Abstract
Purpose: The purpose of this study was to evaluate the outcomes of patients with extracranial chordoma or chondrosarcoma treated by carbon ion radiotherapy (CIRT).
Patients and methods: Between May 2015 and April 2018, 21 consecutive patients with chordoma (n=16) or chondrosarcoma (n=5) treated by CIRT at Shanghai Proton and Heavy Ion Center (SPHIC) were enrolled. The local control (LC), progression free survival (PFS), and overall survival (OS) rates were estimated using the Kaplan-Meier method. Association between each of the candidate prognostic factors and the estimated LC, PFS or OS was tested using the log rank test.
Results: The median gross tumor volume (GTV) was 512.7 ml (range, 142.6-2893.0 ml). The median prescription dose was 69 gray equivalent (GyE) (range, 57-80 GyE). After a median follow-up of 21.8 months (range, 7.2-39.2 months), the 1-year LC, PFS, and OS were 93.8%, 88.4%, and 100%, respectively, whereas the 2-year LC, PFS, and OS were 85.2%, 80.4%, and 100%, respectively. A univariate analysis revealed that age, metal implant status, treatment status, sex, dose, and GTV were not significant prognostic factors for LC, PFS or OS. No grade 2 or higher early and late toxicities were observed within the follow-up.
Conclusion: The results of this retrospective study are encouraging. Patients with extracranial chordoma or chondrosarcoma treated by CIRT in our center achieved a favorable shot-term outcome, without developing severe acute or late adverse events. The long-term results deserve further investigation, even in a prospective randomized trial.
Keywords: carbon ion, chordoma, chondrosarcoma, radiotherapy.
Introduction
Chordoma and chondrosarcoma are rare, slow-growing malignant tumors. Chordoma originates from notochordal remnants 1, 2. The annual incidence of chordoma is 0.1 per 100 000 individuals 2. The most common site of chordoma is at the sacrococcygeal region (50%), followed by the skull base (30-35%) and the mobile spine (15-20%) 2, 3. Chondrosarcoma accounts for 10-20% of all malignant bone tumors 4, 5. The annual incidence of chondrosarcoma is about 0.2/100 000 6. Chondrosarcoma often occurs in the pelvis, femur, and humerus 5. Surgical resection remains the mainstay treatment for these two diseases. Complete radical resection of the tumor ensures longer local control (LC) and disease-free survival compared with partial resection 7-9. However, anatomical complexity, large tumor size, and high complication rates make it rarely possible to achieve R0 resection of these tumors 1, 5, 10. Thus, combining radiotherapy and surgery appears promising. Higher local control rates of primary chordoma or chondrosarcoma are reported in patients who received resection combined with radiotherapy compared with resection only 11-13. However, the majority of chordoma and chondrosarcoma are resistant to conventional photon therapy 14-17.
To overcome the challenge posed by the intrinsic radio-resistance, particle therapy, especially carbon ions, has received considerable attention. Carbon ions provide higher linear energy transfer (LET) and relative biological effectiveness (RBE). This enhanced RBE is driven by a unique DNA damage signature characterized by clustered lesions that overwhelm the DNA repair capacity of tumor cells 18. In addition, carbon ion beams produce the Bragg Peak, which is the release of enormous energy at the end of their range 19, allowing for the maximum destructive energy to be deposited at tumor site while minimizing the damage to the adjacent normal tissues along their path 20, 21. These biological and physical characteristics make carbon ion radiotherapy (CIRT) more advantageous than conventional photon radiotherapy in treating chordoma and chondrosarcoma 5, 7, 9, 22, 23.
As the morbidity of chordoma and chondrosarcoma is low, it will take long time to complete a prospective randomized controlled trial of conventional photon radiotherapy, let alone prospective trials of CIRT. Moreover, very little data about the role of CIRT in treating these tumors has been published. In order to improve the management of these rare diseases, publication of more information is essential. Particle therapy started at Shanghai Proton and Heavy Ion Center (SPHIC) in 2014. Until May 2018, more than 1200 cancer patients (including patients with chordoma or chondrosarcoma) have been treated with particle therapy at our center. In this study, we retrospectively evaluated the efficacy and safety of patients with extracranial chordoma or chondrosarcoma treated by CIRT.
Patients and Methods
Patients
This retrospective study was approved by our institutional review board (approval number, 180620EXP-01). All the patients gave written informed consent for CIRT as well as for future anonymous use of their clinical data. Patients who met the following criteria were included in this study: 1. histologically confirmed extracranial chordoma or chondrosarcoma without metastases; 2. Karnofsky Performance Status (KPS) ≥70; 3. a grossly measurable tumor; 4. no active concomitant malignancy; 5. completed CIRT at our center. Finally, a total of 21 consecutive patients were enrolled in this study.
Treatment planning
To immobilize the patients, an individual vacuum bag and a body mask were used. A CT, without contrast enhancement, was acquired in the treatment position for planning (2 mm slice thickness). To accurately delineate the target volumes and organ at risk (OAR), contrast-enhanced MRI was performed. The target volumes and OAR were delineated through the Siemens Syngo RT planning system (Siemens Healthcare, Erlangen, Germany). The gross tumor volume (GTV) included the visible tumor on the CT and MRI. A 5-mm margin around the GTV was defined as the clinical target volume for the boost dose (CTVboost). The clinical target volume for the primary plan (CTV) was established as a 5-mm margin around the CTVboost. The planning target volume (PTV) was defined as the CTV plus 5-10 mm allowing for setup variability and an internal margin where necessary. All the treatment plans consisted of 2-4 active scanning beams.
The doses were measured by gray equivalent (GyE, defined as carbon ion physical dose multiplied by RBE value). As shown in Table 1, the median prescribed dose to CTVboost was 69 GyE (range: 57-80 GyE). For the patients with sacrococcygeal or pelvis tumor, the dose constraints on the bowel were D-max (maximum dose) <60 GyE; the dose constraints on the rectum were D-max <66 GyE, V60 (volume receiving ≥60 GyE) <1 ml, V50 (volume receiving ≥50 GyE) <10%, and V30 (volume receiving ≥30 GyE) <25%. For the patient with tumor at thoracic vertebra, the dose constraints on the lung, heart, and spinal cord were V20 (volume receiving ≥20 GyE) <20%, D-mean (mean dose) <5 GyE, and D-max <40 GyE, respectively. The dose constraints on the OAR were set at 70% for the patients who received previous photon radiotherapy, disregarding the interval between the two courses of radiotherapy.
Table 1.
Patient Characteristics
| Characteristics | No. (%) |
|---|---|
| Total | 21 (100%) |
| Age (years) | |
| Median (range) | 64 (28-82) |
| Follow-up (months) | |
| Median (range) | 21.8 (7.2-39.2) |
| Sex | |
| male | 10 (47.6%) |
| female | 11 (52.4%) |
| Histology | |
| chordoma | 16 (76.2%) |
| chondrosarcoma | 5 (23.8%) |
| Tumor site | |
| Sacrococcygeal | 19 (90.5%) |
| Thoracic vertebra | 1 (4.8%) |
| Pelvis | 1 (4.8%) |
| Treatment | |
| Primary | 8 (38.1%) |
| Recurrent | 13 (61.9%) |
| Prior radiotherapy | |
| Yes | 6 (28.6%) |
| No | 15 (71.4%) |
| Metal implant | |
| Yes | 8 (38.1%) |
| No | 13 (61.9%) |
| GTV (ml) | |
| Median (range) | 512.7 (142.6-2893.0) |
| Total dose | |
| Median (range) | 69 GyE (57-80 GyE /18-25 Fx) |
| EQD2 (α/β = 2) | |
| Median (range) | 86.25 GyE (65.53-120.0 GyE) |
GyE: gray equivalents; GTV: gross tumor volume; Fx: fractions; EQD2: equivalent doses in 2 GyE fractions calculated using the LQ model.
Follow-up and Statistics
The follow-up period was counted from the first day of the CIRT course. To closely monitor these patients, the follow-up examinations were performed every 3 months in the first two years and every 6 months in the following years. The follow-up examinations included physical examinations, a MRI with contrast enhancement, a chest CT, and abdominal ultrasonography. The treatment efficacy was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 24. Early toxicities, which were defined as side effects occurring within 3 months after the initiation of CIRT, were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v.4.03. Adverse events that occurred 3 months after the initiation of CIRT were considered as late toxicities. The late toxicities were graded using the Radiation Therapy Oncology Group (RTOG) criteria 25.
The local control (LC), progression free survival (PFS), and overall survival (OS) rates were evaluated using the Kaplan-Meier method. The LC rate was defined as the time from the initiation of CIRT to local progression. The PFS was calculated as the time from the initiation of CIRT to local progression, distant metastasis or death due to any cause. The OS was defined as the time from the initiation of CIRT to death due to any cause. The association between each of the candidate prognostic factors and the estimated LC, PFS or OS was tested using the log rank test. The candidate prognostic factors included age, metal implant status, treatment status, sex, dose, and GTV. A two-sided p ≤0.05 was considered statistically significant. All the analyses were performed using R (version 3.3.1).
Results
Patients
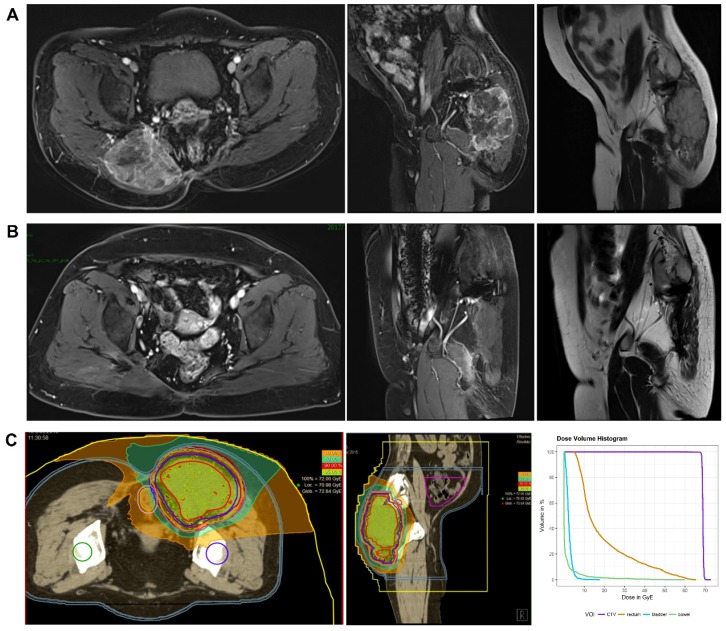
This retrospective study analyzed data of 21 consecutive patients with histologically confirmed chordoma (n=16) or chondrosarcoma (n=5) treated by CIRT between May 2015 and April 2018. All the patients safely and successfully completed CIRT at SPHIC. The median follow-up time was 21.8 months (range, 7.2-39.2 months). The patient characteristics are shown in Table 1. The median age of these patients was 64 years (range, 28-82 years). Tumors occurred in the sacrococcygeal region (n=19), the thoracic vertebra (n=1) or the pelvis (n=1). Eight patients received no previous treatments, and 13 patients had a locally recurrent tumor following previous resections (3 had one surgical resection and 10 had multiple resections). Among the patients with recurrent tumors, 6 received conventional photon radiotherapy in the past, and the median radiation dose was 54 Gy (range, 50-60 Gy). Eight patients had metal implants in their body due to tumor resection. All the patients had a gross tumor before CIRT. The median GTV was 512.7 ml (range, 142.6-2893.0 ml). The median prescribed total dose was 69 GyE (range, 57-80 GyE). The corresponding median equivalent dose calculated for a fraction of 2 GyE was 86.25 GyE (range, 65.53-120.0 GyE, α/β=2 GyE). The MRI images and dose distribution of a representative case are shown in Figure 1.
Figure 1.
The MRI images and dose distribution of a representative case. A 60 years old female patient with sacral chordoma treated with CIRT at our center. A. Dynamic contrast enhanced T1-weighted (axial and sagital view) and T2-weighted MRI (sagital view) images performed before CIRT. B. Dynamic contrast enhanced T1-weighted (axial and sagital view) and T2-weighted MRI (sagital view) images performed one year after CIRT. C. A treatment plan for CIRT using 69 GyE in 23 fractions (the dose distribution and Dose-volume Histogram). Purple line: CTV. Yellow line: rectum. Light blue line: bladder. Green line: bowel. CIRT: carbon ion radiotherapy; GyE: gray equivalents.
Outcome
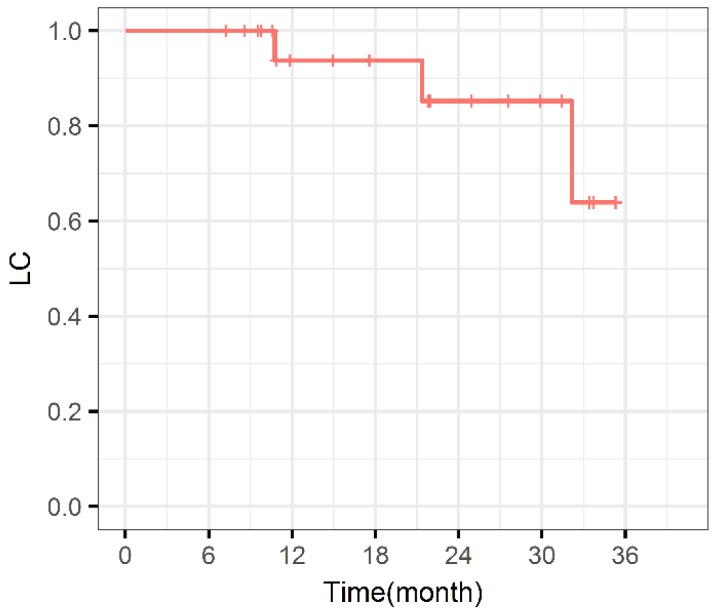
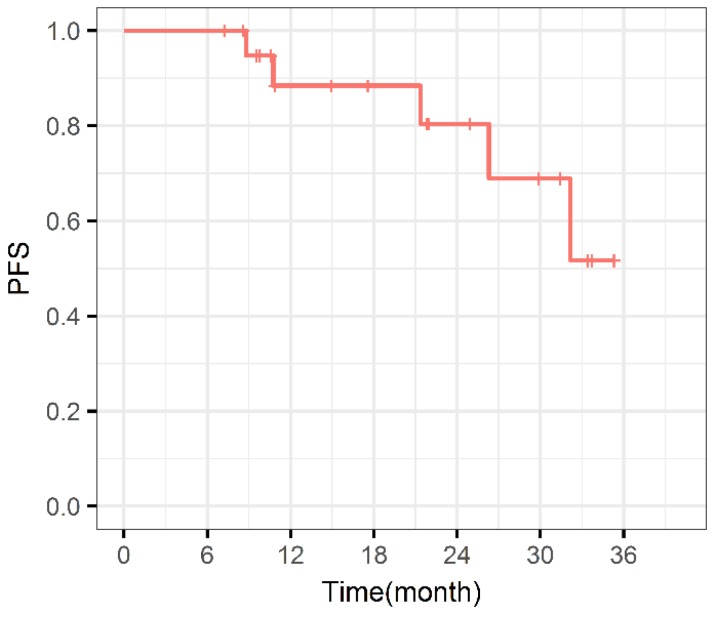
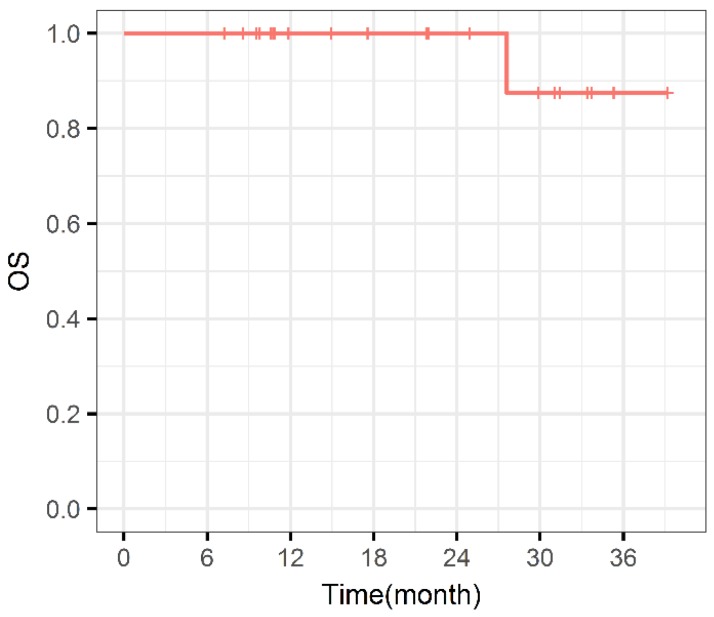
During the entire follow-up, 1 patient with sacral chordoma was evaluated as complete response (CR) at 1 year after CIRT (Figure 1). Three patients (14.3%) experienced progressive disease (PD). A patient with recurrent sacral chordoma who had received surgeries and conventional photon radiotherapy in the past was estimated as PD at 21.4 months after CIRT. In the patients with chondrosarcoma, a patient with primary chondrosarcoma and another with recurrent chondrosarcoma were assessed as PD at 32.2 and 10.8 months after CIRT, respectively. The 1-year and 2-year LC were 93.8% and 85.2%, respectively (Figure 2). Four patients (19.0%) experienced lung metastases. Two patients with chondrosarcoma developed lung metastases at 38.3 and 10.8 months after CIRT. Another two patients with primary sacral chordoma developed lung metastases after CIRT at 8.8 and 26.3 months, while they achieved local tumor control. The 1-year and 2-year PFS were 88.4% and 80.4%, respectively (Figure 3). Among all the patients, 20 patients were alive at the end of follow-up. A patient with primary sacral chordoma died at 27.6 months after CIRT due to lung metastases. The 1-year and 2-year OS were 100% (Figure 4).
Figure 2.
Local control curve. LC: Local control.
Figure 3.
Progression free survival curve. PFS: Progression free survival.
Figure 4.
Overall survival curve. OS: Overall survival.
Pain is the most common symptom that has a major impact on the quality of life of patients with chordoma or chondrosarcoma. Sixteen patients (76.2%) showed a decrease in pain at the end of follow-up. Among these patients, 6 (28.6%) had complete pain relief.
Toxicity
Grade 1 acute skin toxicity occurred in 3 patients (14.2%). The most frequent toxicity was grade 1 myelosuppression in 7 patients (33.3%). None of the patients developed grade 2 or higher acute toxicity. No acute side effects of the gastrointestinal and genitourinary tract were observed (Table 2). Overall, the adverse events of patients treated by CIRT were mild, and no severe late side effects were observed within the follow-up period.
Table 2.
Acute Toxicity
| Grade | Grade 0 | Grade 1 | ≥Grade 2 |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Skin | 18 (85.7%) | 3 (14.2%) | 0 (0%) |
| Myelosuppression | 14 (66.7%) | 7 (33.3%) | 0 (0%) |
| Gastrointestinal tract | 21 (100%) | 0 (0%) | 0 (0%) |
| Genitourinary tract | 21 (100%) | 0 (0%) | 0 (0%) |
| Total | 10 (47.6%) | 0 (0%) |
Predictive parameters
Using the log rank test, we evaluated the predictive value of age, metal implant status, treatment status, sex, dose, and GTV for LC, PFS, and OS (Table 3). Factors, including age, metal implant status, treatment status, sex, and GTV were not significantly associated with the LC, PFS or OS. Although no statistically significant difference was found between the patients treated with a dose ≤70 GyE and those treated with a dose >70 GyE, the patients who received a higher dose tended to have a better PFS (p = .19).
Table 3.
The prognostic significance of the potential factors
| variable | Group | No. (100%) | p-value | ||
|---|---|---|---|---|---|
| LC | PFS | OS | |||
| Age (year) | <64 ≥64 |
10 (47.6%) 11 (52.4%) |
.72 | .76 | .32 |
| Metal implant | Yes No |
8 (38.1%) 13 (61.9%) |
.72 | .31 | .44 |
| Treatment | Primary recurrent |
8 (38.1%) 13 (61.9%) |
.86 | .33 | .20 |
| Sex | Female Male |
11 (52.4%) 10 (47.6%) |
.72 | .98 | .44 |
| Dose (GyE) | ≤70 | 14 (66.7%) | .71 | .19 | 1 |
| >70 | 7 (33.3%) | ||||
| GTV (ml) | <512.7 | 10 (47.6%) | .72 | .48 | .20 |
| ≥512.7 | 11 (52.4%) | ||||
The prognostic significance of the potential factors was tested using the log rank test. A two-sided p <0.05 was considered statistically significant. GyE: gray equivalents; GTV: gross tumor volume; LC: local control; PFS: progression free survival; OS: overall survival.
Discussion
In most cases, patients with chordoma or chondrosarcoma had very large tumor at the time of the first diagnosis. Complete resection of the tumor remains a challenge, hence adjuvant radiotherapy is often recommended, and definitive radiotherapy might be an acceptable alternative to surgery for chordoma and chondrosarcoma 2, 5, 26. Accumulated pre-clinical and clinical evidence demonstrates that CIRT has advantages in some radio-resistant malignancies, including chordoma and chondrosarcoma. However, CIRT has only been used for about 20 years 19. And most of the clinical data are from few institutes in Japan and Germany. The lack of patient data resulting from limited access to carbon ion centers and treatment facilities makes a direct comparison difficult. More clinical data are crucial for future application of CIRT. In this retrospective study, we reported the initial experience of the patients with chordoma or chondrosarcoma treated by CIRT at our center. All the patients refused surgery or were deemed inoperable by the surgeon. The 1-year LC, PFS, and OS were 93.8%, 88.4%, and 100%, respectively, whereas the 2-year LC, PFS, and OS were 85.2%, 80.4%, and 100%, respectively.
To the best of our knowledge, this is the first clinical data from Chinese patients with extracranial chordoma and chondrosarcoma. The biological models applied in treatment planning are different between institutes. One of the main problems with carbon ions is the extreme difficulty in measuring relevant biological effects to produce accurate mathematical models that link dose and linear energy transfer (LET) spectra to clinical response 27. Therefore, it is necessary to assess the treatment efficacy and toxicity between different carbon ion centers. As shown in Table 4, several centers have used carbon ion or proton beams to treat patients with extracranial chordoma or chondrosarcoma in the past years. Data from the Heidelberg Ion Beam Therapy Center (HIT) indicated a 3-year LC rate of 53% and OS of 100% for 56 sacral chordoma patients treated by CIRT in combination with photon therapy or CIRT alone 28. A total of 261 patients with extracranial chondrosarcoma or chordoma were treated with carbon ions at the National Institute of Radiological Sciences (NIRS) in Japan. The delivered total dose ranged from 64 GyE to 73.6 GyE. The reported 5-year LC rates were 53% and 77% for the chondrosarcoma and chordoma patients, respectively 9, 29. CIRT is also available at the Hyogo Ion Beam Medical Center (HIBMC), Hyogo, Japan. Patients with sacral chordomas treated at HIBMC displayed a 3-year LC rate of 94% and OS of 83%, and the delivered total dose was 70.4 GyE 7. Results from the Massachusetts General Hospital (MGH) also show good LC rates and OS for proton radiotherapy 30. In some institutes, the patients were treated by surgery combined with or without photon radiotherapy. Data from the Mayo Clinic and Italy indicate a 5-year LC rate of about 55% for patients with chordoma 31, 32. Although it is difficult to precisely compare the outcomes from multiple institutes due to the retrospective analyses and different patient characteristics, these results may indicate that treatment protocols containing particle therapy, especially CIRT, are expected to achieve better outcomes. Randomized clinical trials are warranted for further comparisons between carbon ion and proton therapy 33.
Table 4.
Comparison with other studies
| Institute | Year | No. | disease | treatment | TD, GyE | Follow-up, months | LC | OS |
|---|---|---|---|---|---|---|---|---|
| HIT 28 | 2015 | 56 | chordoma | C or C+PH | 66 (60-74) | 25 (range: NA) | 2y: 76% 3 y: 53% |
2 y: 100% 3 y: 100% |
| NIRS 9 | 2017 | 73 | ChSa | C | 70.4 (64-73.6) | 49.4 (6.4-146.4) | 5 y: 53% | 5 y: 53% |
| NIRS 29 | 2016 | 188 | Chordoma | C | 67.2 (64-73.6) | 62 (6.8-147.5) | 1 y: 99% 5 y: 77% |
1 y: NA 5 y: 81% |
| HIBMC 7 | 2014 | 23 | chordoma | C or P | 70.4 | 38 (7-78) | 3 y: 94% | 3 y: 83% |
| MGH 30 | 2015 | 126 | chordoma | P | 72.4 (46.3-83.6) | 41 (range: NA) | 5 y: 62% | 5 y: 81% |
| MC 31 | 2005 | 52 | chordoma | Surgery | NA | 93.6 (25.2-276) | 5 y: 59% | 5 y: 74% |
| IOR/INT 32 | 2010 | 130 | Chordoma | Surgery | NA | 142 (76-210) | 5 y: 52% | 5 y: 78% |
| Present | 2018 | 21 | ChSa /chordoma | C | 69 (57-80) | 21.8 (7.2-39.2) | 1 y: 93.8% 2 y: 85.2% |
1 y: 100% 2 y: 100% |
ChSa: chondrosarcoma; C: carbon ion radiotherapy; P: proton radiotherapy; PH: photon radiotherapy; TD: total dose; GyE: gray equivalents; LC: local control; OS: overall survival; HIT: Heidelberg Ion Beam Therapy Center; NIRS: National Institute of Radiological Sciences; HIBMC: Hyogo Ion Beam Medical Center; MGH: Massachusetts General Hospital; MC: Mayo Clinic; IOR: Istituto Ortopedico Rizzoli; INT: Istituto Nazionale Tumori.
Since the follow-up was relatively short in the present study, short-term outcomes are taken into consideration when comparing our results with those of other centers. The reported cumulative 1-year LC rate was 99% for the chordoma patients treated with carbon ions at NIRS 29. Matthias Uhl et al reported a 2-year LC rate of 76% and OS of 100% for chordoma patients 28. In the present study, the 1-year LC and OS were 93.8% and 100%, respectively, whereas the 2-year LC and OS were 85.2% and 100%, respectively. It suggested that CIRT provide short-term efficient tumor control for patients with extracranial chordoma or chondrosarcoma.
Patients treated by CIRT at our center had negligible toxicity. Among all the patients, 3 (14.2%) had grade 1 acute skin side effects, and 7 (33.3%) had grade 1 myelosuppression. None of the patients developed grade 2 or higher acute and late side effects during the follow-up period. The results from HIT and GSI also indicated that no severe toxicity was detected in the patients 28, 34. The data from NIRS showed that late grade 4 skin toxicity was observed in 2 patients, and late grade 3 peripheral nerve injuries occurred in 6 patients. The number of patients who had grade 3 or higher late toxicities accounted for only 4.8% 29. All of these studies, along with our own, demonstrated that carbon ion radiotherapy can be considered safe.
Prognostic factors for patients with chordomas or chondrosarcoma treated with particle therapy have been investigated in previous studies (Table 5). Taking into account the weakness of small patient sample and short follow-up time, we could not found any significant differences of LC and OS in consideration of age, mental implant status, sex, dose, and tumor volume. The results from GSI showed that younger age was significantly associated with an improved LC and OS for patients with chordoma or chondrosarcoma 34, 35. Matthias Uhl et al from HIT demonstrated that males had a better 2-year LC rate than females (p = .03) 28. M MIMA et al from HIBMC also reported that males showed a significantly better PFS (p = .029) 7. The data from PSI showed that patients with chondrosarcoma had better LC (p = .014) and OS rates (p = .014) than those with chordoma 36. In addition, smaller tumor and boost volume were correlated with better LC or OS. The results from the GSI group indicated that a boost volume ≤55 ml was significantly related to better LC rates for patients with chondrosarcoma (p = .039) 35, and a boost volume <75 ml was significantly associated with an improved LC (p = .002) and OS rate (p = .030) for patients with chordoma 34. The results from the NIRS group showed that chondrosarcoma patients with a tumor volume <470 ml had better LC (p = .009) and OS (p = .0008) 9. Damien C. Weber from PSI also reported that a GTV ≤25 mL was related to a better LC (p = .005) and OS (p = .01) 36. The median volume of GTV in the current study was 513 ml (range, 143-2893 ml), which was larger than that of other reports 7, 28, 37, and 2 patients had significantly huge tumor (more than 2000 ml). The Japan and Germany studies showed good results, however, the patients had smaller tumor (the median clinical target volume was 370 ml (range, 47-1468 ml) in Chiba report; the median tumor size was 244 ml (range, 5-1188 ml) in HIT report) 28, 37. And all the patients refused surgery or were deemed inoperable by the surgeon in our study. We evaluated the efficacy and toxicity of CIRT for large or even huge tumors that cannot be totally resected in the present study. Our initial experience would be valuable for the management of these large tumors.
Table 5.
Predictive factors investigated in other studies
| Institute | No. | Treatment | Factors been evaluated |
|---|---|---|---|
| HIT 39 | 101 | primary, n=88 recurrent, n=13 |
age, sex, tumor volume, treatment (primary, recurrence) |
| HIT 28 | 56 | primary, n=41 recurrent, n=15 |
age, dose, resection status, sexa, tumor volume, tumor location, treatment (primary, recurrence)a |
| NIRS 9 | 73 | primary, n=55 recurrent, n=17 Metastatic, n=3 |
age, histologya, tumor volumea, tumor location, treatment (primary, recurrence, metastasis) |
| NIRS 29 | 188 | primary, n=188 | dose, level of proximal invasion, sex, tumor volume |
| GSI 35 | 79 | primary, n=54 recurrent, n=25 |
agea, boost volumea, dose, sex, tumor grade, treatment (primary, recurrence) |
| GSI 34 | 155 | primary, n= 101 recurrent, n=54 |
agea, boost volumea, dose, sex, treatment (primary, recurrence) a |
| HIBMC 7 | 23 | primary, n=23 | age, dose fractionation, ECOG PS, ion type, sexa, spacer placement, tumor volume |
| PSI 36 | 222 | primary, n=171 recurrent, n=51 |
age, compression of the brainstem or optic apparatusa, histologya, number of weekly fractions, sex, GTVa, treatment (primary, recurrence) |
| Present study | 21 | primary, n=8 recurrent, n=13 |
age, metal implantation, sex, treatment (primary, recurrence), dose, tumor volume |
a: factors significantly correlated with LC, PFS, or OS (p ≤0.05). HIT: Heidelberg Ion Beam Therapy Center; NIRS: National Institute of Radiological Sciences; GSI: Society for Heavy Ion Research in Darmstadt; HIBMC: Hyogo Ion Beam Medical Center; PSI: Center for Proton Therapy, Paul Scherrer Institute; GTV: gross tumor volume.
Moreover, treatment for primary or recurrent tumor was the factor investigated. Treatment for recurrent chordoma resulted in a significantly lower LC (p = .001) 28 and OS (p = .025) 34. In our study, we assessed 6 patients who were treated with CIRT as re-irradiation for tumor recurrence. The decreased tolerance of normal tissues, especially vital organs, often limited dose delivered to the tumor in the second course of radiotherapy. Hence, it remains a challenge to treat these patients who failed to respond to the first course of radiotherapy. The physical advantages of carbon ions allows more pronounced sparing of normal tissues 38. The patients with recurrent tumor were safely treated with CIRT at our cancer. In spite of the small sample size, the data indicates that CIRT is a promising and safe treatment alternative for a subgroup of patients who require re-irradiation.
In summary, we reported the use of CIRT in the management of patients with extracranial chordoma or chondrosarcoma in this retrospective study. Although the number of patients is small and the follow-up time is relatively short, the results are encouraging. Patients with extracranial chordoma or chondrosarcoma treated with CIRT at our center achieved a favorable shot-term outcome without developing severe acute or late adverse events. Prospective trials with a longer follow-up time and a larger sample size are still warranted to confirm the local control and survival benefit of this promising treatment technology.
Acknowledgments
We acknowledge the contribution of our colleagues at the Shanghai Proton and Heavy Ion Center. This article has drawn on a program of research funded by the National Key Research and Development Program of China (2017YFC0107600), National Natural Science Foundation of China (81773225), Pudong New area science and technology development fundation (No. PKJ2016-Y43), Science and Technology Commission of Shanghai Municipality (No. 15411950104), and the Shanghai Shen-kang Hospital Development Center (No. 16CR3097B).
References
- 1.Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, Briggs TW. A review of the surgical management of sacral chordoma. Eur J Surg Oncol. 2014;40:1412–20. doi: 10.1016/j.ejso.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Stacchiotti S, Sommer J, Chordoma Global Consensus G. Building a global consensus approach to chordoma: a position paper from the medical and patient community. The Lancet Oncology. 2015;16:e71–83. doi: 10.1016/S1470-2045(14)71190-8. [DOI] [PubMed] [Google Scholar]
- 3.Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–50. doi: 10.1634/theoncologist.12-11-1344. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res; 2001. pp. 224–33. [PubMed] [Google Scholar]
- 5.Outani H, Hamada K, Imura Y, Oshima K, Sotobori T, Demizu Y. et al. Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int J Clin Oncol. 2016;21:186–93. doi: 10.1007/s10147-015-0870-z. [DOI] [PubMed] [Google Scholar]
- 6.Group EESNW. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii100–9. doi: 10.1093/annonc/mds254. [DOI] [PubMed] [Google Scholar]
- 7.Mima M, Demizu Y, Jin D, Hashimoto N, Takagi M, Terashima K. et al. Particle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordoma. The British journal of radiology. 2014;87:20130512. doi: 10.1259/bjr.20130512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preda L, Stoppa D, Fiore MR, Fontana G, Camisa S, Sacchi R. et al. MRI evaluation of sacral chordoma treated with carbon ion radiotherapy alone. Radiother Oncol. 2018;128:203–8. doi: 10.1016/j.radonc.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Imai R, Kamada T, Araki N, Working Group For B, Soft-Tissue S. Clinical Efficacy of Carbon Ion Radiotherapy for Unresectable Chondrosarcomas. Anticancer Res. 2017;37:6959–64. doi: 10.21873/anticanres.12162. [DOI] [PubMed] [Google Scholar]
- 10.Imai R, Kamada T, Tsuji H, Yanagi T, Baba M, Miyamoto T. et al. Carbon ion radiotherapy for unresectable sacral chordomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:5741–6. doi: 10.1158/1078-0432.CCR-04-0301. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed AT, Abdel-Rahman O, Morsi M, Mustafa K, Testini P, Aleem IS. et al. Management of Sacrococcygeal Chordoma: A Systematic Review and Meta-analysis of Observational Studies. Spine (Phila Pa 1976) 2018;43:E1157–E69. doi: 10.1097/BRS.0000000000002638. [DOI] [PubMed] [Google Scholar]
- 12.Jian BJ, Bloch OG, Yang I, Han SJ, Aranda D, Tihan T. et al. Adjuvant radiation therapy and chondroid chordoma subtype are associated with a lower tumor recurrence rate of cranial chordoma. Journal of neuro-oncology. 2010;98:101–8. doi: 10.1007/s11060-009-0068-1. [DOI] [PubMed] [Google Scholar]
- 13.Goda JS, Ferguson PC, O'Sullivan B, Catton CN, Griffin AM, Wunder JS. et al. High-risk extracranial chondrosarcoma: long-term results of surgery and radiation therapy. Cancer. 2011;117:2513–9. doi: 10.1002/cncr.25806. [DOI] [PubMed] [Google Scholar]
- 14.Catton C, O'Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V. et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67–72. doi: 10.1016/s0167-8140(96)91805-8. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–6. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 16.Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE. et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326–38. doi: 10.2106/00004623-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Van Oosterom AT, Dirix LY. Chondrosarcoma and other rare bone sarcomas. Curr Opin Oncol. 1990;2:495–9. [PubMed] [Google Scholar]
- 18.Mohamad O, Sishc BJ, Saha J, Pompos A, Rahimi A, Story MD, Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair. Cancers (Basel); 2017. p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M. et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. The Lancet Oncology. 2015;16:e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 20.Shioyama Y, Tsuji H, Suefuji H, Sinoto M, Matsunobu A, Toyama S. et al. Particle radiotherapy for prostate cancer. International journal of urology: official journal of the Japanese Urological Association. 2015;22:33–9. doi: 10.1111/iju.12640. [DOI] [PubMed] [Google Scholar]
- 21.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953–64. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 22.Imai R, Kamada T, Tsuji H, Sugawara S, Serizawa I, Tsujii H. et al. Effect of Carbon Ion Radiotherapy for Sacral Chordoma: Results of Phase I-II and Phase II Clinical Trials. International Journal of Radiation Oncology*Biology*Physics. 2010;77:1470–6. doi: 10.1016/j.ijrobp.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 23.Outani H, Hamada K, Imura Y, Oshima K, Sotobori T, Demizu Y. et al. Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int J Clin Oncol. 2016;21:186–93. doi: 10.1007/s10147-015-0870-z. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 26.Nishida Y, Kamada T, Imai R, Tsukushi S, Yamada Y, Sugiura H. et al. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int J Radiat Oncol Biol Phys. 2011;79:110–6. doi: 10.1016/j.ijrobp.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 27.Fossati P, Molinelli S, Matsufuji N, Ciocca M, Mirandola A, Mairani A. et al. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Physics in medicine and biology. 2012;57:7543–54. doi: 10.1088/0031-9155/57/22/7543. [DOI] [PubMed] [Google Scholar]
- 28.Uhl M, Welzel T, Jensen A, Ellerbrock M, Haberer T, Jakel O. et al. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlenther Onkol. 2015;191:597–603. doi: 10.1007/s00066-015-0825-3. [DOI] [PubMed] [Google Scholar]
- 29.Imai R, Kamada T, Araki N, Working Group for B, Soft Tissue S. Carbon Ion Radiation Therapy for Unresectable Sacral Chordoma: An Analysis of 188 Cases. Int J Radiat Oncol Biol Phys. 2016;95:322–7. doi: 10.1016/j.ijrobp.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Rotondo RL, Folkert W, Liebsch NJ, Chen YL, Pedlow FX, Schwab JH. et al. High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. J Neurosurg Spine. 2015;23:788–97. doi: 10.3171/2015.3.SPINE14716. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–6. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 32.Stacchiotti S, Casali PG, Lo Vullo S, Mariani L, Palassini E, Mercuri M. et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Annals of surgical oncology. 2010;17:211–9. doi: 10.1245/s10434-009-0740-x. [DOI] [PubMed] [Google Scholar]
- 33.Uhl M, Edler L, Jensen AD, Habl G, Oelmann J, Roder F. et al. Randomized phase II trial of hypofractionated proton versus carbon ion radiation therapy in patients with sacrococcygeal chordoma-the ISAC trial protocol. Radiation oncology (London, England) 2014;9:100. doi: 10.1186/1748-717X-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhl M, Mattke M, Welzel T, Roeder F, Oelmann J, Habl G. et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer. 2014;120:3410–7. doi: 10.1002/cncr.28877. [DOI] [PubMed] [Google Scholar]
- 35.Uhl M, Mattke M, Welzel T, Oelmann J, Habl G, Jensen AD. et al. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer. 2014;120:1579–85. doi: 10.1002/cncr.28606. [DOI] [PubMed] [Google Scholar]
- 36.Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M. et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120:169–74. doi: 10.1016/j.radonc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Imai R, Kamada T, Sugahara S, Tsuji H, Tsujii H. Carbon ion radiotherapy for sacral chordoma. The British journal of radiology; 2011. p. 84. Spec No 1: S48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Combs SE, Kalbe A, Nikoghosyan A, Ackermann B, Jakel O, Haberer T. et al. Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother Oncol. 2011;98:63–7. doi: 10.1016/j.radonc.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Mattke M, Vogt K, Bougatf N, Welzel T, Oelmann-Avendano J, Hauswald H. et al. High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer. 2018;124:2036–44. doi: 10.1002/cncr.31298. [DOI] [PubMed] [Google Scholar]