individualized therapies such as tailored and safe immunosuppression are urgently demanded for organ transplantation. Advanced molecular biological techniques, such as gene array, proteomics researches, mass spectrometry, and genome‐wide association studies (GWAS), are discovering valuable biomarkers, including mRNA, miRNA, protein, small chemical molecules, and genetic signatures [single nucleotide polymorphism (SNP) SSR, CNV et al.] (Hernandez‐Fuentes & Lechler, 2010; Offit, 2011; Rook & Rand, 2011). These objectively detectable or measurable molecules and genetic signatures are biologically or pathogenically involved, and might act as parameters for diagnosis and disease staging, as well as indicators or predictors for disease prognosis and clinical response (Hernandez‐Fuentes & Lechler, 2010). The analysis of genetic characteristics of a patient would assist in interpreting his/her biological and immune response, and help to depict allograft rejection, so that damages to parenchymal tissues can be diagnosed in advance and prevented before irreversible (Hernandez‐Fuentes & Lechler, 2010; Offit, 2011).
SNPs of cytokines and costimulatory molecules are associated with acute rejection (de Reuver et al., 2003; Hernandez‐Fuentes & Lechler, 2010; Kim et al., 2010;); however, none of these findings has been introduced into identifying the risk of rejection. We wondered whether a quantitative risk assessment model could be deduced, by integrating critical biomarkers, such as genetic polymorphism, and other risk factors. And we expected, by using the model, recipients would receive optimized immunosuppression for individualizing their clinical cares.
In conclusion, we discovered SNPs of costimulatory molecule, CD276, as well as its ligand, TREML2, were associated with liver grafts acute rejection. Moreover, HBV (hepatitis B virus) infection was also statistically confirmed as a risk factor for acute rejection. Genetic polymorphism influenced the production of CD276 mRNA. Moreover, by integrating these risk factors, we established a risk assessment model, which categorized recipients into low‐, medium‐, and high‐risk groups.
2. METHODOLOGY
2.1. Population
The diagnoses of enrolled recipients included hepatocellular carcinoma, fulminant hepatitis, and decompensate liver cirrhosis (Table 1). Recipients with autoimmune hepatitis, or drug‐induced hepatitis, or sclerosing cholangitis, or those underwent a second or subsequent liver transplantation, or multiple organ transplantation were excluded. In the retrospective study, 299 recipients who received liver grafts from 2006 to 2011 were enrolled for the clinical aspects analysis (Table 1). However, due to DNA sample quality and limitation of sequencing technology, we used 289 cases, with complete genotype information of total 11 SNPs, to analyze genetic association with acute rejection. The rest 10 cases which lacked genotype information of at least one SNP were excluded in the association analysis. While four of the 10 cases did not lack the genotyping results of rs2127015, rs6915083, and rs7754593, 293 cases were used in the following risk assessment model deduction. Another 89 recipients who received liver grafts from 2011 to 2012 were enrolled for further prospective validation of the risk assessment model. Among them, 11 recipients developed acute rejections. These two cohorts included 345 males and 43 females, aged from 21 to 69 (46.9 ± 9.5) years old.
Table 1.
Relevance of clinical aspects and characteristics of recipient with acute rejection
| Acute rejection group (n = 43) | Nonrejection group (n = 256) | p value | OR (95% CI) | |
|---|---|---|---|---|
| Age | 48.9 ± 10.2 | 46.5 ± 9.0 | 0.895 | |
| Gender (male/female) | 38/5 | 224/32 | 0.872 | 1.086 (0.40–2.96) |
| MELD score | 19.1 ± 8.0 | 19.8 ± 9.4 | 0.776 | |
| Blood type mismatch | 8 (18.6%) | 32 (12.5%) | 0.277 | 1.600 (0.68–3.75) |
| Chronic HBV infection | 32 (74.4%) | 143 (55.9%) | 0.0223 | 2.299 (1.11–4.76) |
| Primary disease | ||||
| Cirrhosis | 36 (83.7%) | 192 (75.0%) | 0.2137 | 1.714 (0.73–4.04) |
| Fulminant Hepatitis | 7 (16.3%) | 50 (19.5%) | 0.6154 | 0.801 (0.34–1.91) |
| HCC | 16 (37.2%) | 102 (39.8%) | 0.7436 | 0.895 (0.46–1.74) |
| Comorbidities | ||||
| Ascites | 20 (46.5%) | 106 (41.4%) | 0.5304 | 1.231 (0.64–2.36) |
| Hepatorenal syndrome | 6 (13.9%) | 22 (8.6%) | 0.2643 | 1.725 (0.66–4.54) |
| Hepatic encephalopathy | 3 (6.9%) | 15 (5.9%) | 0.7756 | 1.205 (0.33–4.35) |
| Portal hypertension | 2 (4.7%) | 10 (3.9%) | 0.8179 | 1.200(0.25–5.68) |
All 388 recipients followed a routine triple combination of immunosuppressive regimen, including tacrolimus, corticosteroid, and mycophenolate mofetil. In brief, the minimum level of tacrolimus blood concentration was maintained at 10–12 ng/ml for the first month after transplantation, at 8–10 ng/ml later in the first year, and at 5–8 ng/ml thereafter. Mycophenolate mofetil was administered 1–2 g per day. Corticosteroid treatment was initiated with 1,000 mg prednisolone once during the operation, continued with gradually reduced methylprednisolone starting at 240 mg on day 1 and ending up at 2.5 mg before discontinuation after 2 months (Xu et al., 2011; Yu et al., 2011).
2.2. Diagnosis of acute rejection
The diagnosis of acute rejection is confirmed by liver biopsy and graded by Banff criteria. Rejection occurred within 6 months was considered as acute rejection (Adeyi, Fischer, & Guindi, 2010; Neuhaus et al., 2002).
2.3. Ethical Compliance
We followed the World Medical Association's Declaration of Helsinki. Written informed consents were obtained. The research procedure was approved and supervised by the Ethical Review Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University.
2.4. DNA extraction and genotyping
Genome DNA was extracted from preoperative peripheral blood. Based on the data from Hapmap (http://www.hapmap.org), the selection of candidate SNPs of CD276 and TREML2 was in accordance with the rule that minor allele frequency and r2 should be no less than 20% and 0.8, respectively. Genotyping was performed by SNaPshot (Applied Biosystems, CA). Data were collected by ABI3130xl Genetic Analyzer (Applied Biosystems, CA), and analyzed on GeneMapper 4.0 (Applied Biosystems, CA).
2.5. Detection of CD276/TREML2 mRNA, and membrane CD276 in PBMCs
Total RNA was extracted from peripheral blood of recipients within 6 months posttransplantation, and cDNA was synthesized by reverse transcription kit (Biorad, CA). We detected the transcripts of CD276 and TREML2 on ABI 7500fast (Applied Biosystems, CA) with iQ SYBR Green Supermix PCR kit (Biorad, CA). The primer pairs used in real‐time PCR reaction were listed as follows; CD276, forward 5′‐CTCCCTACAGCTCCTACCCTC‐3′, reverse 5′‐TGGTCTGTGTATCGCATCCTT‐3′, based on CD276 Genbank sequence (NM_001024736.2); TREML2, forward 5′‐CCCACAGCCTCATAGATAAGACA‐3′, reverse 5′‐CCATATTGCTTTGTTCCCCTT‐3′, based on TREML2 Genbank sequence (NM_024807.4); and GAPDH, forward 5′‐ATGGGGAAGGTGAAGGTCG‐3′, reverse 5′‐GGGGTCATTGATGGCAACAATA‐3′, based on GAPDH Genbank sequence (NM_001256799.2). The relative expression of CD276 and TREML2 mRNA was calculated by ΔΔCT method. mRNA expression of both CD276 and TREML2 was detected three times in each cDNA sample.
To detect membrane CD276, red blood cells were lysed with RBC lysing buffer (eBioscience) from whole blood; subsequently peripheral blood mononuclear cells (PBMCs) were pelleted by density‐gradient centrifugation with Ficoll (Sigma). Cells were incubated with phycoerythrin‐labeled anti‐CD276 antibody (R&D) and fluorescein isothiocyanate labeled anti‐CD3 antibody (eBioscience) in 2% FBS containing PBS for 30 min at 4°C. A Mouse IgG1, κ (eBioscience) and IgG2a, κ (BD Pharmingen) were used as isotype controls for anti‐CD276 and anti‐CD3 antibody, respectively. Finally, cells were quantified on a BD LSRII flow cytometry (BD Bioscience) using CellQuest (BD Bioscience), and data were analyzed by FlowJo (Tree Star, Stanford, CA). Membrane expression of CD276 protein was detected three times in each PBMC sample.
2.6. Comparison of mRNA or protein expression
Unpaired t test or one‐way ANOVA test was used for comparison of two groups or more than two groups with Graphpad Prism 6.0 (Graphpad Software, CA). A two‐tailed p value less than 0.05 was considered statistically significant.
2.7. Association analysis and establishing risk assessment model
Analyses were performed to verify the association between genetic polymorphism and acute rejection by SNPStats (http://bioinfo.iconcologia.net/snpstats) or Haploview (http://www.broad.mit.edu/mpg/haploview). The relevance of clinical characteristics and acute rejection was confirmed by Fisher's exact test by Graphpad Prism 6.0. Variables considered to be statistically significant were subsequently analyzed by multivariable logistic regression using SPSS 20.0 (IBM, IL). The AUROC (area under receiver operating characteristic curve) evaluation was performed by SPSS 20.0 to assess the predictive value of variables and diagnostic accuracy of the model. AUROC value of 0.5 or 1 indicates a bad or good discrimination, respectively (Linden, 2006). A p value less than 0.05 was considered statistically significant.
3. RESULTS
3.1. HBV infection risked acute allograft rejection
The overall incidence of acute rejection within the first half year postoperation was 14.4% (n = 43) in 299 recipients, who received their liver grafts from 2006 to 2009. We did not find any clinical relevance between acute rejection and age, gender, primary diseases, or comorbidities of recipients (Table 1).
We found recipients, positive with HBV infection, were at higher risk than those negatives (Table 1). However, the combination of HBV infection and other diagnoses, or combination between either two of the other diagnoses did not increase the risk of acute rejection (data not shown).
3.2. Genetic polymorphisms of CD276 and TREML2 were both associated with acute allograft rejection
To illustrate the potential association between genetic polymorphisms of costimulatory molecules and acute allograft rejection, seven candidate SNPs of CD276 and four of TREML2 were investigated. Six SNPs of CD276 located in intron, one in 3′ UTR; while two SNPs of TREML2 located in intron, the others in 3′ UTR. We found both genetic polymorphisms of CD276 and TREML2 were associated with acute allograft rejection. Recipients carrying T allele at rs2127015 of CD276, or G allele at rs6915083 or rs7754593 of TREML2 were at high risk of acute rejection (Table 2). Moreover, we performed linkage disequilibrium study to find out haplotype among the SNPs of CD276 and TREML2, or between each other, and identified two haplotype blocks in CD276 and one in TREML2, but none between these two genes (Figure 1). However, no association was observed between haplotypes and acute rejection (Table 3).
Table 2.
Association results for SNPs of CD276 and TREML2 in acute rejection
| SNP | Events | Genotype Count/frequency | p value | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| CD276 | A/A | T/A | T/T | |||
| rs11072431 | AR | 7 (16.3%) | 24 (55.8%) | 12 (27.9%) | 0.1071 | |
| NAR | 75 (30.5%) | 126 (51.2%) | 45 (18.3%) | |||
| CD276 | A/A | G/A | G/G | |||
| rs11574495 | AR | 2 (4.7%) | 18 (41.9%) | 23 (53.5%) | 0.7931 | |
| NAR | 18 (7.3%) | 104 (42.3%) | 124 (50.4%) | |||
| CD276 | C/C | C/T | T/T | |||
| rs12593558 | AR | 5 (11.6%) | 20 (46.5%) | 18 (41.9%) | 0.3265 | |
| NAR | 42 (17.1%) | 128 (52.0%) | 76 (30.9%) | |||
| CD276 | G/G | G/T | T/T | |||
| rs12594627 | AR | 22 (51.2%) | 20 (46.5%) | 1 (2.3%) | 0.2439 | |
| NAR | 113 (45.9%) | 109 (44.3%) | 24 (9.8%) | |||
| C/C | C/T | T/T | ||||
| AR | 2 (4.7%) | 25 (58.1%) | 16 (37.2%) | 0.0733 | ||
| CD276 | NAR | 45 (18.3%) | 130 (52.8%) | 71 (28.9%) | ||
| rs2127015 | C/C | C/T+T/T | ||||
| AR | 2 (4.7%) | 41 (95.3%) | 0.0253 | 0.21 | ||
| NAR | 45 (18.3%) | 201 (81.7%) | (0.05–0.93) | |||
| CD276 | C/C | C/T | T/T | |||
| rs3816661 | AR | 24 (55.8%) | 17 (39.5%) | 2 (4.7%) | 0.6933 | |
| NAR | 124 (50.4%) | 103 (41.9%) | 19 (7.7%) | |||
| CD276 | A/A | G/A | G/G | |||
| rs7176654 | AR | 14 (32.6%) | 23 (53.5%) | 6 (13.9%) | 0.7775 | |
| NAR | 79 (32.1%) | 122 (49.6%) | 45 (18.3%) | |||
| TREML2 | A/A | C/A | C/C | |||
| rs4714431 | AR | 21 (48.8%) | 19 (44.2%) | 3 (7.0%) | 0.2401 | |
| NAR | 91 (37.0%) | 121 (49.2%) | 34 (13.8%) | |||
| A/A | G/A | G/G | ||||
| AR | 3 (7.0%) | 26 (60.5%) | 14 (32.6%) | 0.0436 | ||
| TREML2 | NAR | 48 (19.5%) | 104 (42.3%) | 94 (38.2%) | ||
| rs6915083 | A/A | G/A+G/G | ||||
| AR | 3 (7.0%) | 40 (93.0%) | 0.0467 | 0.30 | ||
| NAR | 48 (19.5%) | 198 (80.5%) | (0.09–1.04) | |||
| G/G | G/T | T/T | ||||
| AR | 23 (53.5%) | 19 (44.2%) | 1 (2.3%) | 0.0887 | ||
| TREML2 | NAR | 101 (41.1%) | 114 (46.3%) | 31 (12.6%) | ||
| rs7754593 | G/G+GT | T/T | ||||
| AR | 42 (97.7%) | 1 (2.3%) | 0.0476 | 6.05 | ||
| NAR | 215 (87.4%) | 31 (12.6%) | (0.80–45.61) | |||
| TREML2 | A/A | G/A | G/G | |||
| rs9394767 | AR | 29 (67.4%) | 13 (30.2%) | 1 (2.3%) | 0.3950 | |
| NAR | 139 (56.5%) | 97 (39.4%) | 10 (4.1%) | |||
AR represents acute rejection, while NAR for nonacute rejection.
Figure 1.
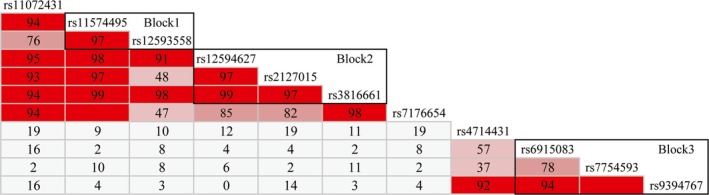
LD plots of CD276 and TREML2. We identified two haplotype blocks, block 1 and block 2, in CD276, and one haplotype block, block 3, in TREML2
Table 3.
Association results of haplotypes in acute rejection
| Block | Haplotype | Events | Haplotype | p value | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Carrier | Noncarrier | |||||
| Block 1 | GT | AR | 54 | 32 | 0.3084 | 1.27 |
| NAR | 280 | 212 | (0.79–2.04) | |||
| AC | AR | 20 | 66 | 0.3025 | 0.754 | |
| NAR | 141 | 351 | (0.44–1.29) | |||
| GC | AR | 10 | 76 | 0.4896 | 0.7802 | |
| NAR | 71 | 421 | (0.38–1.58) | |||
| Block 2 | GTC | AR | 55 | 31 | 0.1258 | 1.44 |
| NAR | 271 | 221 | (0.90–2.32) | |||
| TCT | AR | 19 | 67 | 0.2093 | 0.70 | |
| NAR | 141 | 351 | (0.40–1.21) | |||
| GCC | AR | 8 | 78 | 0.3370 | 0.68 | |
| NAR | 64 | 428 | (0.31–1.48) | |||
| TCC | AR | 2 | 84 | 0.7142 | 0.7571 | |
| NAR | 15 | 477 | (0.17–3.33) | |||
| Block 3 | GA | AR | 63 | 23 | 0.1040 | 1.52 |
| NAR | 316 | 176 | (0.91–2.54) | |||
| TG | AR | 16 | 70 | 0.2927 | 0.73 | |
| NAR | 117 | 375 | (0.40–1.31) | |||
| TA | AR | 7 | 79 | 0.3000 | 0.65 | |
| NAR | 59 | 433 | (0.28–1.47) | |||
AR represents acute rejection, while NAR for nonacute rejection.
3.3. rs2127015 genotype was associated with the expression of CD276
Since rs2127015, rs6915083, and rs7754593 located in either intron or 3′ UTR, these synonymous SNPs might affect mRNA transcription or processing. We detected the expression of both CD276 and TREML2 mRNA in PBMCs, and found recipients carrying T allele in rs2127015 with a higher CD276 mRNA expression (Figure 2). Subsequently, we verified membrane CD276 expression by flow cytometry, and found membrane CD276 expressed on both CD3‐ and CD3 + PBMCs. The percentages of CD276 + cells were positively correlated in the two subpopulations (correlation coefficient = 0.381, P < 0.01). Recipients carrying T allele at rs2127015 were with a higher percentage of CD276 + CD3‐ cells in PBMCs (Figure 3).
Figure 2.
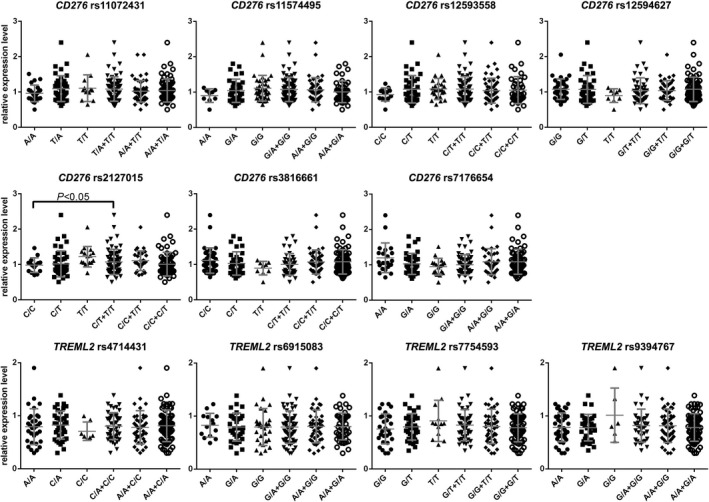
CD276 and TREML2 mRNA expression in PBMCs. Plots represented relative expression level of mRNA of each individual recipient. The result indicated that T allele carriers of rs2127015 expressed more CD276 mRNA than the others (p < 0.05). Bar represented mean ± standard deviation of the scatter plots
Figure 3.
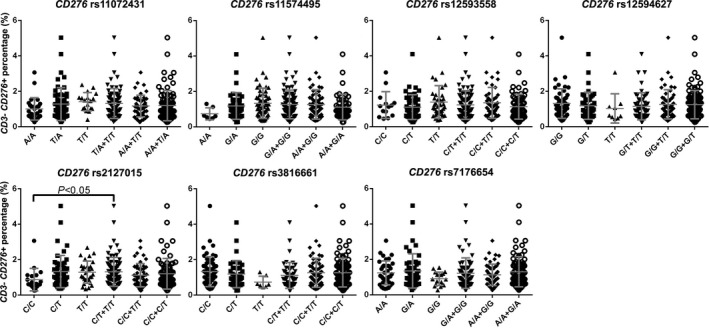
Membrane CD276 expression in CD3‐ PBMCs. Plots represented the percent of CD276 positive cells in CD3‐ PBMCs of each individual recipient. The result indicated that T allele carriers of rs2127015 possessed more CD276 + cell in CD3‐ peripheral blood (p < 0.05). Bar represented mean ± standard deviation of the scatter plots
Figure 4.
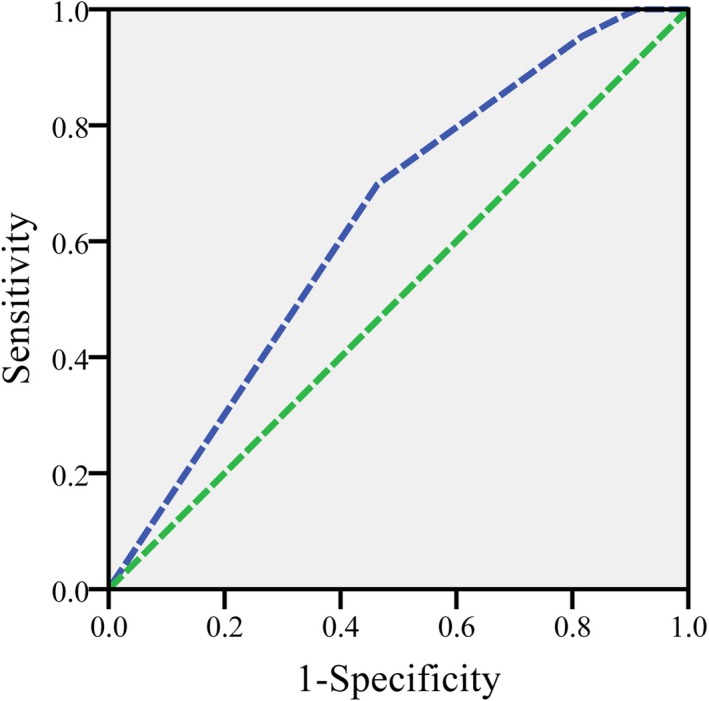
Acute rejection model assessment with ROC curve
3.4. Prediction of acute rejection by combining genetic polymorphisms and HBV infection
Since SNPs of CD276 and TREML2, and HBV infection as well, increased the risk of acute rejection, we performed multivariable logistic regression analysis to verify the possibility of genetic polymorphisms and HBV infection as independent risk factors for acute rejection. Then a risk assessment model of acute rejection was established by the combination between genotype of rs2127015 and HBV infection. The other two associated variables, rs6915083 and rs7754593, which failed the test, were not included. In this model, the risk score was equal to ‐1.122 + 1.493 × rs2127015 + 0.817 × HBV (for rs2127015, T/T and C/T carrier was equal to 1, while C/C was equal to 0; for HBV, HBV infection was equal to 1, otherwise it was equal to 0).
The model exhibited a sensitivity of 69.8%, specificity of 54.7%, and an area under the curve of 0.634 (Figure 2). Subsequently, each recipient could be categorized into three groups: the score > 1, 1 > the score > 0, and the score < 0, which could be considered as high‐, medium‐, and low risk, respectively. According to the model, the incidence of acute rejection was 20.8% (n = 30) in high‐risk group (n = 144), 11.2% (n = 11) in medium‐risk group (n = 98), and 4.3% (n = 2) in low‐risk group (n = 51).
3.5. Validation of the prediction significance of the model by a prospective study
To validate the prediction significance and precision of the assessment model, we performed a prospective cohort study with a population of 89 recipients who received their liver grafts from 2010 to 2011. According to their scores, 89 recipients were divided into three groups (high‐risk group with the score > 1, n = 47; medium‐risk group with 1 > the score > 0, n = 21; low‐risk group with the score < 0, n = 21), then all the recipients were followed up for more than 6 months after transplantation. Finally, 17.0% (n = 8) recipients developed acute rejection in the high‐risk group, while that was 9.5% (n = 2) and 4.8% (n = 1) in the medium‐ and low‐risk group, respectively.
4. DISCUSSION
In our current study, we identified several acute rejection‐associated risk factors, including genetic polymorphisms and HBV infection. And, we also provided a semiquantitative risk assessment model, which would facilitate the individualized immunosuppressive therapy for recipients according to the risk group which he/she belonged to. Recipients categorized into the high‐risk group would be suggested a more optimal regimen and frequent surveillance of immunosuppression, while those in the low‐risk group should avoid excessive immunosuppression.
Although the development of acute rejection is complicated, its initiation requires both allorecognition and T‐cell activation (Ingulli, 2010; Wood & Goto, 2012). Genetic polymorphisms of MHC, costimulatory molecules, and cytokines genes, which involve in the allorecognition and the activation, have been proved to be associated with acute rejection in liver and renal transplantations (Bitetto et al., 2012; Jiang et al., 2007; Tapirdamaz et al., 2006; Zhu, Huang, Liu, & Xie, 2012).
The fact that CD80, CD86, and their ligands involving in acute rejection (Marder et al., 2003; Marin et al., 2005), imply CD276 and TREML2 would be worth studying as well. Unlike other B7s, CD276 expresses in a wide range of human organs both transcriptionally and translationally. Recent studies also proved an upregulation of CD276 in different types of cancers and tumor‐infiltrating blood vessels, including colon cancer, prostate cancer, pancreatic cancer, and liver cancer (Sun et al., 2010; Sun et al., 2012; Yuan et al., 2011; Zhao et al., 2013), suggesting CD276 as an attractive and desirable target for cancer immunotherapy (Seaman et al., 2017). In addition, CD276 promotes invasion and tumor progression (Wang, Kang, & Shan, 2013). CD276 normally expresses on APC cells, and is considered as both a positive and a negative costimulatory molecule (Hofmeyer, Ray, & Zang, 2008). It promotes T cell‐mediated immune responses and development of acute/chronic rejection (Wang et al., 2005), however, diverse functions and contrasting roles of CD276 remain being explored. Thus, we focused on CD276 and its ligand, TREML2, which could provide an intact signal cascade of positive costimulation (Hashiguchi et al., 2008). In this work, we proved that genetic polymorphisms of both CD276 and TREML2 were associated with acute rejection of liver transplantation. The evaluations of mRNA and/or protein expression of CD276 and TREML2 suggested that the association between SNPs and graft rejections might be due to the expression of CD276 in CD3‐ PBMCs. It seemed that individuals with T alleles at rs2127015 would possess higher CD276 mRNA level in PBMC, which might lead to more membrane CD276 expression. We also confirmed a membrane expression of CD276 in CD3 + PBMC, where its ligands normally express. However, TREML2 did not show any mRNA expression difference among alleles. Our results implied the involvement of positive costimulation via CD276 and TREML2 in acute rejection.
The diverse functions and contrasting roles of CD276 may be mainly due to its multiple counterparts on different cells or in different signal pathways. According to the dual role of CD276 in both positive and negative costimulation effects, CD276 might also inhibit acute immune reaction posttransplantation probably. Mouse transplantation model ought to facilitate the studies with gene‐edited mouse. However, some studies suggested that TREML2 might not be a ligand for murine CD276 (Leitner et al., 2009; Yan et al., 2013), which makes it more complicated to elucidate the molecular and immune functions of CD276.
Meanwhile, the regulation of CD276 protein production is also obscure. Three kinds of isoforms of CD276 protein have been reported, transmembrane 4Ig‐CD276(4Ig‐B7‐H3) (Steinberger et al., 2004), transmembrane 2Ig‐CD276(2Ig‐B7‐H3) (Chen, Hou, Li, Xiong, & Liu, 2011), and soluble CD276(sB7‐H3) (Zhang et al., 2008), with different in vivo or cellular distribution patterns, and various biological functions. Meanwhile, soluble 2Ig‐CD276 isoform could be released in serum by matrix metalloproteinase cleavage of 4Ig‐CD276 (Zhang et al., 2008). At least two forms of alternative spliced CD276 mRNAs have been found, one translated into soluble CD276 in hepatocellular carcinoma cells (Chen et al., 2013), another translated into intracytoplasmic 2Ig‐CD276 in monocytes (Yoon et al., 2016). Thus, it is possible that several spliced CD276 mRNAs exist and might be translated into various CD276 isoforms with different biological functions. Besides mutations in UTRs or exons, SNPs in introns were also proved as alternative splicings and fold changes of certain mRNA forms (Suhy et al., 2014; Wang & Sadee, 2016). And in this study, we observed an association of increased expression of CD276 mRNA in rs2127015 T allele carriers. Further studies are needed to elucidate how rs2127015 influences the mRNA expression and whether rs2127015 would lead to alternative splicings.
Several studies confirmed that HBV infection could decrease the incidence of liver graft acute rejection (Crespo, Marino, Navasa, & Forns, 2012; Farges et al., 1996; Neuberger, 1999; Samuel & Kimmoun, 2003); however, the molecular mechanism still remains undiscovered. Generally, to prevent the reinfection of HBV postoperation, most centers applied a long‐term administration of high‐dose intramuscular hepatitis B immunoglobulin (HBIG) (Samuel & Kimmoun, 2003), while we have developed a safe and efficient substitution therapy which combines lamivudine and low‐dose intramuscular HBIG in our center (Zheng et al., 2006). Recent studies suggested that the treatment with immunoglobulin might lead to a decreased risk for rejection and promote allograft acceptance (Bucuvalas, Anand, & Studies of Pediatric Liver Transplantation Research, 2009; Tha‐In, Metselaar, Bushell, Kwekkeboom, & Wood, 2010). The effect of immune tolerance might result from the inhibition of dendritic cells and provoking CD4 + FoxP3 + T cells (Kwekkeboom et al., 2005; Tha‐In et al., 2010). According to these observations, HBIG administration seemed able to benefit the liver transplant recipients with a better immunosuppression and a reduced acute rejection incidence. However, some researchers claimed that further examinations and studies will be needed to draw the conclusion of the survival benefits from HBIG (Ni & Chang, 2006).
The ultimate aim of our risk assessment model was to predict the incidence of acute rejection in advance and individually. The association between risk factors and acute rejection created the opportunity to predict disease risk for principal concern (Kooperberg, LeBlanc, & Obenchain, 2010). Thus, primary diagnosis, genetic background, and some other preoperative variables that clinically related were considered in this work. Although the research community is making great progress in association studies, poor conducts have been discussed (Moons, Kengne, Woodward, et al., 2012). Most genetic association studies only proved a clinical relevance, but not a clue of when the symptom starts and how severe it develops (Bitetto et al., 2012; Jiang et al., 2007; Tapirdamaz et al., 2006; Zhu et al., 2012). It led to a statement for genetic risk prediction studies to strengthen and encompass works with genetic risk factors in translational medicine (Janssens, Ioannidis, van Duijn, Little, & Khoury, 2011). Some other works also advocated successive and consecutive steps for researches (Moons, Kengne, Grobbee, et al., 2012; Moons, Kengne, Woodward, et al., 2012).
So far, our model does not predict whether or when a single recipient will develop acute rejection, however, due to currently limited understanding of genetic factors and other molecules in the immune response of allograft acute rejections. Some works suggested how risk factors would be applied to risk assessment models which follow a procedure of association study, then statistical certification, and finally a formula deduction (Moons, Kengne, Grobbee, et al., 2012; Moons, Kengne, Woodward, et al., 2012). In this study, a “predictor selection strategy” was applied (Moons, Kengne, Woodward, et al., 2012), of which candidate factors that do not contribute usefully will be removed from the model. Then, due to the failure of passing statistical verification, two SNPs of TREML2, which are both associated with acute rejection were not included in the model. The other risk factors, rs2127015 and HBV infection, each assigned to a statistically calculated coefficient, could categorize the recipients into three groups. Nevertheless, the incidence of acute rejection in high‐risk group was as much as four to five times to that in low‐risk group, while the incidence of medium‐risk group was close to normal incidence. We also evaluated our model by internal validation. With this model, we would be allowed to treat recipients with different immunosuppressive regimen. Therefore, recipients categorized into the high‐risk group will receive additional surveillance of immunosuppression both immunologically and pharmacodynamically (Sawitzki, Schlickeiser, Reinke, & Volk, et al., 2011), whereas recipients in the low‐risk group should avoid excessive immunosuppression. To refine our model, integrating more risk factors, statistically or experimentally proved, should be proceeded.
In conclusion, by combining biomarkers, such as genetic polymorphism, with other risk factors, we deduced a semiquantitative risk assessment model, which would benefit recipients with an individualized immunosuppression for clinical cares.
RESEARCH INVOLVING HUMAN PARTICIPANTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants enrolled in the study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation of Zhejiang Province (No. LY18H100003), Innovative Research Groups of National Natural Science Foundation of China (No. 81721091), Major program of National Natural Science Foundation of China (No. 91542205), National S&T Major Project (No. 2017ZX10203205 and 2018ZX10723204‐006), and Zhejiang International Science and Technology Cooperation Project (No. 2016C04003).
Yu X, Wei B, Su R, et al. A risk assessment model of acute liver allograft rejection by genetic polymorphism of CD276 . Mol Genet Genomic Med. 2019;7:e689 10.1002/mgg3.689
REFERENCES
- Adeyi, O. , Fischer, S. E. , & Guindi, M. (2010). Liver allograft pathology: Approach to interpretation of needle biopsies with clinicopathological correlation. Journal of Clinical Pathology, 63(1), 47–74. 10.1136/jcp.2009.068254 [DOI] [PubMed] [Google Scholar]
- Bitetto, D. , Fabris, C. , Falleti, E. , Fornasiere, E. , Avellini, C. , Cmet, S. , & Toniutto, P. (2012). Recipient interleukin‐28B Rs12979860 C/T polymorphism and acute cellular rejection after liver transplantation: Role of the calcineurin inhibitor used. Transplantation, 93(10), 1038–1044. 10.1097/TP.0b013e31824df7f3 [DOI] [PubMed] [Google Scholar]
- Bucuvalas, J. C. , Anand, R. & Studies of Pediatric Liver Transplantation Research, G (2009). Treatment with immunoglobulin improves outcome for pediatric liver transplant recipients. Liver Transplantation, 15(11), 1564–1569. 10.1002/lt.21843 [DOI] [PubMed] [Google Scholar]
- Chen, W. , Hou, Z. , Li, C. , Xiong, S. , & Liu, H. (2011). Cloning and characterization of porcine 4Ig‐B7‐H3: A potent inhibitor of porcine T‐cell activation. PLoS ONE, 6(6), e21341 10.1371/journal.pone.0021341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Liu, P. , Wang, Y. , Nie, W. , Li, Z. , Xu, W. , & Liu, H. (2013). Characterization of a soluble B7‐H3 (sB7‐H3) spliced from the intron and analysis of sB7‐H3 in the sera of patients with hepatocellular carcinoma. PLoS ONE, 8(10), e76965 10.1371/journal.pone.0076965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson, M. R. , & Sayegh, M. H. (2005). T‐cell costimulatory pathways in allograft rejection and tolerance. Transplantation, 80(5), 555–563. 10.1097/01.tp.0000168432.60022.99 [DOI] [PubMed] [Google Scholar]
- Crespo, G. , Marino, Z. , Navasa, M. , & Forns, X . (2012). Viral hepatitis in liver transplantation. Gastroenterology, 142(6), 1373–1383 e1371. 10.1053/j.gastro.2012.02.011 [DOI] [PubMed] [Google Scholar]
- de Reuver, P. , Pravica, V. , Hop, W. , Boor, P. , Metselaar, H. J. , Hutchinson, I. V. , & Kwekkeboom, J. (2003). Recipient ctla‐4 + 49 G/G genotype is associated with reduced incidence of acute rejection after liver transplantation. American Journal of Transplantation, 3(12), 1587–1594. 10.1046/j.1600-6135.2003.00261.x [DOI] [PubMed] [Google Scholar]
- Dell‐Olio, D. , & Kelly, D. A. (2010). Immunosuppressants: What's new? Current Opinion in Organ Transplantation, 15(5), 594–600. 10.1097/MOT.0b013e32833d8936 [DOI] [PubMed] [Google Scholar]
- Farges, O. , Saliba, F. , Farhamant, H. , Samuel, D. , Bismuth, A. , Reynes, M. , & Bismuth, H. (1996). Incidence of rejection and infection after liver transplantation as a function of the primary disease: Possible influence of alcohol and polyclonal immunoglobulins. Hepatology, 23(2), 240–248. 10.1053/jhep.1996.v23.pm0008591847 [DOI] [PubMed] [Google Scholar]
- Hashiguchi, M. , Kobori, H. , Ritprajak, P. , Kamimura, Y. , Kozono, H. , & Azuma, M. (2008). Triggering receptor expressed on myeloid cell‐like transcript 2 (TLT‐2) is a counter‐receptor for B7‐H3 and enhances T cell responses. Proceedings of the National Academy of Sciences of the United States of America, 105(30), 10495–10500. 10.1073/pnas.0802423105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Fuentes, M. P. , & Lechler, R. I. (2010). A ‘biomarker signature’ for tolerance in transplantation. Nature Reviews Nephrology, 6(10), 606–613. 10.1038/nrneph.2010.112 [DOI] [PubMed] [Google Scholar]
- Hofmeyer, K. A. , Ray, A. , & Zang, X. (2008). The contrasting role of B7‐H3. Proceedings of the National Academy of Sciences of the United States of America, 105(30), 10277–10278. 10.1073/pnas.0805458105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher, S. G. (2009). Transplantation Pathology. Seminars in Liver Disease, 29(1), 74–90. 10.1055/s-0029-1192057 [DOI] [PubMed] [Google Scholar]
- Ingulli, E. (2010). Mechanism of cellular rejection in transplantation. Pediatric Nephrology, 25(1), 61–74. 10.1007/s00467-008-1020-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens, A. C. , Ioannidis, J. P. , van Duijn, C. M. , Little, J. , Khoury, M. J. , &Group, G (2011). Strengthening the reporting of genetic risk prediction studies: The GRIPS statement. Genetics in Medicine, 13(5), 453–456. 10.1097/GIM.0b013e318212fa82 [DOI] [PubMed] [Google Scholar]
- Jiang, Z. , Feng, X. , Zhang, W. , Gao, F. , Ling, Q. , Zhou, L. , & Zheng, S. (2007). Recipient cytotoxic T lymphocyte antigen‐4 + 49 G/G genotype is associated with reduced incidence of hepatitis B virus recurrence after liver transplantation among Chinese patients. Liver International: Official Journal of the International Association for the Study of the Liver, 27(9), 1202–1208. 10.1111/j.1478-3231.2007.01553.x [DOI] [PubMed] [Google Scholar]
- Kim, H. J. , Jeong, K. H. , Lee, S. H. , Moon, J. Y. , Lee, T. W. , Kang, S. W. , & Chung, J. H. (2010). Polymorphisms of the CTLA4 gene and kidney transplant rejection in Korean patients. Transplant Immunology, 24(1), 40–44. 10.1016/j.trim.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Kobori, H. , Hashiguchi, M. , Piao, J. , Kato, M. , Ritprajak, P. , & Azuma, M. (2010). Enhancement of effector CD8 + T‐cell function by tumour‐associated B7‐H3 and modulation of its counter‐receptor triggering receptor expressed on myeloid cell‐like transcript 2 at tumour sites. Immunology, 130(3), 363–373. 10.1111/j.1365-2567.2009.03236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooperberg, C. , LeBlanc, M. , & Obenchain, V. (2010). Risk prediction using genome‐wide association studies. Genetic Epidemiology, 34(7), 643–652. 10.1002/gepi.20509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwekkeboom, J. , Tha‐In, T. , Tra, W. M. , Hop, W. , Boor, P. P. , Mancham, S. , & Metselaar, H. J. (2005). Hepatitis B immunoglobulins inhibit dendritic cells and T cells and protect against acute rejection after liver transplantation. American Journal of Transplantation, 5(10), 2393–2402. 10.1111/j.1600-6143.2005.01029.x [DOI] [PubMed] [Google Scholar]
- Leitner, J. , Klauser, C. , Pickl, W. F. , Stockl, J. , Majdic, O. , Bardet, A. F. , & Steinberger, P. (2009). B7‐H3 is a potent inhibitor of human T‐cell activation: No evidence for B7‐H3 and TREML2 interaction. European Journal of Immunology, 39(7), 1754–1764. 10.1002/eji.200839028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden, A. (2006). Measuring diagnostic and predictive accuracy in disease management: An introduction to receiver operating characteristic (ROC) analysis. Journal of Evaluation in Clinical Practice, 12(2), 132–139. 10.1111/j.1365-2753.2005.00598.x [DOI] [PubMed] [Google Scholar]
- Marder, B. A. , Schroppel, B. , Lin, M. , Schiano, T. , Parekh, R. , Tomer, Y. , & Murphy, B. (2003). The impact of costimulatory molecule gene polymorphisms on clinical outcomes in liver transplantation. American Journal of Transplantation, 3(4), 424–431. 10.1034/j.1600-6143.2003.00084.x [DOI] [PubMed] [Google Scholar]
- Marin, L. A. , Moya‐Quiles, M. R. , Miras, M. , Muro, M. , Minguela, A. , Bermejo, J. , & Alvarez‐Lopez, M. R. (2005). Evaluation of CD86 gene polymorphism at + 1057 position in liver transplant recipients. Transplant Immunology, 15(1), 69–74. 10.1016/j.trim.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Moons, K. G. , Kengne, A. P. , Grobbee, D. E. , Royston, P. , Vergouwe, Y. , Altman, D. G. , & Woodward, M . (2012). Risk prediction models: II. External validation, model updating, and impact assessment. Heart, 98(9), 691–698. 10.1136/heartjnl-2011-301247 [DOI] [PubMed] [Google Scholar]
- Moons, K. G. , Kengne, A. P. , Woodward, M. , Royston, P. , Vergouwe, Y. , Altman, D. G. , & Grobbee, D. E . (2012). Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart, 98(9), 683–690. 10.1136/heartjnl-2011-301246 [DOI] [PubMed] [Google Scholar]
- Neuberger, J. (1999). Incidence, timing, and risk factors for acute and chronic rejection. Liver Transplantation and Surgery, 5(4 Suppl 1), S30–S36. 10.1053/JTLS005s00030 [DOI] [PubMed] [Google Scholar]
- Neuhaus, P. , Clavien, P. A. , Kittur, D. , Salizzoni, M. , Rimola, A. , Rimola, A. , … Group, C. I. L. S (2002). Improved treatment response with basiliximab immunoprophylaxis after liver transplantation: Results from a double‐blind randomized placebo‐controlled trial. Liver Transplantation, 8(2), 132–142. 10.1053/jlts.2002.30302 [DOI] [PubMed] [Google Scholar]
- Ni, Y. H. , & Chang, M. H. (2006). The ways paved for prophylaxis against de novo hepatitis B virus infection after liver transplantation: Still many stones left unturned. Pediatric Transplantation, 10(4), 405–407. 10.1111/j.1399-3046.2006.00494.x [DOI] [PubMed] [Google Scholar]
- Offit, K. (2011). Personalized medicine: New genomics, old lessons. Human Genetics, 130(1), 3–14. 10.1007/s00439-011-1028-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, D. J. , Douglas, D. D. , & Mulligan, D. C. (2005). Immunosuppression in liver transplantation. Liver Transplantation, 11(11), 1307–1314. 10.1002/lt.20614 [DOI] [PubMed] [Google Scholar]
- Rook, M. , & Rand, E. (2011). Predictors of long‐term outcome after liver transplant. Current Opinion in Organ Transplantation, 16(5), 499–504. 10.1097/MOT.0b013e32834a945d [DOI] [PubMed] [Google Scholar]
- Rothstein, D. M. , & Sayegh, M. H. (2003). T‐cell costimulatory pathways in allograft rejection and tolerance. Immunological Reviews, 196, 85–108. 10.1046/j.1600-065X.2003.00088.x [DOI] [PubMed] [Google Scholar]
- Samuel, D. , & Kimmoun, E. (2003). Immunosuppression in hepatitis B virus and hepatitis C virus transplants: Special considerations. Clinics in Liver Disease, 7(3), 667–681. 10.1016/S1089-3261(03)00057-6 [DOI] [PubMed] [Google Scholar]
- Sanchez‐Fueyo, A. , & Strom, T. B. (2011). Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology, 140(1), 51–64. 10.1053/j.gastro.2010.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzki, B. , Schlickeiser, S. , Reinke, P. , & Volk, H. D. (2011). Monitoring tolerance and rejection in organ transplant recipients. Biomarkers, 16(Suppl 1), S42–S50. 10.3109/1354750X.2011.578754 [DOI] [PubMed] [Google Scholar]
- Seaman, S. , Zhu, Z. , Saha, S. , Zhang, X. M. , Yang, M. Y. , Hilton, M. B. , … Tessarollo, L. (2017). Eradication of tumors through simultaneous ablation of CD276/B7‐H3‐positive tumor cells and tumor vasculature. Cancer Cell, 31(4), 501–51 e508. 10.1016/j.ccell.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snanoudj, R. , Zuber, J. , & Legendre, C. (2010). Co‐stimulation blockade as a new strategy in kidney transplantation: Benefits and limits. Drugs, 70(16), 2121–2131. 10.2165/11538140-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Steinberger, P. , Majdic, O. , Derdak, S. V. , Pfistershammer, K. , Kirchberger, S. , Klauser, C. , & Knapp, W. (2004). Molecular characterization of human 4Ig‐B7‐H3, a member of the B7 family with four Ig‐like domains. The Journal of Immunology, 172(4), 2352–2359. 10.4049/jimmunol.172.4.2352 [DOI] [PubMed] [Google Scholar]
- Suhy, A. , Hartmann, K. , Newman, L. , Papp, A. , Toneff, T. , Hook, V. , & Sadee, W. (2014). Genetic variants affecting alternative splicing of human cholesteryl ester transfer protein. Biochemical and Biophysical Research Communications, 443(4), 1270–1274. 10.1016/j.bbrc.2013.12.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Chen, L. J. , Zhang, G. B. , Jiang, J. T. , Zhu, M. , Tan, Y. , & Zhang, X. G. (2010). Clinical significance and regulation of the costimulatory molecule B7‐H3 in human colorectal carcinoma. Cancer Immunology, Immunotherapy, 59(8), 1163–1171. 10.1007/s00262-010-0841-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T. W. , Gao, Q. , Qiu, S. J. , Zhou, J. , Wang, X. Y. , Yi, Y. , & Fan, J. (2012). B7‐H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunology, Immunotherapy, 61(11), 2171–2182. 10.1007/s00262-012-1278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapirdamaz, O. , Pravica, V. , Metselaar, H. J. , Hansen, B. , Moons, L. , van Meurs, J. B. , & Kwekkeboom, J. (2006). Polymorphisms in the T cell regulatory gene cytotoxic T lymphocyte antigen 4 influence the rate of acute rejection after liver transplantation. Gut, 55(6), 863–868. 10.1136/gut.2005.080937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha‐In, T. , Metselaar, H. J. , Bushell, A. R. , Kwekkeboom, J. , & Wood, K. J. (2010). Intravenous immunoglobulins promote skin allograft acceptance by triggering functional activation of CD4 + Foxp3 + T cells. Transplantation, 89(12), 1446–1455. 10.1097/TP.0b013e3181dd6bf1 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Fraser, C. C. , Kikly, K. , Wells, A. D. , Han, R. , Coyle, A. J. , & Hancock, W. W. (2005). B7‐H3 promotes acute and chronic allograft rejection. European Journal of Immunology, 35(2), 428–438. 10.1002/eji.200425518 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Kang, F. B. , & Shan, B. E. (2013). B7‐H3‐mediated tumor immunology: Friend or foe? International Journal of Cancer, 134 (12), 2764–2771. 10.1002/ijc.28474 [DOI] [PubMed] [Google Scholar]
- Wang, D. , & Sadee, W. (2016). CYP3A4 intronic SNP rs35599367 (CYP3A4*22) alters RNA splicing. Pharmacogenetics and Genomics, 26(1), 40–43. 10.1097/FPC.0000000000000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, K. J. , & Goto, R. (2012). Mechanisms of rejection: Current perspectives. Transplantation, 93(1), 1–10. 10.1097/TP.0b013e31823cab44 [DOI] [PubMed] [Google Scholar]
- Xu, X. , Tu, Z. , Wang, B. , Ling, Q. , Zhang, L. , Zhou, L. , & Zheng, S. (2011). A novel model for evaluating the risk of hepatitis B recurrence after liver transplantation. Liver International: Official Journal of the International Association for the Study of the Liver, 31(10), 1477–1484. 10.1111/j.1478-3231.2011.02500.x [DOI] [PubMed] [Google Scholar]
- Yan, R. , Yang, S. , Gu, A. , Zhan, F. , He, C. , Qin, C. , & Feng, P. (2013). Murine b7‐h3 is a co‐stimulatory molecule for T cell activation. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 32(6), 395–398. 10.1089/mab.2013.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, B. R. , Chung, Y. H. , Yoo, S. J. , Kawara, K. , Kim, J. , Yoo, I. S. , & Lee, W. W. (2016). Preferential induction of the T cell auxiliary signaling molecule B7‐H3 on synovial monocytes in rheumatoid arthritis. Journal of Biological Chemistry, 291(8), 4048–4057. 10.1074/jbc.M115.680298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Xie, H. , Wei, B. , Zhang, M. , Wang, W. , Wu, J. , & Zhou, L. (2011). Association of MDR1 gene SNPs and haplotypes with the tacrolimus dose requirements in Han Chinese liver transplant recipients. PLoS ONE, 6(11), e25933 10.1371/journal.pone.0025933 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yuan, H. , Wei, X. , Zhang, G. , Li, C. , Zhang, X. , & Hou, J. (2011). B7‐H3 over expression in prostate cancer promotes tumor cell progression. Journal of Urology, 186(3), 1093–1099. 10.1016/j.juro.2011.04.103 [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Hou, J. , Shi, J. , Yu, G. , Lu, B. , & Zhang, X. (2008). Soluble CD276 (B7‐H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology, 123(4), 538–546. 10.1111/j.1365-2567.2007.02723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Li, D. C. , Zhu, X. G. , Gan, W. J. , Li, Z. , Xiong, F. , & Zhao, H. (2013). B7‐H3 overexpression in pancreatic cancer promotes tumor progression. International Journal of Molecular Medicine, 31(2), 283–291. 10.3892/ijmm.2012.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, S. , Chen, Y. , Liang, T. , Lu, A. , Wang, W. , Shen, Y. , & Zhang, M. (2006). Prevention of hepatitis B recurrence after liver transplantation using lamivudine or lamivudine combined with hepatitis B Immunoglobulin prophylaxis. Liver Transplantation, 12(2), 253–258. 10.1002/lt.20701 [DOI] [PubMed] [Google Scholar]
- Zhu, C. L. , Huang, Q. , Liu, C. H. , & Xie, F. (2012). Polymorphisms in the cytotoxic T‐lymphocyte antigen 4 gene and acute rejection risk in transplant recipients. Molecular Biology Reports, 39(9), 8701–8708. 10.1007/s11033-012-1727-4 [DOI] [PubMed] [Google Scholar]


