Abstract
Background. Tobacco companies have actively promoted the substitution of cigarettes with purportedly safer tobacco products (e.g., smokeless tobacco, e-cigarettes) as tobacco harm reduction (THR). Given the tobacco, e-cigarette, and pharmaceutical industries’ substantial financial interests, we quantified industry influence on support for THR.
Objectives. To analyze a comprehensive set of articles published in peer-reviewed journals assessing funding sources and support for or opposition to substitution of tobacco or nicotine products as harm reduction.
Search Methods. We searched PubMed, Embase, Web of Science, and PsycINFO with a comprehensive search string including all articles, comments, and editorials published between January 1, 1992, and July 26, 2016.
Selection Criteria. We included English-language publications published in peer-reviewed journals addressing THR in humans and excluded studies on modified cigarettes, on South Asian smokeless tobacco variants, on pregnant women, on animals, not mentioning a tobacco or nicotine product, on US Food and Drug Administration–approved nicotine replacement therapies, and on nicotine vaccines.
Data Collection and Analysis. We double-coded all articles for article type; primary product type (e.g., snus, e-cigarettes); themes for and against THR; stance on THR; THR concepts; funding or affiliation with tobacco, e-cigarette, pharmaceutical industry, or multiple industries; and each author’s country. We fit exact logistic regression models with stance on THR as the outcome (pro- vs anti-THR) and source of funding or industry affiliation as the predictor taking into account sparse data. Additional models included article type as the outcome (nonempirical or empirical) and industry funding or affiliation as predictor, and stratified analyses for empirical and nonempirical studies with stance on THR as outcome and funding source as predictor.
Main Results. Searches retrieved 826 articles, including nonempirical articles (21%), letters or commentaries (34%), editorials (5%), cross-sectional studies (15%), systematic reviews and meta-analyses (3%), and randomized controlled trials (2%). Overall, 23.9% disclosed support by industry; 49% of articles endorsed THR, 42% opposed it, and 9% took neutral or mixed positions. Support from the e-cigarette industry (odds ratio [OR] = 20.9; 95% confidence interval [CI] = 5.3, 180.7), tobacco industry (OR = 59.4; 95% CI = 10.1, +infinity), or pharmaceutical industry (OR = 2.18; 95% CI = 1.3, 3.7) was significantly associated with supportive stance on THR in analyses accounting for sparse data.
Authors’ Conclusions. Non–industry-funded articles were evenly divided in stance, while industry-funded articles favored THR. Because of their quantity, letters and comments may influence perceptions of THR when empirical studies lack consensus.
Public Health Implications. Public health practitioners and researchers need to account for industry funding when interpreting the evidence in THR debates.
PLAIN-LANGUAGE SUMMARY
Tobacco harm reduction is one of the most divisive issues in tobacco control. Tobacco companies have promoted substitution of potentially safer tobacco products as tobacco harm reduction. Given the tobacco, e-cigarette, and pharmaceutical industries’ substantial financial interests in product substitution, in this study, we quantified industry influence supporting tobacco harm reduction. We systematically reviewed a comprehensive set of articles published in peer-reviewed journals to examine the relationship between industry funding and stance on tobacco harm reduction. We reviewed 4 major databases and all articles, comments, letters, and editorials published between January 1992 and July 2016. Of the 826 articles fitting the study criteria, most were nonempirical articles, letters or commentaries, or editorials. Overall, 23.9% of articles disclosed support by industry; 49% of articles endorsed tobacco harm reduction, 42% opposed it, and 9% took neutral or mixed positions. Financing from the e-cigarette industry (odds ratio [OR] = 20.9; 95% confidence interval [CI] = 5.3, 180.7), tobacco industry (OR = 59.4; 95% CI = 10.1, +infinity), or pharmaceutical industry (OR = 2.18; 95% CI = 1.3, 3.7) was significantly associated with support for tobacco harm reduction. While non–industry-funded articles were evenly divided, industry-funded articles strongly favored tobacco harm reduction. Industry-funded literature, especially letters and comments, may influence tobacco harm reduction scientific dialogue when empirical studies do not provide consensus.
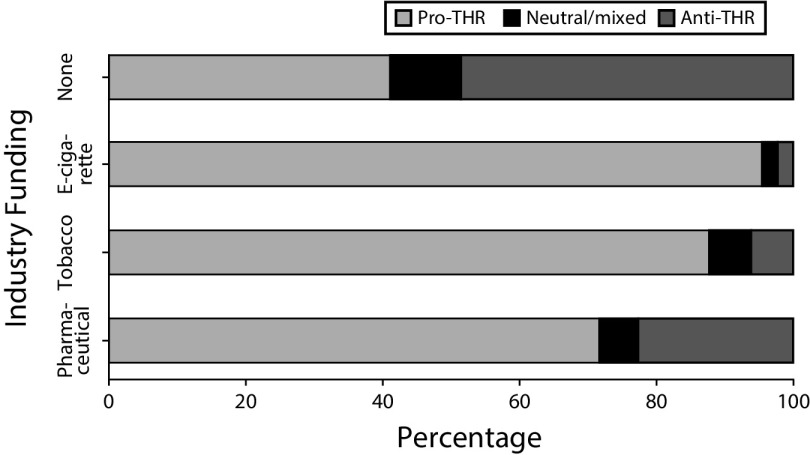
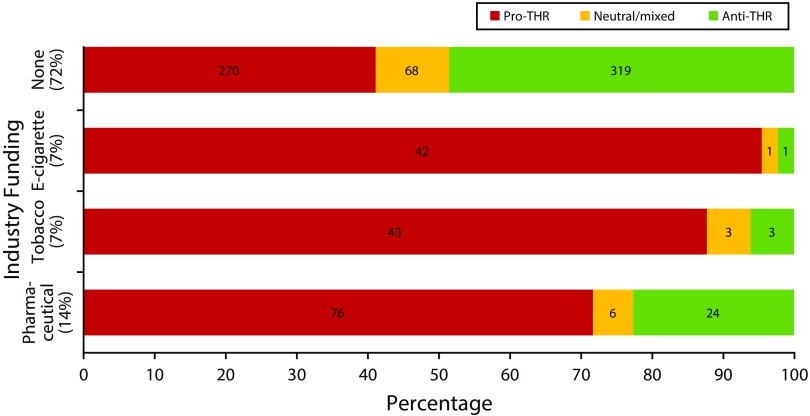
FIGURE 1—
Stance on Tobacco Harm Reduction (THR) by Funding Source
In 2001, the Institute of Medicine conditionally endorsed reduced-harm tobacco products as potentially reducing individual exposure to tobacco toxicants and improving population-level public health outcomes.1 Since then, tobacco harm reduction (THR) has been increasingly interpreted as the complete or partial substitution of cigarettes with purportedly reduced-harm tobacco products (e.g., smokeless tobacco, potentially reduced exposure products, or e-cigarettes).1–3 However, this strategy is supported by few empirical studies: the American College of Physicians, the US Preventive Services Task Force, and the National Academy of Sciences, Engineering, and Medicine all concluded that there is insufficient evidence from randomized controlled trials to support the use of e-cigarettes for smoking cessation.4–6 Nonetheless, THR proponents argue that alternative tobacco products should be promoted for cessation or long-term nicotine maintenance if users prefer such products to established cessation aids such as nicotine replacement therapies.3,7,8 Other public health experts argue that alternative tobacco products should not be endorsed because of a lack of evidence demonstrating these products’ long-term safety and cessation efficacy.9–11
Articles on THR have proliferated since 2001: a search using the keywords “tobacco” and “harm reduction” on PubMed yielded 3 articles in 1999, increasing to 112 in 2016 alone. Published studies, reviews, and opinions exhibit strong differences in their stance on the safety and effectiveness of THR products, and there may be an association between stance and funding or financial conflicts of interest. The tobacco industry has actively promoted new tobacco products including e-cigarettes, embracing the concept and terminology of THR.12–15 Given the tobacco, e-cigarette, and pharmaceutical industries’ substantial financial interest in THR, in this study, we quantified industry influence on stance in published articles regarding THR.
This article presents an analysis of conclusions and stance across a comprehensive set of articles on THR including articles of all formats, funding disclosures, and arguments for and against THR published in peer-reviewed journals between 1992 and 2016. Similar to a previous systematic review,16 this review weighed neither the quality of a particular study type nor evidence for a single clinical outcome. Rather, this review deliberately included all article types (such as nonempirical articles, letters, and comments) so as to systematically evaluate the role of disclosed funding in influencing the academic discourse on THR. Whereas previous studies have reviewed existing literature on the harm of e-cigarettes,17 smokeless tobacco,18,19 and snus,20 each review acknowledged a paucity of reliable randomized controlled trials in the literature.9 This systematic review included a comprehensive broad review of all published articles from peer-reviewed journals on THR and examined whether funding, industry affiliation, or other declared support from the tobacco, e-cigarette, or pharmaceutical industry was associated with support or opposition to THR.
METHODS
We conducted a comprehensive review of all articles, letters, and comments on the topic of THR published in peer-reviewed journals between 1992 and 2016. The study was registered with PROSPERO, protocol CRD42019115435. In addition, the search strings and complete codebook are provided as supplements to the online version of this article at http://www.ajph.org.
Data Sources and Searches
With the help of a knowledge synthesis librarian, we searched PubMed, Embase, Web of Science, and PsycINFO for all articles relevant to THR and associated topics published between January 1, 1992, and July 26, 2016, by using a comprehensive search string (Appendix A, available as a supplement to the online version of this article at http://www.ajph.org). Assessing term frequencies on PubMed, investigators identified 1992 as the first year that the term and concept of “tobacco harm reduction” became popular. This rise in popularity coincided with the introduction of potentially reduced exposure products, followed by cigarette branded Swedish snus products in the United States and other smokeless tobacco products. We iteratively refined the search string to ensure it was able to capture a test set of 17 THR articles of different publication types.21,22
Study Selection
We imported all records obtained through database searches into Covidence,23 a Web-based software platform. We eliminated duplicate citations, and 2 of the authors independently screened 4816 abstracts. We included abstracts agreed on by both reviewers as meeting predetermined criteria for full text review.
We included English-language publications addressing the concept of THR in humans (defined as substituting putatively safer tobacco products) in the full-text review. We excluded studies on modified cigarettes (e.g., Marlboro UltraSmooth, hookah or waterpipe, and South Asian smokeless tobacco variants like chuna, gutka, and betel quid), because these products were generally not considered to be reduced-harm products in the literature.24–26 We excluded studies discussing alternative product use in pregnant women because any form of nicotine consumption (including nicotine replacement therapies) is generally considered harmful for this population.27 We also excluded the following study types: animal studies; theoretical studies that exclusively discussed concepts in THR debates (e.g., “inveterate smokers”) but did not mention a tobacco or nicotine product; studies addressing long-term use of US Food and Drug Administration–approved nicotine replacement therapies; nicotine vaccines; books, theses, reports, and other literature not published in peer-reviewed journals; and abstracts from conference proceedings publications without a corresponding published article.
We excluded studies only when both independent reviewers agreed on exclusion. Disagreements regarding inclusion or exclusion were discussed, with a third reviewer adjudicating if necessary.
Data Extraction
We extracted data on author names, article titles, date of publication, first author’s country, and journal name for all articles included in full-text review. We double-coded all articles for the following: type of article, primary product type (e.g., snus, e-cigarettes), presence of themes for and against THR, the article’s overall stance on THR (5-point Likert scale: strongly pro-, weakly pro-, mixed or neutral, weakly anti-, strongly anti-), and concept of THR present in the article (e.g., long-term product substitution). During this phase of coding, a content-blinded research assistant, who reviewed disclosed author affiliations and conflict of interest statements, determined funding information (tobacco, e-cigarette, pharmaceutical industry, or multiple industries) and other affiliation with industry (such as having authors employed by a tobacco company), and each author’s country. Two investigators subsequently validated this assessment by independently extracting and verifying industry funding and affiliation coding for the entire data set.
Coding Guideline Development and Definitions
We developed a detailed set of coding guidelines (Appendix B, available as a supplement to the online version of this article at http://www.ajph.org) to define the different arguments used to support and oppose THR (to aid in consistent assessments of stance on THR) and to classify different publication types.
We developed the coding guidelines iteratively by reading representative THR articles to generate a list of the most frequently used arguments to promote and criticize tobacco product substitution as harm reduction. We coded a sample of 30 articles using the list to determine reliability, consistency, and the need for new codes. We revised and retested the codebook with an additional 30 sample articles. Through this iterative analysis, we identified and defined 29 “pro-THR” (supporting) and “anti-THR” (criticizing) arguments. All investigators coded test sets of articles to clarify the definitions and revise the codebook and criteria. Investigators assigned each article a score based on the number of pro-THR and anti-THR arguments and the strength of position each article endorsed. The 5-point score was simplified into 3 categories for analysis (i.e., pro-, anti-, neutral). The iterative process of coding test sets continued until the third set of 40 articles where argument saturation and greater-than-70% coding consistency was achieved as measured by Krippendorff’s α. Two investigators double-coded all articles in the data set and differences in coding were resolved through review and consensus, with adjudication by a third investigator as needed.
Similarly, we developed definitions for coding publication type, using both preestablished definitions of empirical studies such as cohort studies and cross-sectional studies and working definitions of nonempirical published articles to differentiate between commentaries, nonsystematic literature reviews, and editorials, and other nonempirical opinion pieces. The complete list of pro-THR and anti-THR arguments and publication types with examples for each definition can be found in the codebook (Appendix B). An external reviewer validated the final codebook by using the coding guidelines to independently code a stratified random sample of 10 articles from the data set for comparison.
Data Synthesis and Analysis
We conducted a descriptive analysis of extracted data to determine the frequency with which products were discussed, the number of THR articles by the first author’s country, difference in THR stance by country, and year of publication (relevant to when e-cigarettes entered the market). We also described stance on THR for each publication type.
To determine association between industry funding or affiliation and stance on THR, we examined only articles classified as pro-THR or anti-THR, excluding neutral articles. First, we used exact logistic regression models with stance on THR as the outcome (pro- vs anti-THR) and source of funding or industry affiliation, which was a categorical variable and indicated whether articles were funded by e-cigarette industry, pharmaceutical industry, and tobacco industry as the predictor. Second, we fit a model with article type as the outcome (nonempirical or empirical). Because of limited numbers of some types of articles (e.g., randomized controlled trials, quasi-experimental studies), we grouped articles classified as letters, commentaries, nonempirical articles, and editorials as nonempirical articles. Other article types were grouped as empirical articles. Lastly, we conducted a stratified analysis by article type with separate models for empirical and nonempirical studies with stance on THR as the outcome and funding source as predictor. We also conducted a sensitivity analysis including the neutral articles, using pro-THR versus neutral or anti-THR as the outcome. We carried out analyses with Stata version 14 (StataCorp LP, College Station, TX) and SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
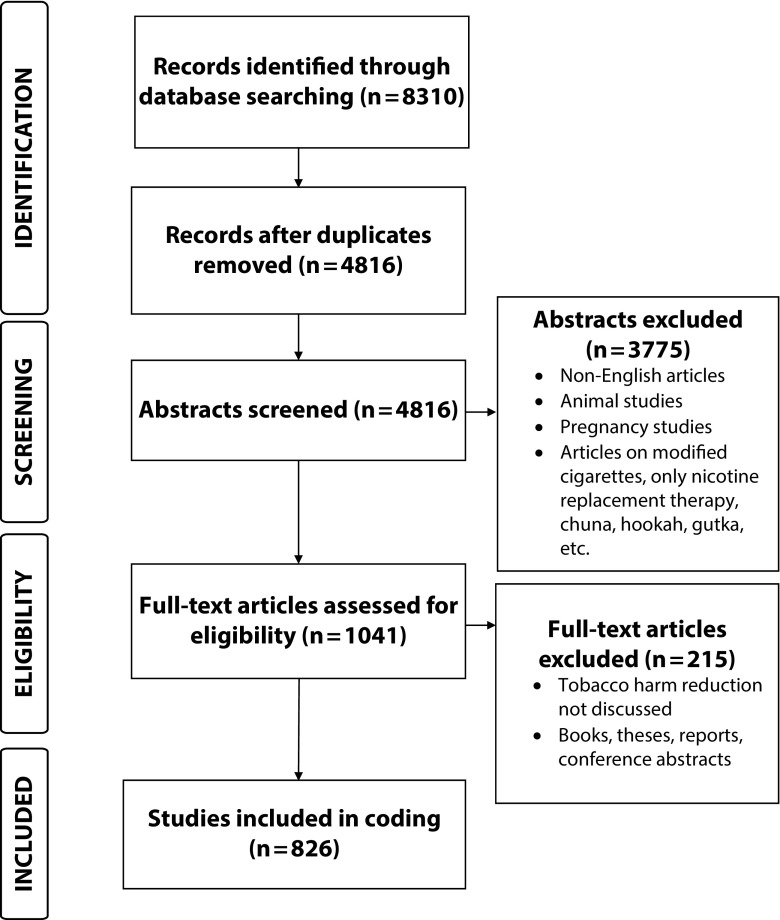
In total, we reviewed 4816 unique abstracts and 1041 full-text articles to address 3 key questions. After full-text review, we excluded 215 articles because they did not fit review criteria, resulting in 826 articles for analysis (Figure 2). The list of included articles can be found in Appendix C (available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 2—
PRISMA Flowchart for Systematic Review of Financial Conflicts of Interest and Stance on Tobacco Harm Reduction
Key Question 1
What types of journal articles on THR were published in peer-reviewed journals? Most THR articles were published after 2004, with 25% of the sample published between 2004 and 2010 and 66% published after 2010. Overall, 49.2% of total articles were pro-THR, 9.3% were mixed or neutral, and 41.5% were anti-THR. E-cigarettes were the most popular product type discussed (appearing in 48.9% of articles), followed by smokeless tobacco (19.5%), snus (17.2%), potentially reduced exposure products (11%), and other products (3.4%). Based on the affiliation of the first author, 48.6% of articles in the data set were from the United States, followed by the United Kingdom (18.3%), Australia (6%), Sweden (5%), and Canada (5%). Among THR articles from the United States, 35% were pro-THR and 54.4% were anti-THR. France and other countries also had a plurality of anti-THR articles. In the United Kingdom, however, this distribution was reversed: 64.9% of articles were pro-THR versus 26.5% anti-THR. The majority of English-language articles from Sweden, Australia, Canada, Italy, New Zealand, and Switzerland were also pro-THR (Table 1).
TABLE 1—
Stance on Tobacco Harm Reduction (THR) of Articles by the Primary Tobacco Product Type, Year of Publication, First Author’s Country, Publication Type, and Funding Source: January 1, 1992, to July 26, 2016
| Total (n = 826), No. (%) | Pro-THR (n = 406), No. (%) | Neutral/Mixed (n = 77), No. (%) | Anti-THR (n = 343), No. (%) | |
| Primary product type | ||||
| E-cigarettes | 404 (48.9) | 199 (49.3) | 38 (9.4) | 167 (41.3) |
| Smokeless tobacco | 161 (19.5) | 75 (46.6) | 12 (7.5) | 74 (45.3) |
| Snus | 142 (17.2) | 59 (41.1) | 17 (12.0) | 66 (46.5) |
| Potentially reduced exposure products | 91 (11.0) | 58 (63.7) | 5 (5.5) | 28 (30.8) |
| Other | 28 (3.4) | 15 (53.6) | 5 (17.9) | 8 (28.6) |
| Year of publication | ||||
| Before 2004 | 75 (9.1) | 39 (52.0) | 4 (5.3) | 32 (42.7) |
| Between 2004 and 2010 | 205 (24.8) | 267 (48.8) | 49 (11.7) | 230 (39.5) |
| After 2010 | 546 (66.1) | 100 (48.9) | 24 (9.0) | 81 (42.1) |
| First author’s country | ||||
| United States | 402 (48.7) | 141 (35.1) | 43 (10.7) | 218 (54.2) |
| United Kingdom | 151 (18.3) | 98 (64.9) | 13 (8.6) | 40 (26.5) |
| Australia | 51 (6.2) | 35 (68.6) | 3 (5.9) | 13 (25.5) |
| Sweden | 40 (4.8) | 24 (60.0) | 4 (10.0) | 12 (30.0) |
| Canada | 40 (4.8) | 27 (67.5) | 2 (5.0) | 11 (27.5) |
| Italy | 25 (3.0) | 22 (88.0) | 1 (4.0) | 2 (8.0) |
| France | 14 (1.7) | 6 (42.9) | 1 (7.1) | 7 (50.0) |
| New Zealand | 17 (2.1) | 12 (70.6) | 3 (17.7) | 2 (11.8) |
| Switzerland | 13 (1.6) | 8 (61.5) | 3 (23.1) | 2 (15.4) |
| Other | 73 (8.3) | 33 (45.2) | 4 (5.5) | 36 (49.3) |
| Publication type | ||||
| Nonempirical studies | 173 (20.9) | 102 (59.0) | 17 (9.8) | 54 (31.2) |
| Letter or commentary | 283 (34.3) | 142 (50.2) | 16 (5.7) | 125 (44.2) |
| Editorial | 44 (5.3) | 18 (40.9) | 8 (18.2) | 18 (40.9) |
| Qualitative | 31 (3.8) | 8 (25.8) | 6 (19.4) | 17 (54.8) |
| Literature review | 38 (4.6) | 15 (39.5) | 7 (18.4) | 16 (42.1) |
| Systematic review or meta-analysis | 24 (2.9) | 10 (41.7) | 4 (16.7) | 10 (41.7) |
| Case studies | 5 (0.6) | 3 (60.0) | 0 (0.0) | 2 (40.0) |
| Cross-sectional studies | 125 (15.1) | 51 (40.8) | 13 (10.4) | 61 (48.8) |
| Case–control studies | 8 (1.0) | 4 (50.0) | 0 (0.0) | 4 (50.0) |
| Cohort studies | 28 (3.4) | 15 (53.6) | 1 (3.6) | 12 (42.9) |
| Quasi-experimental studies | 49 (5.9) | 25 (51.0) | 3 (6.1) | 21 (42.9) |
| Randomized controlled trials | 18 (2.2) | 13 (72.2) | 2 (11.1) | 3 (16.7) |
| Fundinga | ||||
| E-cigarette industry | 57 (29.7) | 53 (93.0) | 2 (3.5) | 2 (3.51) |
| Pharmaceutical industry | 124 (64.6) | 88 (71.0) | 8 (6.5) | 28 (22.6) |
| Tobacco industry | 59 (30.7) | 54 (91.5) | 4 (6.8) | 1 (1.7) |
Of the articles, 4% were funded by multiple industries. Thus, the total number adds up to more than 192 and total percentage also exceeds 100%.
Only 39.4% (326/826) of the included articles were empirical research articles, while 61% (500/826) were nonempirical letters or commentaries (34% of the entire sample), nonempirical articles (21%), and editorials (5%). The most common types of empirical studies identified were cross-sectional studies (15% of the entire sample), followed by quasi-experimental studies (6%). Randomized controlled trials made up 2% of the sample.
Randomized controlled trials, nonempirical studies, and letters or commentaries were more frequently supportive of THR than not (Table 1). Editorials and systematic reviews were the most evenly divided categories, with roughly equal frequency of pro-THR and anti-THR ratings. Cross-sectional studies, literature reviews, and qualitative studies were more frequently anti-THR.
Key Question 2
What proportion of THR articles are funded by the tobacco, pharmaceutical, and e-cigarette industries? About 23.9% (n = 197) of articles were funded by the tobacco, pharmaceutical, and e-cigarette industries or some combination thereof. Overall, 14% of articles were funded by the pharmaceutical industry, 7% by the tobacco industry, and 7% by the e-cigarette industry.
Key Question 3
Does the THR stance of industry-funded THR research differ from non–industry-funded research? Articles reporting any industry funding were significantly more likely to take a pro-THR position. In exact logistic regression models taking into account sparse data, the odds of taking a pro-THR stance was significantly higher for articles funded or supported by the e-cigarette industry (odds ratio [OR] = 20.9; 95% confidence interval [CI] = 5.3, 180.7), the tobacco industry (OR = 59.4; 95% CI = 10.1, +infinity), and the pharmaceutical industry (OR = 2.18; 95% CI = 1.3, 3.7). Ninety-five percent of the e-cigarette industry–funded articles took a pro-THR stance as did 88% of the tobacco industry articles and 72% of the pharmaceutical industry–funded articles. In contrast, only 41.1% of articles without any industry funding or affiliation took a pro-THR stance (Figure 3).
FIGURE 3—
Stance on Tobacco Harm Reduction (THR) by Industry Funding Source: January 1, 1992, to July 26, 2016
Note. Thirty articles were funded by multiple sources.
Among empirical studies, tobacco industry funding perfectly predicted a pro-THR stance, so it was not included in the model. E-cigarette industry funding was significantly associated with a pro-THR stance (OR = 16.4; 95% CI = 2.4, 701.8), as was pharmaceutical funding (OR = 2.1; 95% CI = 1.03, 4.5) for empirical articles. Among nonempirical studies, tobacco industry funding was significantly associated with pro-THR stance (OR = 19.1; 95% CI = 3.0, 799.5), as was e-cigarette industry funding (OR = 20.1; 95% CI = 3.2, 860.2) and pharmaceutical industry funding (OR = 2.4; 95% CI = 1.1, 5.3; Table 2).
TABLE 2—
Exact Logistic Models Fit With Industry Funding or Affiliation Source as Predictors for Pro–Tobacco Harm Reduction (Pro-THR) Stance and Research Article Type: January 1, 1992, to July 26, 2016
| OR (95% CI) | Industry-Funded Pro-THR Articles, No. | |
| Is presence of industry funding or affiliation associated with pro-THR stance? (n = 749)a | ||
| E-cigarette industry | 20.9 (5.3, 180.7) | 53 |
| Tobacco industry | 59.4 (10.1, +Infinity) | 54 |
| Pharmaceutical industry | 2.18 (1.3, 3.7) | 88 |
| Is presence of industry funding or affiliation associated with type of research article (empirical)? (n = 826) | ||
| E-cigarette industry | 0.7 (0.4, 1.3) | 20 |
| Tobacco industry | 2.3 (1.3, 4.2) | 35 |
| Pharmaceutical industry | 1.3 (0.9, 2.2) | 56 |
| Among nonempirical research articles, is industry funding associated with pro-THR stance? (n = 459)b | ||
| E-cigarette industry | 20.1 (3.2, 860.2) | 34 |
| Tobacco industry | 19.1 (3.0, 799.5) | 22 |
| Pharmaceutical industry | 2.4 (1.1, 5.3) | 51 |
| Among empirical research articles, is industry funding associated with pro-THR stance? (n = 258) | ||
| E-cigarette industry | 16.4 (2.46, 701.76) | 19 |
| Tobacco industryc | . . . | 32 |
| Pharmaceutical industry | 2.1 (1.03, 4.45) | 37 |
Note. CI = confidence interval; OR = odds ratio; THR = tobacco harm reduction.
The articles with THR stance coded as neutral or mixed (n = 77) were excluded from this analysis. Models take sparse data into account.
Here, 77 articles taking a neutral THR stance were excluded.
In addition to excluding the 77 neutral THR articles, all 32 articles funded by or affiliated with the tobacco industry were dropped from the analysis as they were all pro-THR (perfect prediction).
DISCUSSION
This systematic review of industry funding in the published literature on THR found that 23.9% of included articles were funded by industries with a financial interest in promoting nicotine-containing products as THR. Industry-funded articles were significantly more likely to support THR. Absent these articles, the published literature did not endorse THR, with 41.1% supporting, 10.4% neutral, and 48.6% against THR. In comparison, 80.9% of industry-funded articles endorsed THR. The impact of industry-funded publications may have skewed the overall debate in favor of product substitution.
These findings are consistent with the scientific literature showing that tobacco industry–funded research favors conclusions supportive to the tobacco industry.28–32 This includes industry research supporting nicotine’s benefits for Alzheimer’s disease,33 neuroenhancement,34 and ulcerative colitis.35 Other studies also documented the influence of pharmaceutical industry funding on multiple types of bias in scientific studies.36 This study suggests that major debates in public health such as THR may be shaped by industry-interested nonempirical articles, letters, and commentaries in the scholarly literature.
The pharmaceutical industry was the most common industry funder of THR articles. This may be because pharmaceutical companies have the most active interest in this area or because there is less stigma associated with disclosure of pharmaceutical funding compared with disclosure of tobacco or e-cigarette funding sources. The tobacco industry’s history of funding scientific research to undermine the evidence of harms from tobacco is well documented.37–40 The higher frequency of disclosed pharmaceutical funding may be the result of undisclosed tobacco and e-cigarette industry funding. Future studies should address undisclosed funding sources, which may be uncovered from internal industry documents41,42 or by tracing disclosed funding from authors’ past publications.43,44
In addition, most published articles were nonempirical research, editorials, letters, and comments. Funding disclosure policies for these types of publications may be more lax than for empirical studies, so the presence of industry funding reported in this study was likely underestimated. Nonempirical studies and reviews can have a substantial impact on scientific debate, particularly when little empiric evidence exists, as has been the case with e-cigarettes. For example, one article, which summarized the result of expert opinion in a Delphi process,45 concluded that e-cigarettes were 95% safer than cigarettes, and it was widely cited8,46 as a definitive scientific consensus, despite its tobacco and e-cigarette funding source47 and lack of any specific empirical evidence supporting the estimate of relative safety.48 Medical practitioners and researchers need to be aware of the presence and influence of industry funding in THR when reviewing this evidence base.
Disclosure of industry funding is necessary but not sufficient to minimize the bias introduced by commercial interests in science.43 Some scientific journals and conferences have adopted policies rejecting articles or abstracts submitted by industry-funded scientists44,49,50 on the grounds that the industry’s financial interests bias their research. This systematic review suggests the wisdom of such a strategy to avoid further industry-induced bias that may perpetuate an industry-induced health epidemic that continues to be the leading preventable cause of death worldwide.
Limitations
This study was limited to disclosed funding sources—undisclosed funding may have had a significant effect on THR position. The study relied on a novel coding instrument to measure position on THR, which was nevertheless subjective. To address this inherent limitation, we established detailed criteria to enumerate the most common THR arguments, investigators double-coded all articles to minimize individual variance in assessment of stance on THR, and an outside coder validated a subset of articles. In addition, while an independent research assistant blinded to the article content extracted all funding disclosures, knowledge of authors’ conflicts of interest might have biased the coders’ assessment of stance. The inclusion of many nonempirical articles and the heterogeneity in the data set precluded the use of meta-analysis or formal analysis of bias other than funding bias. However, the data reflect most of the published literature on THR as of 2016, highlighting how nonempirical articles may influence perceptions of scholarly consensus.
Despite these limitations, this study demonstrates the importance of industry conflict of interest in the THR debate. It also points out several deficiencies in the current body of literature on this topic, chief among which is the lack of empirical studies compared with opinion pieces, letters, and editorials. Future studies may build on this work by analyzing new research with clinical outcomes conducted since 2016, evaluating the risk of bias within different article types, and assessing the role of undisclosed industry funding on investigators’ conclusions.
Conclusions
Industry funding strongly influenced the acceptance of THR as product substitution. The THR scientific literature was dominated by nonempirical articles, which were much more likely to support THR if they received industry funding. Industry funding of scientific research has likely influenced perceptions of consensus on THR as endorsing product substitution as a viable health intervention, when in fact the non–industry-funded scientific literature remained divided on this issue.
ACKNOWLEDGMENTS
This study was supported by the National Cancer Institute (grants R01-141661, R01-87472, and T32- CA113710).
The authors wish to thank Natalie Warren, Carson Benowitz-Fredericks, and Christopher Ackerman for assistance with coding, and Stanton Glantz, PhD, and Nadra Lisha, PhD, for advice on and assistance with data analysis.
Note. The funders had no role in study design, analysis, or publication process. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
HUMAN PARTICIPANT PROTECTION
Institutional review board approval was not needed for this systematic review and secondary data analysis.
REFERENCES
- 1.Committee to Assess the Science Base for Tobacco Harm Reduction. Board on Health Promotion and Disease Prevention, Institute of Medicine. In: Stratton K, Shetty P, Wallace R, Bondurant S, editors. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 2.Terry M, Cummings KM, Erickson A, Shopland D. Core Team on Tobacco Control. 2017. Ending cigarette use by adults in a generation is possible: the views of 120 leaders in tobacco control. Available at: http://www.tobaccoreform.org/wp-content/uploads/2017/03/Executive-Summary-Report-Ending-Cigarette-Use-by-Adults.pdf. Accessed May 10, 2017. [Google Scholar]
- 3.Meier BM, Shelley D. The fourth pillar of the Framework Convention on Tobacco Control: harm reduction and the international human right to health. Public Health Rep. 2006;121(5):494–500. doi: 10.1177/003335490612100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Agency for Healthcare Research and Quality; 2015. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the US Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK321744. Accessed November 18, 2018. [PubMed] [Google Scholar]
- 5.Stratton K, Kwan LY, Eaton DL, editors. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems, Board on Population Health and Public Health Practice, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine. [PubMed] [Google Scholar]
- 6.Crowley RA. Electronic nicotine delivery systems: executive summary of a policy position paper from the American College of Physicians [erratum in Ann Intern Med. 2015;162(12):880] Ann Intern Med. 2015;162(8):583–584. doi: 10.7326/M14-2481. [DOI] [PubMed] [Google Scholar]
- 7.Britton J, Arnott D, McNeill A, Hopkinson N. Tobacco Advisory Group of the Royal College of Physicians. Nicotine without smoke—putting electronic cigarettes in context. BMJ. 2016;353:i1745. doi: 10.1136/bmj.i1745. [DOI] [PubMed] [Google Scholar]
- 8.McNeill A, Brose LS, Calder R . E-Cigarettes: An Evidence Update. London, England: Public Health England; 2015. Public Health England. [Google Scholar]
- 9.Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216. doi: 10.1002/14651858.CD010216.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–128. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry KM, Reynolds LM, Collins JM et al. E-cigarette initiation and associated changes in smoking cessation and reduction: the Population Assessment of Tobacco and Health Study, 2013–2015. Tob Control. 2019;28(1):42–49. doi: 10.1136/tobaccocontrol-2017-054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yach D. Foundation for a smoke-free world. Lancet. 2017;390(10104):1807–1810. doi: 10.1016/S0140-6736(17)32602-8. [DOI] [PubMed] [Google Scholar]
- 13.Peeters S, Gilmore AB. Understanding the emergence of the tobacco industry’s use of the term tobacco harm reduction in order to inform public health policy. Tob Control. 2015;24(2):182–189. doi: 10.1136/tobaccocontrol-2013-051502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan CE, Kyriss T, Glantz SA. Tobacco company efforts to influence the Food and Drug Administration-commissioned Institute of Medicine report Clearing the Smoke: an analysis of documents released through litigation. PLoS Med. 2013;10(5):e1001450. doi: 10.1371/journal.pmed.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.British American Tobacco. Sustainability Report 2016. 2017. Available at: https://www.bat.com/sustainability. Accessed March 21, 2017.
- 16.Wang AT, McCoy CP, Murad MH, Montori VM. Association between industry affiliation and position on cardiovascular risk with rosiglitazone: cross sectional systematic review. BMJ. 2010;340:c1344. doi: 10.1136/bmj.c1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med. 2014;69:248–260. doi: 10.1016/j.ypmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Critchley JA, Unal B. Is smokeless tobacco a risk factor for coronary heart disease? A systematic review of epidemiological studies. Eur J Cardiovasc Prev Rehabil. 2004;11(2):101–112. doi: 10.1097/01.hjr.0000114971.39211.d7. [DOI] [PubMed] [Google Scholar]
- 19.Tam J, Day HR, Rostron BL, Apelberg BJ. A systematic review of transitions between cigarette and smokeless tobacco product use in the United States. BMC Public Health. 2015;15(1):258. doi: 10.1186/s12889-015-1594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans WD, Horn KA, Gray T. Systematic review to inform dual tobacco use prevention. Pediatr Clin North Am. 2015;62(5):1159–1172. doi: 10.1016/j.pcl.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Available at: http://handbook.cochrane.org. Accessed May 6, 2019. [Google Scholar]
- 22.Deyner D, Tranfield D. Producing a systematic review. In: Buchanan D, Bryman A, editors. The SAGE Handbook of Organizational Research Methods. London, England: SAGE Publications Ltd; 2009. pp. 671–689. [Google Scholar]
- 23.Veritas Health Innovation. Covidence systematic review software. 2018. Available at https://www.covidence.org. Accessed July 29, 2016.
- 24.Gupta PC, Ray CS. Epidemiology of betel quid usage. Ann Acad Med Singapore. 2004;33(4 suppl):31–36. [PubMed] [Google Scholar]
- 25.Rees VW, Wayne GF, Connolly GN. Puffing style and human exposure minimally altered by switching to a carbon-filtered cigarette. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2995–3003. doi: 10.1158/1055-9965.EPI-07-2533. [DOI] [PubMed] [Google Scholar]
- 26.Maziak W. The global epidemic of waterpipe smoking. Addict Behav. 2011;36(1-2):1–5. doi: 10.1016/j.addbeh.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickström R. Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol. 2007;5(3):213–222. doi: 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landman A, Glantz SA. Tobacco industry efforts to undermine policy-relevant research. Am J Public Health. 2009;99(1):45–58. doi: 10.2105/AJPH.2007.130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith EA, Malone RE. Philip Morris’s health information web site appears responsible but undermines public health. Public Health Nurs. 2008;25(6):554–564. doi: 10.1111/j.1525-1446.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDaniel PA, Malone RE. British American Tobacco’s partnership with Earthwatch Europe and its implications for public health. Glob Public Health. 2012;7(1):14–28. doi: 10.1080/17441692.2010.549832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith J, Thompson S, Lee K. The Atlas Network: a “strategic ally” of the tobacco industry. Int J Health Plann Manage. 2017;32(4):433–448. doi: 10.1002/hpm.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez C, Fu M, Galán I et al. Conflicts of interest in research on electronic cigarettes. Tob Induc Dis. 2018;16:28. doi: 10.18332/tid/90668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis. 2010;19(2):465–480. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner C, Spilich GJ. Research into smoking or nicotine and human cognitive performance: does the source of funding make a difference? Addiction. 1997;92(11):1423–1426. [PubMed] [Google Scholar]
- 35. Targacept: key messages. RJ Reynolds. March 18, 1999. Available at: https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/szbm0008. Accessed April 13, 2018.
- 36.Sismondo S. How pharmaceutical industry funding affects trial outcomes: causal structures and responses. Soc Sci Med. 2008;66(9):1909–1914. doi: 10.1016/j.socscimed.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Hirshbein L. Scientific research and corporate influence: smoking, mental illness, and the tobacco industry. J Hist Med Allied Sci. 2012;67(3):374–397. doi: 10.1093/jhmas/jrr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muggli ME, Hurt RD, Blanke DD. Science for hire: a tobacco industry strategy to influence public opinion on secondhand smoke. Nicotine Tob Res. 2003;5(3):303–314. doi: 10.1080/1462220031000094169. [DOI] [PubMed] [Google Scholar]
- 39.Barnes DE, Bero LA. Why review articles on the health effects of passive smoking reach different conclusions. JAMA. 1998;279(19):1566–1570. doi: 10.1001/jama.279.19.1566. [DOI] [PubMed] [Google Scholar]
- 40.Mandrioli D, Kearns CE, Bero LA. Relationship between research outcomes and risk of bias, study sponsorship, and author financial conflicts of interest in reviews of the effects of artificially sweetened beverages on weight outcomes: a systematic review of reviews. PLoS One. 2016;11(9):e0162198. doi: 10.1371/journal.pone.0162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glantz SA, Slade J, Bero LA, Hanauer P, Barnes DE, editors. The Cigarette Papers. Berkeley, CA: University of California Press; 1998. [Google Scholar]
- 42.Bero L. Implications of the tobacco industry documents for public health and policy. Annu Rev Public Health. 2003;24(1):267–288. doi: 10.1146/annurev.publhealth.24.100901.140813. [DOI] [PubMed] [Google Scholar]
- 43.Bero LA, Glantz S, Hong M-K. The limits of competing interest disclosures. Tob Control. 2005;14(2):118–126. [PMC free article] [PubMed] [Google Scholar]
- 44.Grundy Q, Dunn AG, Bourgeois FT, Coiera E, Bero L. Prevalence of disclosed conflicts of interest in biomedical research and associations with journal impact factors and altmetric scores. JAMA. 2018;319(4):408–409. doi: 10.1001/jama.2017.20738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nutt DJ, Phillips LD, Balfour D et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res. 2014;20(5):218–225. doi: 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 46.Tobacco Advisory Group. Nicotine without smoke: tobacco harm reduction: a report. London, England: Royal College of Physicians; 2016. [Google Scholar]
- 47.Gornall J. Public Health England’s troubled trail. BMJ. 2015;351:h5826. doi: 10.1136/bmj.h5826. [DOI] [PubMed] [Google Scholar]
- 48.E-cigarettes: Public Health England’s evidence-based confusion. Lancet. 2015;386(9996):829. doi: 10.1016/S0140-6736(15)00042-2. [DOI] [PubMed] [Google Scholar]
- 49.Godlee F, Malone R, Timmis A et al. Journal policy on research funded by the tobacco industry. Thorax. 2013;68(12):1090–1091. doi: 10.1136/thoraxjnl-2013-204531. [DOI] [PubMed] [Google Scholar]
- 50.PLoS Medicine. A new policy on tobacco papers. PLoS Med. 2010;7(2):e1000237. doi: 10.1371/journal.pmed.1000237. [DOI] [PMC free article] [PubMed] [Google Scholar]