Abstract
Background
Increasing evidence indicates that the gut microbiota contributes to the occurrence and development of metabolic diseases. However, little is known about the effects of commonly used antidiabetic agents on the gut microbiota. In this study, we investigated the roles of dipeptidyl peptidase-4 inhibitors (DPP-4i) and α-glucosidase inhibitor in modulating the gut microbiota.
Methods
16S-rDNA sequencing was performed to analyse the effects of DPP-4i and acarbose on the gut microbiota in mice fed a high-fat diet (HFD). Fecal microbiota transplantation (FMT) from type 2 diabetes patients to germ-free mice was performed to investigate the contribution of the altered microbiome to antidiabetic effects of the drugs. Fecal metabolomics was also analysed by untargeted and targeted GC–MS systems.
Findings
Although DPP-4i and α-glucosidase inhibitor both altered the gut microbial composition, only the microbiome modulation of DPP-4i contributed to its hypoglycemic effect. Specifically, the changes of 68.6% genera induced by HFD were rescued by DPP-4i. FMT showed that the DPP-4i-altered microbiome improved glucose tolerance in colonized mice, while acarbose did not. Moreover, DPP-4i increased the abundance of Bacteroidetes, and also promoted a functional shift in the gut microbiome, especially increasing the production of succinate.
Interpretation
Our findings demonstrate an important effect of DPP-4i on the gut microbiota, revealing a new hypoglycemic mechanism and an additional benefit of it. Furthermore, modulating the microbial composition, and the functional shift arising from changes in the microbiome, might be a potential strategy for improving glucose homeostasis.
Fund
This work was supported by grants from the National Natural Science Foundation of China (No. 81700757, No. 81471039, No. 81700714 and No. 81770434), the National Key R&D Program of China (No. 2017YFC1309602, No. 2016YFC1101100, No. 2017YFD0500503 and No. 2017YFD0501001), and the Natural Science Foundation of Chongqing (No. cstc2014jcyjjq10006, No. cstc2016jcyjA0093 and No. cstc2016jcyjA0518).
Keywords: DPP-4i, Gut microbiota, Glucose tolerance, GF mice
Research in context.
Evidence before this study
Previous studies have found that dysbiosis of the gut microbiota is associated with the dysfunction of glucose metabolism, insulin resistance and lipid metabolism. Two major gut phyla, Bacteroidetes and Firmicutes share roles in modulating the response of the host to dynamic changes in the diet, and they are enriched with genes encoding enzymes that govern carbohydrate and lipid metabolism. In addition, the gut metabolite succinate has been indicated as a substrate for intestinal gluconeogenesis to improve glucose tolerance and insulin sensitivity.
Added value of this study
DPP-4i altered the gut microbial composition, predominantly by increasing the abundance of Bacteroidetes, and the alterations contributed to its hypoglycemic effect. Moreover, DPP-4i obviously changed the pattern of fecal metabolites in HFD mice, especially increasing the production of succinate, which improved glucose tolerance.
Implications of all the available evidence
Our study, along with studies by other groups, indicates that alterations of gut microbiota might be a new hypoglycemic mechanism of DPP-4i, and that modulating the bacterial composition and metabolites might be a potential strategy for improving glucose homeostasis.
Alt-text: Unlabelled Box
1. Introduction
The adult human intestine is home for >500 species of microbes [1], and emerging evidence indicates that these microbes are heavily involved in modulating immunity, inflammation, gut-brain neural circuits, and metabolism [2,3]. Aberrant changes in the gut microbiota are associated with the occurrence and development of various diseases, such as immunological diseases [4], inflamed intestinal diseases [5], mental disorders [6], and metabolic diseases [[7], [8], [9], [10]]. In the context of metabolic diseases, the gut microbiota has been reported to be associated with dysfunction of glucose metabolism, insulin resistance and lipid metabolism [11,12]. Consequently, the gut microbiota has been identified to be closely linked to diabetes and obesity, and interventions involving the intestinal bacterial flora are anticipated to be a new therapeutic strategy for these diseases.
Some existing evidence has indicated that gut bacteria can be used in the treatment of metabolic diseases. Prebiotics (oligofructose, inulin-type fructans) and probiotics (Saccharomyces boulardii) have been shown to change the gut microbiota composition, and increase the quantities of Bifidobacterium and Lactobacillus while improving glucose tolerance and lipid metabolism [[13], [14], [15]]. Additionally, as a promising treatment for diabetes and obesity, Roux-en-Y gastric bypass (RYGB) surgery improves the metabolic and inflammatory status partially by modifying the composition of the gut microbiome [16]. Recently, the commonly used antidiabetic drug metformin has been reported to significantly change the composition of the gut microbiome and the concentration of intestinal short chain fatty acids (SCFAs) [[17], [18], [19]], which contributed to its therapeutic effects. Besides, other common hypoglycemic agents, such as acarbose, glucagon-like peptide 1 (GLP-1) agonists and dipeptidyl peptidase–4 inhibitors (DPP-4i), have also been reported to change the gut microbial community and metabolites when improving glucose metabolism [[20], [21], [22], [23], [24]]. While, the role of alterations of the microbiome and metabolites in the hypoglycemic effect of these agents is not completely clear. As one fermentation product of bacterium strains derived from Actinoplanes sp. SE50, α-glucosidase inhibitor slows carbohydrate uptake and reduces postprandial hyperglycemia by inhibiting α-glucosidase activity in the brush border of small intestinal mucosa [25]. DPP-4i is the most extensively used oral hypoglycemic agent worldwide, and it targets the DPP-4 enzyme and inhibits the degradation of GLP-1 to reduce blood glucose levels [26]; its target DPP-4 has a high expression level in the small intestine [27]. As such, these drugs might also have the ability to improve glucose homeostasis through affecting gut microbial composition.
In the current study, we investigated the effects of DPP-4i and α-glucosidase inhibitor on the intestinal microbial flora, and the relationship between the altered microbiomes and the antidiabetic effects of the drugs. Additionally, although it is not clear how alterations in the gut microbiota improve glucose homeostasis in the host, a potential mechanism includes increased production of SCFAs and other organic acids [[28], [29], [30]]. Therefore, changes in fecal metabolomics were also observed in the present study.
Here, we report that the DPP-4i-altered gut microbiota participates in its effect on glucose metabolism, while α-glucosidase inhibitor does not, indicating a novel hypoglycemic mechanism and an additional benefit of DPP-4i. Moreover, DPP-4i obviously altered the abundance of Bacteroidetes, and promoted a functional shift in the gut microbiome, which might be a potential therapeutic strategy for type 2 diabetes (T2D).
2. Materials and methods
2.1. Clinical study design
Thirty newly diagnosed T2D patients were recruited at Xinqiao Hospital, Third Military Medical University (Chongqing, China), and were randomized into four groups: one group treated with acarbose (n = 8), one group with sitagliptin (Sit, n = 7), and the other two groups with the corresponding placebos (n = 7–8). The two drugs are in tablet and are available in the pharmaceutical market. Acarbose (Bayer Pharmaceutical Co., Germany) was administered at a start dose of 150 mg/d. Sitagliptin (Merck Sharp & Dohme, USA) was administered at a dose of 100 mg/d. Furthermore, we recommended that all individuals maintain a reduced daily caloric intake of 25 kcal/kg, and perform regular physical exercise (2.5 h/week) throughout the entire treatment as in previous studies [18]. We collected peripheral blood and fecal samples from the enrolled patients after treatment for two months. Each sample was frozen immediately at −80 °C or stored in personal −20 °C freezers before transport to the laboratory within 24 h.
Inclusion criteria were as follows: (i) age between 18 and 70 years; (ii) newly diagnosed T2D, based on World Health Organization 1998 diagnostic criteria [31]; (iii) HbA1c lower than 9%, and body mass index (BMI) <30 kg/m2; (iv) not treatment with any antidiabetic agents before recruitment, and absence of other metabolic diseases; (v) native of Chongqing. Exclusion criteria were as follows: (i) pregnancy; (ii) current or recent cancer (1 year); (iii) use of antibiotics, prebiotics, probiotics or fiber supplements during the 3 months prior to enrollment; (iv) treatment with bariatric surgery; (iv) diagnosis of hypertension, coronary heart disease, cerebral infarction or other vascular related chronic diseases; (v) presence of gastrointestinal disorders or a history of chronic physical/mental disease, such as Alzheimer's disease or Parkinson's disease.
Informed written consent was obtained from all participants. The experiment was approved by the Ethics Committee of Xinqiao Hospital of the Third Military Medical University. Complete clinical trial registration is deposited in the Chinese Clinical Trials Registry (http://www.chictr.org.cn/index.aspx), and the registration number is ChiCTR-OPC-17010757.
2.2. Animal study
C57BL/6 male mice (3–4 weeks old) were purchased from the Model Animal Research Center of Nanjing University. After a 2-week acclimatization period, mice were fed a normal diet (ND) or a high fat diet (HFD, 60% fat, 20% protein, 20% carbohydrate (kcal/100 g), D12492; Research Diets, New Brunswick, New Jersey, USA) for 14 weeks. The mice were then divided into different groups based on matched weights and fasting blood glucose levels. The different treatment groups were administered 400 mg/kg of acarbose [32], 4 g/kg of Sit [33], or 300 mg/kg of saxagliptin [34] (Sax, Onglyza®, AstraZeneca, Wilmington, DE) mixed with HFD for 4 weeks.
For GLP-1 receptor agonist treatment, HFD mice received 200 μg/kg/day liraglutide (Novo Nordisk, HFD_Lira) or normal saline (HFD_NaCl) as a control through subcutaneous injection for 4 weeks. For antibiotic treatment, HFD mice were administered vancomycin (MedChemExpress, 0.5 g/l) and bacitracin (Aladdin, 1.0 g/l) in drinking water for one week before and four weeks during Sit treatment (Ab_Sit), or administered Sit alone for 4 weeks as a control (C_Sit). The depletion of bacteria by antibiotics was verified using quantitative real-time PCR (qPCR) as previously described [23]. For succinate treatment, 5–6 weeks old germ-free (GF) Kunming male mice (Third Military Medical University Experimental Animal Research Center, Chongqing, China) were fed a HFD and administered sodium succinate (BBI Life Sciences) in drinking water (2.5%) for 6 weeks. After treatment, fecal samples were collected from the treated mice and frozen immediately at −80 °C. The experimental protocol was approved by the Third Military Medical University Institutional Animal Care and Use Committee.
2.3. Fecal genomic DNA extraction and 16S-rDNA sequencing
Fecal genomic DNA was extracted from 0.1 g frozen fecal samples using an E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) according to the manufacturer's protocol. The DNA concentration and purification were measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, USA), and the DNA quality was detected by 1% agarose gel electrophoresis. Amplicon libraries covering the V3-V4 hypervariable regions of the bacterial 16S-rDNA gene were amplified using primers 341F: 5′-ACTCCTACGGGRSGCAGCAG-3′, and 806R: 5′-GGACTACVV GGGTATCTAATC-3′. PCR was performed in a 20 μl mixture containing 4 μl of 5 × FastPfu Buffer, 2 μl of 2.5 mmol/l dNTPs, 0.8 μl of each primer (5 μmol/l), 0.4 μl of FastPfu Polymerase and 10 ng of template DNA. PCR was conducted with an initial denaturation for 3 min at 95 °C, followed by 27 cycles of 30 s at 95 °C, 30 s for annealing at 55 °C, and 45 s for elongation at 72 °C, and a final extension at 72 °C for 10 min. The reactions were performed on a thermocycler PCR system (GeneAmp 9700, ABI, USA). All PCR products were purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluor™-ST (Promega, USA). Purified and pooled amplicon libraries were paired-end sequenced (2 × 300) on the Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
Raw sequence reads were demultiplexed, quality-filtered, merged and clustered into OTUs with a 97% similarity cutoff using UPARSE (version 7.1, http://drive5.com/uparse/), and chimeric sequences were identified and removed using UCHIME. The taxonomy of the acquired OTUs was analysed using the RDP Classifier Bayesian algorithm (http://rdp.cme.msu.edu/) against the SILVA database (version128) with a confidence threshold of 70%.
2.4. Untargeted metabolomics analyses
Fecal samples (60 mg) were spiked with 40 μl of internal standards (0.3 mg/ml 2-chlorophenylalanine in methanol) and 360 μl of cold methanol (Merck, Darmstadt, Germany) solution, and then extracted according to the manufacturer's instructions (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). The extracted samples were detected using a 7890A-5975C gas chromatograph-mass spectrometry (GC–MS) detection system (Agilent Technologies, Santa Clara, CA). The QC sample was a pooled sample in which aliquots of all the extracted samples were mixed, and then analysed using the same method as used for the analytic samples.
2.5. Targeted SCFAs analyses
Fecal samples (10 mg) were supplemented with 10 μl of internal standards (0.0125 μl/μl 2-ethylbutyric acid, Sigma-Aldrich) and 500 μl of methanol, and then extracted according to the manufacturer's protocol (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China). The extracted samples were detected using a 6890A-5973C GC–MS system (Agilent Technologies, Santa Clara, CA). SCFAs standards were mixtures of acetate, propionate, butyrate, isobutyrate, valerate and isovalerate. All the standards, excluding isovalerate (Sigma-Aldrich), were purchased from Merck (Darmstadt, Germany).
2.6. Fecal microbiota transplantation (FMT)
5–6 weeks old GF Kunming male mice were fed a HFD (60% fat, 20% protein, 20% carbohydrate, kcal/100 g) for 8 weeks before FMT. The transplant materials were derived from 12 donors: 3 from Sit-treated T2D patients, 3 from acarbose-treated patients, and 6 from matched placebo-treated patients. Each fecal sample (100 mg) was suspended in 1.0 ml sterile phosphate-buffered saline under anaerobic condition (80% N2:10% CO2:10% H2), and colonized to 3 GF mice by oral gavage with 200 μl of the suspended fecal microbiota as in our previous study [6]. After FMT, the colonized mice were separately housed in different gnotobiotic isolators to prevent normalization of the gut microbiota, and continued feeding with a HFD. Body weight was measured for all the colonized mice every week and glucose tolerance 2 weeks after FMT. One mouse transplanted with Sit-matched placebo-treated microbiota, and another transplanted with acarbose-treated microbiota died after FMT, and both were excluded from the analysis. The experimental protocol was approved by the Third Military Medical University Institutional Animal Care and Use Committee.
2.7. Glucose tolerance test (GTT) and insulin secretion test
The treated mice and colonized mice were fasted overnight hours for GTT as in previous studies [17,30], and then administered glucose at 1 g/kg of body weight via i.p. injection. Tail blood glucose concentrations were measured at 0, 15, 30, 60 and 120 min after glucose injection using an Accu-Check glucometer (Roche, Basel, Switzerland). Insulin secretion levels were measured at 0, 15 and 30 min during a GTT using a Rat/Mouse Insulin ELISA kit (Millipore, Bedford, MA, USA). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously reported: HOMA-IR = (fasting glucose (mmol/l) × fasting insulin (mU/l))/22.5 [35].
2.8. Statistical analysis
Data are expressed as the mean ± SEM. The significance of differences between two groups was evaluated using Student's t-test. For comparing multiple groups, the differences were analysed by one-way ANOVA with FDR correction or Tukey's test. 't- A p value <.05 was defined as statistically significant.
3. Results
3.1.1. DPP-4i and acarbose alter the composition of the gut microbial community
To observe the effects of these drugs on the gut microbiota, HFD mice were treated with the α-glucosidase inhibitor acarbose or DPP-4i at corresponding doses according to previous studies [32,33]. As expected, in comparison with ND mice, HFD mice showed increased weight gain, and impaired glucose tolerance (Supplementary Fig. S1). Compared with HFD control mice, HFD_Sit and HFD_AC mice showed a significant improvement in glucose tolerance (Supplementary Fig. S1b, c). In contrast to their effects on glucose metabolism, acarbose and Sit treatment had no effect on body weight (Supplementary Fig. S1a), indicating that the improvements in glucose tolerance are not related to body weight.
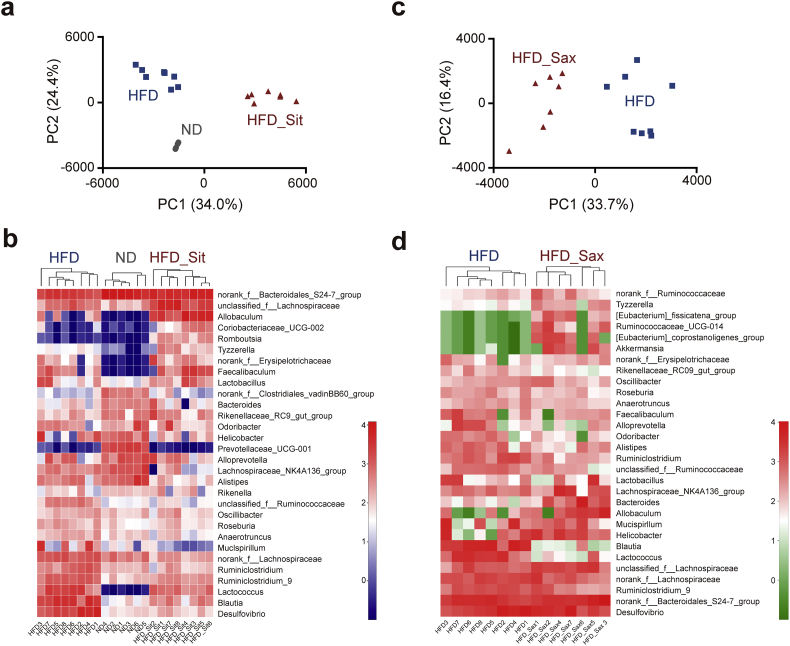
We next determined the effect of acarbose and Sit on the composition of the gut microbiota using 16S-rDNA sequencing. The results showed that >80% of the obtained operational taxonomic units (OTUs) were assigned to Bacteroidetes and Firmicutes, consistent with previous studies [36]. As shown in Supplementary Fig. S2a, principal components analysis (PCA) revealed a difference in distribution of the gut microbial community between the acarbose treated (HFD_AC) and HFD groups. However, in the heatmap analysis, although the two groups were roughly clustered, two acarbose-treated samples (HFD_AC2 and HFD_AC7, labeled with green color) were classified into the cluster that included all samples of the HFD group (Supplementary Fig. S2b). Interestingly, although acarbose is generally considered most likely to affect the microbiome based on its functional mechanism [25], Sit actually showed a more pronounced effect on the gut microbiota compared with acarbose. The HFD_Sit samples formed a cluster that was completely distinct from that of the HFD samples (Fig. 1a). The HFD and ND groups also formed different clusters, which is consistent with previous reports [17,37]. Moreover, hierarchical clustering of the heatmap revealed striking changes in the genera resulting in distinct clustering of the samples (Fig. 1b), further suggesting that Sit has a more definitive regulatory effect on the microbiota compared with acarbose. Currently, several kinds of DPP-4i are applied clinically, and we then sought to investigate whether the effect on the gut microbiota is universal for all DPP-4i as a pharmaceutical class effect. Administration of Sax to HFD mice also led to similar changes in gut microbial community structure (Fig. 1c, d). Taken together, these results demonstrate that DPP-4i and acarbose both alter the composition of the gut microbiota, while the effects of DPP-4i seemed to be more pronounced. Additionally, the modulatory effects of DPP-4i on the gut microbiota may not be restricted to one inhibitor of the family, but is rather a class effect.
Fig. 1.
DPP-4i alters the composition of the gut microbiota in HFD mice. (a) Cluster analysis of the ND (n = 6), HFD (n = 8) and HFD_Sit (n = 8) groups using PCA. The first two principal components (PC1 and PC2) from PCA are plotted for each sample. The percentage variation covered in the plotted principal components is marked on the axes. Each spot represents one sample, and each group of mice is labeled by a different symbol. (b) Heatmap analysis of species abundance clustering at the genus level in the ND, HFD and HFD_Sit groups. The heatmap shows the top 30 genera ranked on the basis of abundance. Each column in the heatmap represents one sample, and each row represents one genus. The color bar showing blue to red indicates the relative abundance of each genus. (c) Cluster analysis of the HFD (n = 8) and HFD_Sax (n = 7) groups by PCA. (d) Heatmap analysis of species abundance clustering at the genus level in the HFD and HFD_Sax groups. The heatmap shows the top30 ranked genera. The range of colors from green to red indicates the relative abundance of each genus. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
As DPP-4i lowers blood glucose levels by inhibiting GLP-1 degradation, we sought to clarify whether the effect of DPP-4i on gut microbiota is associated with GLP-1; HFD mice were treated with GLP-1 receptor agonist liraglutide (HFD_Lira) or normal saline as control (HFD_NaCl). After liraglutide treatment, the weight and fasting blood glucose level were significantly decreased (Supplementary Fig. S4a, b). The microbial composition in HFD_Lira mice was different from that in HFD_NaCl and HFD_Sit mice (Supplementary Fig. S4c). In addition, a previous study has also reported that the microbial composition in HFD mice was changed after liraglutide treatment, and that it was different from that in Sax-treated HFD mice [38]. Combined with our results, this finding suggested that the effect of DPP-4i on gut microbiota is not mediated by GLP-1, at least not mainly by GLP-1.
3.1.2. DPP-4i- altered gut microbiota improves glucose tolerance
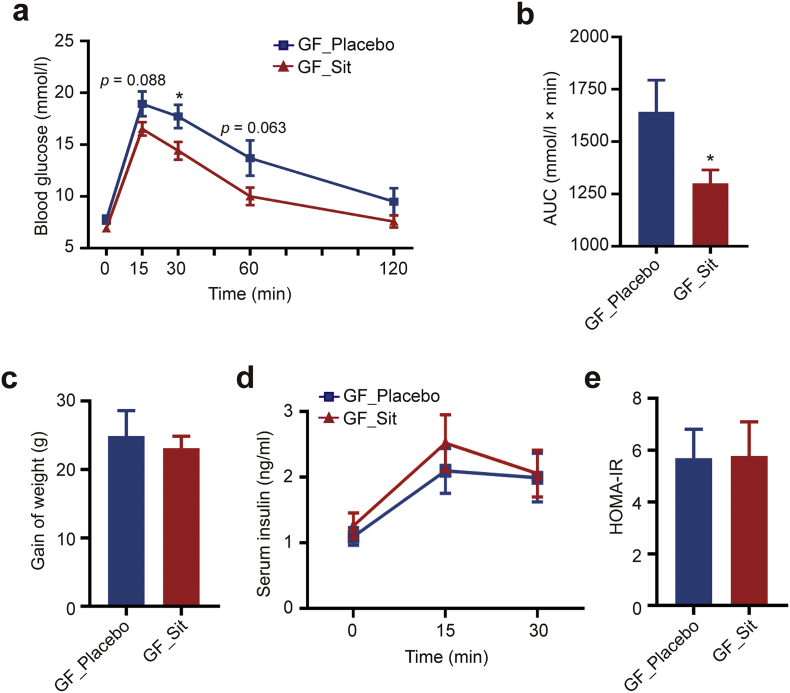
Next, to investigate whether the effects of α-glucosidase inhibitor and DPP-4i on the gut microbiota contribute to their hypoglycemic effects, FMT was performed in GF mice. To maximize the translational relevance, the transplanted fecal microbiota was obtained from T2D patients, who had not yet received other potentially confounding medications, and were treated with acarbose or Sit monotherapy at a therapeutic dose, or with the corresponding placebo. All groups were recommended to consume a calorie-restricted diet and undergo regular physical activity as in a previous study [18]. The clinical characteristics of the patients before and after acarbose or Sit treatment are listed in Supplementary Table S1 and Supplementary Table S2, respectively. Two weeks after FMT, the mice colonized with microbiota from acarbose-treated T2D patients showed no significant change in glucose tolerance as compared with the controls (Supplementary Fig. S2c). While, the transplanted microbiota from Sit-treated T2D patients improved HFD-induced glucose intolerance in the colonized mice (Fig. 2a, b), even though there were no obvious differences in weight, insulin secretion, or HOMA-IR (Fig. 2c–e). These results suggest that the alterations of the gut microbiota induced by DPP-4i at least partially contribute to its hypoglycemic effect.
Fig. 2.
DPP-4i-altered gut microbiota improves glucose tolerance in GF mice. The GF mice were colonized with fecal microbiota obtained from 3 Sit-treated T2D patients (GF_Sit) or 3 control patients treated with placebo (GF_Placebo). Each donor sample was transplanted to 3 GF mice, and the GF mice were fed a HFD for 8 weeks before and 2 weeks after FMT. (a) Blood glucose concentrations of colonized mice were measured during a GTT 2 weeks after FMT. (b) The area under the curve (AUC) for the GTT curves. (c) Body weight gain of colonized mice. (d-e) Insulin secretion levels during GTT (d) and HOMA-IR (e) were measured 2 weeks after FMT. All mice were fasted for 16 h before detecting blood glucose and insulin concentrations. GF_Placebo, n = 8; GF_Sit, n = 9. Data are presented as the means ± SEM. * p < .05, t-test.
To determine whether differences in the gut microbiota between Sit-treated T2D patients and placebo-treated controls were maintained in the colonized mice, fecal samples from T2D donors and colonized mice were subjected to 16S-rDNA sequencing. The principal coordinate analysis (PCoA) plots revealed that the key characteristic discriminative gut microbiota observed in T2D donors (Supplementary Fig. S5a) were also represented in the recipient mice (Supplementary Fig. S5c). Ninety percent of the OTUs sequenced in the colonized mice were assigned to the phyla Firmicutes and Bacteroidetes (Supplementary Fig. S5d), which was similar to the characteristic observed in the microbiome of T2D donors (Supplementary Fig. S5b). Besides, consistent with the T2D_Sit donors, GF_Sit mice exhibited increased abundance of the phylum Bacteroidetes (Supplementary Fig. S5e) compared with GF_Placebo mice. Moreover, the characteristics of gut microbial composition observed in colonized mice were also similar to DPP-4i- treated HFD mice (Fig. 1). These findings suggest that the different microbial communities detected in the two groups of colonized mice may be responsible for the improvement of glucose tolerance.
In addition to FMT, we also treated HFD mice with antibiotics (vancomycin and bacitracin) to verify the role of gut microbiota in the hypoglycemic effect of DPP-4i from another point of view. Vancomycin and bacitracin have strong sterilizing effects and selectively reduce two major gut phyla, Firmicutes and Bacteroidetes [28]. As shown in the results, the antibiotic treatment distinctly reduced the abundance of specific bacteria, such as Bacteroides and Oscillibacter (Supplementary Fig. S6a), and attenuated the effect of Sit on fasting blood glucose and glucose tolerance in HFD mice (Supplementary Fig. S6c-e). Taken together, these results demonstrate that the gut microbiota plays an important role in the hypoglycemic effect of DPP-4i.
3.1.3. DPP-4i increases the abundance of Bacteroidetes
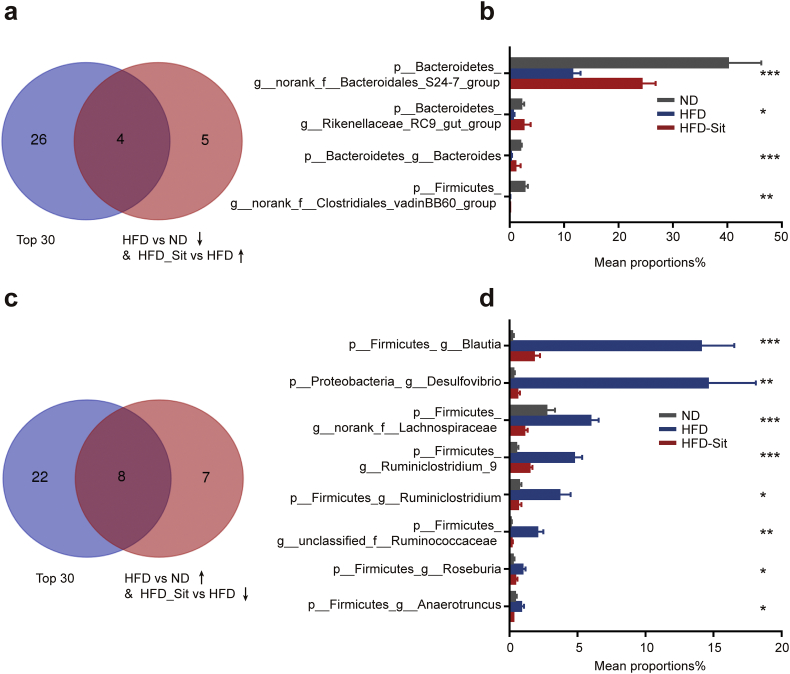
To understand the mechanism underlying the regulation of gut microbiota by DPP-4i and its hypoglycemic effect, the primary changes in the microbiome induced by Sit were analysed. At the genus level, noticeable changes in the abundance of 35 genera accounted for the differences in the gut microbial communities observed in mice fed different diets or treated with Sit (Supplementary Table S3). Interestingly, the alterations of 24 genera (68.6%) induced by HFD were rescued by Sit (Fig. 3a, c). Further stratification of genera per abundance (top 30, Supplementary Table S4) revealed that 12 genera with high abundance had opposite changes between the HFD_Sit and HFD mice (Fig. 3), suggesting that the hypoglycemic effect of Sit might be mediated by a specific subset of bacterial taxa. Intriguingly, 3/4 of the Sit-increased genera with high abundance belonged to Bacteroidetes (Fig. 3b); and 7/8 of the Sit-decreased genera with high abundance belonged to Firmicutes (Fig. 3d). In addition, 17 bacteria classified at the family level were significantly changed after Sit treatment (Supplementary Table S5). Sit treatment reversed the HFD-induced alterations of 8 families (47.1%) with high abundance (top 15), such as Bacteroidales S24–7 group, Bacteroidaceae, Ruminococcaceae, Desulfovibrionaceae and Streptococcaceae (Supplementary Fig. S7), most of which have been correlated with obesity and its related disorders [[39], [40], [41]]. Moreover, the changes at the family level were similar to those observed at the genus level, meaning that most of the Sit-reversed families and genera belong to the phyla Firmicutes and Bacteroidetes. Previous studies have reported that Bacteroidetes and Firmicutes are important in the regulation of carbohydrate and lipid metabolism [12,13,16,36]. In Bacteroidetes, Sit treatment obviously increased S24–7 group (ranked top1) and Bacteroides, which have been reported to be able to modulate glucose and lipid metabolism [42,43]. Therefore, these results suggest that the hypoglycemic effect of DPP-4i mediated by the gut microbiota might be closely associated with Bacteroidetes and Firmicutes.
Fig. 3.
DPP-4i reverses the alterations of the bacterial genera induced by HFD. Combined analysis of the Sit-reversed genera with the high abundance genera (top 30 genera ranked on the basis of abundance). (a-b) The Venn diagram (a) and bar plots (b) show that the 4 genera with high abundance (top 30) were decreased by HFD but increased in the HFD_Sit group. (c–d) The Venn diagram (c) and bar plots (d) show that the 8 genera with high abundance (top 30) were increased by HFD but decreased in the HFD_Sit group. In A and C, the blue circle represents the top 30 ranked genera based on abundance, and the red circle represents the genera showing the opposite change between the HFD_Sit group and HFD group. n = 6–8 mice per group. Data in the bar plots are presented as the means ± SEM. * p < .05, ** p < .01, *** p < .001, one-way ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
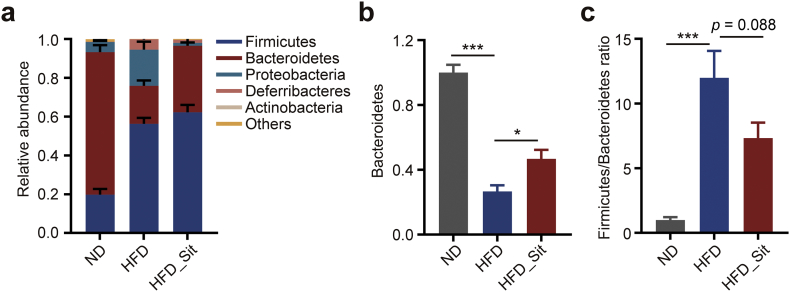
Bacteroidetes and Firmicutes consist of >80% of all phylogenetic types in both humans and mice [1,12]. To directly observe the modulatory effect of DPP-4i on the two phyla, we further analysed the changes in the gut microbiota at the phylum level. As shown in Fig. 4a, HFD resulted in an increase in Firmicutes and a decrease in Bacteroidetes, consistent with previous studies [44,45]. However, Sit rescued these changes, predominantly by increasing the abundance of Bacteroidetes (Fig. 4b–c). Furthermore, we also observed the same trend of changes in Bacteroidetes (increased by almost 63%) and Firmicutes (reduced by almost 34%) in T2D patients after Sit treatment (Supplementary Fig. S8), although there was no significant difference, which might be due to the considerable individual differences among the patients. Taken together, these results indicate that the hypoglycemic effect of DPP-4i mediated by the gut microbiota might be primarily related to Bacteroidetes and Firmicutes, suggesting a potentially distinct strategy for regulating glucose metabolism.
Fig. 4.
DPP-4i increases the abundance of Bacteroidetes in HFD mice. (a) The relative abundance of bacterial phyla in ND, HFD and HFD_Sit groups. (b) The relative abundance of Bacteroidetes in each group. (c) The ratio of Firmicutes to Bacteroidetes based on the relative abundance in each group. n = 6–8 mice each group. Data are presented as the means ± SEM. * p < .05, *** p < .001, one-way ANOVA with Tukey correction for multiple comparisons.
3.1.4. DPP-4i promotes a functional shift in the gut microbiome
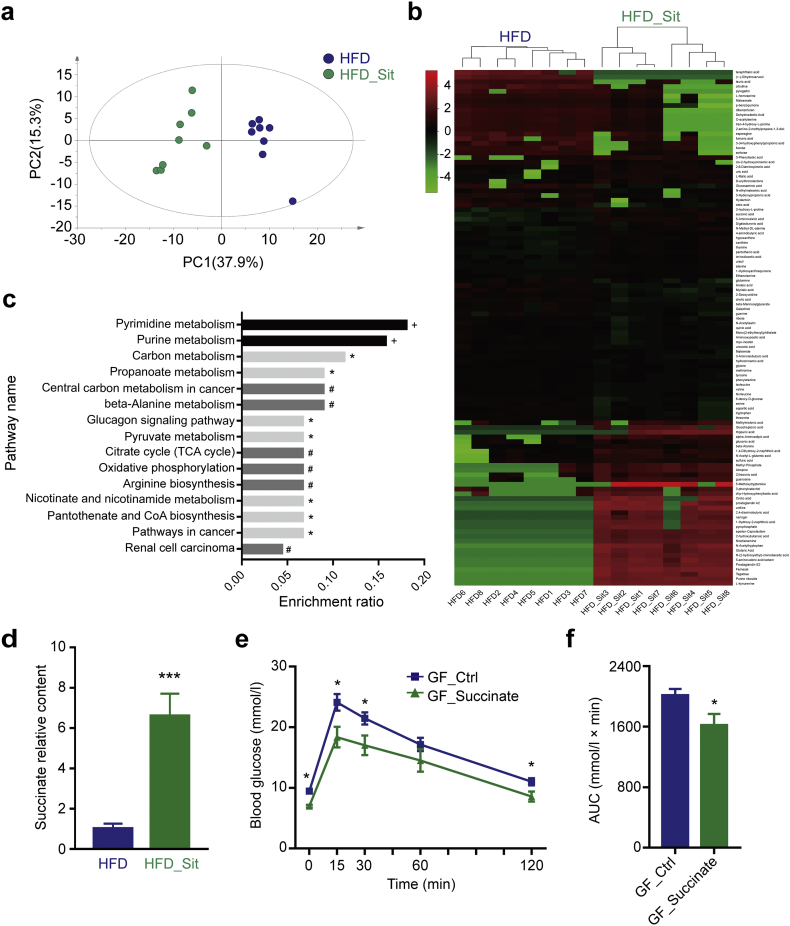
Although it is now commonly accepted that the gut microbiota is closely associated with glucose metabolism in the host, the molecular mechanisms underlying the microbiome regulation of glucose metabolism are still not clear. Some studies have suggested that SCFAs, bile acids and some organic acids may be the potential mechanism [29,30,46]; therefore, we further performed a corresponding metabolomics analysis of the fecal samples. As shown in Fig. 5a-b, PCA and heatmap analysis revealed distinct patterns of metabolites in the HFD and HFD_Sit groups, with 109 of the 249 metabolites detected to be significantly changed after Sit treatment (Supplementary Table S6). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis based on the changed metabolites showed that 15 biological pathways were significantly affected by Sit (Supplementary Table S7), and that these changes were primarily linked to carbohydrate (carbon, propanoate and pyruvate metabolism, glucagon signaling pathway, oxidative phosphorylation and citrate cycle), amino acid (beta-alanine metabolism and arginine biosynthesis) and nucleic acid metabolism (pyrimidine and purine metabolism) (Fig. 5c). Besides, Sit treatment resulted in a trend towards an increase in the content of SCFAs, (Supplementary Fig. S9). Importantly, in the analysis of other organic acids, we observed a significant increase in the content of succinate (increased by almost 6-fold, Fig. 5d), which has been reported to be a key substrate of intestinal gluconeogenesis (IGN) in the improvement of glucose metabolism [30]. To confirm the effect of succinate on glucose metabolism, GF mice were administered succinate while fed a HFD. Mice supplemented with succinate showed significant improvement of glucose tolerance compared with GF mice fed without succinate (Fig. 5e, f). These results suggested that DPP-4i promoted a functional shift in the gut microbiome, which might also contribute to its hypoglycemic effects.
Fig. 5.
DPP-4i promotes a functional shift in the gut microbiome. (a) Cluster analysis of the HFD and HFD_Sit groups based on the fecal metabolites using PCA analysis. The first two principal components (PC1 and PC2) from the PCA are plotted for each sample. The percentage variation in the plotted principal components is marked on the axes. Each spot represents one sample, and each group of mice is indicated by a different color. (b) Heatmap analysis of the fecal metabolite patterns in the HFD and HFD_Sit groups. The heatmap shows the 109 significantly changed metabolites between the two groups. Each column in the heatmap represents one sample, and each row represents one metabolite. The color bar showing green to red indicates the relative content of metabolites. (c) KEGG pathway enrichment analysis based on the changed metabolites. * p < .05, # p < .01, + p < .001, Fisher's exact test with BH correction. (d) The relative content of succinate in the fecal samples of HFD and HFD_Sit mice. (e) GF mice were fed a HFD and administered succinate in drinking water (2.5%) for 6 weeks. Blood glucose concentrations were measured during a GTT after succinate treatment. (f) The AUC for the GTT curves. All mice were fasted for 6 h before detecting blood glucose. a-d, n = 8; e-f, n = 5. Data in d-f are presented as the means ± SEM. * p < .05, *** p < .001, t-test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
DPP-4i is currently recommended by the American Association of Clinical Endocrinologists as a first-line hypoglycemic treatment in T2D [47], and has been proposed to lower blood glucose primarily through inhibiting the degradation of GLP-1 [48]. The DPP-4 enzyme has a wide tissue distribution [49], and exerts pleiotropic physiologic functions [[50], [51], [52]], which might determine the multiplicity of the effects of DPP-4i. Previous studies by our teams and others have revealed that DPP-4i reduces atherosclerosis and inflammation [53], and accelerates wound healing [54], but it may also promote various tumor metastases [55]. Here we report the effects of DPP-4i on modulating the gut microbiota, which is a new hypoglycemic mechanism and an additional benefit of this drug.
Our findings demonstrated that DPP-4i could substantially reverse the changes in the gut microbiota induced by HFD. Previous studies have revealed that HFD results in a shift of the gut microbiota that is correlated with increasing plasma glucose and glucose intolerance [17,56,57]. In our current study, DPP-4i Sit and Sax significantly changed the composition of the gut microbial community. More importantly, after DPP-4i treatment, 24 genera showed a reversal of changes induced by HFD, among the 35 genera accounting for the differences in the gut microbial communities. Many of the reversed genera have been reported to have potential benefits for maintaining regular glucose and lipid metabolism, such as Bacteroides and S24–7 group. Bacteroides is the most abundant genus in the human gastrointestinal tract and comprises >30% of the bacteria [58]. Its abundance can be increased by resveratrol, and plays an important role in the mechanism underlying resveratrol-induced improvements of glucose homeostasis [42]. S24–7 group was the most abundant genus in mice in our observations, and it has been reported to partially mediate the exercise-driven prevention of obesity [43]. In our analysis, the two genera were both increased by DPP-4i, suggesting a potential benefit of DPP-4i on glucose and lipid metabolism. DPP-4i also rescued the abundance of Blautia and Anaerotruncus in HFD mice, which was similar to the effects of metformin on the bacteria in a previous study [17]. Additionally, our previous study suggested that the structural similarity of Sit and Sax is only 26.6% [55], but their impact on the microbial community here was similar. Therefore, the effects of DPP-4i on the gut microbiota may not be restricted to one of the family, but instead extend to the entire class.
Overall, our results suggested that the modulation of gut microbiota community by DPP-4i is a new hypoglycemic mechanism and an additional benefit of this drug. Fecal samples from DPP-4i- treated T2D patients transferred to HFD-fed GF mice improved the glucose intolerance of the recipients, suggesting that the altered microbiome contributes to hypoglycemic effects of DPP-4i even in the absence of additional treatments. Although the α-glucosidase inhibitor also changed the composition of the bacterial flora, the microbiome does not seem to be linked to its hypoglycemic effect. Furthermore, the modulation of the gut microbiome by DPP-4i might provide benefits in addition to its hypoglycemic effect. Recently, additional effects of DPP-4i have been emerging. DPP-4i exerts anti-atherosclerotic effects and reduces inflammation via inhibition of monocyte activation/chemotaxis [53]. Our previous study disclosed that it can also improve wound healing via promoting epithelial-mesenchymal transition [54]. On the other hand, the negative effect of DPP-4i on promoting metastasis of multiple cancers by activating NRF2 signaling has also been revealed [55]. Consequently, rational clinical application of DPP-4i (maximizing benefits while minimizing negative effects) depends on an in-depth and comprehensive understanding of its effects and mechanisms. Here we reported its modulation of the gut microbiota, which may not only be a new mechanism underlying its hypoglycemic effects, but also indicate additional benefits, such as the regulation of immunity, inflammation, or gut-brain neural circuit through alterations of the microbiome. Therefore, considerable research should attract interest to explore the potential effects of DPP-4i in these fields.
The alteration of the gut microbiota induced by DPP-4i is dominated by an increase in the abundance of Bacteroidetes, which might indicate a potential strategy for regulating glucose metabolism. Our results showed that Bacteroidetes was reduced while Firmicutes was increased in HFD mice, while DPP-4i rescued these changes primarily by increasing the abundance of Bacteroidetes. These effects were also observed in T2D patients. Bacteroidetes and Firmicutes share roles in modulating the response of the host to dynamic changes in the diet [59], and they are enriched with genes encoding enzymes that govern carbohydrate and lipid metabolism, such as α-glucosidases and α-amylases [36]. Therefore, the regulatory effect of DPP-4i on Bacteroidetes and Firmicutes might be important for its hypoglycemic effects, which indicates a potential strategy for improving glucose metabolism in the future. This concept can be further strengthened by some current treatments for diabetes and obesity, such as prebiotics [13,30], probiotics [15], resveratrol [42], and RYGB surgery [16], in which Bacteroidetes and Firmicutes also have prominent roles in improving glucose metabolism. However, the abundance of Firmicutes and Bacteroidetes showed no obvious change after acarbose treatment, which might be the potential reason for the difference in efficacy between DPP-4i and acarbose (Supplementary Fig. S3).
Our results showed that DPP-4i also improved the functional shift in the gut microbiome. Metabolomics analysis demonstrated that DPP-4i changed the pattern of metabolites, and KEGG pathway enrichment analysis suggested that the changes were mainly linked to carbohydrate, amino acid and nucleic acid metabolism. Microbiota metabolites, such as SCFAs, have been identified to play important roles in the regulation of metabolic homeostasis. Previous studies have found that propionate and butyrate, generated by the fermentation of soluble fiber by gut bacteria, improve insulin sensitivity and glucose tolerance by promoting IGN [30,60,61]. Our data showed a trend towards an increase in SCFAs and other organic acids, especially succinate (increased by almost 6-fold), after DPP-4i treatment. As a key intermediate in microbial propionate synthesis [62], succinate has been indicated to be a substrate for IGN to improve glucose tolerance and insulin sensitivity [30]. Metformin- and dietary fibers-mediated improvements in glucose and lipid metabolism are closely associated with an increase in succinate [18,30,63]. Our data confirmed that succinate supplementation improved the glucose intolerance induced by HFD in GF mice. Therefore, the upregulation of succinate by DPP-4i observed in our study is important for the antidiabetic effect of DPP-4i. Interestingly, previous studies have reported that succinate can be produced by Bacteroides [64,65], which in turn confirmed our observation of the increase in Bacteroides induced by DPP-4i. Additionally, our KEGG analysis suggested that DPP-4i also influenced amino acid metabolism, which has been reported to be associated with GLP-1 resistance [66], glucose tolerance and obesity [67,68]). In addition, the change in bile acids induced by antidiabetic medication has also been reported to be correlated with clinical outcomes, and could improve metabolic health [69]. Therefore, additional research is needed to explore these possible mechanisms.
In summary, our study has demonstrated that DPP-4i modulates the composition of the gut microbiota, which is a new hypoglycemic mechanism and an additional benefit of this drug. In addition, it reduced the ratio of Firmicutes to Bacteroidetes and improved the functional shift in the microbiome, indicating a potential strategy for regulating glucose metabolism in the future.
Author contributions
XY.L., LY.S. and BH.Z.: acquisition of data, analysis and interpretation of data; XY.L., LY.S.: drafting of the manuscript; YY.Q., BY.L., H.Q. and Y.Z.: acquisition and analysis of data; M.L., HD.Z., Y.W., YX.D., J.X., RF.S., Q.T., LQ.C. and X.L.: acquisition of data; SD.G., GY.Y., ZM.Z. and XY.P.: critical revision of the manuscript for important intellectual content; HT.Z. and H.W.: study concept and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, acquisition of funding, and study supervision.
Funding sources
This work was supported by grants from the National Natural Science Foundation of China (No.81700757, No.81471039, No.81700714 and No.81770434), the National Key R&D Program of China (No.2017YFC1309602, No.2016YFC1101100, No.2017YFD0500503 and No.2017YFD0501001), and the Natural Science Foundation Project of Chongqing (No. cstc2014jcyjjq10006, No. cstc2016jcyjA0093 and No. cstc2016jcyjA0518).
Declaration of interests
The authors declare no conflicts interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.057.
Contributor Information
Hong Wei, Email: weihong63528@163.com.
Hongting Zheng, Email: fnf7703@hotmail.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill S.R., Pop M., Deboy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S. Metagenomic analysis of the human distal gut microbiome. Science (New York, NY) 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce-Keller A.J., Salbaum J.M., Berthoud H.R. Harnessing gut microbes for mental health: getting from here to there. Biol Psychiatry. 2018;83(3):214–223. doi: 10.1016/j.biopsych.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher J.U., Abramson S.B. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7(10):569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu H., Khosravi A., Kusumawardhani I.P., Kwon A.H., Vasconcelos A.C., Cunha L.D. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science (New York, NY) 2016;352(6289):1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21(6):786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 7.Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro H., Suez J., Elinav E. Personalized microbiome-based approaches to metabolic syndrome management and prevention. J Diabetes. 2017;9(3):226–236. doi: 10.1111/1753-0407.12501. [DOI] [PubMed] [Google Scholar]
- 9.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson F.H., Tremaroli V., Nookaew I., Bergstrom G., Behre C.J., Fagerberg B. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 11.Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everard A., Lazarevic V., Derrien M., Girard M., Muccioli G.G., Neyrinck A.M. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cani P.D., Neyrinck A.M., Fava F., Knauf C., Burcelin R.G., Tuohy K.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 15.Everard A., Matamoros S., Geurts L., Delzenne N.M., Cani P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio. 2014;5(3) doi: 10.1128/mBio.01011-14. [e01011-14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furet J.P., Kong L.C., Tap J., Poitou C., Basdevant A., Bouillot J.L. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 18.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Manneras-Holm L. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 19.Bauer P.V., Duca F.A., Waise T.M.Z., Rasmussen B.A., Abraham M.A., Dranse H.J. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab. 2018;27(1):101–117. doi: 10.1016/j.cmet.2017.09.019. [e5] [DOI] [PubMed] [Google Scholar]
- 20.Zhao L., Chen Y., Xia F., Abudukerimu B., Zhang W., Guo Y. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front Endocrinol (Lausanne) 2018;9:233. doi: 10.3389/fendo.2018.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreira G.V., Azevedo F.F., Ribeiro L.M., Santos A., Guadagnini D., Gama P. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143–154. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Yan X., Feng B., Li P., Tang Z., Wang L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J Diabetes Res. 2016;2016 doi: 10.1155/2016/2093171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares M., Neyrinck A.M., Potgens S.A., Beaumont M., Salazar N., Cani P.D. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018;61(8):1838–1848. doi: 10.1007/s00125-018-4647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su B., Liu H., Li J., Sunli Y., Liu B., Liu D. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7(5):729–739. doi: 10.1111/1753-0407.12232. [DOI] [PubMed] [Google Scholar]
- 25.Hirsh A.J., Yao S.Y., Young J.D., Cheeseman C.I. Inhibition of glucose absorption in the rat jejunum: a novel action of alpha-D-glucosidase inhibitors. Gastroenterology. 1997;113(1):205–211. doi: 10.1016/s0016-5085(97)70096-9. [DOI] [PubMed] [Google Scholar]
- 26.Holst J.J., Deacon C.F. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47(11):1663–1670. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- 27.Mentzel S., Dijkman H.B., Van Son J.P., Koene R.A., Assmann K.J. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem. 1996;44(5):445–461. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- 28.Hwang I., Park Y.J., Kim Y.R., Kim Y.N., Ka S., Lee H.Y. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015;29(6):2397–2411. doi: 10.1096/fj.14-265983. [DOI] [PubMed] [Google Scholar]
- 29.Perry R.J., Peng L., Barry N.A., Cline G.W., Zhang D., Cardone R.L. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Backhed F., Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016;24(1):151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Carrascosa J.M., Molero J.C., Fermin Y., Martinez C., Andres A., Satrustegui J. Effects of chronic treatment with acarbose on glucose and lipid metabolism in obese diabetic Wistar rats. Diabetes Obes Metab. 2001;3(4):240–248. doi: 10.1046/j.1463-1326.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.J., Nian C., Doudet D.J., McIntosh C.H. Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes. 2008;57(5):1331–1339. doi: 10.2337/db07-1639. [DOI] [PubMed] [Google Scholar]
- 34.Birnbaum Y., Bajaj M., Qian J., Ye Y. Dipeptidyl peptidase-4 inhibition by Saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res Care. 2016;4(1) doi: 10.1136/bmjdrc-2016-000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Zhou J., Liu Q.Z., Wang L.L., Shang J. Simvastatin and Bezafibrate ameliorate emotional disorder induced by high fat diet in C57BL/6 mice. Sci Rep. 2017;7(1):2335. doi: 10.1038/s41598-017-02576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L., Li P., Tang Z., Yan X., Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. 2016;6 doi: 10.1038/srep33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim K.A., Gu W., Lee I.A., Joh E.H., Kim D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao S., Fei N., Pang X., Shen J., Wang L., Zhang B. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol Ecol. 2014;87(2):357–367. doi: 10.1111/1574-6941.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng H., Ishaq S.L., Zhao F.Q., Wright A.G. Colonic inflammation accompanies an increase of beta-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J Nutr Biochem. 2016;35:30–36. doi: 10.1016/j.jnutbio.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Sung M.M., Kim T.T., Denou E., Soltys C.M., Hamza S.M., Byrne N.J. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes. 2017;66(2):418–425. doi: 10.2337/db16-0680. [DOI] [PubMed] [Google Scholar]
- 43.Evans C.C., LePard K.J., Kwak J.W., Stancukas M.C., Laskowski S., Dougherty J. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anhe F.F., Roy D., Pilon G., Dudonne S., Matamoros S., Varin T.V. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 45.Zheng X., Huang F., Zhao A., Lei S., Zhang Y., Xie G. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017;15(1):120. doi: 10.1186/s12915-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 47.Handelsman Y., Bloomgarden Z.T., Grunberger G., Umpierrez G., Zimmerman R.S., Bailey T.S. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(Suppl. 1):1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 49.Bae E.J. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch Pharm Res. 2016;39(8):1114–1128. doi: 10.1007/s12272-016-0813-x. [DOI] [PubMed] [Google Scholar]
- 50.Wesley U.V., Hatcher J.F., Ayvaci E.R., Klemp A., Dempsey R.J. Regulation of dipeptidyl peptidase IV in the post-stroke rat brain and in vitro ischemia: implications for chemokine-mediated neural progenitor cell migration and angiogenesis. Mol Neurobiol. 2017;54(7):4973–4985. doi: 10.1007/s12035-016-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maes M., Bonaccorso S., Marino V., Puzella A., Pasquini M., Biondi M. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6(4):475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 52.dos Santos L., Salles T.A., Arruda-Junior D.F., Campos L.C., Pereira A.C., Barreto A.L. Circulating dipeptidyl peptidase IV activity correlates with cardiac dysfunction in human and experimental heart failure. Circ Heart Fail. 2013;6(5):1029–1038. doi: 10.1161/CIRCHEARTFAILURE.112.000057. [DOI] [PubMed] [Google Scholar]
- 53.Shah Z., Kampfrath T., Deiuliis J.A., Zhong J., Pineda C., Ying Z. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124(21):2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long M., Cai L., Li W., Zhang L., Guo S., Zhang R. DPP-4 inhibitors improve diabetic wound healing via direct and indirect promotion of epithelial-mesenchymal transition and reduction of scarring. Diabetes. 2018;67(3):518–531. doi: 10.2337/db17-0934. [DOI] [PubMed] [Google Scholar]
- 55.Wang H., Liu X., Long M., Huang Y., Zhang L., Zhang R. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci Transl Med. 2016;8(334):334ra51. doi: 10.1126/scitranslmed.aad6095. [DOI] [PubMed] [Google Scholar]
- 56.Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hildebrandt M.A., Hoffmann C., Sherrill-Mix S.A., Keilbaugh S.A., Hamady M., Chen Y.Y. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5) doi: 10.1053/j.gastro.2009.08.042. [1716–24.e1-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 59.Muegge B.D., Kuczynski J., Knights D., Clemente J.C., Gonzalez A., Fontana L. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science (New York, NY) 2011;332(6032):970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Soty M., Gautier-Stein A., Rajas F., Mithieux G. Gut-brain glucose signaling in energy homeostasis. Cell Metab. 2017;25(6):1231–1242. doi: 10.1016/j.cmet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 62.Reichardt N., Duncan S.H., Young P., Belenguer A., McWilliam Leitch C., Scott K.P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8(6):1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Everard A., Lazarevic V., Gaia N., Johansson M., Stahlman M., Backhed F. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8(10):2116–2130. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischbach M.A., Sonnenburg J.L. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011;10(4):336–347. doi: 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheifinger C.C., Wolin M.J. Propionate formation from cellulose and soluble sugars by combined cultures of Bacteroides succinogenes and Selenomonas ruminantium. Appl Microbiol. 1973;26(5):789–795. doi: 10.1128/am.26.5.789-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grasset E., Puel A., Charpentier J., Collet X., Christensen J.E., Terce F. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab. 2017;25(5):1075–1090. doi: 10.1016/j.cmet.2017.04.013. [e5] [DOI] [PubMed] [Google Scholar]
- 67.Org E., Blum Y., Kasela S., Mehrabian M., Kuusisto J., Kangas A.J. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol. 2017;18(1):70. doi: 10.1186/s13059-017-1194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng S., Rhee E.P., Larson M.G., Lewis G.D., McCabe E.L., Shen D. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu Y., Wang X., Li J., Zhang Y., Zhong H., Liu R. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1785. doi: 10.1038/s41467-017-01682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material