Abstract
Hepatic encephalopathy (HE) is a common and serious neuropsychiatric complication of cirrhosis, acute liver failure, and porto-systemic shunting. HE largely contributes to the morbidity of patients with liver disease, severely affecting the quality of life of both patients and their relatives and being associated with poor prognosis. Its presentation is largely variable, manifesting with a broad spectrum of cognitive abnormalities ranging from subtle cognitive impairment to coma. The pathogenesis of HE is complex and has historically been linked with hyperammonemia. However, in the last years, it has become evident that the interplay of multiple actors, such as intestinal dysbiosis, gut hyperpermeability, and neuroinflammation, is of crucial importance in its genesis. Therefore, HE can be considered a result of a dysregulated gut-liver-brain axis function, where cognitive impairment can be reversed or prevented by the beneficial effects induced by “gut-centric” therapies, such as non-absorbable disaccharides, non-absorbable antibiotics, probiotics, prebiotics, and fecal microbiota transplantation. In this context dietary modifications, by modulating the intestinal milieu, can also provide significant benefit to cirrhotic patients with HE. This review will provide a comprehensive insight into the mechanisms responsible for gut-liver-brain axis dysregulation leading to HE in cirrhosis. Furthermore, it will explore the currently available therapies and the most promising future treatments for the management of patients with HE, with a special focus on the dietary approach.
Keywords: Cirrhosis, Hepatic encephalopathy, Diet therapy, Gut microbiota, Leaky gut, Hyperammonemia, Prebiotics, Probiotics, Gluten-casein free diet, Gut microbiota transplantation
Core tip: Hepatic encephalopathy (HE) is a serious complication of cirrhosis resulting from a multifactorial impairment of gut-liver-brain axis functioning. Multiple interrelated factors (e.g., intestinal hyperpermeability, dysbiosis, hyperammonemia, inflammation) cooperate in its development. “Gut-centric” therapies, including non-absorbable disaccharides, antibiotics, prebiotics, probiotics, and fecal microbiota transplantation have been successfully employed to manage HE: pertinent current knowledge will be reviewed. Furthermore, the utility of dietary modifications in this context is increasingly recognized, thus opening a new promising research path. This review sheds light on dietary therapeutic strategies for HE, exploring how they can target the mechanisms underlying gut-liver-brain axis dysregulation.
INTRODUCTION
Hepatic encephalopathy (HE) is one of the most debilitating complications of liver cirrhosis and represents a relevant cause of hospitalization[1]; it is associated with both direct and indirect costs to health services. HE is a predictor of poor prognosis and severely affects patients’ quality of life, often entailing a heavy burden for relatives and caregivers[2]. HE consists of a brain dysfunction caused by liver insufficiency and porto-systemic shunting, and it manifests as a wide spectrum of neurological or psychiatric abnormalities, ranging from subclinical alterations to coma[3].
Based on the variable severity of its manifestations, HE has been arbitrarily classified in five stages, from minimal HE (MHE) to grade IV according to the West-Haven criteria[3]. These stages can be further divided into two categories: overt HE (OHE), including grades II-IV, in which diagnosis can be established through a physical examination detecting evident neurologic and neuropsychiatric abnormalities, and covert HE (CHE), including MHE (no clinical evidence of mental dysfunction but presence of abnormalities in psychometric tests) and grade I according to West-Haven criteria (i.e., a trivial lack of awareness, a discreet psychomotor retardation, or a subtle lack of attention)[4,5]. As per International Society for HE and Nitrogen Metabolism consensus, the presence of disorientation in time or asterixis identifies the onset of OHE[4,6].
Although the variety of clinical presentations and the difficulty in detecting MHE make it hard to quantify the exact prevalence of HE, it is estimated that approximately 30%-40% of patients with cirrhosis will develop OHE during their disease course[7,8], whereas MHE or CHE occur in 20%-80% of patients[9]. Subjects with a previous episode of OHE have a 40% cumulative risk of recurrence at 1 year, and subjects with recurrent OHE have a 40% cumulative risk of another episode within 6 months[1,10].
Although the pathogenesis of this condition has not been fully elucidated yet, progress in research has led to the identification of several potential determinants of HE, among which intestinal dysbiosis, gut permeability alterations, inflammation, and oxidative stress seem to play a key role[11]. In particular, HE can be regarded as a model for impaired gut-liver-brain axis functioning: specific microbiota changes in the gut of cirrhotic patients, along with altered intestinal permeability, have been associated with endotoxemia and bacterial translocation, leading to increased inflammatory response both at a systemic level and in the central nervous system (CNS), which finally induces impaired cognition and favors the onset of HE. Although the mechanisms underlying this gut-brain interplay are far from being fully clarified, the importance of the gut in HE pathogenesis is corroborated by the beneficial effects that gut-centric therapies such as lactulose and lactitol, non-absorbable antibiotics such as rifaximin and neomycin, probiotics, and prebiotics exert on patients’ cognitive function[12].
In this context, available data suggest that dietary modifications too might exert relevant conditioning on several factors involved in the gut-liver-brain axis, including gut microbiota, intestinal permeability, and inflammation.
This review will give insight into the mechanisms responsible for gut-liver-brain axis dysregulation that leads to HE development in the context of cirrhosis. Furthermore, we will explore how the different therapeutic approaches investigated so far are supposed to act in this complex network. A special focus will be given to dietary interventions.
PATHOGENESIS
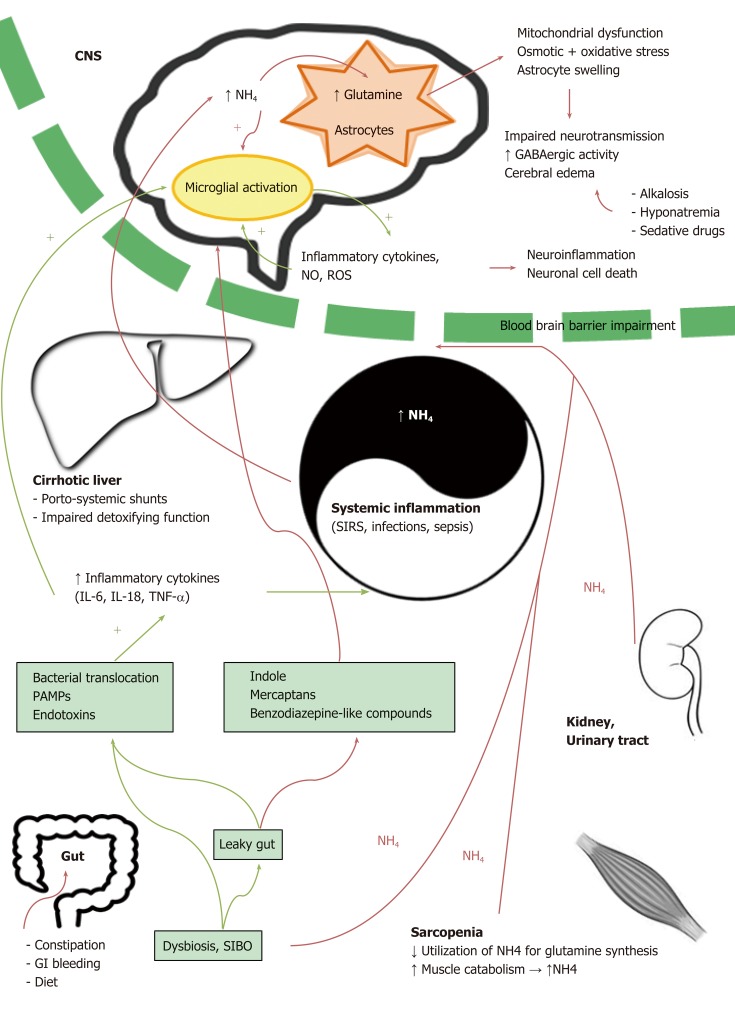
The pathogenesis of HE is a complex entity in which multiple factors cooperate in determining the functional impairment of neuronal cells[13], as illustrated in Figure 1.
Figure 1.
Multifactorial pathogenesis of hepatic encephalopathy. The figure summarizes the pathogenetic mechanisms at all levels of the gut-liver-brain axis underlying the development of hepatic encephalopathy. In this context, the interplay between systemic inflammation and hyperammonemia plays a central role (see text for details). CNS: Central nervous system; GABA: Gamma-aminobutyric acid; GI: Gastrointestinal; IL: Interleukin; NH4: Ammonia; NO: Nitric oxide; PAMPs: Pathogen associated molecular patterns; ROS: Reactive oxygen species; SIBO: Small intestine bacterial overgrowth; SIRS: Systemic inflammatory response syndrome; TNF-α: Tumor necrosis factor-alpha.
In patients with liver cirrhosis, it is believed that high levels of gut-derived toxins and endogenous neurotoxic substances escape from liver catabolism, due to the impaired detoxifying function of the cirrhotic liver and to the presence of porto-systemic shunts, and that these toxins reach the brain through the blood-brain barrier (BBB). In this context, a number of different factors, including gut dysbiosis and small intestine bacterial overgrowth, leaky intestinal barrier, cirrhosis-related systemic inflammation and neuroinflammation, oxidative stress, nitrogen metabolism, changes in neurotransmission, gamma-amino butyric acid (GABA)ergic or benzodiazepine pathway abnormalities, as well as BBB disturbances, appear to contribute to the development of HE[14-16].
Ammonia and other neurotoxic compounds
Increased blood ammonia is a cornerstone in HE development[17-19]. Ammonia, a by-product of nitrogen metabolism, is derived from gut and kidneys[20]. In the gut, both the small intestine and colon are sources of great amounts of ammonia as a product of the enzyme glutaminase and a large number of urease-producing bacteria.
Ammonia-rich blood normally reaches the liver through the portal vein, where it is detoxified through the urea cycle[21,22]. In patients with portosystemic shunts or liver failure, gut-derived blood bypasses the liver, and the liver itself has impaired capacity for detoxification. As a consequence, nitrogenous waste products accumulate in the systemic circulation. Excess ammonia crosses the BBB and is subsequently absorbed and used by astrocytes to synthesize glutamine; intracellular accumulation of excess glutamine causes osmotic and oxidative stress, mitochondrial dysfunction, and, finally, astrocyte swelling. This can lead to cerebral edema (with the extreme consequences of increased intracranial pressure and brain herniation often seen in acute liver failure) as well as to increased GABAergic activity[21,23].
Apart from the gut, also kidneys, urinary tract, and muscles are involved in nitrogen metabolism and contribute in determining ammonia circulating levels. In this setting, muscle tissue is of particular interest because: 1- sarcopenia is a recognized risk factor for HE, due to the reduced utilization of ammonia for glutamine synthesis in the context of muscular tissue deficiency[24-27]; 2- protein catabolism, which is enhanced in fasting conditions, can contribute to hyper-ammonemia through the release of nitrogen compounds.
Nowadays, the relevance of ammonia per se in the pathogenesis of HE has been partially questioned, in light of evidence that ammonia levels in chronic liver failure do not reliably correlate with HE severity[28-30] and the identification of the synergistic role of inflammatory mediators and a number of other potentially neurotoxic compounds, including mercaptans, benzodiazepine-like substances, and indole, a tryptophan derivative that is produced by gut microbes and transformed into oxindole in the brain, where it displays sedative properties[31-34].
Inflammation
Inflammation has been suggested to play a synergistic role in HE pathophysiology, increasing the effect of ammonia and thus partially explaining the weak correlation between ammonia circulating levels and HE severity. Inflammation is both systemic and localized to the CNS[35-37]. At a local level, proinflammatory cytokines are produced by the brain in the presence of ammonia, giving rise to neuroinfla-mmation[21,38].
Decompensated cirrhosis is characterized by a chronic systemic inflammatory state that concurs to the maintenance of characteristic clinical features such as generalized vasodilation and hyperdynamic circulation[21,39]. The genesis of systemic inflammation in cirrhosis is multifactorial: an impaired intestinal permeability caused by portal hypertension allows pathological bacterial translocation from the intestinal lumen to the splanchnic and systemic circulation. Translocated bacteria and bacterial products (pathogen-associated molecular patterns) stimulate the immune response, leading to the release of inflammatory cytokines, causing in turn oxidative stress[40,41].
Systemic inflammatory response syndrome and sepsis are recognized as key players in precipitating and exacerbating HE, possibly by rendering the brain more susceptible to concurrent hyperammonemia[23]. HE patients show high levels of inflammatory cytokines, such as interleukin (IL)-6, IL-18, and tumor necrosis factor alpha (TNF-α).
Tranah et al[38] reported that the presence and severity of HE are not associated with ammonia concentration alone but with serum levels of inflammatory cytokines such as TNF-α and IL-6. In another study, induced hyperammonemia in cirrhotic patients resulted in worse neuropsychiatric test scores only when inflammation was present[42].
It is now widely accepted that sepsis can trigger HE in cirrhotic patients by releasing proinflammatory mediators in the context of altered nitrogen meta-bolism[43,44], thus indicating that systemic inflammation is a critical determinant of the presence and severity of HE in chronic liver failure[23,45].
Moreover, patients with acute and chronic liver failure are functionally immuno-suppressed and prone to infections, which are well-known precipitants of HE. The innate immune response, comprising phagocytic cells such as monocytes and neutrophils, was impaired both in acute liver failure and cirrhosis in different preclinical studies and animal models[43,46,47]. Hyperammonemia itself appears to have a role in worsening immune function. Ammonia-fed rats and cirrhotic patients given amino acid drinks to induce hyperammonemia develop impaired neutrophil phagocytic activity with neutrophils spontaneously producing reactive oxygen species[48].
Hence, on the one hand the aberrant activation of neutrophils contributes to systemic inflammation and bystander damage to host organs, whereas on the other hand their impaired microbicidal capacity predisposes to infections with further worsening of the inflammatory milieu and induction of clinical decompensation of cirrhosis[23,47].
Systemic inflammation can also affect neuroinflammation: proinflammatory cytokines are transported across the BBB from the systemic circulation. However, there is good evidence that inflammatory mediators can also be produced by the brain itself[21].
Microglial cells, which are essentially CNS resident macrophages, can be activated by systemic inflammation and in turn release proinflammatory cytokines. Chronic hyperammonemia is sufficient to induce microglial activation[49], and this activation results in brain-derived proinflammatory cytokines[50], in particular TNF-α, IL-6, and IL-1β[51]. This inflammatory state leads to neuronal death in vitro and in vivo[52]. In this context, the extent of microglial activation was found to be predictive of the level of HE as well as of the presence of cerebral edema in acute liver failure[53].
Furthermore, BBB contains endothelial cells that can induce the release of proinflammatory mediators when stimulated by systemic inflammation: endothelial cells are provided with TNF-α and IL-1β receptors that convey signals able to induce the synthesis of secondary messengers in the brain, such as nitric oxide and prostanoids[54,55].
Leaky gut and bacterial translocation
The intestinal barrier is a functional unit composed of the intestinal epithelial cells, the immune effectors (immune cells and immunoglobulins), the mucus layer, and the intercellular junctions (tight junctions and gap junctions), which allow selective passage of substances through the paracellular pathway[56]. The paracellular transport regulated by the tight junctions is a dynamic system that can be modulated by several factors, such as neurotransmitters, cytokines, food components, and other signaling molecules such as zonulin, a protein synthesized in the intestinal and liver cells that reversibly increases intestinal permeability[57-59].
Patients with liver cirrhosis exhibit structural and functional changes in the intestinal barrier, the so-called “leaky gut”[60-62], which can lead to increased intestinal permeability to bacteria and their products[63,64]. The impaired expression of tight junction proteins, a common finding in patients with cirrhosis[65,66], is one of the main mechanisms underlying the disruption of the intestinal barrier[67].
Tight junctions are composed by different families of transmembrane proteins, among which occludins, claudins, and junctional adhesion molecules are the most important. The intracellular domains of these proteins interact with cytoplasmic proteins called “zonula occludens”, which allow the anchorage of the protein complex to the cytoskeleton[68].
The increased levels of proinflammatory cytokines, particularly TNF-α, as well as other inflammatory mediators were found to be responsible for the decreased expression of occludin and claudin-1 in the intestinal epithelium of cirrhotic patients[69-71]. This downregulation was more significant in the phase of decom-pensated cirrhosis (Child-Pugh classes B and C)[72-75].
Furthermore, several other factors cooperate in affecting the integrity of the intestinal barrier, such as portal hypertension (by slowing down mucosal blood flow with consequent vascular congestion), gut dysbiosis, short-chain fatty acids (SCFAs), oxidative stress, endotoxemia, and alcohol consumption[76,77], as illustrated in Figure 2.
Figure 2.
Leaky gut in liver cirrhosis. Multiple factors are involved in the increase of intestinal permeability found in cirrhotic patients. SCFA: Short-chain fatty acids.
A recent interesting study conducted by Muñoz et al[78] in rat models of cirrhosis demonstrated that the presence of ascites (identifying a phase of decompensated disease) correlates with significant damage of the tight and adherens junctions, increased intestinal permeability, and enhanced bacterial translocation, which can be normalized by antibiotics administration. This reinforced the hypothesis that coexistent dysbiosis and immune dysregulation play a pivotal role in disrupting the intestinal barrier. Hence, the homeostasis of the intestinal barrier is likely to be modulated by a dynamic symbiotic relationship between the gut microbiome and the immune system[78]. Due to increased gut permeability, bacteria can pass the intestinal barrier and migrate to mesenteric lymph nodes and other organs, a process known as bacterial translocation[79]. This phenomenon is responsible for increased levels of circulating bacterial products and endotoxins, which directly correlate with the severity of liver disease and lead to the development of several complications, especially infections and HE[80,81] .
GUT MICROBIOTA
The human gut contains 1014 bacteria, more than ten times the number of somatic cells in the human body[82]. Microorganisms start colonizing the gut after birth, and their density and types vary among different parts of the intestines, among individuals, and in the same individual during periods of illness and following dietary changes[83-85]. In the healthy individual, the host/microbiota relationship is characterized by a homeostatic symbiosis: the host provides nutrients, and the microbiota influences the correct epithelial function and nutrient absorption. Normally, anaerobes are more represented than aerobes, and the majority of species belong to the genera Bacteroidetes and Firmicutes[86].
The liver receives blood supply from the intestine through the portal circulation and is therefore exposed to gut-derived toxins, including bacteria and bacterial products, which are normally eliminated by the inflammatory response orchestrated by a large number of resident macrophages, dendritic cells, lymphocytes, and natural killer cells[87,88].
In cirrhotic patients with impaired immune response and altered intestinal barrier, it is clear how gut microflora can play a major role in triggering systemic inflammation, even in the absence of overt infection[15,89]. Furthermore, the increase of translocated bacterial products is believed to be responsible for the cognitive impairment found in HE[90].
A growing number of studies is trying to identify the existence of specific “microbiome signatures” related to cirrhosis and its complications, but the heterogeneity in study designs, investigated populations, bacterial taxonomic levels considered, origin of the microbiome samples (fecal microbiota or mucosa samples), the different methodologies used, along with the lack of standardization, make it difficult to obtain clear-cut results. Yet, some common findings in the gut microbiota of patients with cirrhosis can be highlighted, consisting in a higher proportion of Enterobacteriaceae, Alcaligenaceae, Streptococcaceae, Veillonellaceae, and Fusobacteriaceae, along with a reduction of Bacteroidetes, Ruminococcaceae, and Lachnospiraceae in comparison with healthy controls[91-93].
Of note, Ruminococcaceae and Lachnospiraceae are butyrate-producing bacteria[77]. Butyrate is a SCFA used as a source of energy by enterocytes and able to influence the intestinal barrier function through the stimulation of tight junctions and mucus production. SCFAs play a role in increasing anti-bacterial peptides and reducing colonic inflammation; therefore, their reduction may have a detrimental role in the whole setting of systemic inflammation[94,95].
As a result of these findings, further studies were designed to search for associations between gut flora alterations and development of HE or other complications of cirrhosis and to evaluate how gut-centric therapies may help treat them. Hence, specific changes in the gut microbiome have been correlated with cognitive function and systemic inflammation.
In patients with HE, a higher proportion of Veillonellaceae was linked to increased circulating inflammatory cytokines (IL-6, TNF-α, IL-2, and IL-13) and poor cognition when compared to cirrhotic patients without HE[95].
Alcaligenaceae abundance was associated with poor cognitive performance[12]. These organisms are Proteobacteria responsible for opportunistic infections that degrade urea to produce ammonia, thus explaining their association with loss of cognitive functions.
In another study by Bajaj et al[96], microbiome testing was performed on stool and sigmoid mucosa tissue of cirrhotic patients with concurrent HE, cirrhotic patients with normal cognitive function, and healthy controls. Blautia, Fecalibacterium, Roseburia, and Dorea were associated with good cognition and decreased in-flammation in both HE/non-HE, whereas genera overrepresented in HE (Enterococcus, Megasphaera, and Burkholderia) were linked to poor cognition and inflammation.
Zhang et al[97] found an overrepresentation of Streptococcaceae and Veillonellaceae in stools of cirrhotic patients with and without HE compared with normal individuals. In addition, the abundance of Streptococcus salivarius was significantly higher in cirrhotic patients with HE than in those without, and increased levels of this bacteria were correlated with ammonia accumulation in patients with HE.
A recent study by Ahluwalia et al[98] aimed to evaluate the contribution of specific gut bacteria to neuronal changes in cirrhotic patients with HE. Cirrhotic patients without HE, cirrhotic patients with HE, and healthy controls underwent stool microbiota analysis, systemic inflammatory assessment, and magnetic resonance imaging analysis. Cirrhotic patients with HE had a higher abundance of Staphylococcaceae, Enterococcaceae, Porphyromonadaceae, and Lactobacillaceae compared to controls and cirrhotics without HE. These microbial populations were linked to increased endotoxin and ammonia production as well as with worse cognitive performance. Specific microbial families such as Enterobacteriaceae positively correlated with hyperammonemia-associated astrocytic changes diagnosed through magnetic resonance imaging spectroscopy. Porphyromonadaceae only correlated with neuronal changes without linkages with ammonia levels.
Other regions of the gastrointestinal tract have been associated with dysbiosis in cirrhotic patients with HE[73]. Bajaj et al[99] studied oral and distal gut microbiota in both patients with and without HE. Salivary microbiota in cirrhotic subjects with HE showed an increased proportion of Enterobacteriaceae and lower amounts of autochthonous bacteria and Erysipelothricaceae compared to non-HE and healthy controls. The alterations of oral microbiota in cirrhotic subjects were correlated with an increased potential for endotoxins synthesis and with the existence of both a local salivary proinflammatory milieu (expressed by higher levels of IL-1β, IL-6, and immunoglobulin A secretion), and a systemic inflammatory status, thus suggesting a contribution of oral microbiota in the overall inflammation found in cirrhosis. Hence, dysbiosis, represented by a reduction in autochthonous bacterial abundance in favor of other microorganisms, is present in saliva as well as in the stools of cirrhotic patients, and this change could reflect a globally impaired mucosal-immune function. As a result, it has been postulated that the identification of specific stool and salivary microbial signatures associated with better cognitive function could potentially be used to predict the absence of MHE thus avoiding cognitive testing[100].
These findings suggest that microbiome composition is strictly correlated with cognition and inflammation in cirrhotic patients, especially in those who develop HE.
Small intestinal bacterial overgrowth
Small intestinal bacterial overgrowth (SIBO), a manifestation of gut microbial dysbiosis, represents a common finding in cirrhosis, affecting up to 59% of patients and correlating with the severity of liver disease[101-103]. Quantitative cultures of proximal jejunal aspirate with bacterial counts ≥ 105 colony forming units per milliliter are considered the diagnostic gold standard[104]. However, non-invasive tests such as glucose breath test and lactulose breath test have been developed to investigate SIBO with no need for endoscopic examination and at lower costs[105].
Gram-negative bacteria, and particularly Escherichia coli and Klebsiella pneumoniae, are found to be overrepresented in SIBO[106,107], and this condition favors bacterial translocation and endotoxemia, thus representing a risk factor for the development of clinical decompensation events, such as spontaneous bacterial peritonitis or HE[77].
The results of a recent meta-analysis[108] showed an overall prevalence of 41% for SIBO in cirrhosis, significantly higher than the prevalence among control subjects (11%). The prevalence did not differ according to etiology of liver disease, but did vary according to the diagnostic test used (lactulose vs glucose breath test vs aspirate culture) and according to Child-Pugh class, with higher prevalence in patients with worse liver function. Cirrhotics with SIBO more often had ascites, spontaneous bacterial peritonitis, and MHE compared to those without SIBO [75.6% vs 33.5% for MHE; OR 6.28 (95% confidence interval: 2.10–18.80; P = 0.001)]. Furthermore, two of the studies included in the meta-analysis evaluated orocecal transit time, demon-strating a significant prolongation in cirrhotics with SIBO compared to those without[109,110].
Therefore, HE appears to be significantly more frequent in cirrhosis when SIBO coexists; in this case, increased amounts of intestinal bacteria in the context of an altered intestinal permeability and disrupted immune function can lead to increased endotoxemia, inflammation, and hyperammonemia, finally eliciting the development of decompensation[111,112].
Future studies are needed to clarify the causes of SIBO in cirrhosis. A cooperation of several factors can be hypothesized, including impaired intestinal motility leading to stasis of luminal content, local and systemic immune dysregulation leading to reduced secretion of luminal immunoglobulins A, the presence of gastric hypochlorhydria (particularly in case of therapy with proton pump inhibitors), and alterations in bile acids metabolism[113].
At present, no clear evidence is available showing that the elimination of SIBO in cirrhosis could lead to clinical improvement of the disease course. Large, randomized controlled trials (RCTs) exploring this issue are required.
THERAPY
As previously described, the accumulation of gut-derived toxic substances in patients with impaired liver function induces a systemic inflammatory response as well as detrimental effects on the CNS, ultimately leading to the development of HE.
Several conditions can precipitate acute episodes of HE, among them: constipation, concomitant infections, gastrointestinal bleeding, administration of sedative drugs, dehydration following liquid losses or excess of diuretics, hyponatremia, and alkalosis. These so-called “precipitating factors” can act at various levels of the gut-liver-brain axis, amplifying the intestinal production of ammonia and absorption of toxins, boosting the inflammatory response or enhancing the negative effects of hyperammonemia on the CNS. Consequently, the initial management of an acute episode of HE should always include an exhaustive search for any precipitating factor and its elimination or correction[3,114]. Secondly, general treatment for HE should be initiated.
Currently, available therapies for HE primarily target the reduction of ammonia and the modulation of gut microbiota. The efficacy of these gut-centric therapeutic approaches further supports the pathogenetic relevance of the alterations of gut microflora and intestinal barrier. See Table 1 for an overview on the available therapeutic approaches for HE, their mechanisms of action, and the corresponding levels of evidence.
Table 1.
Therapeutic strategies in hepatic encephalopathy
| Therapeutic approach | Mechanism of action | Gut microbiota modulation | Level of evidence according to EASL/AASLD guidelines | |||
| Non absorbable disaccharides (lactulose and lactilol) | Decrease serum ammonia levels by: | - Accelerating intestinal transit | Yes | - Treatment: GRADE II-1, B, 1 [3] | ||
| - Reducing ammonia synthesis in the gut | - Secondary prophylaxis: GRADE II-1, A, 1 [3] | |||||
| Rifaximin | Decreases serum ammonia levels and proinflammatory cytokines release by: | - Modifying intestinal bacterial metabolism and abundance | Yes | Secondary prophylaxis: | ||
| GRADE I, A, 1 [3] | ||||||
| - Inhibiting bacterial translocation | ||||||
| Adequate protein intake (1.2-1.5 g/kg per day) | Decrease serum ammonia levels by: | - Balancing nitrogen metabolism | - | Treatment: | ||
| - GRADE I, A, 1 [3] | ||||||
| - GRADE II-2, B, 1 [136] | ||||||
| - Preventing sarcopenia | ||||||
| Dairy proteins | Decrease serum ammonia levels (process unclear) | Yes | Treatment: | |||
| - GRADE II-3, B, 1 [136] | ||||||
| Vegetable proteins | Decrease serum ammonia levels by: | - Increasing ammonia detoxification (urea cycle) | Yes | Treatment: | ||
| - Accelerating intestinal transit (high fiber content) | ||||||
| - GRADE II-3, B, 1 [136] | ||||||
| Reduce circulating mercaptans and indoles | ||||||
| Oral branched-chain amino acids (BCAA) | Unclear. Postulated: | - Increasing ammonia detoxification (glutamine synthesis) | - | Treatment: | ||
| - GRADE I, B, 2 [3] | ||||||
| Decrease serum ammonia levels by: | ||||||
| - GRADE I-1, A, 1 [136] | ||||||
| Rebalance of CNS system neurotransmitters synthesis | ||||||
| L-ornithine-L-aspartate (LOLA) | Decreases serum ammonia levels by: | - Increasing ammonia detoxification (urea cycle and glutamine synthesis) | - | Treatment: | ||
| - GRADE I, B, 2 [3] | ||||||
| Zinc | Decrease serum ammonia levels by: | - Increasing ammonia detoxification (urea cycle and glutamine synthesis) | - | No recommendations | ||
| Prebiotics | Decrease proinflammatory cytokines release and serum ammonia levels by: | - Reducing intestinal permeability | Yes | No recommendations | ||
| - Reducing luminal pH | ||||||
| - Reducing ammonia absorption | ||||||
| - Accelerating intestinal transit | ||||||
| Probiotics | Decrease proinflammatory cytokines release and serum ammonia levels by: | - Reducing intestinal permeability | Yes | No recommendations | ||
| - Reducing luminal pH | ||||||
| - Reducing ammonia absorption | ||||||
| Gluten-casein free diet | Unclear. Postulated: | - reducing absorption of gluten- and casein-derived peptides | Yes | No recommendations | ||
| Rebalance of CNS dysfunction by: | ||||||
| - decreasing proinflammatory cytokines production | ||||||
| Fecal microbiota transplantation | Rebalance of gut microbiota | Yes | No recommendations | |||
Criteria used to classify recommendations (EASL/AASLD guidelines)[3,136]: Level of evidence: I: Randomized, controlled trials, II-1 controlled trials without randomization, II-2) cohort or case-control analytical studies, II-3 multiple time series, dramatic uncontrolled experiments, III opinions of respected authorities, descriptive epidemiology. Quality of evidence: A: High: further research is very unlikely to change our confidence in the estimated effect; B: Moderate: further research is likely to have an important impact on our confidence in the estimated effect and may change the estimate; C: Low: further research is likely to have an important impact on our confidence in the estimated effect and is likely to change the estimate. Any change of estimate is uncertain. Grade of recommendation: 1: Strong: factors influencing the strength of recommendation included the quality of evidence, presumed patient-important outcomes, and costs, 2: Weak: variability in preferences and values or more uncertainty. Recommendation is made with less certainty, higher costs, or resource consumption. CNS: Central nervous system.
NON-DIETARY APPROACH
Non-absorbable disaccharides
At present, non-absorbable disaccharides, such as lactulose and lactitol, represent the first-line standard of care treatment recommended by international guidelines for use in OHE as well as in secondary prophylaxis[3]. The main mechanisms explaining their efficacy in the management of HE can be summarized as a cathartic effect, reducing intestinal transit time and content of toxic compounds, together with the ability to modulate the intestinal flora, and finally resulting in a reduction of ammonia levels[115-117].
In detail, these synthetic disaccharides pass through the intestine without being absorbed and are partially metabolized by gut bacteria, with the production of lactic and acetic acid. The consequent acidification of the gut content inhibits bacterial production of ammonia and converts ammonia into non-absorbable ammonium, trapping it in the intestinal lumen and preventing its passage in the blood[114-116]. Non-absorbable disaccharides can also inhibit glutaminase activity, thus reducing the intestinal production of ammonia[118]. Besides, lactulose and lactitol act as prebiotics, favoring the growth of beneficial saccharolytic bacteria, such as Bifidobacteria and Lactobacilli, and counteracting the growth of harmful, ammonia-producing bacteria[15,91,114,115,119].
Moreover, the promotion of microbial growth by non-absorbable disaccharides prompts bacterial uptake of ammonia as a nitrogen source for protein synthesis[120].
Furthermore, it has been demonstrated that lactulose reduces bacterial DNA translocation, with a consequent decrease in serum ammonia and levels of in-flammatory mediators[121].
Rifaximin
In patients experiencing recurrent bouts of HE despite administration of non-absorbable disaccharides, it is recommended to implement secondary prophylaxis by adding rifaximin[3].
Rifaximin is a non-absorbable antibiotic that has been shown to reduce serum ammonia and improve cognitive function in patients with HE, thus preventing recurrences and decreasing hospitalization rates[122,123]. Several studies proved rifaximin efficacy in both prevention of recurrences and treatment of acute bouts of HE, and its beneficial effects on neuropsychiatric and neuromotor abnormalities have been observed[124,125]. Rifaximin is thought to act through a number of mechanisms, including the modulation of gut microbiota, reduction of ammonia circulating levels and bacterial translocation, and reduced release of endotoxins and proinflammatory cytokines with consequent anti-inflammatory effects[126-128]. It also directly affects intestinal barrier and gut bacteria function[129-131].
The effect of rifaximin on the gut-liver-brain axis was investigated by Bajaj et al[132], who observed improved cognition and reduced endotoxemia after 8 weeks of rifaximin administration in 20 cirrhotic patients with MHE. Despite only slight modifications of microbiota composition were observed (namely a reduction in Veillonellaceae and an increase in Eubacteriaceae), serum metabolomics analysis suggested that rifaximin significantly altered bacterial functioning. In fact, there was an increase in serum saturated and unsaturated fatty acids, as well as other bacterial end-products, with a potentially beneficial impact on cognitive functions. The authors postulated that rifaximin might positively affect cognitive function mainly through a beneficial modulation of bacterial metabolism rather than by reducing absolute or relative bacterial abundances.
Rifaximin efficacy appears to be further increased when used in addition to lactulose: a double-blind prospective study by Sharma et al[10] revealed a significant decrease in OHE and length of hospital stay with combination therapy compared to lactulose alone. These data reveal how synergistic strategies may enhance treatment efficacy.
Other non-dietary therapies
Several other non-dietary treatments have been proposed for the management of HE in cirrhosis, many of which are still under investigation. They basically aim to lower serum ammonia levels (ornithine phenylacetate, glycerol phenylbutyrate, AST-120, polyethylene glycol) and to scavenge inflammatory and reactive oxygen species (albumin administration and dialysis)[133,134]. At present, the evidence of their efficacy in patients with HE is scarce or limited, and they cannot be recommended in this setting. As modulation of intestinal microbiota or dietary interventions is not the target of these therapies, their literature analysis is beyond the scope of this review.
DIETARY APPROACH
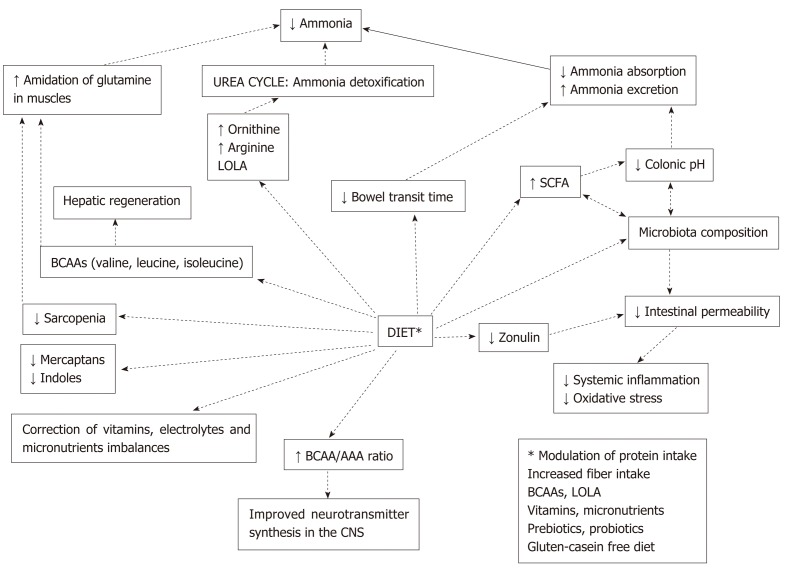
Therapeutic strategies used in the management of HE aimed to treat its main pathogenetic factors: increased ammonia levels, inflammation, and alterations of gut microbiota. Along with pharmaceutical products, diet plays a role of primary importance in addressing this condition. As illustrated in Figure 3, changes in food habits may modulate nitrogen metabolism and exert beneficial effects on gut microbiota, thus interrupting the chain of events that leads to inflammation and development of cognitive impairment[135]. Different nutritional strategies have been proposed in order to correctly manage HE, including modulation of protein intake (regarding both avoidance of protein restriction and selection of specific protein sources), increased fiber intake, and use of foods with prebiotic and probiotic effects.
Figure 3.
Potential benefits of dietary modulation in hepatic encephalopathy. AAA: Aromatic amino acids; BCAA: Branched-chain amino acids; CNS: Central nervous system; LOLA: L-ornithine–L-aspartate; SCFA: Short-chain fatty acids.
Current evidence strongly suggests that specific dietary approaches can largely contribute to the treatment and prevention of HE, and several recommendations regarding dietary changes have already been included in the main clinical guidelines.
The European Association for the Study of the Liver (EASL) Clinical Practice Guidelines on nutrition in chronic liver disease[136], the American Association for the Study of Liver Diseases (AASLD) and EASL Practice Guidelines for HE[3], and the ESPEN Guidelines on nutrition in liver disease[137] recommend daily energy intakes of 35-40 kcal/kg and that high-calorie diets should be implemented in cirrhotic patients in conditions of increased energy expenditure (e.g., in cases of acute decompensation). Carbohydrates should make up for 40%-60% of total caloric intake, and complex carbohydrates should be preferred. Lipids, which should account for 25%-50% of dietary calories, are particularly useful in HE patients as they have been demonstrated to exert beneficial effects on gut flora and on bowel transit time[138].
Proteins
Adequate nutrition is of utmost importance in all cirrhotic patients, who exhibit protein-energy malnutrition and muscle wasting in up to 60% of cases. As muscle tissue contributes to the removal of circulating ammonia by increasing glutamine synthesis, sarcopenia is not only associated with worsening of clinical conditions and increased mortality in cirrhotic patients[139] but also represents an independent risk factor for HE[25,140,141]. Adequate protein intake is therefore extremely important in cirrhotic patients with HE[142], both in terms of timing and quality of nutrient ingestion.
Firstly, it is mandatory to define a pattern of dietary intake in order to grant a correct substrate utilization; this is a very relevant issue, as catabolism of amino acids for glucose production depletes tissues of proteins and increases ammonia levels[143]. Cirrhotic patients should have frequent meals during the day, avoiding fasting for longer than 3-6 h. It has been demonstrated that a late-evening snack, containing approximately 50 g of carbohydrates, has a beneficial effect on substrate utilization and nitrogen production[144], therefore preventing HE and reducing HE severity[2,145,146,147]. It is recommended that breakfast and late-evening snack also include some proteins in order to fulfill energy and protein requirements[136].
As dysregulated nitrogen metabolism plays a key role in the development of HE, protein intake requirement in patients with HE has been widely investigated. Early evidence suggested that episodes of HE could be controlled by reducing protein intake[148,149], but these observations have been largely debunked by several subsequent studies.
In 1995, a study by Morgan et al[150] questioned for the first time the real usefulness of protein restriction in HE, demonstrating that patients with alcoholic hepatitis whose diet provided a higher protein intake experienced an improvement in mental status, suggesting that the lack of an adequate protein intake could favor HE. A study found that restriction of protein intake has no beneficial effect on the evolution of episodic HE and that it can worsen the nutritional status of these patients by exacerbating protein breakdown from muscles[151]. Furthermore, they showed that patients with HE could safely follow a normal protein diet.
Additional studies confirmed that normal protein intake is well tolerated and useful in HE to ensure sufficient substrate for energy synthesis and hepatocyte function[152,153]. Hence, avoidance of protein restriction in patients with HE is now strongly recommended. The International Society for HE and Nitrogen Meta-bolism[145], the EASL Clinical Practice Guidelines on nutrition in chronic liver disease[136], the AASLD and EASL Practice Guidelines for HE[3], and the ESPEN Guidelines on nutrition in liver disease[137] recommend for patients with HE a daily protein intake of 1.2-1.5 g/kg per day.
The amount of protein is not the only important factor to take into consideration; in cirrhosis, tolerance to dietary proteins (in terms of the development of HE after protein ingestion) seems to vary among different protein sources; dairy and vegetable proteins have been suggested to be better tolerated than animal proteins.
The evidence regarding dairy proteins is limited. An old study by Fenton et al[154] described a consistent reduction of plasma ammonia in three patients when meat was replaced by dairy protein and hypothesized that this improvement might be due to gut flora modifications. Bessman et al[155] administered intragastrically blood and milk-protein preparations to patients with liver disease, observing significantly higher elevations of circulating ammonia after administration of blood rather than after milk-protein preparations. More recently, a 14 day high-protein casein-vegetable diet was shown to improve cognitive performance and lower serum ammonia levels in 150 patients with OHE, thus confirming the irrationality of dietary protein restriction and the usefulness of a casein-vegetable based diet[152].
The beneficial effects of vegetable proteins have been widely studied among cirrhotic patients. Bianchi et al[156] tested the effect of a vegetable versus animal protein diet on nitrogen metabolism and cognitive function in cirrhotic patients with persistent HE, and the results showed that ammonia levels, as well as clinical severity of HE, significantly improved during vegetable protein diet. Uribe et al[157] also demonstrated improved mental state and encephalogram results in patients with HE undergoing vegetable protein diet compared to those on animal protein diet. Finally, Maharshi et al[158], in a recent RCT, showed that 6 months of 1-1.5 g/d of vegetable protein was effective in treating MHE, preventing OHE episodes, and improving patients’ quality of life.
Multiple reasons may explain the superiority of vegetable proteins: they contain lower quantities of methionine and cysteine compared to animal proteins. These amino acids are precursors of mercaptans and indoles, which, as mentioned before, have been implicated in HE development[159]. On the other hand, vegetable-derived proteins contain high ornithine and arginine levels, which are implicated in ammonia detoxification through the urea cycle[160]. Another advantage of vegetable proteins is their high fiber content[145], which favors intestinal transit and consequently a more efficient ammonia excretion. Moreover, fiber digestion operated by intestinal bacteria produces the SCFAs acetic, propionic, and butyric acid, therefore reducing colonic pH, which improves ammonia excretion[161,162]. This may result in favorable changes in microbiota composition, with associated enhanced anti-inflammatory and antioxidant properties[163,164]. Although additional benefits of vegetable protein diets on intestinal microbiota in HE patients have been hypothesized, to our knowledge no studies exploring the effects of this dietary approach on gut microorganisms in HE patients have been published so far.
To summarize, although vegetable proteins may be better than animal proteins for patients with HE and should therefore be encouraged, they can also cause bloating, flatulence, and diarrhea, which may consequently reduce patients’ compliance to the dietary regimen. In order to make the diet palatable and tolerable in the long run, a strategy of protein intake from different sources (dairy, vegetable, and high-quality animal proteins) seems the most reasonable one and should be recommended[136,165,166].
Branched-chain amino acids
Another way to prevent excessive protein catabolism and reduce ammonia levels in HE patients is through the administration of branched-chain amino acids (BCAAs): valine, leucine, and isoleucine.
These essential amino acids are used by skeletal muscles for the amidation of glutamine, a process that allows ammonia detoxification[167]. Due to the combination of impaired hepatic function, portosystemic shunting, and skeletal muscle loss, with hyperinsulinemia and hyperglucagonemia, BCAA levels in cirrhotic patients are usually reduced[145,168,169], whereas a concomitant rise in the levels of aromatic amino acids (AAA: phenylalanine, tyrosine, and tryptophan) has been observed[170-172].
Decreased breakdown of AAA due to impaired liver function and increased utilization of BCAAs in the muscle are thought to be the main causes for the observed decrease in the BCAA/AAA ratio, also called the Fischer-ratio[145,167,173]. The consequent increase of AAA influx in the CNS has been postulated to be responsible for im-balances in neurotransmitter synthesis, contributing to HE[168-170,174].
Oral supplementation of BCAAs in HE patients could therefore improve their clinical condition through the facilitation of ammonia detoxification, and their possible use in these patients has been widely studied[168].
There is accumulating evidence showing that long-term oral supplementation of BCAAs may confer nutritional benefits and improve survival in HE patients, probably due in part to the effect of leucine, which stimulates hepatic regeneration[175] and muscle protein synthesis[176]. Furthermore, BCAAs promote correction of plasmatic amino acid imbalance and counteract the harmful brain influx of AAA across the impaired BBB[145,177].
A meta-analysis[178] performed on nine RCTs demonstrated a significant improvement in the grade of HE with the administration of oral BCAAs compared to other nutritional supplements, but no difference was found in terms of resolution of HE. Another recent Cochrane meta-analysis on 16 RCTs indicated that oral administration of BCAAs had a beneficial impact on HE, without effect on mortality, quality of life, and nutritional status[179]. Considering prophylaxis of HE, several studies showed that oral BCAAs do not prevent development or recurrence of HE in cirrhotic patients[180-182].
Furthermore, it should be mentioned that many of these trials have methodology issues that limit their value and that oral BCAAs supplements are not used in many countries because of their cost (they are not reimbursed) and scarce palatability[137]. As a consequence, even if the use of oral BCAAs should be considered in this clinical setting, there is still a need for additional high-quality RCTs to confirm their efficacy in preventing and treating HE.
L-ornithine–L-aspartate
L-ornithine–L-aspartate (LOLA) is a mixture of two endogenous amino acids with the capacity to fix ammonia in the form of urea or glutamine. They are substrates for the urea cycle and can also activate glutamine production by activating glutamine synthetase in hepatocytes and muscle cells. Therefore, LOLA can be used as a supplement to reduce serum ammonia levels[136,137,183].
The efficacy of LOLA in patients with HE was addressed in three recent reviews and meta-analyses. The first one, a Cochrane review[184], suggested a possible beneficial effect of LOLA on mortality and HE, without increased serious adverse events in comparison with placebo or no intervention and a possible favorable impact on HE when compared with probiotics. The authors, however, considered the beneficial profile of LOLA uncertain, due to the low quality of the available studies. The second study[185] showed that LOLA was significantly more effective compared to placebo/no intervention for improvement of mental state in all types of HE and for lowering of blood ammonia[185]. The last and very recent meta-analysis highlighted the benefit of LOLA in a wide range of clinical presentations of HE, including OHE as well as MHE, where the oral formulation of LOLA was particularly effective[186]. The concomitant reduction of blood ammonia levels was reported in all RCTs that investigated this issue. Network meta-analysis showed that LOLA appears to be comparable (or superior) in efficacy to other ammonia-lowering agents, including non-absorbable disaccharides and probiotics. Furthermore, LOLA seems to be effective also for the treatment of post-transjugular intrahepatic portosystemic shunt HE and secondary HE prophylaxis. The authors concluded supporting the use of LOLA in the treatment of HE.
Vitamins and micronutrients
Generally, patients suffering from liver disease present vitamin deficiencies due to altered hepatic function, reduction of reserves, as well as inadequate dietary intake or malabsorption[187]. Deficiencies of vitamins and electrolytes can potentially cause a variety of neuropsychiatric symptoms, hence mimicking or worsening HE.
Among vitamins potentially affecting cognitive function, cirrhotic patients often present vitamin B deficiency, probably due to intestinal malabsorption and decreased liver storage. Although the consequences of vitamin B deficiency in patients with advanced liver disease are not fully understood (except vitamin B1 deficiency), it is known that this group of vitamins is linked to cognitive function[188,189], and its reduction may cause additional CNS alterations in patients with HE[190].
Patients with cirrhosis may also have reduced levels of micronutrients; among them, zinc has been implicated in the pathogenesis of HE, as glutamine synthetase and ornithine transcarbamylase, which are involved in ammonia detoxification, are both zinc-dependent. Zinc administration has been suggested to improve psychometric tests in some studies[191,192], but overall results are conflicting[193-195].
Furthermore, clinicians should always pay attention to electrolyte imbalances, as they can both trigger the development of HE and worsen pre-existing abnormalities of mental function. In particular, hyponatremia, hypomagnesemia, and hy-percalcemia, if present, should be promptly corrected in cirrhotic patients with altered mental status, bearing in mind the importance of a slow rebalancing in sodium levels, because of the risk of developing central pontine myelinolysis[2,136,196].
Currently, supplementation of vitamins and micronutrients is recommended by the EASL[136] and ESPEN guidelines[137] in patients with documented deficiencies or during the first 2 weeks of nutritional support when the deficiency is clinically suspected.
DIETARY APPROACH, INTESTINAL MICROBIOTA MODULATION, AND GUT-LIVER AXIS
Since the influence of diet on gut microbiota composition in both healthy and unhealthy populations has been abundantly demonstrated[197-199], and the connection between gut, liver, and brain plays a fundamental role in the development of HE in cirrhotic patients[21,69,82], it has been hypothesized that specific dietary approaches targeting the gut-liver-brain axis may be implemented in the therapeutic management of HE.
Prebiotics and probiotics
Prebiotics are food substrates that are selectively used by host microorganisms causing alterations in the composition and activity of gut microbiota and thus conferring a health benefit[200]. Probiotics are live microorganisms that, when ingested in adequate amounts, alter the microflora conferring a favorable effect on the health of the host[201]. Synbiotics are defined as a combination of both pre- and probiotics. They produce beneficial alterations in gut microbiota and may be, at least in theory, helpful in the management of HE thanks to their gut-centric action[145,202]. In fact, the modulation of gut microbiota operated through supplementation of pre- and probiotics decreases pathogenic bacteria and reduces luminal pH, thus lowering ammonia absorption, improving nutritional status of gut epithelium, and decreasing intestinal permeability; all these changes reduce systemic inflammation and oxidative stress and lower circulating ammonia levels[203-205].
Prebiotics
At present, lactulose, lactitol, fructo-oligosaccharides, and galacto-oligosaccharides are the most commonly used prebiotics. Malaguarnera et al[206,207] demonstrated that a combination of probiotics and fructo-oligosaccharides was effective in treating MHE, improving neuropsychiatric function when compared both to placebo and lactulose. Liu et al[198] showed that the administration of a synbiotic preparation composed by probiotics and four fermentable fibers induced reversal of MHE in 50% of patients. This study also revealed that fermentable fibers alone could be beneficial in a substantial proportion of patients. Soluble fibers have prebiotic properties, as they are usually a substrate for fermentation. According to these data, Sitkin et al[208] suggested that dietary fibers supplementation modified gut microbiota and improved psychometric tests in patients with MHE. To summarize, although treatment with prebiotics seems to be promising in cirrhotic patients with HE, their efficacy (except lactulose) has still to be established; and therefore, they cannot be recommended as part of the conventional therapy.
Probiotics
In a recent meta-analysis of 21 trials with 1420 participants, Dalal et al[209] compared the effects of probiotics vs placebo or no intervention or lactulose in MHE or OHE. The meta-analysis showed that probiotics, when compared to placebo or no intervention, probably improved recovery and may confer an advantage in terms of the development of OHE, quality of life, and plasma ammonia concentrations, with little or no difference on mortality. When compared to lactulose, probiotics did not show any statistically significant advantage in terms of recovery, development of OHE, quality of life, plasma ammonia concentration, or mortality. The authors highlighted that whether probiotics are better than lactulose for HE is uncertain due to the very low quality of the available evidence, and they claimed for new high-quality RCTs to clarify further the efficacy of probiotics on HE. Therefore, at present, the use of probiotics cannot be routinely recommended for treating patients with HE.
Lunia et al[210] evaluated the usefulness of probiotics as primary prophylaxis for HE in cirrhotic patients, showing that a 3 month course of probiotics reduced levels of arterial ammonia, improved psychometric tests, and reduced the risk of developing HE compared to placebo. Regarding the setting of secondary prophylaxis for HE, a clinical trial by Agrawal et al[211] compared the efficacy of probiotics and lactulose in this field. Probiotics were revealed to be as effective as lactulose in preventing new episodes of HE. Dhiman et al[212] further strengthened these data, demonstrating that probiotics, compared to placebo, reduced the risk of HE-related hospitalization in patients who recovered from a previous episode of HE. The results of these studies are promising, but further standardized trials performed with optimal methodological quality are needed in order to define the role of probiotics in the context of both primary and secondary prophylaxis for HE[213,214].
Probiotic yogurt supplementation
The modulation of gut flora through dietary interventions in cirrhotic patients has been studied by Bajaj et al[215], who investigated whether the supplementation of probiotic yogurt in cirrhotic patients could be useful in treating MHE and preventing OHE. Cirrhotic patients were randomized to receive 12 oz of yogurt daily vs no treatment for 60 days. The study demonstrated a higher rate of MHE reversal in patients treated with yogurt as well as a better rate of prevention of OHE de-velopment. A subsequent study by Liu et al[216] displayed that probiotic yogurt could modify intestinal microflora in patients with chronic liver disease, increasing the number of beneficial bacteria and reducing levels of Escherichia coli. This research field seems therefore promising, but further evidence is needed to confirm these preliminary results.
Gluten-casein free diet
The gut-liver-brain axis has been widely studied as a possible therapeutic target for other conditions in which gut microbiota alterations and intestinal barrier impairment are thought to have a pathogenetic role, such as celiac disease and autism spectrum disorders (ASD). In these settings, altered intestinal permeability may favor leakage of gut-derived toxic substances, which in turn may trigger systemic inflammation through cytokine production and may reach CNS to induce neurological damage[46,217-219]. This model recalls the mechanisms involved in HE, where gut-derived substances also induce an inflammatory response and can cross the BBB and cause cognitive impairment. In ASD, casein and gluten-derived peptides passing through the altered intestinal barrier have been suggested to play a pathogenetic role[220], potentially eliciting inflammatory responses both at a systemic and CNS level, where these peptides are believed to act as neuropeptides and alter neurological fun-ctions[221].
Hence, gluten-casein free diet has been postulated to confer beneficial effects on patients with ASD by reducing both systemic inflammation and circulating opioid peptides levels (β-gliadomorphine and β-caseomorphine). Even if the efficacy of this therapeutic approach remains controversial[217], there is increasing evidence that the elimination or reduction of gluten and casein from the diet may confer some advantages in patients with ASD, in terms of both gastrointestinal and cognitive benefits[222,223]. Furthermore, also in contexts other than celiac disease and ASD, gluten has been shown to impair intestinal permeability through zonulin upregulation, and a gluten-free diet has been shown to influence positively microbiota composition[224-226].
In light of these data, on the basis of the common ground of altered gut-liver-brain axis and increased intestinal permeability, a similar dietary approach could be implemented in the management of cirrhotic patients with HE. This intriguing possibility has been investigated in a pilot study performed by Balzola et al[227]. Sixteen patients awaiting liver transplantation for end-stage cirrhosis with chronic HE were enrolled and clinical, neurological, and gastroenterological evaluations were performed. A normoproteic gluten-casein free diet was undertaken, along with maintenance of previously ongoing therapies targeting HE. Clinical and neurological evaluation was performed after 1 and 3 months; cognitive function (arithmetic, memory, and orientation) and memory skills measured with Mini Mental Test and Rey Auditory Verbal Learning Test showed a statistically significant improvement in 14/16 (88%) patients both at 1 and 3 months. Executive functions and attention evaluated by Trial Making Test significantly increased at 3 months. Baseline and 3 month electroencephalograms did not correlate with the improvement of mental status. Only one hospitalization for HE was necessary among the 16 patients during the 3 month follow-up, whilst a mean hospitalization rate of 1 to 3 episodes per month was observed in a control group made of 10 cirrhotic patients with the same clinical background (chronic HE). Notably, a transient HE episode was reported in a patient who accidentally introduced gluten during the study, and a HE recurrence was experienced by one patient who decided to reintroduce gluten after the 3 month follow-up.
Although this experience was very limited, it introduced an element of novelty that, if replicated, could add a simple therapeutic tool in the management of cirrhotic patients with HE, at least for those affected by the most severe forms. Investigators should therefore address this dietary approach as a potential adjunctive therapy in patients with severe liver disease and HE in order to verify its efficacy.
Fecal microbiota transplantation
Another approach targeting gut dysbiosis in HE patients is fecal microbiota transplantation (FMT): this innovative treatment was investigated by Bajaj et al[228], who performed an RCT comparing its efficacy, in terms of cognitive improvement, adverse events, microbiota, and metabolomic changes, versus standard of care in patients with recurrent HE. A suitable stool donor was selected through cross-sectional microbiome data, and patients enrolled in the FMT arm were then administered a 90 mL enema after a 5 day broad-spectrum antibiotic course. After 150 days of follow-up, there was a statistically significant cognitive improvement in the FMT group, together with increased microbial overall diversity and expansion of beneficial taxa. No severe adverse events were registered.
A very recent study by the same authors has strengthened this approach, suggesting long-term (12-15 months) safety and sustained improvement in clinical and cognitive function parameters with prevention of HE recurrence among patients who received FMT[229].
CONCLUSION
HE is a serious complication of cirrhosis that significantly impacts on the quality of life of both patients and caregivers and heavily contributes to hospitalizations and mortality in these patients. The association among HE, malnutrition, sarcopenia, and poor prognosis is nowadays sound, and there is accumulating evidence that in this context intestinal dysbiosis and gut hyperpermeability play a pivotal role, being part of an altered interaction between the gut, the liver, and the brain. The findings discussed in this review show in their entirety and complexity the fundamental implication of the gut-liver-brain axis in the development of HE, as well as the important role that dietary modifications and modulation of microbiota may play in preventing and treating HE. If it is true that further research is surely necessary to achieve stronger scientific evidence in the very complex field of HE, it is equally true that current data suggest that the path taken is the right one.
Footnotes
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement:Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Peer-review started: March 15, 2019
First decision: April 22, 2019
Article in press: May 21, 2019
P-Reviewer: Garbuzenko DV, Kreisel W, Park YM, Ruiz-Margáin A, Zapater P S-Editor: Cui LJ L-Editor: Filipodia E-Editor: Zhang YL
Contributor Information
Daniela Campion, Division of Gastroenterology and Hepatology, Città della Salute e della Scienza di Torino Hospital, University of Turin, 10126 Turin, Italy.
Ilaria Giovo, Division of Gastroenterology and Hepatology, Città della Salute e della Scienza di Torino Hospital, University of Turin, 10126 Turin, Italy.
Paola Ponzo, Division of Gastroenterology and Hepatology, Città della Salute e della Scienza di Torino Hospital, University of Turin, 10126 Turin, Italy.
Giorgio M Saracco, Division of Gastroenterology and Hepatology, Città della Salute e della Scienza di Torino Hospital, University of Turin, 10126 Turin, Italy.
Federico Balzola, Division of Gastroenterology and Hepatology, Città della Salute e della Scienza di Torino Hospital, University of Turin, 10126 Turin, Italy.
Carlo Alessandria, Division of Gastroenterology and Hepatology, Città della Salute e della Scienza di Torino Hospital, University of Turin, 10126 Turin, Italy. carloalessandria@libero.it.
References
- 1.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:3–9. doi: 10.1111/j.1746-6342.2006.03215.x. [DOI] [PubMed] [Google Scholar]
- 2.Amodio P. Hepatic encephalopathy: Diagnosis and management. Liver Int. 2018;38:966–975. doi: 10.1111/liv.13752. [DOI] [PubMed] [Google Scholar]
- 3.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, Morgan MY International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739–747. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014–2021. doi: 10.1002/hep.23216. [DOI] [PubMed] [Google Scholar]
- 6.Weissenborn K. Hepatic Encephalopathy: Definition, Clinical Grading and Diagnostic Principles. Drugs. 2019;79:5–9. doi: 10.1007/s40265-018-1018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridola L, Nardelli S, Gioia S, Riggio O. Quality of life in patients with minimal hepatic encephalopathy. World J Gastroenterol. 2018;24:5446–5453. doi: 10.3748/wjg.v24.i48.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shawcross DL, Dunk AA, Jalan R, Kircheis G, de Knegt RJ, Laleman W, Ramage JK, Wedemeyer H, Morgan IE New Insights Steering Committee. How to diagnose and manage hepatic encephalopathy: a consensus statement on roles and responsibilities beyond the liver specialist. Eur J Gastroenterol Hepatol. 2016;28:146–152. doi: 10.1097/MEG.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridola L, Cardinale V, Riggio O. The burden of minimal hepatic encephalopathy: from diagnosis to therapeutic strategies. Ann Gastroenterol. 2018;31:151–164. doi: 10.20524/aog.2018.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885–891, 891.e1. doi: 10.1053/j.gastro.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 11.Butterworth RF. Hepatic Encephalopathy in Cirrhosis: Pathology and Pathophysiology. Drugs. 2019;79:17–21. doi: 10.1007/s40265-018-1017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397–403. doi: 10.4161/gmic.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochoa-Sanchez R, Rose CF. Pathogenesis of Hepatic Encephalopathy in Chronic Liver Disease. J Clin Exp Hepatol. 2018;8:262–271. doi: 10.1016/j.jceh.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcovich M, Zocco MA, Roccarina D, Ponziani FR, Gasbarrini A. Prevention and treatment of hepatic encephalopathy: focusing on gut microbiota. World J Gastroenterol. 2012;18:6693–6700. doi: 10.3748/wjg.v18.i46.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S29–S36. doi: 10.1016/j.jceh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liere V, Sandhu G, DeMorrow S. Recent advances in hepatic encephalopathy. F1000Res. 2017;6:1637. doi: 10.12688/f1000research.11938.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima LCD, Miranda AS, Ferreira RN, Rachid MA, Simões E Silva AC. Hepatic encephalopathy: Lessons from preclinical studies. World J Hepatol. 2019;11:173–185. doi: 10.4254/wjh.v11.i2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayakumar AR, Norenberg MD. Hyperammonemia in Hepatic Encephalopathy. J Clin Exp Hepatol. 2018;8:272–280. doi: 10.1016/j.jceh.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ninan J, Feldman L. Ammonia Levels and Hepatic Encephalopathy in Patients with Known Chronic Liver Disease. J Hosp Med. 2017;12:659–661. doi: 10.12788/jhm.2794. [DOI] [PubMed] [Google Scholar]
- 20.Dasarathy S, Mookerjee RP, Rackayova V, Rangroo Thrane V, Vairappan B, Ott P, Rose CF. Ammonia toxicity: from head to toe? Metab Brain Dis. 2017;32:529–538. doi: 10.1007/s11011-016-9938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturgeon JP, Shawcross DL. Recent insights into the pathogenesis of hepatic encephalopathy and treatments. Expert Rev Gastroenterol Hepatol. 2014;8:83–100. doi: 10.1586/17474124.2014.858598. [DOI] [PubMed] [Google Scholar]
- 22.Parekh PJ, Balart LA. Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin Liver Dis. 2015;19:529–537. doi: 10.1016/j.cld.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7–S20. doi: 10.1016/j.jceh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, Merli M, Riggio O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt Placement. Clin Gastroenterol Hepatol. 2017;15:934–936. doi: 10.1016/j.cgh.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Dasarathy S, Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care. 2018;21:30–36. doi: 10.1097/MCO.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanai T, Shiraki M, Watanabe S, Kochi T, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Res. 2017;47:1359–1367. doi: 10.1111/hepr.12873. [DOI] [PubMed] [Google Scholar]
- 27.Chang KV, Chen JD, Wu WT, Huang KC, Lin HY, Han DS. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? A systematic review and meta-analysis. J Formos Med Assoc. 2019;118:833–842. doi: 10.1016/j.jfma.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Ferenci P. Hepatic encephalopathy. Gastroenterol Rep (Oxf) 2017;5:138–147. doi: 10.1093/gastro/gox013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kundra A, Jain A, Banga A, Bajaj G, Kar P. Evaluation of plasma ammonia levels in patients with acute liver failure and chronic liver disease and its correlation with the severity of hepatic encephalopathy and clinical features of raised intracranial tension. Clin Biochem. 2005;38:696–699. doi: 10.1016/j.clinbiochem.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Flamm SL. Complications of Cirrhosis in Primary Care: Recognition and Management of Hepatic Encephalopathy. Am J Med Sci. 2018;356:296–303. doi: 10.1016/j.amjms.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Baraldi M, Avallone R, Corsi L, Venturini I, Baraldi C, Zeneroli ML. Natural endogenous ligands for benzodiazepine receptors in hepatic encephalopathy. Metab Brain Dis. 2009;24:81–93. doi: 10.1007/s11011-008-9111-8. [DOI] [PubMed] [Google Scholar]
- 32.Mannaioni G, Carpenedo R, Corradetti R, Carlà V, Venturini I, Baraldi M, Zeneroli ML, Moroni F. Tryptophan metabolism and hepatic encephalopathy. Studies on the sedative properties of oxindole. Adv Exp Med Biol. 1999;467:155–167. [PubMed] [Google Scholar]
- 33.Odeh M. Pathogenesis of hepatic encephalopathy: the tumour necrosis factor-alpha theory. Eur J Clin Invest. 2007;37:291–304. doi: 10.1111/j.1365-2362.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 34.Riggio O, Mannaioni G, Ridola L, Angeloni S, Merli M, Carlà V, Salvatori FM, Moroni F. Peripheral and splanchnic indole and oxindole levels in cirrhotic patients: a study on the pathophysiology of hepatic encephalopathy. Am J Gastroenterol. 2010;105:1374–1381. doi: 10.1038/ajg.2009.738. [DOI] [PubMed] [Google Scholar]
- 35.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera-Pastor A, Llansola M, Montoliu C, Malaguarnera M, Balzano T, Taoro-Gonzalez L, García-García R, Mangas-Losada A, Izquierdo-Altarejos P, Arenas YM, Leone P, Felipo V. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications. Acta Physiol. 2019;226:e13270. doi: 10.1111/apha.13270. [DOI] [PubMed] [Google Scholar]
- 37.Azhari H, Swain MG. Role of Peripheral Inflammation in Hepatic Encephalopathy. J Clin Exp Hepatol. 2018;8:281–285. doi: 10.1016/j.jceh.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tranah TH, Vijay GK, Ryan JM, Shawcross DL. Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis. 2013;28:1–5. doi: 10.1007/s11011-012-9370-2. [DOI] [PubMed] [Google Scholar]
- 39.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol. 1983;244:G52–G57. doi: 10.1152/ajpgi.1983.244.1.G52. [DOI] [PubMed] [Google Scholar]
- 41.Piano S, Brocca A, Angeli P. Renal Function in Cirrhosis: A Critical Review of Available Tools. Semin Liver Dis. 2018;38:230–241. doi: 10.1055/s-0038-1661372. [DOI] [PubMed] [Google Scholar]
- 42.Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247–254. doi: 10.1016/j.jhep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Coltart I, Tranah TH, Shawcross DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophys. 2013;536:189–196. doi: 10.1016/j.abb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Wright G, Jalan R. Ammonia and inflammation in the pathogenesis of hepatic encephalopathy: Pandora's box? Hepatology. 2007;46:291–294. doi: 10.1002/hep.21843. [DOI] [PubMed] [Google Scholar]
- 45.Odeh M, Sabo E, Srugo I, Oliven A. Serum levels of tumor necrosis factor-alpha correlate with severity of hepatic encephalopathy due to chronic liver failure. Liver Int. 2004;24:110–116. doi: 10.1111/j.1478-3231.2004.0894.x. [DOI] [PubMed] [Google Scholar]
- 46.Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011;54:640–649. doi: 10.1016/j.jhep.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 47.Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062–1069. doi: 10.1002/hep.23367. [DOI] [PubMed] [Google Scholar]
- 48.Shawcross DL, Wright GA, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, Pitsillides AA, Jalan R. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology. 2008;48:1202–1212. doi: 10.1002/hep.22474. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo JM, Felipo V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139:675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 50.Jiang W, Desjardins P, Butterworth RF. Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J Cereb Blood Flow Metab. 2009;29:944–952. doi: 10.1038/jcbfm.2009.18. [DOI] [PubMed] [Google Scholar]
- 51.Bezzi P, Domercq M, Vesce S, Volterra A. Neuron-astrocyte cross-talk during synaptic transmission: physiological and neuropathological implications. Prog Brain Res. 2001;132:255–265. doi: 10.1016/S0079-6123(01)32081-2. [DOI] [PubMed] [Google Scholar]
- 52.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang W, Desjardins P, Butterworth RF. Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J Neurochem. 2009;109:485–493. doi: 10.1111/j.1471-4159.2009.05981.x. [DOI] [PubMed] [Google Scholar]
- 54.Saper CB. The dance of the perivascular and endothelial cells: mechanisms of brain response to immune signaling. Neuron. 2010;65:4–6. doi: 10.1016/j.neuron.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 55.Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci. 2003;8:s1321–s1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- 56.Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukui H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm Intest Dis. 2016;1:135–145. doi: 10.1159/000447252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. 2012;10:1096–1100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 60.Nagpal R, Yadav H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann Nutr Metab. 2017;71:11–16. doi: 10.1159/000479918. [DOI] [PubMed] [Google Scholar]
- 61.Fujii T, Seki T, Maruoka M, Tanaka J, Kawashima Y, Watanabe T, Sawamura T, Inoue K. Lactulose-L-rhamnose intestinal permeability test in patients with liver cirrhosis. Hepatol Res. 2001;19:158–169. doi: 10.1016/s1386-6346(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 62.Zuckerman MJ, Menzies IS, Ho H, Gregory GG, Casner NA, Crane RS, Hernandez JA. Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci. 2004;49:621–626. doi: 10.1023/b:ddas.0000026307.56909.21. [DOI] [PubMed] [Google Scholar]
- 63.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 64.Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol. 1999;11:755–759. doi: 10.1097/00042737-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Alexopoulou A, Agiasotelli D, Vasilieva LE, Dourakis SP. Bacterial translocation markers in liver cirrhosis. Ann Gastroenterol. 2017;30:486–497. doi: 10.20524/aog.2017.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutiérrez A, Carnices F, Palazón JM, Sola-Vera J, Pérez-Mateo M. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482–1486. [PubMed] [Google Scholar]
- 67.Aguirre Valadez JM, Rivera-Espinosa L, Méndez-Guerrero O, Chávez-Pacheco JL, García Juárez I, Torre A. Intestinal permeability in a patient with liver cirrhosis. Ther Clin Risk Manag. 2016;12:1729–1748. doi: 10.2147/TCRM.S115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 69.Karthikeyan A, Mohan P, Chinnakali P, Vairappan B. Elevated systemic zonula occludens 1 is positively correlated with inflammation in cirrhosis. Clin Chim Acta. 2018;480:193–198. doi: 10.1016/j.cca.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 70.Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN, Scopa CD, Thomopoulos KC. Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439–446. doi: 10.1111/j.1365-2362.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- 71.Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, Triantos CK, Thomopoulos KC. Intestinal barrier dysfunction in cirrhosis: Current concepts in pathophysiology and clinical implications. World J Hepatol. 2015;7:2058–2068. doi: 10.4254/wjh.v7.i17.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalaitzakis E, Johansson JE, Bjarnason I, Björnsson E. Intestinal permeability in cirrhotic patients with and without ascites. Scand J Gastroenterol. 2006;41:326–330. doi: 10.1080/00365520510024278. [DOI] [PubMed] [Google Scholar]
- 73.Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M, Ghirlanda G, Gasbarrini A. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323–327. doi: 10.1038/ajg.2009.558. [DOI] [PubMed] [Google Scholar]
- 74.Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, Miranda A, Portincasa P, Carbonara V, Palasciano G, Martorelli L, Esposito P, Cartenì M, Del Vecchio Blanco C, Loguercio C. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42:200–204. doi: 10.1016/j.dld.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Benjamin J, Singla V, Arora I, Sood S, Joshi YK. Intestinal permeability and complications in liver cirrhosis: A prospective cohort study. Hepatol Res. 2013;43:200–207. doi: 10.1111/j.1872-034X.2012.01054.x. [DOI] [PubMed] [Google Scholar]
- 76.Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 2013;33:1457–1469. doi: 10.1111/liv.12271. [DOI] [PubMed] [Google Scholar]
- 77.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 78.Muñoz L, Borrero MJ, Úbeda M, Conde E, Del Campo R, Rodríguez-Serrano M, Lario M, Sánchez-Díaz AM, Pastor O, Díaz D, García-Bermejo L, Monserrat J, Álvarez-Mon M, Albillos A. Intestinal Immune Dysregulation Driven by Dysbiosis Promotes Barrier Disruption and Bacterial Translocation in Rats With Cirrhosis. Hepatology. 2018 doi: 10.1002/hep.30349. [DOI] [PubMed] [Google Scholar]
- 79.Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24–33. doi: 10.1007/s12072-017-9798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 81.Ponziani FR, Zocco MA, Cerrito L, Gasbarrini A, Pompili M. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol. 2018;12:641–656. doi: 10.1080/17474124.2018.1481747. [DOI] [PubMed] [Google Scholar]
- 82.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 83.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 84.O'Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51–58. doi: 10.1097/MOG.0b013e3282f323f3. [DOI] [PubMed] [Google Scholar]
- 85.Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 89.Mancini A, Campagna F, Amodio P, Tuohy KM. Gut : liver : brain axis: the microbial challenge in the hepatic encephalopathy. Food Funct. 2018;9:1373–1388. doi: 10.1039/c7fo01528c. [DOI] [PubMed] [Google Scholar]
- 90.Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 91.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 93.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Usami M, Miyoshi M, Yamashita H. Gut microbiota and host metabolism in liver cirrhosis. World J Gastroenterol. 2015;21:11597–11608. doi: 10.3748/wjg.v21.i41.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–G685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Z, Zhai H, Geng J, Yu R, Ren H, Fan H, Shi P. Large-scale survey of gut microbiota associated with MHE Via 16S rRNA-based pyrosequencing. Am J Gastroenterol. 2013;108:1601–1611. doi: 10.1038/ajg.2013.221. [DOI] [PubMed] [Google Scholar]
- 98.Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. doi: 10.1038/srep26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ, Sikaroodi M, Gillevet PM. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260–1271. doi: 10.1002/hep.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bajaj JS, Fagan A, White MB, Wade JB, Hylemon PB, Heuman DM, Fuchs M, John BV, Acharya C, Sikaroodi M, Gillevet PM. Specific Gut and Salivary Microbiota Patterns Are Linked With Different Cognitive Testing Strategies in Minimal Hepatic Encephalopathy. Am J Gastroenterol. 2019 doi: 10.14309/ajg.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah A, Shanahan E, Macdonald GA, Fletcher L, Ghasemi P, Morrison M, Jones M, Holtmann G. Systematic Review and Meta-Analysis: Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Liver Disease. Semin Liver Dis. 2017;37:388–400. doi: 10.1055/s-0037-1608832. [DOI] [PubMed] [Google Scholar]
- 102.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 103.Fukui H. Gut Microbiota and Host Reaction in Liver Diseases. Microorganisms. 2015;3:759–791. doi: 10.3390/microorganisms3040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adike A, DiBaise JK. Small Intestinal Bacterial Overgrowth: Nutritional Implications, Diagnosis, and Management. Gastroenterol Clin North Am. 2018;47:193–208. doi: 10.1016/j.gtc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 105.Rezaie A, Pimentel M, Rao SS. How to Test and Treat Small Intestinal Bacterial Overgrowth: an Evidence-Based Approach. Curr Gastroenterol Rep. 2016;18:8. doi: 10.1007/s11894-015-0482-9. [DOI] [PubMed] [Google Scholar]
- 106.Ponziani FR, Gerardi V, Gasbarrini A. Diagnosis and treatment of small intestinal bacterial overgrowth. Expert Rev Gastroenterol Hepatol. 2016;10:215–227. doi: 10.1586/17474124.2016.1110017. [DOI] [PubMed] [Google Scholar]
- 107.Quigley EMM. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO) Curr Gastroenterol Rep. 2019;21:3. doi: 10.1007/s11894-019-0671-z. [DOI] [PubMed] [Google Scholar]
- 108.Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018;12:567–576. doi: 10.1007/s12072-018-9898-2. [DOI] [PubMed] [Google Scholar]
- 109.Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 110.Lunia MK, Sharma BC, Sachdeva S. Small intestinal bacterial overgrowth and delayed orocecal transit time in patients with cirrhosis and low-grade hepatic encephalopathy. Hepatol Int. 2013;7:268–273. doi: 10.1007/s12072-012-9360-9. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y, Feng Y, Cao B, Tian Q. The effect of small intestinal bacterial overgrowth on minimal hepatic encephalopathy in patients with cirrhosis. Arch Med Sci. 2016;12:592–596. doi: 10.5114/aoms.2015.55675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fukui H, Wiest R. Changes of Intestinal Functions in Liver Cirrhosis. Inflamm Intest Dis. 2016;1:24–40. doi: 10.1159/000444436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Revaiah PC, Kochhar R, Rana SV, Berry N, Ashat M, Dhaka N, Rami Reddy Y, Sinha SK. Risk of small intestinal bacterial overgrowth in patients receiving proton pump inhibitors versus> proton pump inhibitors plus prokinetics. JGH Open. 2018;2:47–53. doi: 10.1002/jgh3.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Strauss E, Tramote R, Silva EP, Caly WR, Honain NZ, Maffei RA, de Sá MF. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39:542–545. [PubMed] [Google Scholar]
- 115.Elwir S, Rahimi RS. Hepatic Encephalopathy: An Update on the Pathophysiology and Therapeutic Options. J Clin Transl Hepatol. 2017;5:142–151. doi: 10.14218/JCTH.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bothe MK, Maathuis AJH, Bellmann S, van der Vossen JMBM, Berressem D, Koehler A, Schwejda-Guettes S, Gaigg B, Kuchinka-Koch A, Stover JF. Dose-Dependent Prebiotic Effect of Lactulose in a Computer-Controlled In Vitro Model of the Human Large Intestine. Nutrients. 2017:9. doi: 10.3390/nu9070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016:CD003044. doi: 10.1002/14651858.CD003044.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Leeuwen PA, van Berlo CL, Soeters PB. New mode of action for lactulose. Lancet. 1988;1:55–56. doi: 10.1016/s0140-6736(88)91033-1. [DOI] [PubMed] [Google Scholar]
- 119.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 120.Weber FL, Jr, Banwell JG, Fresard KM, Cummings JH. Nitrogen in fecal bacterial, fiber, and soluble fractions of patients with cirrhosis: effects of lactulose and lactulose plus neomycin. J Lab Clin Med. 1987;110:259–263. [PubMed] [Google Scholar]
- 121.Moratalla A, Ampuero J, Bellot P, Gallego-Durán R, Zapater P, Roger M, Figueruela B, Martínez-Moreno B, González-Navajas JM, Such J, Romero-Gómez M, Francés R. Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int. 2017;37:212–223. doi: 10.1111/liv.13200. [DOI] [PubMed] [Google Scholar]
- 122.Hudson M, Schuchmann M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. Eur J Gastroenterol Hepatol. 2019;31:434–450. doi: 10.1097/MEG.0000000000001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H, Yoo JJ, Cho YY, Cho EJ, Yu SJ, Kim MY, Kim YJ, Baik SK, Yoon JH. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther. 2017;46:845–855. doi: 10.1111/apt.14275. [DOI] [PubMed] [Google Scholar]
- 124.Kang DJ, Kakiyama G, Betrapally NS, Herzog J, Nittono H, Hylemon PB, Zhou H, Carroll I, Yang J, Gillevet PM, Jiao C, Takei H, Pandak WM, Iida T, Heuman DM, Fan S, Fiehn O, Kurosawa T, Sikaroodi M, Sartor RB, Bajaj JS. Rifaximin Exerts Beneficial Effects Independent of its Ability to Alter Microbiota Composition. Clin Transl Gastroenterol. 2016;7:e187. doi: 10.1038/ctg.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40:123–132. doi: 10.1111/apt.12803. [DOI] [PubMed] [Google Scholar]
- 126.DuPont HL. Therapeutic Effects and Mechanisms of Action of Rifaximin in Gastrointestinal Diseases. Mayo Clin Proc. 2015;90:1116–1124. doi: 10.1016/j.mayocp.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 127.Ponziani FR, Gerardi V, Pecere S, D'Aversa F, Lopetuso L, Zocco MA, Pompili M, Gasbarrini A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J Gastroenterol. 2015;21:12322–12333. doi: 10.3748/wjg.v21.i43.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ponziani FR, Scaldaferri F, Petito V, Paroni Sterbini F, Pecere S, Lopetuso LR, Palladini A, Gerardi V, Masucci L, Pompili M, Cammarota G, Sanguinetti M, Gasbarrini A. The Role of Antibiotics in Gut Microbiota Modulation: The Eubiotic Effects of Rifaximin. Dig Dis. 2016;34:269–278. doi: 10.1159/000443361. [DOI] [PubMed] [Google Scholar]
- 129.Ponziani FR, Zocco MA, D'Aversa F, Pompili M, Gasbarrini A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol. 2017;23:4491–4499. doi: 10.3748/wjg.v23.i25.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther. 2016;43 Suppl 1:11–26. doi: 10.1111/apt.13435. [DOI] [PubMed] [Google Scholar]
- 131.Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, Kitade M, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol. 2017;23:8355–8366. doi: 10.3748/wjg.v23.i47.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12:135–147. doi: 10.1007/s12072-017-9812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swaminathan M, Ellul MA, Cross TJ. Hepatic encephalopathy: current challenges and future prospects. Hepat Med. 2018;10:1–11. doi: 10.2147/HMER.S118964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Merli M, Iebba V, Giusto M. What is new about diet in hepatic encephalopathy. Metab Brain Dis. 2016;31:1289–1294. doi: 10.1007/s11011-015-9734-5. [DOI] [PubMed] [Google Scholar]
- 136.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pazgan-Simon M, Zuwała-Jagiełło J, Serafińska S, Simon K. Nutrition principles and recommendations in different types of hepatic encephalopathy. Clin Exp Hepatol. 2016;1:121–126. doi: 10.5114/ceh.2016.57759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naseer M, Turse EP, Syed A, Dailey FE, Zatreh M, Tahan V. Interventions to improve sarcopenia in cirrhosis: A systematic review. World J Clin Cases. 2019;7:156–170. doi: 10.12998/wjcc.v7.i2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pearlman M, Akpotaire O. Diet and the Role of Food in Common Gastrointestinal Diseases. Med Clin North Am. 2019;103:101–110. doi: 10.1016/j.mcna.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 141.Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. 2002;36:1163–1171. doi: 10.1053/jhep.2002.36497. [DOI] [PubMed] [Google Scholar]
- 142.Jindal A, Jagdish RK. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019 doi: 10.3350/cmh.2019.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Perumpail BJ, Li AA, Cholankeril G, Kumari R, Ahmed A. Optimizing the Nutritional Support of Adult Patients in the Setting of Cirrhosis. Nutrients. 2017;9 doi: 10.3390/nu9101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen JC, Wang LC, Kuo HT, Fang YC, Lee HF. Late-evening snacking improves liver functions in patients with liver cirrhosis: A meta-analysis of randomized controlled trials. J Gastroenterol and Hepatol. 2019 doi: 10.1111/jgh.14665. [DOI] [PubMed] [Google Scholar]
- 145.Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, Uribe M, Vilstrup H, Morgan MY. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 146.Maki H, Yamanaka-Okumura H, Katayama T, Ozawa Y, Hosoda A, Kurata N, Amemiya F. Late evening snacks with branched-chain amino acids improve the Fischer ratio with patients liver cirrhosis at fasting in the next morning. Clin Nutr ESPEN. 2019;30:138–144. doi: 10.1016/j.clnesp.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Jurado García J, Costán Rodero G, Calañas-Continente A. [Importance of nutritional support in patients with hepatic encephalopathy] Nutr Hosp. 2012;27:372–381. doi: 10.1590/S0212-16112012000200006. [DOI] [PubMed] [Google Scholar]
- 148.PHILLIPS GB, SCHWARTZ R, GABUZDA GJ, Jr, DAVIDSON CS. The syndrome of impending hepatic coma in patients with cirrhosis of the liver given certain nitrogenous substances. N Engl J Med. 1952;247:239–246. doi: 10.1056/NEJM195208142470703. [DOI] [PubMed] [Google Scholar]
- 149.Summerskill WH, Wolfe SJ, Davidson CS. The management of hepatic coma in relation to protein withdrawal and certain specific measures. Am J Med. 1957;23:59–76. doi: 10.1016/0002-9343(57)90358-3. [DOI] [PubMed] [Google Scholar]
- 150.Morgan TR, Moritz TE, Mendenhall CL, Haas R. Protein consumption and hepatic encephalopathy in alcoholic hepatitis. VA Cooperative Study Group #275. J Am Coll Nutr. 1995;14:152–158. doi: 10.1080/07315724.1995.10718487. [DOI] [PubMed] [Google Scholar]
- 151.Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 152.Gheorghe L, Iacob R, Vădan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol. 2005;14:231–238. [PubMed] [Google Scholar]
- 153.Campollo O, Sprengers D, Dam G, Vilstrup H, McIntyre N. Protein tolerance to standard and high protein meals in patients with liver cirrhosis. World J Hepatol. 2017;9:667–676. doi: 10.4254/wjh.v9.i14.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fenton JC, Knight EJ, Humpherson PL. Milk-and-cheese diet in portal-systemic encephalopathy. Lancet. 1966;1:164–166. doi: 10.1016/s0140-6736(66)90696-9. [DOI] [PubMed] [Google Scholar]
- 155.BESSMAN AN, MIRICK GS. Blood ammonia levels following the ingestion of casein and whole blood. J Clin Invest. 1958;37:990–998. doi: 10.1172/JCI103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bianchi GP, Marchesini G, Fabbri A, Rondelli A, Bugianesi E, Zoli M, Pisi E. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J Intern Med. 1993;233:385–392. doi: 10.1111/j.1365-2796.1993.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 157.Uribe M, Márquez MA, Garcia Ramos G, Ramos-Uribe MH, Vargas F, Villalobos A, Ramos C. Treatment of chronic portal--systemic encephalopathy with vegetable and animal protein diets. A controlled crossover study. Dig Dis Sci. 1982;27:1109–1116. doi: 10.1007/BF01391449. [DOI] [PubMed] [Google Scholar]
- 158.Maharshi S, Sharma BC, Sachdeva S, Srivastava S, Sharma P. Efficacy of Nutritional Therapy for Patients With Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin Gastroenterol Hepatol. 2016;14:454–460.e3; quiz e33. doi: 10.1016/j.cgh.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 159.Zieve L, Doizaki WM, Zieve J. Synergism between mercaptans and ammonia or fatty acids in the production of coma: a possible role for mercaptans in the pathogenesis of hepatic coma. J Lab Clin Med. 1974;83:16–28. [PubMed] [Google Scholar]
- 160.de Bruijn KM, Blendis LM, Zilm DH, Carlen PL, Anderson GH. Effect of dietary protein manipulation in subclinical portal-systemic encephalopathy. Gut. 1983;24:53–60. doi: 10.1136/gut.24.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yao CK, Fung J, Chu NHS, Tan VPY. Dietary Interventions in Liver Cirrhosis. J Clin Gastroenterol. 2018;52:663–673. doi: 10.1097/MCG.0000000000001071. [DOI] [PubMed] [Google Scholar]
- 162.Lattanzi B, D´Ambrosio D. Merli M Hepatic Encephalopathy and Sarcopenia: Two Faces of the Same Metabolic Alteration. J Clin Exp Hepatol. 2019;9:125–130. doi: 10.1016/j.jceh.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uribe M, Dibildox M, Malpica S, Guillermo E, Villallobos A, Nieto L, Vargas F, Garcia Ramos G. Beneficial effect of vegetable protein diet supplemented with psyllium plantago in patients with hepatic encephalopathy and diabetes mellitus. Gastroenterology. 1985;88:901–907. doi: 10.1016/s0016-5085(85)80006-8. [DOI] [PubMed] [Google Scholar]
- 164.Bosscher D, Breynaert A, Pieters L, Hermans N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J Physiol Pharmacol. 2009;60 Suppl 6:5–11. [PubMed] [Google Scholar]
- 165.Dasarathy S. Nutrition and Alcoholic Liver Disease: Effects of Alcoholism on Nutrition, Effects of Nutrition on Alcoholic Liver Disease, and Nutritional Therapies for Alcoholic Liver Disease. Clin Liver Dis. 2016;20:535–550. doi: 10.1016/j.cld.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anand AC. Nutrition and Muscle in Cirrhosis. J Clin Exp Hepatol. 2017;7:340–357. doi: 10.1016/j.jceh.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fischer JE, Baldessarini RJ. False neurotransmitters and hepatic failure. Lancet. 1971;2:75–80. doi: 10.1016/s0140-6736(71)92048-4. [DOI] [PubMed] [Google Scholar]
- 168.Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013;28:580–588. doi: 10.1177/0884533613496432. [DOI] [PubMed] [Google Scholar]
- 169.Jawaro T, Yang A, Dixit D, Bridgeman MB. Management of Hepatic Encephalopathy: A Primer. Ann Pharmacother. 2016;50:569–577. doi: 10.1177/1060028016645826. [DOI] [PubMed] [Google Scholar]
- 170.Morgan MY, Milsom JP, Sherlock S. Plasma ratio of valine, leucine and isoleucine to phenylalanine and tyrosine in liver disease. Gut. 1978;19:1068–1073. doi: 10.1136/gut.19.11.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hiraoka A, Michitaka K, Kiguchi D, Izumoto H, Ueki H, Kaneto M, Kitahata S, Aibiki T, Okudaira T, Tomida H, Miyamoto Y, Yamago H, Suga Y, Iwasaki R, Mori K, Miyata H, Tsubouchi E, Kishida M, Ninomiya T, Kohgami S, Hirooka M, Tokumoto Y, Abe M, Matsuura B, Hiasa Y. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416–1423. doi: 10.1097/MEG.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 172.Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015;31:14–20. doi: 10.1016/j.nut.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 173.Dejong CH, van de Poll MC, Soeters PB, Jalan R, Olde Damink SW. Aromatic amino acid metabolism during liver failure. J Nutr. 2007;137:1579S–1585S; discussion 1597S-1598S. doi: 10.1093/jn/137.6.1579S. [DOI] [PubMed] [Google Scholar]
- 174.Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond) 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holecek M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition. 2010;26:482–490. doi: 10.1016/j.nut.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 176.Kimball SR, Jefferson LS. Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem Biophys Res Commun. 2004;313:423–427. doi: 10.1016/j.bbrc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 177.Hagenfeldt L, Eriksson LS, Wahren J. Amino acids in liver disease. Proc Nutr Soc. 1983;42:497–506. doi: 10.1079/pns19830056. [DOI] [PubMed] [Google Scholar]
- 178.Metcalfe EL, Avenell A, Fraser A. Branched-chain amino acid supplementation in adults with cirrhosis and porto-systemic encephalopathy: systematic review. Clin Nutr. 2014;33:958–965. doi: 10.1016/j.clnu.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 179.Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kobayashi M, Ikeda K, Arase Y, Suzuki Y, Suzuki F, Akuta N, Hosaka T, Murashima N, Saitoh S, Someya T, Tsubota A, Kumada H. Inhibitory effect of branched-chain amino acid granules on progression of compensated liver cirrhosis due to hepatitis C virus. J Gastroenterol. 2008;43:63–70. doi: 10.1007/s00535-007-2122-0. [DOI] [PubMed] [Google Scholar]
- 181.Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 182.Ruiz-Margáin A, Macías-Rodríguez RU, Ríos-Torres SL, Román-Calleja BM, Méndez-Guerrero O, Rodríguez-Córdova P, Torre A. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev Gastroenterol Mex. 2018;83:9–15. doi: 10.1016/j.rgmx.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 183.Kircheis G, Lüth S. Pharmacokinetic and Pharmacodynamic Properties of L-Ornithine L-Aspartate (LOLA) in Hepatic Encephalopathy. Drugs. 2019;79:23–29. doi: 10.1007/s40265-018-1023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2018;5:CD012410. doi: 10.1002/14651858.CD012410.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Butterworth RF, Kircheis G, Hilger N, McPhail MJW. Efficacy of l-Ornithine l-Aspartate for the Treatment of Hepatic Encephalopathy and Hyperammonemia in Cirrhosis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Exp Hepatol. 2018;8:301–313. doi: 10.1016/j.jceh.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Butterworth RF, McPhail MJW. L-Ornithine L-Aspartate (LOLA) for Hepatic Encephalopathy in Cirrhosis: Results of Randomized Controlled Trials and Meta-Analyses. Drugs. 2019;79:31–37. doi: 10.1007/s40265-018-1024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moore C, Stein AC. Assessment and Management of Nutrition Status in the Hospitalized Patient With Cirrhosis. Clin Liver Dis (Hoboken) 2018;12:113–116. doi: 10.1002/cld.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wendołowicz A, Stefańska E, Ostrowska L. Influence of selected dietary components on the functioning of the human nervous system. Rocz Panstw Zakl Hig. 2018;69:15–21. [PubMed] [Google Scholar]
- 189.Martínez García RM, Jiménez Ortega AI, López Sobaler AM, Ortega RM. Nutrition strategies that improve cognitive function. Nutr Hosp. 2018;35:16–19. doi: 10.20960/nh.2281. [DOI] [PubMed] [Google Scholar]
- 190.Bémeur C, Butterworth RF. Nutrition in the management of cirrhosis and its neurological complications. J Clin Exp Hepatol. 2014;4:141–150. doi: 10.1016/j.jceh.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chavez-Tapia NC, Cesar-Arce A, Barrientos-Gutiérrez T, Villegas-López FA, Méndez-Sanchez N, Uribe M. A systematic review and meta-analysis of the use of oral zinc in the treatment of hepatic encephalopathy. Nutr J. 2013;12:74. doi: 10.1186/1475-2891-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mousa N, Abdel-Razik A, Zaher A, Hamed M, Shiha G, Effat N, Elbaz S, Elhelaly R, Hafez M, El-Wakeel N, Eldars W. The role of antioxidants and zinc in minimal hepatic encephalopathy: a randomized trial. Therap Adv Gastroenterol. 2016;9:684–691. doi: 10.1177/1756283X16645049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bresci G, Parisi G, Banti S. Management of hepatic encephalopathy with oral zinc supplementation: a long-term treatment. Eur J Med. 1993;2:414–416. [PubMed] [Google Scholar]
- 194.Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi H. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010;32:1080–1090. doi: 10.1111/j.1365-2036.2010.04448.x. [DOI] [PubMed] [Google Scholar]
- 195.Katayama K, Saito M, Kawaguchi T, Endo R, Sawara K, Nishiguchi S, Kato A, Kohgo H, Suzuki K, Sakaida I, Ueno Y, Habu D, Ito T, Moriwaki H, Suzuki K. Effect of zinc on liver cirrhosis with hyperammonemia: a preliminary randomized, placebo-controlled double-blind trial. Nutrition. 2014;30:1409–1414. doi: 10.1016/j.nut.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 196.Guevara M, Baccaro ME, Torre A, Gómez-Ansón B, Ríos J, Torres F, Rami L, Monté-Rubio GC, Martín-Llahí M, Arroyo V, Ginès P. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382–1389. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]
- 197.Shen TD. Diet and Gut Microbiota in Health and Disease. Nestle Nutr Inst Workshop Ser. 2017;88:117–126. doi: 10.1159/000455220. [DOI] [PubMed] [Google Scholar]
- 198.Liu X, Cao S, Zhang X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J Agric Food Chem. 2015;63:7885–7895. doi: 10.1021/acs.jafc.5b02404. [DOI] [PubMed] [Google Scholar]
- 199.Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. 2018;47:192–202. doi: 10.1111/apt.14397. [DOI] [PubMed] [Google Scholar]
- 200.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 201.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 202.Sharma BC, Singh J. Probiotics in management of hepatic encephalopathy. Metab Brain Dis. 2016;31:1295–1301. doi: 10.1007/s11011-016-9826-x. [DOI] [PubMed] [Google Scholar]
- 203.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 204.Viramontes Hörner D, Avery A, Stow R. The Effects of Probiotics and Symbiotics on Risk Factors for Hepatic Encephalopathy: A Systematic Review. J Clin Gastroenterol. 2017;51:312–323. doi: 10.1097/MCG.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 205.Marlicz W, Wunsch E, Mydlowska M, Milkiewicz M, Serwin K, Mularczyk M, Milkiewicz P, Raszeja-Wyszomirska J. The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis. J Physiol Pharmacol. 2016;67:867–877. [PubMed] [Google Scholar]
- 206.Malaguarnera M, Greco F, Barone G, Gargante MP, Malaguarnera M, Toscano MA. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 2007;52:3259–3265. doi: 10.1007/s10620-006-9687-y. [DOI] [PubMed] [Google Scholar]
- 207.Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, Pennisi G, Li Volti G, Galvano F. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2010;22:199–206. doi: 10.1097/MEG.0b013e328330a8d3. [DOI] [PubMed] [Google Scholar]
- 208.Sitkin S. Minimal hepatic encephalopathy: possibilities of dietary fibers treatment. J Hepatol. 2012;56:67. [Google Scholar]
- 209.Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. doi: 10.1002/14651858.CD008716.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003–8.e1. doi: 10.1016/j.cgh.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 211.Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107:1043–1050. doi: 10.1038/ajg.2012.113. [DOI] [PubMed] [Google Scholar]
- 212.Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, Khattri A, Malhotra S, Duseja A, Chawla YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–37.e3. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 213.McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2011:CD008716. doi: 10.1002/14651858.CD008716.pub2. [DOI] [PubMed] [Google Scholar]
- 214.Kornerup LS, Gluud LL, Vilstrup H, Dam G. Update on the Therapeutic Management of Hepatic Encephalopathy. Curr Gastroenterol Rep. 2018;20:21. doi: 10.1007/s11894-018-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, Pleuss JA, Krakower G, Hoffmann RG, Binion DG. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103:1707–1715. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 216.Liu JE, Zhang Y, Zhang J, Dong PL, Chen M, Duan ZP. Probiotic yogurt effects on intestinal flora of patients with chronic liver disease. Nurs Res. 2010;59:426–432. doi: 10.1097/NNR.0b013e3181fa4dc6. [DOI] [PubMed] [Google Scholar]
- 217.Campion D, Ponzo P, Alessandria C, Saracco GM, Balzola F. The role of microbiota in autism spectrum disorders. Minerva Gastroenterol Dietol. 2018;64:333–350. doi: 10.23736/S1121-421X.18.02493-5. [DOI] [PubMed] [Google Scholar]
- 218.Piwowarczyk A, Horvath A, Łukasik J, Pisula E, Szajewska H. Gluten- and casein-free diet and autism spectrum disorders in children: a systematic review. Eur J Nutr. 2018;57:433–440. doi: 10.1007/s00394-017-1483-2. [DOI] [PubMed] [Google Scholar]
- 219.Heyman M, Abed J, Lebreton C, Cerf-Bensussan N. Intestinal permeability in coeliac disease: insight into mechanisms and relevance to pathogenesis. Gut. 2012;61:1355–1364. doi: 10.1136/gutjnl-2011-300327. [DOI] [PubMed] [Google Scholar]
- 220.Harris C, Card B. A pilot study to evaluate nutritional influences on gastrointestinal symptoms and behavior patterns in children with Autism Spectrum Disorder. Complement Ther Med. 2012;20:437–440. doi: 10.1016/j.ctim.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 221.Janecka A, Staniszewska R, Gach K, Fichna J. Enzymatic degradation of endomorphins. Peptides. 2008;29:2066–2073. doi: 10.1016/j.peptides.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 222.Alessandria C, Caviglia GP, Campion D, Nalbone F, Sanna C, Musso A, Abate ML, Rizzetto M, Saracco GM, Balzola F. HLA-DQ Genotyping, Duodenal Histology, and Response to Exclusion Diet in Autistic Children With Gastrointestinal Symptoms. J Pediatr Gastroenterol Nutr. 2019 doi: 10.1097/MPG.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 223.Ghalichi F, Ghaemmaghami J, Malek A, Ostadrahimi A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: a randomized clinical trial. World J Pediatr. 2016;12:436–442. doi: 10.1007/s12519-016-0040-z. [DOI] [PubMed] [Google Scholar]
- 224.Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, Saieva L, Cypers H, Stampone T, Di Benedetto P, Gabrielli A, Fasano A, Elewaut D, Triolo G. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76:1123–1132. doi: 10.1136/annrheumdis-2016-210000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Martin VJ, Leonard MM, Fiechtner L, Fasano A. Transitioning From Descriptive to Mechanistic Understanding of the Microbiome: The Need for a Prospective Longitudinal Approach to Predicting Disease. J Pediatr. 2016;179:240–248. doi: 10.1016/j.jpeds.2016.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dieterich W, Schuppan D, Schink M, Schwappacher R, Wirtz S, Agaimy A, Neurath MF, Zopf Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin Nutr. 2018;38:697–707. doi: 10.1016/j.clnu.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 227.Balzola F, Sanna C, Ottobrelli A, Torrani M, Martini S. Leotta D, Marchet A, Veneziani A, Capellero B, Boggio D, Galletti R, Mollo A, Bianco B, Rizzetto M. Chronic hepatic encephalopathy (HE) in patients with severe liver cirrhosis: efficacy of wheat and milk protein free diet in the reduction of clinical episodes. J Hep. 2011;54:64. [Google Scholar]
- 228.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term Outcomes of Fecal Microbiota Transplantation in Patients With Cirrhosis. Gastroenterology. 2019;156:1921–1923.e3. doi: 10.1053/j.gastro.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]