Abstract
BACKGROUND
The formation of liver fibrosis is mainly caused by the activation of hepatic stellate cells (HSCs) and the imbalance of extracellular matrix (ECM) production and degradation. The treatment of liver fibrosis mainly includes removing the cause, inhibiting the activation of HSCs, and inhibiting inflammation. NOD-like receptor (NLR) family, caspase activation and recruitment domain (CARD) domain containing 5/NOD27/CLR16.1 (NLRC5) is a highly conserved member of the NLR family and is involved in inflammation and immune responses by regulating various signaling pathways such as nuclear factor-κB (NF-κB) signaling. It has been found that NLRC5 plays an important role in liver fibrosis, but its specific effect and possible mechanism remain to be fully elucidated.
AIM
To investigate the role of NLRC5 in the activation and reversion of HSCs induced with transforming growth factor-β (TGF-β) and MDI, and to explore its relationship with liver fibrosis.
METHODS
A total of 24 male C57BL/6 mice were randomly divided into three groups, including normal, fibrosis, and recovery groups. Twenty-four hours after a liver fibrosis and spontaneous reversion model was established, the mice were sacrificed and pathological examination of liver tissue was performed to observe the degree of liver fibrosis in each group. LX-2 cells were cultured in vitro and treated with TGF-β1 and MDI. Real-time quantitative PCR (qPCR) and Western blot were used to analyze the expression levels of NLRC5, α-smooth muscle actin (α-SMA), and collagen type I alpha1 (Col1a1) in each group. The activity of NF-κB in each group of cells transfected with NLRC5-siRNA was detected.
RESULTS
Compared with the normal mice, the expression level of NLRC5 increased significantly (P < 0.01) in the fibrosis group, but decreased significantly in the recovery group (P < 0.01). In in vitro experiments, the content of NLRC5 was enhanced after TGF-β1 stimulation and decreased to a lower level when treated with MDI (P < 0.01). The expression of α-SMA and Col1a1 proteins and mRNAs in TGF-β1-mediated cells was suppressed by transfection with NLRC5-siRNA (P < 0.01). Western blot analysis showed that the expression of NF-κB p65 protein and phosphorylated IκBα (p-IκBα) was increased in the liver of mice in the fibrosis group but decreased in the recovery group (P < 0.01), and the protein level of nuclear p65 and p-IκBα was significantly increased after treatment with NLRC5-siRNA (P < 0.01).
CONCLUSION
NLRC5 may play a key role in the development and reversal of hepatic fibrosis through the NF-κB signaling pathway, and it is expected to be one of the clinical therapeutic targets.
Keywords: NLRC5, Hepatic stellate cells, Liver fibrosis, Recovery
Core tip: Liver fibrosis is a pathological tissue repair process characterized by excessive deposition of extracellular matrix in the liver, accompanied by inflammation, and eventually progresses to cirrhosis. Several recent reports have shown that effective treatment can reverse liver fibrosis, which is associated with inactivation of hepatic stellate cells (HSCs) and multiple signaling pathways. NOD-like receptor (NLR) family, caspase activation and recruitment domain (CARD) domain containing 5/NOD27/CLR16.1 (NLRC5) is the largest member of the NLR family and is highly expressed in immune tissues or organs such as the spleen, lung, thymus, and liver, mediating inflammation inhibition and antiviral response. This study aimed to investigate the role of NLRC5 in activating and devitalization of HSCs and its mechanism. The results demonstrate that NLRC5 may be involved in the development and reversal of liver fibrosis by negative regulation of nuclear factor-κB.
INTRODUCTION
Liver fibrosis is a common consequence of long-term liver repair after injury caused by different etiologies[1-3]. The condition of liver fibrosis assumes the chronic process and continually progresses in the presence of injury factors. Ultimately, it will lead to a series of serious complications and increase the mortality rate related to liver cirrhosis[4,5]. With the gradual understanding of the fibrosis mechanism, a series of treatment measures such as removing the cause and inhibiting the activation of hepatic stellate cell (HSC) have been applied to clinical therapy[6-9]. Several recent reports have shown that frequent activation of HSCs for a long time will lead to extensive deposition of collagen fibers, resulting in liver fibrosis and liver dysfunction[10]. Transforming growth factor-β1 (TGF-β1) plays an important role in the activation of HSCs and is considered to be one of the most important fibrotic factors because it can inhibit extracellular matrix (ECM) degradation while promoting ECM synthesis[11-14]. It is well known that clearly knowing genes related to hepatic fibrosis, blocking the persistent damage of the liver, and then reversing liver fibrosis can be helpful in the treatment of this disease. The reversal of liver fibrosis is controversial for a long time. Studies in recent years, however, have found that liver fibrosis is reversible, and even patients with advanced liver fibrosis may return to normal, which has a correlation with the degradation of ECM and various signaling pathways[15-21]. Moreover, Abidali et al[22] confirmed that rat liver fibrosis can be automatically reversed after stopping the injection of carbon tetrachloride (CCl4), but the possible mechanism of progression and reversal of liver fibrosis was not analyzed. Our previous study found that NLRC5 protein levels fluctuated abnormally during progression and reversal of liver fibrosis. As the largest member of the NOD-like receptor (NLR) family, NLRC5 is widely expressed in various tissues and cell lines of humans and mice, but is mainly concentrated in immune cells and immune-related tissues[23,24]. Current studies on NLRC5 are mostly focused on the regulation of major histocompatibility complex (MHC) I gene expression and the participation in the antiviral innate immune response[25,26]. In the study by Catalano et al[27], NLRC5 can negatively regulate the nuclear factor-κB (NF-κB) signaling pathway by inhibiting the phosphorylation of κB-inhibiting protein kinase alpha antibody (IKKα) and κB-inhibiting protein kinase beta antibody (IKKβ), while the activation of this signaling pathway is closely related to the activation of HSCs. These results suggest that NLRC5 is involved in the development of liver fibrosis, however, there are few reports on the role of NLRC5 in liver fibrosis. Therefore, this study aimed to investigate the role and mechanism of NLRC5 in HSC activation and reversal, and to explore its relationship with liver fibrosis to evaluate its clinical application value.
MATERIALS AND METHODS
Animal model
A total of 24 male C57BL/6 mice weighing 16-18 g were provided as the CCl4 liver injury model by the First Affiliated Hospital of Zhengzhou University. Animals were randomly divided into the following groups: normal mice (normal group), mice with liver fibrosis (fibrosis group), and mice with reversal of liver fibrosis (recovery group). Before the experiments, they were placed in a clean animal room at 24 °C with free access to water and food for one week. Fibrosis was induced in mice by subcutaneous administration of CCl4 (10% CCl4 in olive oil, Shanghai Zhanyun Chemical Co., Ltd.) at a dose of 2 mL/kg, 3 times a week for 6 wk, and in the recovery group, it was discontinued for another 4 wk. The normal group was injected with the same dose of olive oil for the same treatment time. The mice were sacrificed at 24 h after the last injection of CCl4 and liver tissues were excised to confirm the successful establishment of the fibrosis model.
Cell culture
Human hepatic stellate cell line LX-2 (Shanghai Yaji Biotechnology Co., Ltd.) was cultured in DMEM medium (Gibco) containing 10% fetal bovine serum (FBS, Gibco), 100 IU/mL penicillin/streptomycin, and 1% glutamine. Then, the medium was placed in an incubator with 5% CO2 and 70%-80% humidity at 37 °C. In accordance with the routine culture, the medium was changed daily. Digestion and passage were performed until 80% confluence of cells. Cells in the logarithmic growth phase were used in experiments. Four groups of LX-2 cells were studied: (1) TGF-β1: treated with recombinant human 5 ng/mL TGF-β1 (PeproTech, United States) and cultivated for 24 h; (2) Control: normal cells; and (3) TGF-β1 + MDI: MDI (50 μL 3-isobutyl-1-methylxanthine + 10 μL dexamethasone + 576.7 μL insulin, Sigma, United States) was added into culture of LX-2 cells for 48 h following treatment with 2.5 ng/mL TGF-β1.
Histological examination
The fresh liver tissues of mice were removed and fixed in fixative solution prepared (10% formalin, Bouin's fixative) to denature and coagulate tissue and cellular proteins. Then, the tissue blocks were dehydrated using graded ethanol, cleared in xylene, embedded in paraffin, and sectioned at 5 μm thickness. After xylene dewaxing and gradient ethanol hydration, hematoxylin and eosin staining and Masson staining (Shanghai Ruchuang Biotechnology Co., Ltd.) were performed to observe the histopathological changes of the liver.
Immunohistochemistry
Routine xylene dewaxing, gradient ethanol hydration, and citric thermal remediation for 15 min were done and paraffin-embedded liver tissue was handled with 3% H2O2 for 10 min to block endogenous peroxidase activity. Then, normal sheep serum was used to seal up the nonspecific sites. After that, the sections were incubated with primary antibody against α-SMA (dilution, 1:400; Shanghai Ru Chuang Biotechnology Co., Ltd.) overnight at 4 °C, followed by incubation with a biotinylated secondary antibody (Shanghai Ruchuang Biotechnology Co., Ltd.) for 60 min at room temperature. The sections were stained with DAB, counterstained with haematoxylin, finally mounted, and observed under a microscope.
SiRNA transfection
The design and synthesis of specific siRNA for NLRC5 were assisted by Shanghai Hengfei Biotechnology Co., Ltd. The NLRC5-siRNA sense sequence was 5'-GGG ACTGAGAGCTTTGTAT-3', and the antisense sequence was 5'-CGC ACCCTAGACTGAAA-3'. The sense sequence of the NLRC5-siRNA negative control was 5'-UUCUCCGAACGUGUCACGUTT-3', and the antisense was 5'-ACG UGACACGUUCGGAGAATT-3'. LX-2 cells were cultured in DMEM medium with 10% FBS at a density of 2 × 105 cells/mL. The diluted LipofectamineTM 2000 (Invitrogen, United States) were mixed with the diluted siRNA oligomer and incubated for 20 min, then the mixture was added into the medium. The medium was changed at 6 h post-transfection, and TGF-β1 was added at a concentration of 10 ng/mL to culture for an additional 48 h. Q-PCR and Western blot were used to detect the expression of NLRC5, and normal LX-2 cells transfected with NLRC5-siRNA negative control were used as controls.
Real-time fluorescent quantitative PCR
Total RNA was extracted from liver tissue and LX-2 cells using Trizol reagent (Invitrogen, United States) according to the manufacturer’s instructions. cDNA was synthesized using a reverse transcription kit (Dalian Bao Bioengineering Co., Ltd.), and the target gene was detected using an SYBR Green Fluorescence Quantitation Kit (Dalian Bao Bioengineering Co., Ltd.). The 25-μL PCR reaction system consisted of 1 μL of upstream and downstream primers (1 μmol/L), 1 μL of cDNA product, 2.5 μL of Taq 10 × Buffer, 1 μL of Taq DNA polymerase, and 3 μL of dNTP mixture, and the remaining volume was supplemented with deionized water. The amplification parameters were pre-denaturation at 94 °C for 5 min and 35 cycles of denaturation at 94 °C for 5 s, annealing at 59 °C for 30 s, and extension at 72 °C for 1 min. β-actin was used as an internal reference gene, and the Ct value of each sample was analyzed. The primer sequences were designed according to the Genbank database and using Prime 5 software, and the sequences used are as follows: NLRC5 forward, 5’-CTATCAACTGCCCTTCCACAAT-3’ and reverse, 5’-TCTCTATCTGCCCACAGCC-TAC-3’; α-SMA forward, 5’-CTATTCCTTCGTGACTACT-3’ and reverse, 5’-ATGCTGTTATAGGTGGTGGTT-3’; Col1a1 forward, 5’-CCCGGGTTTCAGAG-ACAACTTC-3’ and reverse, 5’-TCCACATGCTTTATTCCAGCAATC-3’; β-actin forward, 5’-GAGGCACTCTTCCAGCCTTC-3’ and reverse, 5’-GGATGTC CACGTCACACTTC-3’.
Western blot
Nuclear and cytoplasmic proteins were obtained from mouse liver tissues and cells using radioimmunoprecipitation assay (RIPA) lysis buffer and PMSF (100:1 Beijing Saibaisheng Gene Technology Co., Ltd.). The protein contents of the samples were determined by the bicinchoninic acid (BCA, Beijing Saibaisheng Gene Technology Co., Ltd.) method. Twenty micrograms of protein samples were separated by 10% SDS-PAGE, and transferred to polyvinylidene fluoride membranes. Following blocking with 5% skim milk for 1 h at room temperature, the membranes were incubated with primary antibodies against NLRC5 (Shanghai Youningwei Biotechnology Co., Ltd.) at 1:3000 dilution, α-SMA (Shanghai Gefan Biotechnology Co., Ltd.) at 1:3000 dilution, Col1a1 (Shanghai Gefan Biotechnology Co., Ltd.) at 1:3000 dilution, β-actin (Santa Cruz) at 1:1000 dilution, IκBα (Cell Signal) at 1:2000 dilution, p-IκBα (Cell Signal) at 1:2000 dilution, and p65 (Cell Signal) at 1:2000 dilution overnight at 41 °C. After washing with TBST, diluted IgG antibody conjugated with horseradish peroxidase (1:2000 Santa Cruz) was added to incubate for 2 h at room temperature. The membranes were developed with an enhanced chemiluminescence detection kit (Beijing Saibaisheng Gene Technology Co., Ltd.).
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software, and the experimental data are expressed as the mean ± standard deviation. One-way ANOVA was applied to determine the significant difference, and the differences between groups were tested using the LSD test. P < 0.05 was considered statistically significant.
RESULTS
Histopathological results of liver fibrosis
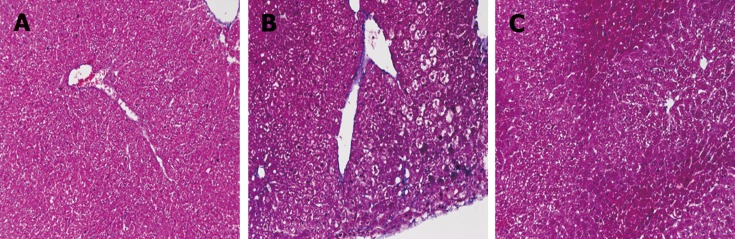
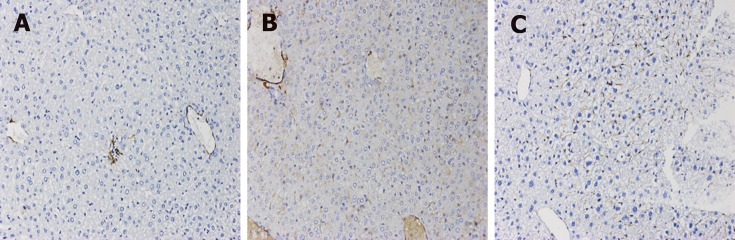
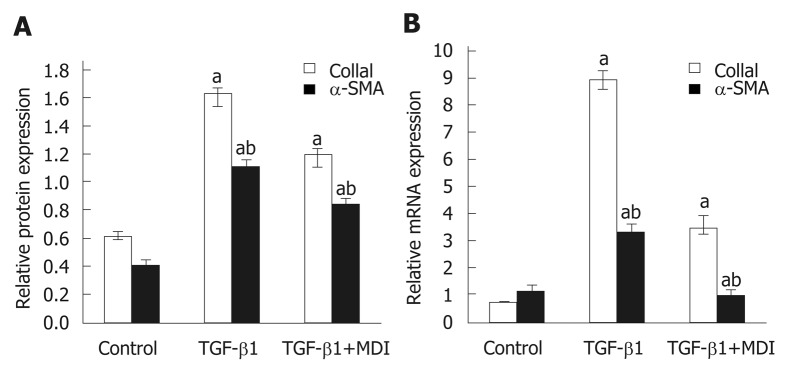
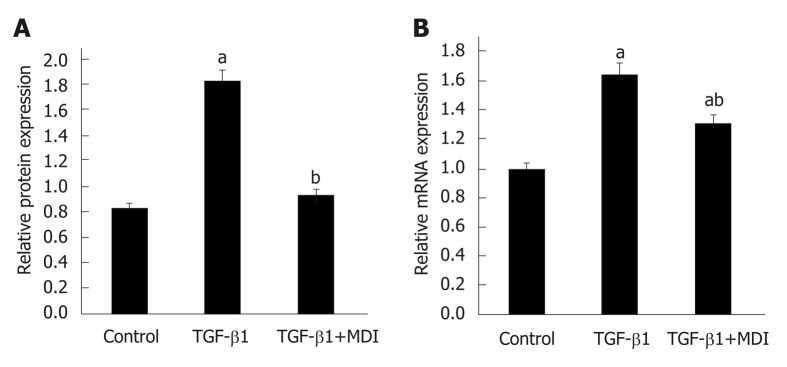
Fibrosis was determined by HE staining and Masson staining. After 6 wk of CCl4 induction, the mouse liver become coarse and dark gray in color. The liver tissue showed relative swelling, punctate necrosis, and regeneration. Infiltration of inflammatory cells, fibrous hyperplasia, steatosis, and ballooning changes were found in the central vein and the portal area, and the structure of partial hepatic lobules was not clear. In contrast, the liver of the normal mice was red in color and well marginated, the structure of the hepatic lobule was normal and integral, the cords of liver cells were arranged orderly, and there was no degeneration, necrosis, or inflammatory cells infiltration. In the recovery group, the contour of liver tissue was fine and the structure became normal (Figure 1). In addition, the results of immunohistochemistry showed that the expression of α-SMA was high in the experimental group, distributed in the cytoplasm of hepatocytes, and aggregated in the portal area, but only a little was observed in the normal and recovery groups (Figure 2). The relative protein and mRNA levels of α-SMA and Col1a1 were increased in LX-2 cells under TGF-β1 stimulation (P < 0.01), while decreased after MDI treatment (P < 0.05). As shown in Figure 3, the successful establishment and reversal of fibrosis model were confirmed.
Figure 1.
Masson staining for assessment of liver tissue in each group (×200). A: Liver tissue from normal mice (normal group); B: Mice with liver fibrosis (fibrosis group); C: Reversal of liver fibrosis in mice (recovery group).
Figure 2.
Immunohistochemical staining for α-SMA in liver tissue in each group (×200). A: Liver tissue from normal mice (normal group); B: Mice with liver fibrosis (fibrosis group); C: Reversal of liver fibrosis in mice (recovery group).
Figure 3.
Expression of α-SMA and Col1α1 in LX-2 cells. A: Relative mRNA expression; B: Quantification of Western blot. aP < 0.05 vs control; bP < 0.05 vs transforming growth factor-β1. TGF-β1: Transforming growth factor-β1.
Expression of NLRC5 in liver tissue and LX-2 cells
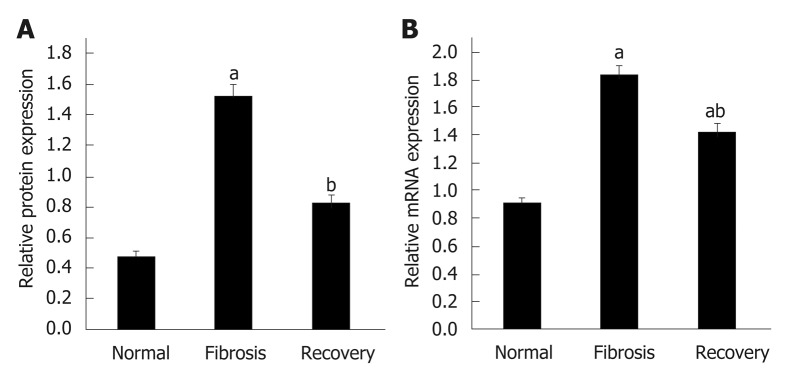
To investigate the differential expression of NLRC5 during fibrosis and reversal model, Western blot and qPCR were used to analyze the relative protein and mRNA expression levels of NLRC5. In contrast with the normal group, the expression level of NLRC5 increased significantly in the fibrosis group (P < 0.01), but significantly decreased in the recovery group (P < 0.01) (Figure 4). For the in vitro experiments, the expression level of NLRC5 was enhanced after TGF-β1 stimulation (P < 0.01), and decreased to a lower level when treated with MDI (P < 0.01) (Figure 5).
Figure 4.
Expression level of NLRC5 in each group of mice. A: Relative mRNA expression; B: Quantification of Western blot. aP < 0.05 vs normal; bP < 0.05 vs fibrosis.
Figure 5.
Expression level of NLRC5 in LX-2 cells. A: Relative mRNA expression; B: Quantification of Western blot. aP < 0.05 vs control; bP < 0.05 vs transforming growth factor-β1. TGF-β1: Transforming growth factor-β1.
Effect of NLRC5-siRNA transfection on the expression of NLRC5 in LX-2 cells
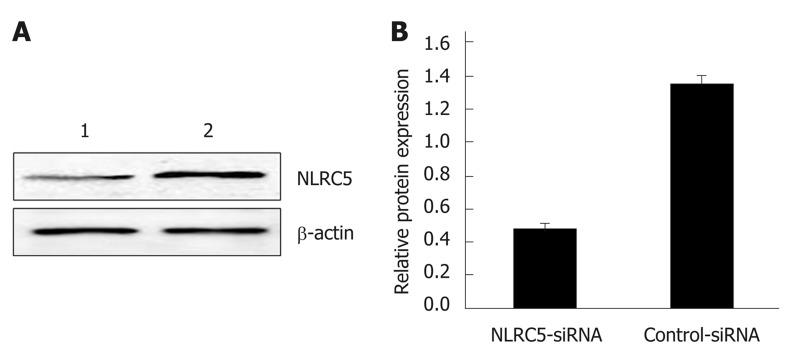
Western blot was applied to confirm whether NLRC5-siRNA was successfully transfected into LX-2 cells. The results showed that the NLRC5 protein level in LX-2 cells transfected with NLRC5-specific siRNA was obviously lower compared with control cells and NLRC5-siRNA negative control (P < 0.01) (Figure 6).
Figure 6.
Effect of NLRC5 knockdown in LX-2 cells. A: Western blot; B: Quantification of Western blot. Lane 1: LX-2 cells transfected with NLRC5-siRNA; Lane 2: LX-2 cells transfected with control-siRNA; aP < 0.05 vs control-siRNA.
Effect of NLRC5 on the expression of α-SMA and Col1a1 in LX-2 cells
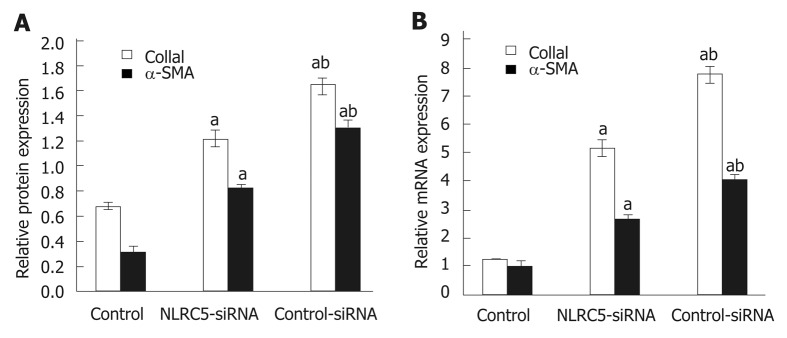
α-SMA is one of the markers of activated HSCs. Q-PCR and Western blot were used to analyze the relationship between the change of NLRC5 in LX-2 cells and the expression of α-SMA. As shown in Figure 7, it was observed that the down-regulation of NLRC5 decreased the expression levels of α-SMA protein and mRNA in LX-2 cells stimulated with TGF-β1 (P < 0.01), and Col1a1 expression was also suppressed by transfection of NLRC5-siRNA (P < 0.01).
Figure 7.
Effect of NLRC5 knockdown on α-SMA and Col1a1 expression in LX-2 cells. A: Relative mRNA expression; B: Quantification of Western blot. aP < 0.05 vs control; bP < 0.05 vs NLRC5-siRNA.
Effect of NLRC5 on NF-κB activity in hepatic fibrosis
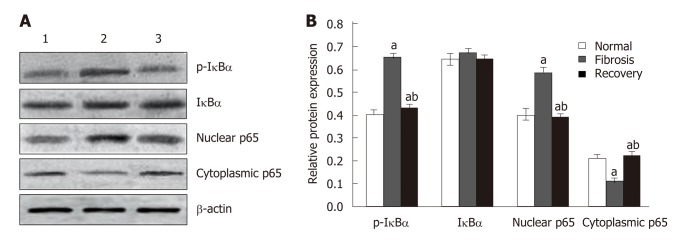
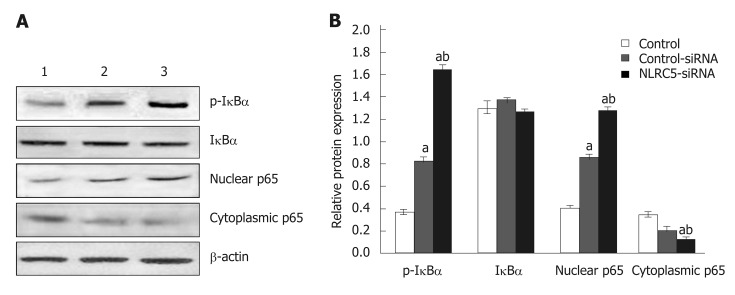
Western blot analysis showed that the expression of p65 and p-IκBα increased in the liver of mice in the fibrosis group (P < 0.01), but decreased in the recovery group (P < 0.05). There was no significant change in total protein of IκBα (Figure 8). To detect the relationship between NLRC5 and the NF-κB signaling pathway during the activation of HSCs, the effect of NLRC5-siRNA on LX-2 cells induced with TGF-β1 was investigated. The results showed that the protein levels of nuclear p65 and p-IκBα were significantly increased after treatment with NLRC5-siRNA (P < 0.01), while the level of cytoplasmic p65 was decreased (P < 0.05), and the expression of IκBα protein was almost unchanged (Figure 9).
Figure 8.
Effect of NLRC5 on nuclear factor-κB activity in mice hepatocytes. A: Western blot; B: Quantification of Western blot. Lane 1: Normal mice (normal group); Lane 2: Mice with liver fibrosis (fibrosis group); Lane 3: Reversal of liver fibrosis in mice (recovery group); aP < 0.05 vs normal; bP < 0.05 vs fibrosis.
Figure 9.
Effect of NLRC5 on nuclear factor-κB activity in LX-2 cells. A: Western blot; B: Quantification of Western blot. Lane 1: Control; Lane 2: LX-2 cells transfected with NLRC5-siRNA; Lane 3: LX-2 cells transfected with control-siRNA; aP < 0.05 vs control; bP < 0.05 vs NLRC5-siRNA.
DISCUSSION
Hepatic fibrosis is a common pathological change in chronic liver injury, involving multiple types of molecules, cells, and tissues[28]. If treated not appropriately, the condition would develop to histological cirrhosis or hepatocellular carcinoma, and result in serious consequences. From the pathological mechanism, liver fibrosis is mainly induced by the accumulation of ECM protein in the hepatic lobules and portal area. Excessive deposition of ECM destroys the normal structure of the liver, leading to hepatic function damage[29,30]. In this process, activation of HSCs is the key point in hepatic fibrosis. Phenotypic modulation of activated HSCs is enhanced, then these cells are transformed into myofibroblast-like cells, express α-SMA, produce ECM, and accelerate liver fibrosis following hepatic damage[31-33]. Meanwhile, TGF-β1 inhibits HSC apoptosis by means of autocrine and paracrine mechanisms, induces matrix protein expression, and therefore becomes one of the most important cytokines in the process of liver fibrosis[34]. Since the promoter of the TGF-β1 activating factor tissue transglutaminase gene contains a binding site for NF-κB, the synthesis of TGF-β1 is regulated by NF-κB[35,36]. Additionally, experimental studies have shown that NF-κB can amplify the liver inflammatory reaction by enhancing the expression of some factors related to HSC activation including inflammatory factors (IL-1, TNF-α, and IL-6), cell adhesion factors, and transforming growth factors, aggravating the disease[37-40]. Currently, NLRC5 is a new direction in the study of the NF-κB signaling pathway. It has been confirmed that NLRC5 plays an important role in regulating the NF-κB signaling pathway, type I interferon (INF-I) signaling pathway, and MHC-I gene expression[41,42]. As a member of the NLR family, NLRC5 can respond to various pathogens and intracellular danger signals and participates in the body's innate and adaptive immune responses. It is worth mentioning that NLRC5 is related to immune responses, besides that it may be a key regulator during development and reversal of hepatic fibrosis[43], but little is known about the role of NLRC5 in hepatic fibrosis. Therefore, this article analyzed the relationship between NLRC5 and hepatic fibrosis and its related mechanism, in order to evaluate its clinical application value.
In this study, the expression levels of NLRC5 in liver tissue and LX-2 cells were analyzed to explore the role of NLRC5 in liver fibrosis, and the results showed that the expression of NLRC5 in the fibrosis group was significantly higher than that in the normal and recovery groups. Moreover, NLRC5 was increased in LX-2 cells treated with TGF-β1 while decreased in cells following treatment with MDI as compared with the control cells. Based on these results, it was suggested that NLRC5 may play an important role during the occurrence and reversal of liver fibrosis. Transfection with NLRC5-siRNA in activated LX-2 cells can reduce the expression of α-SMA and Col1a1, suggesting a decrease in the number of activated HSCs.
Large animal experiments indicated that the major pathophysiological mechanism of HSC activation is transcriptional activation mediated by NF-κB[44,45]. The expression and secretion of various inflammatory factors and adhesion molecules can be induced and involved in the formation of liver fibrosis during increased NF-κB activity. In the resting state, p65 subunit binds to the IκB monomer and is located in the cytoplasm, and NF-κB in the nucleus is deficient. When suffering the outsider incitement, p65 translocates into the nucleus and activates NF-κB through the classical pathway following the phosphorylation and degradation of IκBα[46]. The results of this study showed that the expression levels of p65 and p-IκBα in the nucleus were significantly increased after NLRC5-siRNA treatment, suggesting that NLRC5 may be involved in liver fibrosis by negative regulation of NF-κB. In the study by Chang G et al[47], the high expression of NLRC5 protein in mouse hepatic fibrosis after activation of the NF-κB signaling pathway was presented. NLRC5 protein, because of its high expression level and large relative molecular mass, can inhibit the binding of NEMO to IKKα and IKKβ and down-regulate the expression of nuclear p65 and p-IκBα, and it is also activated by IKKα and IKKβ, forming a negative regulation cycle[48,49]. The inactive NF-κB stays within the cytoplasm, close to IκB family members in the inhibitory protein family. The interaction of NLRC5 with IKKα and IKKβ blocks the phosphorylation of the inhibitory protein IκB and eventually inhibits the activation of NF-κB signaling pathway[50]. More importantly, it was found that specific knockout of NLRC5 not only enhanced NF-kB and type I interferon signaling and target gene expression, but also modulated antiviral immune responses of multicellular lines and primary cells in a study of NLRC5 and NF-κB[51]. With conserved biological functions in humans and mice, as well as in various cell types, NLRC5 may be critical in the maintenance of immune homeostasis, particularly in regulation of innate immune responses[52]. However, the relationship between NLRC5 and the NF-kB signaling pathway and its roles in many types of diseases have yet to be determined. According to the results of this study, we infer that NLRC5 itself is induced by NF-κB-dependent mechanisms in LX-2 cells. Activation of TGF-β1 increases the expression of phosphorylated IκBα, stimulates the translocation of p65 from the cytoplasm to nucleus, and promotes the expression of NLRC5 in HSCs. The interaction of NLRC5 and NF-κB accelerates the development of liver fibrosis. There are still some limitations that need to be improved in this paper. The research on the NF-κB signaling pathway is not deep enough and whether there are other signaling pathways involved requires further research.
In conclusion, NLRC5 may participate in the process of HSC activation and ECM synthesis through the NF-κB signaling pathway. It can intervene in the key processes related to liver fibrosis, which provides a theoretical basis for clinical treatment of liver fibrosis.
ARTICLE HIGHLIGHTS
Research background
Continuous progression of liver fibrosis is the key to the development of chronic liver disease to cirrhosis. The nuclear factor-κB (NF-κB) signaling pathway is closely related to the formation and reversion of hepatic fibrosis. It has been found that NLRC5 is involved in the development of liver fibrosis by regulating the NF-κB signaling pathway and some studies suggest that NLRC5 is a key regulator of liver fibrosis and its reversal, but the role of NLRC5 in liver fibrosis remains unclear.
Research motivation
NLRC5 is highly expressed in immune tissues or organs and involved in the regulation of innate and adaptive immunity by inducing inflammation and cell death. Researches have shown that it may play an important role in the activation and inactivation of hepatocytes, but the research on the mechanism of action fell far behind the immunological study. Our study aimed to investigate the role and mechanism of NLRC5 in liver fibrosis to evaluate its clinical application value.
Research objectives
In this study, we analyzed the expression levels of NLRC5 in liver tissue and LX-2 cells and the activity of NF-κB in hepatic fibrosis after treatment with NLRC5-siRNA. The purpose of this study was to explore the relationship between NLRC5 and the NF-κB signaling pathways during the development and reversal of hepatic fibrosis.
Research methods
Eight-week-old male C57BL/6 mice were randomly divided into groups to establish liver fibrosis and its reversal model. Meanwhile, human hepatic stellate cell (HSC) line LX-2 was cultured in vitro and treated with transforming growth factor-β1 (TGF-β1) and MDI to activate and inactivate the cells. The degree of liver fibrosis and the expression of NLRC5 in mouse tissues and LX-2 cells were detected by qPCR and Western blot. After interfering with NLRC5 by siRNA, the activity of NF-κB in liver fibrosis was detected.
Research results
The expression level of NLRC5 was higher in liver tissue of fibrosis mice and activated HSCs, but decreased in mice with hepatic fibrosis with spontaneous reversion and inactivated HSC cells (P < 0.01). After treatment with NLRC5-siRNA, the activity of the NF-κB signaling pathway was increased in the liver of fibrosis mice and activated HSCs (P < 0.05).
Research conclusions
NLRC5 may play a key role in regulating the progression and reversal of liver fibrosis by negatively regulating the NF-κB signaling pathway, and it is expected to be one of the clinical therapeutic targets.
Research perspectives
NLRC5 plays a physiologically important role in maintaining immune homeostasis, particularly in regulating innate immune responses. Exploring the role and mechanism of NLRC5 and NF-κB in liver fibrosis can provide an important reference for the treatment of liver fibrosis.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University.
Institutional animal care and use committee statement: The study was approved by the institutional animal care and use committee of the First Affiliated Hospital of Zhengzhou University.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Peer-review started: March 20, 2019
First decision: April 4, 2019
Article in press: June 1, 2019
P-Reviewer: Faerch K, LaRue S, Rukavina M S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Yan-Zhen Zhang, Department of Second Gastroenterology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China. zhangyz8848@163.com.
Jian-Ning Yao, Department of Second Gastroenterology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Lian-Feng Zhang, Department of Second Gastroenterology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Chun-Feng Wang, Department of Second Gastroenterology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Xue-Xiu Zhang, Department of Second Gastroenterology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Bing Gao, Department of Second Gastroenterology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
References
- 1.Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis. 2017;37:1–10. doi: 10.1055/s-0036-1597816. [DOI] [PubMed] [Google Scholar]
- 2.Poilil Surendran S, George Thomas R, Moon MJ, Jeong YY. Nanoparticles for the treatment of liver fibrosis. Int J Nanomedicine. 2017;12:6997–7006. doi: 10.2147/IJN.S145951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alegre F, Pelegrin P, Feldstein AE. Inflammasomes in Liver Fibrosis. Semin Liver Dis. 2017;37:119–127. doi: 10.1055/s-0037-1601350. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Luo Z, Pan Y, Zheng W, Li W, Zhang Z, Xiong P, Xu D, Du M, Wang B, Yu J, Zhang J, Liu J. H19/miR-148a/USP4 axis facilitates liver fibrosis by enhancing TGF-β signaling in both hepatic stellate cells and hepatocytes. J Cell Physiol. 2019;234:9698–9710. doi: 10.1002/jcp.27656. [DOI] [PubMed] [Google Scholar]
- 5.Ji D, Chen GF, Wang JC, Cao LH, Lu F, Mu XX, Zhang XY, Lu XJ. Identification of TAF1, HNF4A, and CALM2 as potential therapeutic target genes for liver fibrosis. J Cell Physiol. 2019;234:9045–9051. doi: 10.1002/jcp.27579. [DOI] [PubMed] [Google Scholar]
- 6.Cui G, Chen J, Wu Z, Huang H, Wang L, Liang Y, Zeng P, Yang J, Uede T, Diao H. Thrombin cleavage of osteopontin controls activation of hepatic stellate cells and is essential for liver fibrogenesis. J Cell Physiol. 2019;234:8988–8997. doi: 10.1002/jcp.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Hashem F, Al-Humayed S, Amin SN, Kamar SS, Mansy SS, Hassan S, Abdel-Salam LO, Ellatif MA, Alfaifi M, Haidara MA, Al-Ani B. Metformin inhibits mTOR-HIF-1α axis and profibrogenic and inflammatory biomarkers in thioacetamide-induced hepatic tissue alterations. J Cell Physiol. 2019;234:9328–9337. doi: 10.1002/jcp.27616. [DOI] [PubMed] [Google Scholar]
- 8.Bavaro DF, Saracino A, Fiordelisi D, Bruno G, Ladisa N, Monno L, Angarano G. Influence of HLA-B18 on liver fibrosis progression in a cohort of HIV/HCV coinfected individuals. J Med Virol. 2019;91:751–757. doi: 10.1002/jmv.25385. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khabori M, Daar S, Al-Busafi SA, Al-Dhuhli H, Alumairi AA, Hassan M, Al-Rahbi S, Al-Ajmi U. Noninvasive assessment and risk factors of liver fibrosis in patients with thalassemia major using shear wave elastography. Hematology. 2019;24:183–188. doi: 10.1080/10245332.2018.1540518. [DOI] [PubMed] [Google Scholar]
- 10.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512–10522. doi: 10.3748/wjg.v22.i48.10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 13.Yang D, Li L, Qian S, Liu L. Evodiamine ameliorates liver fibrosis in rats via TGF-β1/Smad signaling pathway. J Nat Med. 2018;72:145–154. doi: 10.1007/s11418-017-1122-5. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Wei C, Liu N, Wu F, Chen J, Wang C, Sun Z, Wang Y, Liu L, Zhang X, Wang B, Zhang Y, Zhong H, Han Y, He X. GP73, a novel TGF-β target gene, provides selective regulation on Smad and non-Smad signaling pathways. Biochim Biophys Acta Mol Cell Res. 2019;1866:588–597. doi: 10.1016/j.bbamcr.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Manohar M, Kandikattu HK, Verma AK, Mishra A. IL-15 regulates fibrosis and inflammation in a mouse model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G954–G965. doi: 10.1152/ajpgi.00139.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Deng X, Liang J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp Cell Res. 2017;352:420–426. doi: 10.1016/j.yexcr.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Hu C, Zhao L, Duan J, Li L. Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med. 2019;23:1657–1670. doi: 10.1111/jcmm.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu HX, Yao Y, Bu FT, Chen Y, Wu YT, Yang Y, Chen X, Zhu Y, Wang Q, Pan XY, Meng XM, Huang C, Li J. Blockade of YAP alleviates hepatic fibrosis through accelerating apoptosis and reversion of activated hepatic stellate cells. Mol Immunol. 2019;107:29–40. doi: 10.1016/j.molimm.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Brenner DA, Kisseleva T. Combatting Fibrosis: Exosome-Based Therapies in the Regression of Liver Fibrosis. Hepatol Commun. 2018;3:180–192. doi: 10.1002/hep4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bond A, Hayes S, Abraham A, Teubner A, Farrer K, Pironi L, Lal S. Reversal of intestinal failure associated liver disease fibrosis in a patient receiving long term home parenteral nutrition. Clin Nutr ESPEN. 2018;28:228–231. doi: 10.1016/j.clnesp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Abidali H, Cole L, Seetharam AB. Rapid reversal of colonic pneumatosis with restoration of mesenteric arterial supply. Clin J Gastroenterol. 2018;11:461–464. doi: 10.1007/s12328-018-0872-2. [DOI] [PubMed] [Google Scholar]
- 22.Vijayan S, Sidiq T, Yousuf S, van den Elsen PJ, Kobayashi KS. Class I transactivator, NLRC5: a central player in the MHC class I pathway and cancer immune surveillance. Immunogenetics. 2019;71:273–282. doi: 10.1007/s00251-019-01106-z. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Li H, Xiao C, Zeng X, Xiao X, Zhou Q, Xiao P. NLRC5: potential novel non-invasive biomarker for predicting and reflecting the progression of IgA nephritis. J Transl Med. 2018;16:317. doi: 10.1186/s12967-018-1694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan L, Yao S, Sun S, Su Q, Li J, Wei B. NLRC5 and autophagy combined as possible predictors in patients with endometriosis. Fertil Steril. 2018;110:949–956. doi: 10.1016/j.fertnstert.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Wang Z, Zhang Y, Zhu J, Li L, Wang X, Cui X, Sun Y, Tang W, Gao C, Ma C, Yi F. NLRC5 deficiency protects against acute kidney injury in mice by mediating carcinoembryonic antigen-related cell adhesion molecule 1 signaling. Kidney Int. 2018;94:551–566. doi: 10.1016/j.kint.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Zhao X, Yang S, Chen B, Shi J. Knockdown of NLRC5 inhibits renal fibroblast activation via modulating TGF-β1/Smad signaling pathway. Eur J Pharmacol. 2018;829:38–43. doi: 10.1016/j.ejphar.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 27.Catalano C, da Silva Filho MI, Jiraskova K, Vymetalkova V, Levy M, Liska V, Vycital O, Naccarati A, Vodickova L, Hemminki K, Vodicka P, Weber ANR, Försti A. Short article: Influence of regulatory NLRC5 variants on colorectal cancer survival and 5-fluorouracil-based chemotherapy. Eur J Gastroenterol Hepatol. 2018;30:838–842. doi: 10.1097/MEG.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 28.Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64:157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Sun D, Wang G, Cui S, Field RA, Li J, Zang Y. Alogliptin alleviates liver fibrosis via suppression of activated hepatic stellate cell. Biochem Biophys Res Commun. 2019;511:387–393. doi: 10.1016/j.bbrc.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 30.Eulenberg VM, Lidbury JA. Hepatic Fibrosis in Dogs. J Vet Intern Med. 2018;32:26–41. doi: 10.1111/jvim.14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Lu Y, Yang P, Chen Q, Wang Y, Ding Q, Xu T, Li X, Li C, Huang C, Meng X, Li J, Zhang L, Wang X. MicroRNA-145 induces the senescence of activated hepatic stellate cells through the activation of p53 pathway by ZEB2. J Cell Physiol. 2019;234:7587–7599. doi: 10.1002/jcp.27521. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald AJ, Nadim MK, Durand F, Karvellas CJ. Acute kidney injury in cirrhosis: implications for liver transplantation. Curr Opin Crit Care. 2019;25:171–178. doi: 10.1097/MCC.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 33.Zayed N, Darweesh SK, Mousa S, Atef M, Ramzy E, Yosry A. Liver stiffness measurement by acoustic radiation forced impulse and transient elastography in patients with intrahepatic cholestasis. Eur J Gastroenterol Hepatol. 2019;31:520–527. doi: 10.1097/MEG.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 34.Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol. 2019;13:361–374. doi: 10.1080/17474124.2019.1579641. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Ding Y, Bi Y, Wang Y, Zhi Y, Wang J, Wang F. Staphylococcus aureus induces TGF-β1 and bFGF expression through the activation of AP-1 and NF-κB transcription factors in bovine mammary gland fibroblasts. Microb Pathog. 2016;95:7–14. doi: 10.1016/j.micpath.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Kong X, Jiang S, Liao W, Zhang Z, Song J, Liang Y, Zhang W. miR-627/HMGB1/NF-κB regulatory loop modulates TGF-β1-induced pulmonary fibrosis. J Cell Biochem. 2019;120:2983–2993. doi: 10.1002/jcb.27038. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Shen W, Zhang JY, Jia CH, Xie ML. Stevioside attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-κB/TGF-β1/Smad signaling pathway. Food Funct. 2019;10:1179–1190. doi: 10.1039/c8fo01663a. [DOI] [PubMed] [Google Scholar]
- 38.Xianyuan L, Wei Z, Yaqian D, Dan Z, Xueli T, Zhanglu D, Guanyi L, Lan T, Menghua L. Anti-renal fibrosis effect of asperulosidic acid via TGF-β1/smad2/smad3 and NF-κB signaling pathways in a rat model of unilateral ureteral obstruction. Phytomedicine. 2019;53:274–285. doi: 10.1016/j.phymed.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Bai F, Huang Q, Wei J, Lv S, Chen Y, Liang C, Wei L, Lu Z, Lin X. Gypsophila elegans isoorientin-2″-O-α-l-arabinopyranosyl ameliorates porcine serum-induced immune liver fibrosis by inhibiting NF-κB signaling pathway and suppressing HSC activation. Int Immunopharmacol. 2018;54:60–67. doi: 10.1016/j.intimp.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Pan X, Cao H, Shu X, Sun H, Lu J, Liang J, Zhang K, Zhu F, Li G, Zhang Q. IL-32γ promotes integrin αvβ6 expression through the activation of NF-κB in HSCs. Exp Ther Med. 2017;14:3880–3886. doi: 10.3892/etm.2017.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu YR, Yan X, Yu HX, Yao Y, Wang JQ, Li XF, Chen RN, Xu QQ, Ma TT, Huang C, Li J. NLRC5 promotes cell proliferation via regulating the NF-κB signaling pathway in Rheumatoid arthritis. Mol Immunol. 2017;91:24–34. doi: 10.1016/j.molimm.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Ma SR, Xie XW. NLRC5 deficiency promotes myocardial damage induced by high fat diet in mice through activating TLR4/NF-κB. Biomed Pharmacother. 2017;91:755–766. doi: 10.1016/j.biopha.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 43.Xu T, Ni MM, Huang C, Meng XM, He YH, Zhang L, Li J. NLRC5 Mediates IL-6 and IL-1β Secretion in LX-2 Cells and Modulated by the NF-κB/Smad3 Pathway. Inflammation. 2015;38:1794–1804. doi: 10.1007/s10753-015-0157-6. [DOI] [PubMed] [Google Scholar]
- 44.Han M, Liu X, Liu S, Su G, Fan X, Chen J, Yuan Q, Xu G. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces hepatic stellate cell (HSC) activation and liver fibrosis in C57BL6 mouse via activating Akt and NF-κB signaling pathways. Toxicol Lett. 2017;273:10–19. doi: 10.1016/j.toxlet.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Ramos-Tovar E, Flores-Beltrán RE, Galindo-Gómez S, Vera-Aguilar E, Diaz-Ruiz A, Montes S, Camacho J, Tsutsumi V, Muriel P. Stevia rebaudiana tea prevents experimental cirrhosis via regulation of NF-κB, Nrf2, transforming growth factor beta, Smad7, and hepatic stellate cell activation. Phytother Res. 2018;32:2568–2576. doi: 10.1002/ptr.6197. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa MM, Chen H, Rathinam CV. Constitutive Activation of NF-κB Pathway in Hematopoietic Stem Cells Causes Loss of Quiescence and Deregulated Transcription Factor Networks. Front Cell Dev Biol. 2018;6:143. doi: 10.3389/fcell.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang G, Liu X, Ma T, Xu L, Wang H, Li Z, Guo X, Xu Q, Chen G. A mutation in the NLRC5 promoter limits NF-κB signaling after Salmonella Enteritidis infection in the spleen of young chickens. Gene. 2015;568:117–123. doi: 10.1016/j.gene.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Cao L, Wu XM, Hu YW, Xue NN, Nie P, Chang MX. The discrepancy function of NLRC5 isoforms in antiviral and antibacterial immune responses. Dev Comp Immunol. 2018;84:153–163. doi: 10.1016/j.dci.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Luan P, Zhuang J, Zou J, Li H, Shuai P, Xu X, Zhao Y, Kou W, Ji S, Peng A, Xu Y, Su Q, Jian W, Peng W. NLRC5 deficiency ameliorates diabetic nephropathy through alleviating inflammation. FASEB J. 2018;32:1070–1084. doi: 10.1096/fj.201700511RR. [DOI] [PubMed] [Google Scholar]
- 50.Fekete T, Bencze D, Szabo A, Csoma E, Biro T, Bacsi A, Pazmandi K. Regulatory NLRs Control the RLR-Mediated Type I Interferon and Inflammatory Responses in Human Dendritic Cells. Front Immunol. 2018;9:2314. doi: 10.3389/fimmu.2018.02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu L, Ma T, Chang G, Liu X, Guo X, Xu L, Zhang Y, Zhao W, Xu Q, Chen G. Expression patterns of NLRC5 and key genes in the STAT1 pathway following infection with Salmonella pullorum. Gene. 2017;597:23–29. doi: 10.1016/j.gene.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Benkő S, Kovács EG, Hezel F, Kufer TA. NLRC5 Functions beyond MHC I Regulation-What Do We Know So Far? Front Immunol. 2017;8:150. doi: 10.3389/fimmu.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]