Abstract
Glial cell line-derived neurotrophic factor (GDNF) has been reported to be involved in negatively regulating the effects of addictive disorders. The objective of this study was to investigate alterations in the levels of GDNF in patients with Internet gaming disorder (IGD) and to assess the relationship between GDNF levels and the severity of IGD indices. Nineteen male patients with IGD and 19 sexmatched control subjects were evaluated for alteration of plasma GDNF levels and for relationship between GDNF levels and clinical characteristics of Internet gaming, including the Young’s Internet Addiction Test (Y-IAT). The GDNF levels were found to be significantly low in patients with IGD (103.2±62.0 pg/mL) compared with the levels of controls (245.2±101.6 pg/mL, p<0.001). GDNF levels were negatively correlated with Y-IAT scores (Spearman’s rho=-0.645, p=<0.001) and this negative correlation remained even after controlling for multiple variables (r=-0.370, p=0.048). These findings support the assumed role of GDNF in the regulation of IGD.
Keywords: Glial cell line-derived neurotrophic factor, Behavior, Addictive, Internet, Game, Recreational
INTRODUCTION
Glial cell line-derived neurotrophic factor (GDNF) is neuropeptide and it is essential for the maintenance of dopaminergic neurons [1] and has been shown to enhance re-growth of adult dopamine neurons following neural insult [2].
Furthermore, several preclinical studies indicate that GDNF plays an important role in the behavioral effects of abused drugs [3] and neuroadaptation induced by repeated exposure to drugs, including cocaine [4,5], methamphetamine [6,7], morphine [8], and alcohol [9-11] in the mesolimbic dopamine pathway. Intriguingly, most of the studies suggest that activation of the GDNF system results in the prevention of behavioral adaptation to drugs of abuse [3]. Intra-ventral tegmental area (VTA) infusion of GDNF was effective in decreasing conditioned place preference (CPP) to cocaine [3]; GDNF heterozygote knockout mice, which express lower GDNF levels than their wild-type counterparts, show increased methamphetamine [6] and morphine CPP [8]. In addition, previous studies have suggested that GDNF reduces ethanol consumption and relapse [9-11].
Internet gaming disorder (IGD) is currently included in Section III of the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the DSM-5 proposed that IGD is a pattern of excessive and prolonged Internet gaming that results in a cluster of cognitive and behavioral symptoms, including loss of control over gaming, tolerance, and withdrawal symptoms [12]. These characteristics are traditionally associated and shared with substance-related addictions [12,13] and gambling disorder [14,15]. Furthermore, from a neurobiological perspective, recent neuroimaging studies have shown that IGD may have an impact on the dopaminergic system [13]. Dopaminergic neurons in the VTA are a critical element of the neural circuitry implicated in addictive behavior and the mesolimbic dopaminergic circuit has also been implicated in addiction [16]. In studies using positron emission tomography scanning [17,18], increased release of dopamine to D2 receptors, especially in the ventral striatum, was suggested following a game play in a healthy male adult. Another study using single photon emission computed tomography [19] suggests that the level of dopamine release in the ventral striatum during an Internet game is comparable to that induced by psychostimulant drugs [20,21].
These findings suggest that GDNF, which plays a role in mesolimbic dopamine circuits, is associated with IGD. However, there are no clinical study results that show altered levels of GDNF in IGD. Therefore, the aims of the present study were to investigate alterations in the plasma levels of GDNF in IGD patients compared to that in healthy controls and to assess the relationship between GDNF levels and the severity of IGD indices.
METHODS
Participants
Nineteen male IGD patients were recruited and diagnosed according to the DSM-5 criteria. The patients were still playing Internet games at the time of enrollment and had not previously received any treatment for IGD. IGD group subjects with past or current medical disorders, neurological disorders, or other psychiatric disorders including substance use disorders were excluded. The control group consisted of 19 healthy male subjects and was negative for medical, neurological, and psychiatric disorders and regular use of any medication.
Measures and procedure
Participants were assessed using the Alcohol Use Disorders Identification Test (AUDIT) [22], Fagerström Test for Nicotine Dependence (FTND) [23], Beck Depression Inventory (BDI)-II [24], Beck Anxiety Inventory (BAI)-II [25], and Barratt Impulsiveness Scale-11 (BIS-11) [26]. The characteristics of their Internet gaming were measured by the first period of Internet gaming, weekday/weekend average Internet gaming usage hours for the past year, elapsed time after the last game, and the Korean version of Young’s Internet Addiction Test (Y-IAT) to assess the severity of IGD. The Y-IAT comprised 20 items with 5 Likert scales ranging from 1 (Not at all) to 5 (Always) with higher scores indicating a greater tendency toward addiction [27]. Y-IAT scores of 20–39 are regarded as an average user, 40–69 as a possible addicted user, and more than 70 as an addicted user [27]. In a previous study to explore the psychometric properties of the Y-IAT, six factors were identified; salience, excessive use, neglecting work, anticipation, lack of control, and neglecting social life [28]. All subjects were also examined using the Korean Wechsler Adult Intelligence Scale (K-WAIS) to measure their intelligence quotient (IQ) and cognitive ability.
GDNF concentration was measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Wuhan EIAab Science Co., Ltd., China). Further explanation on the measurement of plasma GDNF are provided in the Supplementary Materials (in the onlineonly Data Supplement).
This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary’s Hospital (IRB number: KC15EISI0103). All subjects were informed about the study and all provided informed consent.
Statistical analyses
Differences in sociodemographic, clinical variables, and plasma GDNF levels between the IGD and control groups were tested using the independent t-test, Mann-Whitney U test, or Fisher’s exact test. The correlation between GDNF levels and clinical characteristics in all participants was examined using Spearman’s rho and partial correlation that controlled for age, AUDIT, FTND, BDI-II, BAI-II and BIS-11 scores, and total IQ. All statistical analyses were conducted using SPSS, version 24.0 (IBM Corp., Armonk, NY, USA).
RESULTS
The sociodemographic, clinical characteristics, and GDNF levels of the IGD patients and control subjects are listed in Table 1. In the IGD group, the average playing duration per weekday or weekend day was 2.5±2.0 hours and 4.0±1.9 hours, respectively. The Y-IAT score of IGD patients was 52.1±10.0, which is regarded as possibly addicted. There was no difference in mean age, marital and employment status, smoking and alcohol drinking status, beginning of Internet gaming, weekday and weekend average usage hours of Internet gaming, elapsed time after the last game, and AUDIT, FTND, BDI-II, BAI-II, and K-WAIS scores between the two groups. However, the mean Y-IAT and BIS-11 scores were significantly higher in the IGD group (75.1±26.1 at BIS-11) than in the normal control group (38.8±5.1 and 54.9±17.0, respectively). With regard to the six factors of Y-IAT, the scores were higher in the IGD group compared to normal controls except for the salience factor. The mean plasma GDNF levels were found to be significantly low in the patients with IGD (103.2±62.0 pg/mL) when compared with those of the healthy controls (245.2±101.6 pg/mL, p<0.001) (Table 1).
Table 1.
Sociodemographics, clinical characteristics, and GDNF levels of subjects
| Variables | IGD (N=19, male) | Controls (N=19, male) | p-value |
|---|---|---|---|
| Age, mean years (SD) | 31.2 (8.0) | 31.3 (4.2) | 0.817 |
| Marital status, N (%) | 0.495 | ||
| Unmarried | 14 (73.7) | 11 (57.9) | |
| Married | 5 (26.3) | 8 (42.1) | |
| Employment status, N (%) | 0.313 | ||
| Yes | 10 (52.6) | 14 (73.7) | |
| No | 9 (47.4) | 5 (26.3) | |
| Smoking, N (%) | 1.000 | ||
| Non-smoker | 6 (31.6) | 6 (31.6) | |
| Current smoker | 13 (68.4) | 13 (68.4) | |
| Alcohol, N (%) | 1.000 | ||
| Non-drinker | 7 (36.8) | 6 (31.6) | |
| Drinker | 12 (63.2) | 13 (68.4) | |
| AUDIT, mean (SD) | 9.4 (11.5) | 10.6 (9.0) | 0.452 |
| FTND, mean (SD) | 2.4 (4.7) | 2.8 (4.8) | 0.977 |
| First period of Internet gaming, N (%) | 0.831 | ||
| Lower grade of elementary school | 1 (5.3) | 0 (0.0) | |
| Upper grade of elementary school | 2 (10.5) | 3 (15.8) | |
| Middle school | 6 (31.6) | 5 (26.3) | |
| High school | 3 (15.8) | 6 (31.6) | |
| 20 s | 5 (26.3) | 4 (21.0) | |
| 30 s | 2 (10.5) | 1 (5.3) | |
| Weekday average Internet game usage hours, mean hours/day (SD) | 2.5 (2.0) | 1.9 (1.1) | 0.181 |
| Weekend average Internet game usage hours, mean hours/days (SD) | 4.0 (1.9) | 3.3 (1.7) | 0.271 |
| Elapsed time after the last game, mean hours (SD) | 16.7 (9.2) | 19.0 (12.3) | 0.311 |
| Y-IAT, mean (SD) | 52.1 (10.0) | 38.8 (5.1) | <0.001* |
| Salience | 11.0 (2.0) | 9.8 (2.6) | 0.172 |
| Excessive use | 15.1 (3.3) | 11.1 (1.3) | <0.001* |
| Neglecting work | 7.9 (2.8) | 5.0 (1.6) | <0.001* |
| Anticipation | 4.6 (1.6) | 3.3 (1.5) | 0.023* |
| Lack of control | 8.5 (2.5) | 6.3 (1.5) | 0.003* |
| Neglecting social life | 5.1 (1.3) | 3.4 (1.1) | <0.001* |
| BDI-II, mean (SD) | 21.1 (5.5) | 17.8 (7.9) | 0.142 |
| BAI-II, mean (SD) | 20.7 (5.0) | 17.1 (11.4) | 0.109 |
| BIS–11, mean (SD) | 75.1 (26.1) | 54.9 (17.0) | 0.019* |
| GDNF, pg/mL (SD) | 103.2 (62.0) | 245.2 (101.6) | <0.001* |
| K-WAIS, full scale IQ, mean (SD) | 104.9 (17.0) | 108.4 (11.6) | 0.460 |
p<0.05 in independent t-test, Mann-Whitney U test, or Fisher’s exact test.
IGD: Internet gaming disorder patients, SD: standard deviation, N: numbers, AUDIT: alcohol use disorders identification test, FTND: Fagerström test for nicotine dependence, Y-IAT: Young’s Internet addiction test, BDI: Beck depression inventory, BAI: Beck anxiety inventory, BIS: Barratt Impulsiveness Scale, GDNF: glial cell line-derived neurotrophic factor, K-WAIS: Korean Wechsler Adult Intelligence Scale, IQ: Intelligence quotient
GDNF plasma levels were negatively correlated with BIS-11 scores (Spearman’s rho=-0.526, p=0.001) but not significantly correlated with age (p=0.536), AUDIT (p=0.084), FTND (p=0.497), BDI-II (p=0.164) and BAI-II (p=0.138) scores, and total IQ (p=0.884). Regarding gaming characteristics and IGD severity, GDNF levels were negatively correlated with Y-IAT scores (Spearman’s rho=-0.645, p=<0.001) and positively correlated with elapsed time after the last game (Spearman’s rho=0.420, p=0.009) but not significantly correlated with weekday (p=0.126) and weekend average Internet gaming usage hours (p=0.074). After controlling age, AUDIT, FTND, BDI-II, BAI-II and BIS-11 scores, and total IQ, a negative correlation remained between plasma GDNF levels and Y-IAT scores (r=-0.370, p=0.048) and a negatively correlated trend was observed between GDNF levels and Y-IAT salience sub-factor scores (r=-0.339, p=0.055) (Figure 1).
Figure 1.
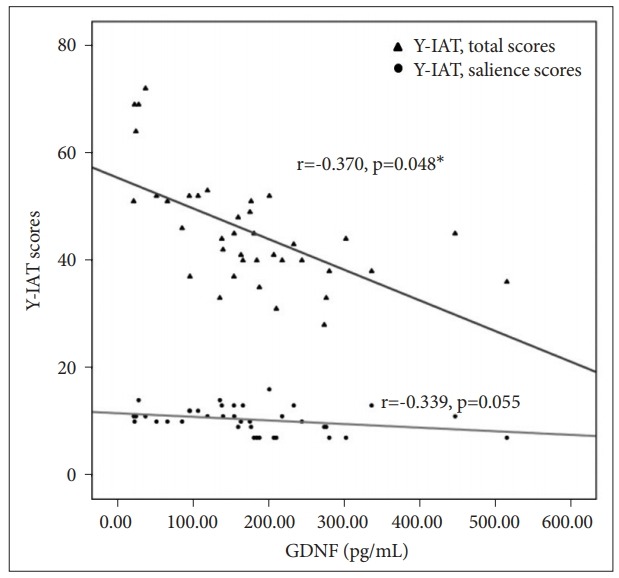
Partial correlation between plasma GDNF levels and Y-IAT total and salience scores. *p<0.05. GDNF: glial cell line-derived neurotrophic factor, Y-IAT: Young’s Internet addiction test.
DISCUSSION
In this study, we found significantly lower plasma GDNF levels among patients with IGD than in healthy controls. This finding is consistent with a previous study in which GDNF levels were lower in alcohol-dependent patients [29]. In addition, we found a negative correlation between GDNF levels and IGD severity. Similar to the present results, a previous study with heavy alcohol drinkers (years of drinking: 8.85±7.38, daily alcohol consumption: 193.27±58.59 grams) reported that GDNF levels were negatively associated with tolerance [29].
We also found that salience showed a negative correlated trend with GDNF levels. Salience, specifically incentive salience, is a cognitive process that produces a bias of attentional processing toward reward-associated stimuli [30-32] and regulated primarily by dopamine neurotransmission in the mesocorticolimbic pathway [32,33]. GDNF is associated with the mesolimbic dopaminergic system; the main area of action of GDNF is the midbrain [11,34,35] and the main site of GDNF in the midbrain is the striatum [36-38]. Moreover, signaling proteins for GDNF, GFRα1 (GDNF family receptor α1) and Ret (Rearranged during transfection receptor), are highly expressed in midbrain dopamine neurons [39,40]. Additionally, motivation to self-administer and amphetamine-seeking behavior related to incentive salience is enhanced and sensitivity to the rewarding activity is increased in GDNF heterozygote knockout mice [3,7].
The mechanisms of action of GDNF associated with addiction remain unknown at present. However, several studies suggest that increased GDNF levels are associated with a reduction in the activity of tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis, in the midbrain [3,41,42]. GDNF VTA injections antagonized cocaine or morphine-induced induction of tyrosine hydroxylase, which its activation enhances dopamine release in terminal areas [3]. These changes could result in a synaptic alteration, changing the responsiveness of the mesolimbic dopaminergic system and subsequently blunting the incentive and/or rewarding property, and the neuroadaptations associated with addiction.
The present study has the following limitations; first, our sample size was modest, and we investigated plasma levels of GDNF in only male patients. Previous animal studies have shown gender differences in the regulation of neurotrophic factors [43,44]. Second, our sample did not include IGD patient with severe levels of severity such as patients who require hospitalization. Third, plasma GDNF levels were measured in peripheral blood rather than in the central nervous system although a prior study reported that reduced GDNF plasma levels may imply a decreased ability to repair neuronal injury [45].
Despite these limitations, it is worth noting that this research is the first clinical study to investigate alterations in GDNF levels in IGD. The present study showed that GDNF levels are significantly lower in IGD patients compared to controls and GDNF levels have a negative correlation with IGD severity. The findings of this study support the assumed role of GDNF in the regulation of addiction including IGD as observed in preclinical studies. Our findings show the possibility of the GDNF pathway as a promising target for understanding and treatment of addictions including IGD.
Acknowledgments
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT (NRF–2014M3C7A1062893).
Footnotes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jo-Eun Jeong, Hyun Cho, Jung-Seok Choi, Sam-Wook Choi, Dai-Jin Kim. Data curation: Jo-Eun Jeong, Hyun Cho. Formal analysis: Jo-Eun Jeong, Funding acquisition: Dai-Jin Kim. Investigation: Jo-Eun Jeong, Soo-Hyun Paik, Mi Ran Choi, Hyun Cho, Jung-Seok Choi, Sam-Wook Choi, Dai-Jin Kim. Methodology: Jo-Eun Jeong, Hyun Cho, Jung-Seok Choi, Sam-Wook Choi, Dai-Jin Kim. Project administration: Dai-Jin Kim. Resources: Jo-Eun Jeong, Hyun Cho. Software: Jo-Eun Jeong, Soo-Hyun Paik, Mi Ran Choi, Hyun Cho. Supervision: Jung-Seok Choi, Sam-Wook Choi, Dai-Jin Kim. Validation: Jung-Seok Choi, Sam-Wook Choi, Dai-Jin Kim. Visualization: Jo-Eun Jeong. Writing—original draft: Jo-Eun Jeong. Writing—review & editing: Jo-Eun Jeong.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2019.04.02.2.
REFERENCES
- 1.Carnicella S, Ron D. GDNF--a potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- 3.Messer CJ, Eisch AJ, Carlezon WA, Jr, Whisler K, Shen L, Wolf DH, et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green-Sadan T, Kinor N, Roth-Deri I, Geffen-Aricha R, Schindler CJ, Yadid G. Transplantation of glial cell line-derived neurotrophic factorexpressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur J Neurosci. 2003;18:2093–2098. doi: 10.1046/j.1460-9568.2003.02943.x. [DOI] [PubMed] [Google Scholar]
- 5.Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, Kinor N, Boguslavsky Y, Margel S, et al. Glial cell line-derived neurotrophic factorconjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194:97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, et al. An inducer for glial cell line-derived neurotrophic factor and tumor necrosis factor-alpha protects against methamphetamine-induced rewarding effects and sensitization. Biol Psychiatry. 2007;61:890–901. doi: 10.1016/j.biopsych.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. FASEB J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- 8.Niwa M, Nitta A, Shen L, Noda Y, Nabeshima T. Involvement of glial cell line-derived neurotrophic factor in inhibitory effects of a hydrophobic dipeptide Leu-Ile on morphine-induced sensitization and rewarding effects. Behav Brain Res. 2007;179:167–171. doi: 10.1016/j.bbr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: The American Psychiatric Association; 2013. [Google Scholar]
- 13.Yau YH, Crowley MJ, Mayes LC, Potenza MN. Are Internet use and video-game-playing addictive behaviors? Biological, clinical and public health implications for youths and adults. Minerva Psichiatr. 2012;53:153–170. [PMC free article] [PubMed] [Google Scholar]
- 14.Müller KW, Beutel ME, Egloff B, Wölfling K. Investigating risk factors for Internet gaming disorder: a comparison of patients with addictive gaming, pathological gamblers and healthy controls regarding the big five personality traits. Eur Addict Res. 2014;20:129–136. doi: 10.1159/000355832. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Kim YK, Gwak AR, Lim JA, Lee JY, Jung HY, et al. Restingstate regional homogeneity as a biological marker for patients with Internet gaming disorder: A comparison with patients with alcohol use disorder and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:104–111. doi: 10.1016/j.pnpbp.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Baik SH, Park CS, Kim SJ, Choi SW, Kim SE. Reduced striatal dopamine D2 receptors in people with internet addiction. Neuroreport. 2011;22:407–411. doi: 10.1097/WNR.0b013e328346e16e. [DOI] [PubMed] [Google Scholar]
- 18.Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein AM. Computer and video game addiction-a comparison between game users and non-game users. Am J Drug Alcohol Abuse. 2010;36:268–276. doi: 10.3109/00952990.2010.491879. [DOI] [PubMed] [Google Scholar]
- 20.Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- 22.Kim CG, Kim JS, Jung JG, Kim SS, Yoon SJ, Suh HS. Reliability and validity of alcohol use disorder dentification test-Korean revised version for screening at-risk drinking and alcohol use disorders. Korean J Fam Med. 2014;35:2–10. doi: 10.4082/kjfm.2014.35.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn HK, Lee HJ, Jung DS, Lee SY, Kim SW, Kang JH. The Reliability and validity of Korean version of questionnaire for nicotine dependence. J Korean Acad Fam Med. 2002;23:999–1008. [Google Scholar]
- 24.Sung H, Kim J, Park Y, Bai D, Lee S, Ahn H. A study on the reliability and the validity of Korean version of the Beck Depression Inventory-II (BDI-II) J Korean Soc Biol Ther Psychiatry. 2008;14:201–212. [Google Scholar]
- 25.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 26.Chung Y, Lee C. A study of factor structures of the Barratt impulsiveness scale in Korean university students. Korean J Clin Psychol. 1997;16:117–129. [Google Scholar]
- 27.Young KS. Internet addiction: the emergence of a new clinical disorder. Cyberpsychol Behav. 1998;1:237–244. [Google Scholar]
- 28.Widyanto L, McMurran M. The psychometric properties of the internet addiction test. Cyberpsychol Behav. 2004;7:443–450. doi: 10.1089/cpb.2004.7.443. [DOI] [PubMed] [Google Scholar]
- 29.Heberlein A, Muschler M, Wilhelm J, Frieling H, Lenz B, Gröschl M, et al. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1060–1064. doi: 10.1016/j.pnpbp.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Puglisi-Allegra S, Ventura R. Prefrontal/accumbal catecholamine system processes high motivational salience. Front Behav Neurosci. 2012;6:31. doi: 10.3389/fnbeh.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev. 2015;95:853–951. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- 35.Semba J, Akanuma N, Wakuta M, Tanaka N, Suhara T. Alterations in the expressions of mRNA for GDNF and its receptors in the ventral midbrain of rats exposed to subchronic phencyclidine. Brain Res Mol Brain Res. 2004;124:88–95. doi: 10.1016/j.molbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, et al. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- 38.Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodríguez-Díaz M, Afonso-Oramas D, Lanciego JL, et al. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21:1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- 39.Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 40.Trupp M, Belluardo N, Funakoshi H, Ibáñez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- 43.Cirulli F, Francia N, Branchi I, Antonucci MT, Aloe L, Suomi SJ, et al. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34:172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roeding RL, Perna MK, Cummins ED, Peterson DJ, Palmatier MI, Brown RW. Sex differences in adolescent methylphenidate sensitization: effects on glial cell-derived neurotrophic factor and brain-derived neurotrophic factor. Behav Brain Res. 2014;273:139–143. doi: 10.1016/j.bbr.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Choudhry MA, Chaudry IH. Alcohol, burn injury, and the intestine. J Emerg Trauma Shock. 2008;1:81–87. doi: 10.4103/0974-2700.43187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


