Abstract
Objectives
Tocotrienols and tocopherols are members of the vitamin E family, with similar structures; however, only tocotrienols have been reported to achieve potent anti‐cancer effects. The study described here has evaluated anti‐cancer activity of vitamin E to elucidate mechanisms of cell death, using human breast cancer cells.
Materials and methods
Anti‐cancer activity of a tocotrienol‐rich fraction (TRF) and a tocotrienol‐enriched fraction (TEF) isolated from palm oil, as well as pure vitamin E analogues (α‐tocopherol, α−, δ− and γ−tocotrienols) were studied using highly aggressive triple negative MDA‐MB‐231 cells and oestrogen‐dependent MCF‐7 cells, both of human breast cancer cell lines. Cell population growth was evaluated using a Coulter particle counter. Cell death mechanism, poly(ADP‐ribose) polymerase cleavage and levels of NF‐κB were determined using commercial ELISA kits.
Results
Tocotrienols exerted potent anti‐proliferative effects on both types of cell by inducing apoptosis, the underlying mechanism of cell death being ascertained using respective IC 50 concentrations of all test compounds. There was marked induction of apoptosis in both cell lines by tocotrienols compared to treatment with Paclitaxel, which was used as positive control. This activity was found to be associated with cleavage of poly(ADP‐ribose) polymerase (a DNA repair protein), demonstrating involvement of the apoptotic cell death signalling pathway. Tocotrienols also inhibited expression of nuclear factor kappa‐B (NF‐κB), which in turn can increase sensitivity of cancer cells to apoptosis.
Conclusion
Tocotrienols induced anti‐proliferative and apoptotic effects in association with DNA fragmentation, poly(ADP‐ribose) polymerase cleavage and NF‐κB inhibition in the two human breast cancer cell lines.
Introduction
Breast cancer is the second leading cause of cancer‐related death in women after lung/bronchial cancer 1. It is also the most common cancer that incapacitates Malaysian women from of ethnicities 2, 3 as it does all over the world 1. Currently, it is possible to successfully treat most patients with breast cancer using hormonal manipulation; however, it is a major challenge to achieve a cure for women with highly aggressive triple negative breast cancers [lacking over‐expression of oestrogen‐receptor (ER), progesterone receptor (PR) and Her‐2/neu (ERB‐B2) 4]. It has been shown that in the order of 15–20% of breast cancers can be classified as of the triple negative type 4. Most triple negative breast cancers are basal cell carcinomas and vice versa; most basal cancers are usually triple negative. Basal‐like breast cancer is associated with high‐grade metastasis, poor prognosis and often affects younger patients 4. Most triple negative breast cancers appear to share similar gene expression profiles and DNA repair deficiencies with BRCA1‐associated breast carcinomas 4.
Most current chemotherapeutic agents aim to enhance apoptotic cell death 5. Apoptosis is cell suicide or programmed cell death, mediated by activation of a progression‐conserved intracellular pathway 6. Systematic removal of apoptotic cells by macrophages can actively result in anti‐inflammatory and immunosuppressive responses 7. Presence of cleaved poly(ADP‐ribose) polymerase (PARP‐1) enzyme is commonly used to detect apoptosis in many cell types. Cleavage of PARP‐1 into two fragments (89 and 24 kDa) is reported to correlate with functional activation of caspases 8. In addition, cell death mediated by apoptosis‐inducing factor (AIF), independent of caspases, is also modulated by PARP‐1 9. PARP appears to be an important substrate for a large number of suicide proteases involved in the cell death process 10. Moreover, ability of PARP‐1 to interact and modulate the transcription factor NF‐κB makes it crucial in decisions of life and death of a cell 11. This protein plays a major role in a number of cell processes involving DNA repair and programmed cell death 12.
The physiological role of PARP‐1 is as a DNA damage sensor and is to assist in repair of single‐strand DNA nicks, known as ‘base excision repair’. PARP‐1 can also regulate gene expression as it can also function as a transcriptional co‐factor and protein through polyADP ribosylation 13; cleavage of PARP reduces inflammatory responses and apoptotic cell death 14. The majority of breast carcinomas have been found to express high levels of PARP‐1 15. NF‐κB is a protein complex that controls transcription of DNA, a transcription factor regulating several genes that are involved in cell proliferation and apoptosis 16. Due to its critical role in cell proliferation, differentiation, survival, adhesion and inflammation, NF‐κB has been implicated in carcinogenesis.
Palm oil contains vitamin E, of which 70% are various isomers of tocotrienols and the remaining 30% α‐tocopherols 17. It has been suggested that vitamin E is the key constituent of palm oil that inhibits growth of tumours, as vitamin E‐stripped palm oil fraction enhances tumorigenesis 17. Tocotrienols have been shown to have the ability to induce apoptosis selectively in cancers, but not in normal cells 18. It has been proposed that selective TRF‐mediated cell cycle perturbation and apoptosis may be associated with p53 and Akt/NF‐κB signalling pathways 19. Several studies have found that α‐tocopherol does not demonstrate any anti‐proliferative and/or pro‐apoptotic activity in cancer 20, 21, 22, but, TRF and δ‐tocotrienol have been shown to inhibit proliferation of cancer cells by arresting them in G2 phase, and by inducing apoptosis via expression of Fas receptor, Fas ligand, caspase‐8, caspase‐3 and bax 23. It has been reported that that γ‐tocotrienol induces activation of caspase‐8 and caspase‐3, but not caspase‐9 in neoplastic mammary epithelial cells 24.
To date, many studies have been performed that demonstrate potent anti‐proliferative effects of tocotrienols on human breast cancer cells 17, 18, 20, 21, 22, 23, 25, however, molecular and therapeutic targets of tocotrienols await further investigation 26. In this study, we have compared anti‐malignant effects of the various forms of vitamin E (tocotrienol analogues (α−, δ− and γ−) and α‐tocopherol, TRF, TEF) isolated from palm oil to (i) induce apoptosis, PARP‐1 cleavage and inhibition of NF‐κB on two human breast cancer cell lines.
Materials and methods
Reagents
Cell culture: Dulbecco's modified Eagle's medium (DMEM) with high glucose (GIBCO, Invitrogen, Grand Island, NY USA); TrypLE™ Express Stable Trypsin‐like (GIBCO, Invitrogen); l‐glutamine (GIBCO, Invitrogen); foetal bovine serum (GIBCO, Invitrogen); penicilin‐streptomycin (GIBCO, Invitrogen); β‐oestradiol (Sigma Chemicals, San Louis, USA); RPMI medium 1640 (1X) (GIBCO, Invitrogen); phosphate‐buffered saline (Sigma Chemicals).
Test compounds
Tocotrienol‐rich fraction (TRF), standardized composition of palm vitamin E containing 32% α‐tocopherol, 25% α‐tocotrienol, 29% γ‐tocotrienol and 14% δ‐tocotrienol (Golden Hope Plantations, Selangor, Malaysia); TEF, tocotrienol fraction free from α‐tocopherol, containing 45.3% α‐tocotrienol, 29.4% γ‐tocotrienol and 25.3% δ‐tocotrienol (Davos Life Sciences Ptd Ltd, Singapore); α‐tocopherol (Aldrich Chemical Company Inc, Milwaukee, WI, USA) and pure tocotrienol analogues (α−, δ‐, γ‐) (Eisai Food & Chemicals Co. Ltd., Tokyo, Japan). Stock solutions (10 mg/ml) of all vitamin E treatments were prepared and quantified using high‐performance liquid chromatography (HPLC). These treatments to be diluted with medium in the various experiments.
Culture of cell lines
Highly aggressive triple negative MDA‐MB‐231, and oestrogen‐dependent MCF‐7 human breast cancer cells, were purchased from ATCC. MDA‐MB‐231 cells were cultured as monolayers in culture flasks (Orange Scientific, Braine‐l' Alleud, Belgium) in DMEM containing 10% foetal bovine serum (FBS) (GIBCO, Invitrogen), 1% l‐glutamine (GIBCO, Invitrogen) and 1% penicillin‐streptomycin (GIBCO, Invitrogen) in humidified atmosphere of 5% CO2 in air at 37 °C. MCF‐7 cells were cultured under identical conditions and medium, except that 10−8 M β‐oestradiol was added to the medium. Culture media were changed routinely every 2–3 days.
Determination of cell population expansion
MCF‐7 and MDA‐MB‐231 cells were harvested and counted using a haemocytometer. Cell numbers were adjusted to 0.5 × 105 cells/ml using medium, and 0.5 ml cell suspension was plated in 24‐well tissue culture plates (Orange Scientific). After 24 h, medium was changed and relevant test compounds were added to cultures in a range of concentrations (0–20 μg/ml). Concentration range for this study was chosen as per guidelines of standard National Cancer Institute (NCI) criteria, which recommend that natural bioactive compounds with IC50 less than 20 μg/ml are considered to be sufficient for anti‐cancer agents 27, 28. Cells were cultured in presence of the various forms of vitamin E in humidified atmosphere of 5% CO2 in air at 37 °C for 72 h. Control cultures contained cells and medium alone. After 72 h, cells were washed in 0.9% NaCl to remove non‐adherent dead cells and were lysed [2.5 mm HEPES (Calbiochem, San Diego, CA, USA) buffer, 1.5 m MgCl2 (MERCK, Darmstadt, Germany) and zap‐oglobin ΙΙ lytic (Beckman Coulter, Brea, CA, USA)] for 15 min. Released nuclei were suspended in isoton III (Beckman Coulter) and counted using a Coulter particle counter ZI (Beckman Coulter) with particle size set at >5 μm. All cell counts were carried out in triplicate on triplicate well treatments.
Determination of cell death mechanisms
A commercial cell death detection ELISA kit (Roche Diagnostic Gmbh, Mannheim, Germany) was used to detect mechanisms of cell death as previously described 29. These human breast cancer cells were seeded at 1 × 104cells/well and incubated overnight in humidified atmosphere of 5% CO2 in air at 37 °C. Following this, 100 μl vitamin E at required concentrations was added and incubated for 24 or 48 h. Treatment with Paclitaxel, a widely used chemotherapeutic compound, was used as positive control. For this part of the study, IC50 concentrations of the various vitamin E forms were used (i) MDA‐MB‐231(TRF: 8.5 μg/ml, TEF: 3.7, α‐tocotrienol: 9.6 μg/ml, δ‐tocotrienol: 6.9 μg/ml, γ‐tocotrienol: 4.7 μg/ml, Paclitaxel: 1.3 μg/ml) and (ii) MCF‐7 (TRF: 4.6 μg/ml, TEF: 3.4, α‐tocotrienol: 11.1 μg/ml, δ‐tocotrienol: 6.8 μg/ml, γ‐tocotrienol: 6.4 μg/ml, paclitaxel: 1.3 μg/ml). As IC50 value could not be determined for α‐tocopherol here, we used the highest concentration tested in the study −20 μg/ml. Higher concentration of α‐tocopherol was not evaluated as natural compounds with IC50 greater than 20 μg/ml are considered to be unsuitable as anti‐cancer agents 27, 28. Where apoptotic versus necrotic effects of the various forms of vitamin E were compared, fixed concentration (10 μg/ml) was used. This concentration of vitamin E was chosen on the basis of previous studies, which had shown that it had an effect on cancer cells 20, but produced no adverse effect on normal cells 30. Results are presented as enrichment factor of mono and oligonucleosomes calculated using the following formula, provided by the manufacturer:
[mU= absorbance]
Determination of PARP cleavage by western blot analysis
The MDA‐MB‐231 and MCF‐7 cells (5 × 106) were seeded in suitable culture flasks overnight, then, were cultured with the various test compounds at 10 μg/ml for 24 h. Following this, cells were treated with 1 nM TNF‐α (Miltenyl Biotec GmbH, Bergisch Gladbach, Germany) for 30 min, followed by harvesting and washing in ice‐cold PBS and re‐suspension in ice‐cold whole cell lysis buffer (10 mm Tris‐HCl pH7.4, 1.92 mm MgCl2, 1 mm EDTA pH 8.0, 50 mm NaCl, 6 mm β‐mercaptoethanol and 2% Triton X‐100) containing 1% protease inhibitor cocktail (Calbiochem, San Diego, CA, USA). Suspension were incubated on ice for 30 min with intermittent vortexing before centrifugation (10 000 rpm, 12 min). Supernatants containing whole cell extract were transferred to pre‐chilled microfuge tubes. Protein estimation was performed using the Bradford method, then 40 μg whole cell protein was resolved on 10% SDS‐PAGE gels and electroblotted on to nitrocellulose membranes (Bio‐Rad, Hercules, CA, USA) using a semi‐dry transfer system (Bio‐Rad). Membranes were then blocked with 3% milk (Bio‐Rad) in PBST for 45 min before probing with rabbit polyclonal antibody to PARP (ab6079, Abcam, Cambridge, MA, USA) in 3% BSA overnight at 4 °C with constant agitation. Following a washing step, membranes were incubated in HRP‐conjugated goat anti‐rabbit polyclonal secondary IgG (ab6721, Abcam) in 3% BSA for 2 h at room temperature with constant agitation. Membranes were developed for visualization using ECL solution according to manufacturer's instructions (GE Healthcare Bio‐Science, LittleChalfont, UK). Following primary antibody visualization, membranes were used for loading control GAPDH detection after stripping. Membranes were incubated in stripping buffer, re‐blocked and probed with primary rabbit polyclonal anti‐GAPDH (ab9385, Abcam), followed by steps as described above. Bands on autoradiography hyperfilms were quantified using ImageJ software and normalized against GAPDH.
Determination of PARP cleavage by ELISA
A commercial ELISA kit was used to determine levels of cleaved PARP‐1 (Asp214), according to the manufacturer's protocol (PathScan, Cell Signaling Technology Inc Beverly, USA). This assay detects endogenous cleaved PARP‐1, using a sandwich immunoassay format. Both MDA‐MB‐231 and MCF‐7 human breast cancer cells (1 × 107) were harvested and seeded in 10 cm petri dishes. Cells were incubated overnight, then treated with test compounds at 10 μg/ml for 24 h. This was followed by treatment with 1 nM TNF‐α for 30 min. Then, cells were washed briefly in ice‐cold PBS and re‐suspended in ice‐cold cell lysis buffer (20 mm Tris (pH 7.5), 150 mm NaCl,1 mm ethylene diamine tetraacetate (EDTA), 1 mm ethylene glycol‐bis(2 aminoethyl)‐N,N,N′,N′‐tetraacetic acid (EGTA), 1% Triton X‐100, 2.5 mm sodium pyrophosphate, 1 mm β‐glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin) containing 1 mm PMSF (Calbiochem). Cell lysate was prepared according to the manufacturer's protocol. Cells were scraped and cell suspensions were incubated on ice, and sonicated before centrifugation to collect cell lysate. Protein estimation was preformed using DC protein assay, according to manufacturer's protocol (Bio‐Rad Laboratories). Lysate with 1 μg/μl protein concentration was added to microwells of 96‐well plates coated with cleaved PARP (Asp214) antibody (provided with the kit), and incubated overnight to allow binding of endogenous cleaved PARP to antibody‐coated wells. After a washing step, PARP detection antibody was added, then HRP‐conjugated antibody. Finally, chromagen TMB substrate was added to allow development of colour, which was then halted by addition of the stop solution. Spectrometric absorbance of samples was measured at 450 nm; results are presented as percentage of cleaved PARP.
Determination of NF‐κB activation by ELISA
MDA‐MB‐231 and MCF‐7 cells were harvested and seeded at 2 × 106cells/well in 6‐well plates; these were incubated overnight and treated with 10 μg/ml of test compounds for 24 h. Then cells were exposed to 1 nM TNF‐α for 30 min; media used for treatment was discarded and fresh media without treatment solutions were added to allow cells to stabilize and prevent cell stress. Following this, nuclear protein was extracted using a commercial nuclear extraction kit according to manufacturer's protocol (Panomics, Affymetrix Inc, Santa Clara, CA, USA). Protein estimation was performed using DC Protein Assay (Bio‐Rad laboratories) according to manufacturer's protocol. NF‐κB transcription factor activity was analysed using a commercial ELISA kit to quantify the NF‐κB p65, according to the manufacturer's recommended procedure (Panomics) as previously described 31. Briefly, activated NF‐κB p65 molecules from 1 μg/μl nuclear protein were applied to NF‐κB p65 TF binding probe on biotinylated oligonucleotide to form TF‐DNA complexes for 30 min. To capture TF‐DNA complexes, these samples were then transferred to streptavidin‐coated plates and incubated for an hour, followed by primary antibody directed to NF‐κB p65, and incubated a further hour. Subsequently, HRP‐conjugated secondary antibody was left to react for another hour before addition of tetramethylbenzidine (TMB) substrate. TMB chromagen becomes blue in colour upon oxidation with hydrogen peroxide catalysed by HRP. Yellow colouration is then formed after addition of the specific stop solution, containing phosphoric acid. Spectrometric absorbance of samples was measured at 450 nm; results are presented as fold change against control.
Statistical analysis
Results were calculated as average of mean ± standard deviation; one‐way analysis of variance (ANOVA) was used to assess differences between groups. Differences among treatments were tested by Tukey HSD. Results were considered statistically significant when P < 0.05.
Results
Anti‐proliferative activity of vitamin E isomers on human breast cancer cells
Treatment with TRF, TEF and tocotrienol analogues inhibited proliferation of both MDA‐MB‐231(Fig. 1a) and MCF‐7 (Fig. 1b) cells in a dose‐dependent manner. In contrast, α‐tocopherol did not show any inhibition of cell proliferation at concentrations tested (0 to 20 μg/ml). Complete suppression of expansion was observed when MDA‐MB‐231 cells were cultured in the presence of 15 μg/ml of TRF, TEF and the three natural forms (α‐, δ‐ and γ‐) of tocotrienol. With MCF‐7 cells, complete growth suppression was also achieved when concentrations of 15 μg/ml of TEF, δ‐tocotrienol and γ‐tocotrienol. In some cases, cell numbers fell below the plating density. Based on IC50 values obtained (Table 1), efficiency of palm oil vitamin E to suppress proliferation of MDA‐MB‐231 cells reduced in the following order: TEF > γ‐tocotrienol > δ‐tocotrienol > TRF > α‐tocotrienol, whilst for MCF‐7 cells, it reduced as: TEF > TRF > γ‐tocotrienol ≥ δ‐tocotrienol > α‐tocotrienol were used.
Figure 1.
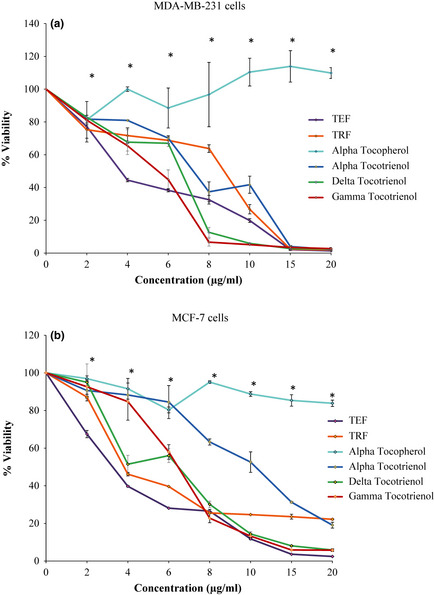
Antiproliferative activity of palm vitamin E on human breast cancer cells. The (a) MDA‐MB‐231 and (b) MCF‐7 human breast cancer cells were co‐cultured with increasing concentrations (0–20 μg/ml) of the test compounds at 37 °C in a humidified 5% CO 2 incubator for 72 h. The viable cells were assayed using Coulter Particle Counter. Points indicate triplicate the mean of triplicate counts/well ± STD for triplicates in each treatment group. All values are significantly different (P < 0.05) from the control group except for *.
Table 1.
Effect of Various Individual Test Compounds on Human Breast Cancer Cell Lines
| IC50 values (μg/ml) | ||
|---|---|---|
| Test Compound | MDA‐MB‐231 | MCF‐7 |
| TRF | 8.5 ± 0.2 | 4.55 ± 0.7 |
| TEF | 3.7 | 3.4 ± 0.2 |
| Alpha Tocopherol | * | * |
| Alpha Tocotrienol | 9.55 ± 1.1 | 11.05 ± 0.45 |
| Delta Tocotrienol | 6.9 ± 0.3 | 6.8 ± 0.3 |
| Gamma Tocotrienol | 4.7 ± 0.8 | 6.35 ± 0.15 |
| Paclitaxel | 1.25 | 1.265 ± 0.005 |
IC50 indicates treatment dose that induced a 50% cell growth inhibition as compared to controls over the 3 days treatment period. The experiment was done in replicates and the values are given as average.
not achieved
Cell death mechanisms of palm vitamin E
For this part of the study, IC50 concentration of the various test compounds we had initially determined, was used. As IC50 value could not be determined for α‐tocopherol, we used the highest concentration tested (20 μg/ml) for this isomer. Paclitaxel, a well‐established anti‐cancer drug that has been reported to have anti‐proliferative effects on both MDA‐MB‐231 and MCF‐7 human breast cancer cell lines 32, was used as positive control in this assay, untreated cancer cells served as negative control. Following 24 h treatment, marked induction of apoptosis was observed following treatments with tocotrienol analogues on both MDA‐MB‐231 (Fig. 2a) and MCF‐7 (Fig. 2a) cells, compared to Paclitaxel. Following 48 h treatment (Fig. 2b), degrees of apoptosis induced in both cancer cell lines by the tocotrienol analogues was greater (P < 0.05) than that of Paclitaxel. Efficiency of tocotrienols to induce apoptosis at their respective IC50 values in MDA‐MB‐231 cells reduced in the following order: γ‐tocotrienol > α‐tocotrienol > δ‐tocotrienol, whilst for MCF‐7 cells, it was: γ‐tocotrienol > δ‐tocotrienol > α‐tocotrienol.
Figure 2.
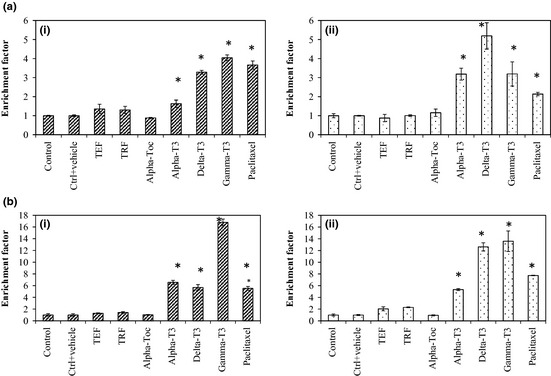
Apoptotic effect of vitamin E on human breast cancer cells treated at IC50 concentrations. The apoptotic effect of of vitamin E (IC 50 concentrations) on (i) MDA‐MB‐231 and (ii) MCF‐7 cells at after a) 24 h and b) 48 h. Results are shown as the mean ± STD from triplicate cultures. (*) values are significantly different from the control group, P < 0.05 (one‐way ANOVA).
Using the same kit, we also evaluated apoptotic versus necrotic cell death at a high concentration (10 μg/ml) for all test compounds studied. In MDA‐MB‐231 cells (Fig 3a), it appeared that cell death could happen via both apoptosis and necrosis when the cells were treated with Paclitaxel, TEF or any of the three tocotrienol analogues (α‐, δ‐ or γ‐) compared to untreated control cells. However, levels of apoptotic death were notably higher compared to necrotic death. For MCF‐7 cells (Fig 3b), there was significant (P < 0.05) increase in apoptotic death in all treated [Paclitaxel], TEF or any of the three tocotrienol forms (α‐, δ‐ or γ‐) cells and levels of necrotic death were not significantly different (P > 0.05) compared to the control group. The three tocotrienol analogues (α‐, δ‐ or γ‐) and TEF, exhibited higher apoptotic effects compared to Paclitaxel, whilst necrotic patterns between these vitamin E analogues and Paclitaxel were similar. Hence, it appears that execution of death of the cancer cells by these compounds was predominantly apoptotic. Efficiency of palm oil vitamin E to induce apoptosis at 10 μg/ml in MDA‐MB‐231 cells reduced in the following order: TEF > α‐tocotrienol >δ‐tocotrienol > γ‐tocotrienol whilst for MCF‐7 cells, it was: α‐tocotrienol > δ‐tocotrienol > TEF > γ‐tocotrienol.
Figure 3.

Mode of cell death of human breast cancer cells treated with vitamin E isomers for 72 h. The mode of cell death induced in (a) MDA‐MB‐231 and (b) MCF‐7 cells co‐cultured with various treatments of vitamin E (10 μg/ml) for 72 h. Results are shown as the mean ± STD from triplicate cultures. (*) values are significantly different from the control group, P < 0.05 (one‐way ANOVA).
Poly(ADP‐ribose) polymerase cleaving activity of tocotrienols
Poly(ADP‐ribose) polymerase (PARP) is a protein involved in a number of cell processes involving mainly DNA repair and programmed cell death. Its cleavage indicates presence of an apoptotic event. As here, tocotrienols were seen to induce apoptosis by formation of inter‐nucleosomal DNA fragments in both MDA‐MB‐231 and MCF‐7 cells, we further studied mechanisms of apoptosis‐induction by PARP cleavage, a well‐known apoptotic marker. To observe PARP cleavage activity, the ELISA method was employed to detect the 89 kDa cleaved fragment ‐ the catalytic domain. PARP cleavage was evident as early as 24 h using 10 μg/ml of tocotrienols (Fig 4). Here, efficacy of palm oil vitamin E to induce PARP cleavage at 10 μg/ml at 24 h in MDA‐MB‐231 cells reduced in the following order: TEF > TRF > γ‐tocotrienol / α‐tocotrienol > δ‐tocotrienol, whilst for MCF‐7cells, it was: TEF > γ‐tocotrienol / α‐tocotrienol > δ‐tocotrienol > TRF. For further assessment of the observed effect, we conducted western blot analysis. This was found to be consistent with the appearance of cleaved PARP bands by western blot assay, further confirming that tocotrienols were indeed an effective agent in inducing apoptosis via PARP cleavage. Fig. 5 shows that 116 kDa PARP was cleaved revealing the 26 kDa cleaved fragment of the DNA‐binding domain, on treatment with tocotrienols. In this study, we report that MCF‐7 cells were more sensitive towards tocotrienols compared to MDA‐MB‐231 cells, based on appearance of 116 kDa PARP bands.
Figure 4.

Apoptosis induction by PARP cleavage following Vitamin E treatment. The (a) MDA‐MB‐231 and (b) MCF‐7 human breast cancer cells were co‐cultured for 24 h with 10 μg/ml of various isomers of vitamin E. These cells were briefly exposed to 1 nm TNF‐α for 30 min. The PARP cleavage activity was determined using ELISA method. Results are shown as the mean ± STD from duplicate cultures. (*) values are significantly different from the control group, P < 0.05 (one‐way ANOVA).
Figure 5.
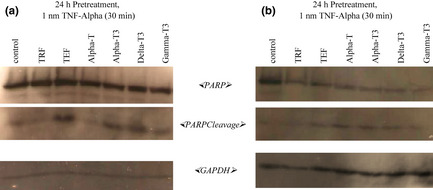
Apoptosis induction by PARP cleavage with Vitamin E treatment. The (a) MDA‐MB‐231 and (b) MCF‐7 human breast cancer cells were co‐cultured for 24 h with 10 μg/ml of various isomers of vitamin E. These cells were briefly exposed to 1 nm TNF‐α for 30 min. The PARP cleavage activity was determined using the western blotting method.
Effect of palm oil vitamin E on TNF‐alpha induced NF‐κB expression
Following the above, we studied effects of test compounds on NF‐κB expression by ELISA, as γ‐tocotrienol has been reported to suppress NF‐κB activation in various cell lines 20. Thus, we speculated that tocotrienols could lead to suppression of NF‐κB expression, leading to anti‐proliferative effects and potentiation of apoptosis. Interestingly, tocotrienols as well as α‐tocopherols equally suppressed TNF‐α‐induced NF‐κB expression in both MDA‐MB‐231 and MCF‐7 cell lines (Fig 6).
Figure 6.
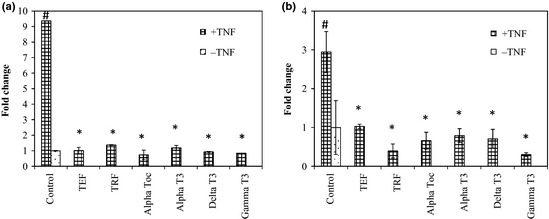
Effect of Vitamin E on TNF‐α activated NF‐κB expression. The (a) MDA‐MB‐231 and (b) MCF‐7 human breast cancer cells were co‐cultured for 24 h with 10 μg/ml of various isomers of vitamin E. These cells were briefly exposed to 1 nM TNF‐α for 30 min. Nuclear extracts were prepared and analysed for NF‐κB expression by ELISA. Results are shown as the mean ± STD from duplicate cultures. (*) values are significantly different from the control +TNF group, (#) values are significantly different from the control group, p < 0.05 (one‐way ANOVA).
Discussion
There are numerous studies on tocotrienols that have provided convincing and prominent evidence on their anti‐proliferative properties on breast cancer cells 18, 19, 22, 33, liver 34, 35, prostate 21, 25, 36, skin 37, colon 38, blood 38, lung 37, 40, lymph gland 39 and myeloid 20 malignancies. Tocotrienols have been found to inhibit expansion of human breast cancer cell cultures, irrespective of oestrogen status, whilst alpha‐tocopherol displays no inhibitory effect on the same cell lines 21. Recently, it was shown that tocotrienols preferentially inhibited androgen‐independent human prostate cancer cells and α‐tocopherols were found to have no effect 24. There is also compelling evidence from experimental models of breast cancer that tocotrienols display greater biopotency compared to tocopherols 18.
In the first part of this study, we evaluated anti‐proliferative effects of various natural forms of vitamin E found in palm oil, on two human breast cancer cell lines, namely the highly aggressive triple negative oestrogen‐independent MDA‐MB‐231 and oestrogen‐dependent MCF‐7 cells. Most initial anti‐cancer studies had used the TRF form of vitamin E–a mixture of various forms of tocotrienols (70%), and α‐tocopherol (30%). Although TRF showed anti‐cancer activity, contributions of various analogues of vitamin E were not clear. Hence here, anti‐cancer effects of TRF, TEF, α‐tocopherol and three analogues (α‐, δ‐ and γ‐) of tocotrienol as anti‐cancer agents, were compared. Results showed that the ability of palm vitamin E to suppress proliferation of the human breast cancer cells reduced in (i) MDA‐MB231 cells: TEF > γ‐tocotrienol > δ‐tocotrienol > TRF >α‐tocotrienol and in (ii) MCF‐7 cells: TEF > TRF > γ‐tocotrienol ≥ δ‐tocotrienol > α‐tocotrienol. Similar anti‐proliferative and cytotoxic patterns had been previously demonstrated in other studies 18, 41. In addition, a number of studies have shown that δ‐tocotrienol and γ‐tocotrienol possess higher anti‐proliferative activity 33, 42, 43, supporting the findings of this study.
In nature, many natural compounds have cytotoxic and chemopreventative effects, but not all are able to trigger apoptosis. Findings from the current study show that tocotrienols inhibited cell proliferation by inducing apoptosis in both human breast cancer cell lines tested. This was determined by use of a commercial ELISA kit for cell death detection, based on the quantitative sandwich‐enzyme‐immunoassay‐principle, using mouse monoclonal antibodies directed against DNA component of nucleosomes and histones, respectively 44.
Compared to vehicle‐treated controls, tocotrienol analogue‐treated groups at their respective IC50 values, showed significant differences in inducing apoptosis in cells of these two lines, after 48 h treatment. The findings show that ability of the palm tocotrienols to induce apoptosis at their respective IC50 values reduced in the following order for (i) MDA‐MB‐231 cells: γ‐ > α‐ > δ‐tocotrienols and for (ii) MCF‐7 cells: γ‐ > δ‐ > α‐tocotrienols. When levels of apoptotic and necrotic cell death induced in these were compared, we found that apoptotic death was induced notably higher compared to necrosis at the concentration tested (10 μg/ml). This confirmed that the mode of cell death induced by palm oil vitamin E was apoptosis. Also, at the concentration tested (10 μg/ml), there was no detectable cell death activity observed in the cells treated with α‐tocopherol. This finding supports the data that we obtained from anti‐proliferative work where no cell death was observed in both the human breast cancer cells when treated with α‐tocopherol at the concentration tested (0–20 μg/ml).
Poly(ADP‐ribose) polymerase is a protein involved in a number of cell processes involving mainly DNA repair, and programmed cell death, mediated by PARP cleavage, as well as by PARP inhibition. It is also a well‐known apoptotic marker. Majorities of breast carcinoma cells have been found to express high levels of PARP‐1 15. In the study described here, a PARP cleavage assay was used to elucidate that these proteins play a role in induction of apoptosis by vitamin E analogues in the two breast cancer cell lines studied. Results show that the vitamin E analogues induced apoptosis in MCF‐7 and MDA‐MB231 cells via PARP cleavage. The ability of palm oil vitamin E to induce PARP cleavage appeared to reduce in this order in (i) MDA‐MB‐231 cells: TEF > TRF > γ‐tocotrienol/α‐tocotrienol > δ‐tocotrienol and in (ii) MCF‐7 cells: TEF > γ‐tocotrienol/α‐tocotrienol > δ‐tocotrienol > TRF.
Although PARP‐1 is the major target protein for polyADP‐ribosylation, there are other acceptor proteins, such as p53, NF‐κB, histones, DNA ligases, DNA polymerases and DNA‐topoisomerases 45. NF‐κB actvity has been implicated in carcinogenesis because of its critical roles in cell proliferation, differentiation, survival, adhesion and inflammation 20. A dual role for NF‐κB inhibition and apoptosis, has been described for many cell systems. This supports the role of this transcription factor in cell survival. In this study, we found that low concentrations (1 nM) TNF‐alpha can induce expression of NF‐κB in both MCF‐7 and MDA‐MB‐231 cells, and all palm oil vitamin E used in this study can inhibit this activation.
It was somewhat surprising to note that although alpha‐tocopherol did not inhibit proliferation or induce apoptosis in the two human breast cancer cell lines, it potently reduced expression of NF‐κB in both cell type. Levels of inhibition observed were comparable to those observed with the tocotrienols. This could be that at low concentrations (10 μg/ml), α‐tocopherol may be exerting its antioxidant role that promotes cell survival pathways, but negates pro‐apoptotic effects. Perhaps, if higher concentration of α−tocopherol, one that exhibits anti‐cancer effects, were used, a different outcome might be observed. So, there is need to carry out more work in this area to further elucidate the pathways affected by α‐tocopherol. Activities of TEF and TRF mixtures were found to be higher than of pure tocotrienols. This could be because the various analogues of vitamin E found in TRF and TEF may be working via different pathways and hence, by using these compounds, we may be targeting different pathways, which could act synergistically to produce higher effects observed when these compounds are used compared to single forms of the vitamin E.
In summary, palm tocotrienols induce apoptosis in human breast cancer cells, most probably through pathways that involve PARP cleavage and NF‐κB inhibition.
Competing Interests
The authors declare that they have no competing interests.
Authors’ contributions
RL was a postgraduate student working on this project, who did all the laboratory work. AR, RL, KN and KS were involved in project conceptualization and design. RL and AR prepared the manuscript. KN and KS helped to edit the manuscript. All authors had read and approved the manuscript.
Acknowledgements
This work was supported by research grants from the International Medical University (IMU) and the Malaysian Palm Oil Board (MPOB).
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P (2001) Estimating the world cancer burden: Globocan 2000. Int. J. Cancer 94, 153–156. [DOI] [PubMed] [Google Scholar]
- 2. Hisham AN, Yip CH (2004) Overview of breast cancer in Malaysian women: a problem with late diagnosis. Asian J Surg. 27, 130–133. [DOI] [PubMed] [Google Scholar]
- 3. Hisham AN, Yip CH (2003) Spectrum of breast cancer in Malaysian women: overview. World J. Surg. 27, 921–923. [DOI] [PubMed] [Google Scholar]
- 4. Kaufman HL, Wadler S, Antman K (2011) Molecular Targeting in Oncology (Cancer Drug Discovery and Development). Humana Press, Totowa, Nj: 1617376531. [Google Scholar]
- 5. Ricci MS, Wei‐Xing Z (2006) Chemotherapeutic approaches for targeting cell death pathways. Oncologist 11, 342–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fulda S, Gorman Am, Hori O, Samali A (2010) Cellular stress responses: cell survival and cell death. Int. J. Cell Biol. 2010, Article ID 214074, 1–23; doi: 10.1155/2010/214074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tassiulas I, Park‐Min K, Hu Y, Kellerman L, Mevorach D, Ivashkiv LB (2007) Apoptotic cells inhibit LPS‐induced cytokine and chemokine production and IFN responses in macrophages. Hum. Immunol. 68, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koh DW, Dawson TM, Dawson VL (2005) Mediation of cell death by poly(ADP‐ribose) polymerase‐1. Pharmacol. Res. 52, 5–14. [DOI] [PubMed] [Google Scholar]
- 9. Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL (2006) Apoptosis‐inducing factor mediates poly(ADP‐ribose) (PAR) polymer‐induced cell death. Proc. Natl Acad. Sci. USA 103, 18314–18319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaitanya GV, Babu PP (2009) Differential PARP Cleavage: an indication of heterogeneous forms of cell death and involvement of multiple proteases in the infarct of focal cerebral ischemia in rat. Cell. Mol. Neurobiol. 29, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hassa PO, Hottiger MO (2002) The functional role of poly(ADP‐ribose)polymerase 1 as novel coactivator of NF‐κB in inflammatory disorders. Cell. Mol. Life Sci. 59, 1534–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sodhi RK, Singh N, Jaggi AS (2010) Poly(ADP‐ribose) polymerase‐1 (PARP‐1) and its therapeutic implications. Vascul. Pharmacol. 53, 77–87. [DOI] [PubMed] [Google Scholar]
- 13. Devalaraja‐Narashimha K, Padanilam BJ (2010) PARP1 deficiency exacerbates diet‐induced obesity in mice. J. Endocrinol. 205, 243–252. [DOI] [PubMed] [Google Scholar]
- 14. Virag L, Szabo E, Bakondi E, Bai P, Gergely P, Hunyadi J, Szabo C (2002) Nitric oxide‐peroxynitrite‐poly(ADP‐ribose) polymerase pathway in the skin. Exp. Dermatol. 11, 189–202. [DOI] [PubMed] [Google Scholar]
- 15. Domagala P, Huzarski T, Lubinski J, Gugala K, Domagala W (2011) PARP‐1 expression in breast cancer including BRCA1‐associated, triple negative and basal‐like tumors: possible implications for PARP‐1 inhibitor therapy. Breast Cancer Res. Treat. 127, 861–869. [DOI] [PubMed] [Google Scholar]
- 16. Westerheide SD, Mayo MW, Anest V, Hanson JL, Baldwin AS Jr (2001) The putative oncoprotein Bcl‐3 induces cyclin D1 to stimulate G1 Transition Mol. Cell Biol. 21, 8428–8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nesaretnam K, Guthrie N, Chambers AF, Carroll KK (1995) Effect of tocotrienols on the growth of a human breast cancer cell line in culture. Lipids 30, 1139–1142. [DOI] [PubMed] [Google Scholar]
- 18. McIntyre BS, Briski KP, Gapor A, Sylvester PW (2000) Antiproliferative and Apoptotic Effects of Tocopherols and Tocotrienols on Preneoplastic and Neoplastic Mouse Mammary Epithelial Cells. PSEBM 224, 292–301. [DOI] [PubMed] [Google Scholar]
- 19. Srivastava JK, Gupta S (2006) Tocotrienol‐rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem. Biophys. Res. Commun. 346, 447–453. [DOI] [PubMed] [Google Scholar]
- 20. Ahn KS, Sethi G, Krishnan K, Aggarwal BB (2007) γ‐Tocotrienol inhibits nuclear factor‐κb signaling pathway through inhibition of receptor‐interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 282, 809–820. [DOI] [PubMed] [Google Scholar]
- 21. Nesaretnam K, Stephen R, Dils R, Darbre P (1998) Tocotrienols inhibits the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids 33, 461–469. [DOI] [PubMed] [Google Scholar]
- 22. Sylvester PW, Russell M, Ip MM, Ip C (1986) Comparative effects of different animal and vegetable fats before and during carcinogenesis administration on mammary tumorigenesis, sexual maturation and endocrine function in rats. Cancer Res. 46, 757–762. [PubMed] [Google Scholar]
- 23. Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Canali R, Virgili F (2004) Tocotrienol‐Rich Fraction from palm oil affects gene expression in tumors resulting from MCF‐7 cell inoculation in athymic mice. Lipids 39, 459–467. [DOI] [PubMed] [Google Scholar]
- 24. Nesaretnam K, Koon TH, Selvaduray KR, Bruno RS, Ho E (2008) Modulation of cell growth and apoptosis response in human prostate cancer cells supplemented with tocotrienols. Eur. J. Lipid Sci. Technol. 110, 23–31. [Google Scholar]
- 25. Yu FL, Gapor A, Bender W (2005) Evidence for the preventive effect of the polyunsaturated phytol side chain in tocotrienols on 17β‐estradiol epoxidation. Cancer Detect. Prev. 29, 383–388. [DOI] [PubMed] [Google Scholar]
- 26. Aggarwal BB, Sundaram C, Prasad S, Kannappan R (2010) Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem. Pharmacol. 80, 1613–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cordell GA, Kinghorn AD, Pezzuto JM (1996) Separation, structure elucidation, and bioassay of cytotoxic natural products In: Colegate SM, Molyneux RJ, eds. Bioactive natural products: Detection, isolation and structural determination, pp. 196–216. London: CRC Press. [Google Scholar]
- 28. Chiang LC, Chiang W, Chang MY, Ng LT, Lin CC (2003) Antileukemic activity of selected natural products in Taiwan. Am. J. Chin. Med. 31, 37–46. [DOI] [PubMed] [Google Scholar]
- 29. Er HM, Cheng EH, Radhakrishnan AK (2007) Anti‐proliferative and mutagenic activities of aqueous and methanol extracts of leaves from Pereskia bleo (Kunth) DC (Cactaceae). J. Ethnopharmacol. 113, 448–456. [DOI] [PubMed] [Google Scholar]
- 30. Yam ML, Abdul Hafid SR, Cheng HW, Nesaretnam K (2009) Tocotrienols suppress proinflammatory markers and cyclooxygenase‐2 expression in RAW264.7 macrophages. Lipids, 44, 787–789. [DOI] [PubMed] [Google Scholar]
- 31. Zenhom M, Hyder A, de Vrese M, Heller KJ, Roeder T, Schrezenmeir J (2011) Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco‐2 cells via activation of PPARg and peptidoglycan recognition protein 3. J. Nutr. 141, 971–977. [DOI] [PubMed] [Google Scholar]
- 32. Clarke RB, Laidlaw IJ, Jones LJ, Howell A, Anderson E (1993) Effect of tamoxifen on Ki67 labelling index in human breast tumours and its relationship to oestrogen and progesterone receptor status. Br. J. Cancer 67, 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester PW (2000) Antiproliferative and apoptotic effects of tocopherols and tocotrienols on normal mouse mammary epithelial cells. Lipids 35, 171–180. [DOI] [PubMed] [Google Scholar]
- 34. Sakai M, Okabe M, Yamasaki M, Tachibana H, Yamada K (2004) Induction of apoptosis by tocotrienol in rat hepatoma dRLh‐84 cells. Anticancer Res. 24, 1683–1688. [PubMed] [Google Scholar]
- 35. Sakai M, Okabe M, Tachibana H, Yamada K (2006) Apoptosis induction by γ‐tocotrienol in human hepatoma Hep3B cells. J. Nutr. Biochem. 17, 672–676. [DOI] [PubMed] [Google Scholar]
- 36. Yap WN, Chang PN, Han HY, Lee TW, Ling M, Wong YC, Yap YL (2008) γ‐Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple‐signalling pathways. Br. J. Cancer 99, 1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McAnally JA, Gupta J, Sodhani S, Bravo L, Mo H (2007) Tocotrienols potentiate lovastatin‐mediated growth suppression in vitro and in vivo. Exp Biol Med. 232, 523–531. [PubMed] [Google Scholar]
- 38. Mo H, Elson CE (1999) Apoptosis and cell‐cycle arrest in human and murine tumor cells are initiated by isoprenoids. J. Nutr. 129, 804–813. [DOI] [PubMed] [Google Scholar]
- 39. Birringer M, Lington D, Vertuani S, Manfredini S, Scharlau D, Glei M, Ristow M (2010) Proapoptotic effects of long‐chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free Radic. Biol. Med. 49, 1315–1322. [DOI] [PubMed] [Google Scholar]
- 40. Yano Y, Satoh H, Fukumoto K, Kumadaki I, Ichikawa T, Yamada K, Hagiwara K, Yano T (2005) Induction of cytotoxicity in human lung adenocarcinoma cells by 6‐O‐carboxypropyl‐alpha‐tocotrienol, a redox silent derivative of alpha‐tocotrienol. Int. J. Cancer 115, 839–846. [DOI] [PubMed] [Google Scholar]
- 41. Guthrie N, Gapor A, Chambers AF, Carroll KK (1997) Palm oil tocotrienols and plant flavonoids act synergistically with each other and with Tamoxifen in inhibiting proliferation and growth of estrogen receptor‐negative MDA‐MB‐435 and ‐positive MCF‐7 human breast cancer cells in culture. Asia Pacific J. Clin. Nutr. 6, 41–45. [PubMed] [Google Scholar]
- 42. Viola V, Pilolli F, Piroddi M, Pierpaoli E, Orlando F, Provinciali M, Betti M, Mazzini F, Galli F (2012) Why tocotrienols work better: insights into the in vitro anti‐cancer mechanism of vitamin E. Genes Nutr. 7, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pierpaoli E, Viola V, Pilolli F, Piroddi M, Galli F, Provinciali M (2010) γ‐ and δ‐tocotrienols exert a more potent anticancer effect than α‐tocopheryl succinate on breast cancer cell lines irrespective of HER‐2/neu expression. Life Sci. 86, 668–675. [DOI] [PubMed] [Google Scholar]
- 44. Wu D, Ingram A, Lahti JH, Mazza B, Grenet J, Kapoor A, Liu L, Kidd VJ, Tang D (2002) Apoptotic release of histones from nucleosomes. J. Biol. Chem. 277, 12001–12008. [DOI] [PubMed] [Google Scholar]
- 45. Jagtap P, Szabo C (2005) Poly (ADP‐ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov. 4, 421–440. [DOI] [PubMed] [Google Scholar]


