Abstract
BACKGROUND
Esophageal adenocarcinoma (EAC) and high-grade dysplasia (HGD) may appear in young patients with Barrett’s esophagus (BE). However, characteristics of Barrett’s-related neoplasia in this younger population remain unknown.
AIM
To identify clinical characteristics that differ between young and old patients with early-stage Barrett’s-related neoplasia.
METHODS
We conducted a retrospective analysis of a prospectively maintained database comprised of consecutive patients with early-stage EAC (pT1) and HGD at a tertiary-referral center between 2001 and 2017. Baseline characteristics, drug and risk factor exposures, clinicopathological staging of EAC/HGD and treatment outcomes [complete eradication of neoplasia (CE-N), complete eradication of intestinal metaplasia (CE-IM), recurrence of neoplasia and recurrence of intestinal metaplasia] were retrieved. Multivariate analyses were performed to identify factors that differed significantly between older and younger (≤ 50 years) patients.
RESULTS
We identified 450 patients with T1 EAC and HGD (74% and 26%, respectively); 45 (10%) were ≤ 50 years. Compared to the older group, young patients were more likely to present with ongoing gastroesophageal reflux disease (GERD) symptoms (55% vs 38%, P = 0.04) and to be obese (body mass index > 30, 48% vs 32%, P = 0.04). Multivariate logistic regression analysis showed that young patients were significantly more likely to have ongoing GERD symptoms [odds ratio (OR) 2.00, 95% confidence interval (CI) 1.04-3.85, P = 0.04] and to be obese (OR 2.06, 95%CI 1.07-3.98, P = 0.03) whereas the young group was less likely to have a smoking history (OR 0.39, 95%CI 0.20-0.75, P < 0.01) compared to the old group. However, there were no significant differences regarding tumor histology, CE-N, CE-IM, recurrence of neoplasia and recurrence of intestinal metaplasia (mean follow-up, 44.3 mo).
CONCLUSION
While guidelines recommend BE screening in patients > 50 years of age, younger patients should be considered for screening endoscopy if they suffer from obesity and GERD symptoms.
Keywords: Barrett’s Esophagus, Gastroesophageal reflux disease, Obesity, Esophageal adenocarcinoma, High-grade dysplasia, Guideline, Young patient
Core tip: Esophageal adenocarcinoma (EAC) and high-grade dysplasia (HGD) may appear in young patients with Barrett’s esophagus (BE). To identify clinical characteristics of young patients with Barrett’s neoplasia, we conducted a retrospective analysis. 450 patients with T1 EAC and HGD were identified; 45 (10%) were young patients at age ≤ 50 years. Compared to the older group, young patients were more likely to present with ongoing gastroesophageal reflux disease (GERD) symptoms and to be obese on multivariate analysis. While guidelines recommend BE screening in patients > 50 years of age, younger patients should be considered for screening endoscopy if they suffer from obesity and GERD symptoms.
INTRODUCTION
Barrett’s esophagus (BE) is a premalignant condition for esophageal adenocarcinoma (EAC)[1], which has a poor prognosis with a 5-year survival rate below 20%[2]. However, screening endoscopy of the general population for BE or EAC is not recommended because of the low incidence of EAC and the lack of randomized controlled trials supporting its efficiency[3-6]. According to recent guidelines, screening should be considered only in patients with multiple risk factors for BE or EAC; such as long standing gastroesophageal reflux disease (GERD) symptoms, age > 50 years, white race, male sex, obesity, family history of BE or EAC, and smoking[3-6].
Older age is one of the most important risk factors for BE[7,8]. Most guidelines set the cut-off at age 50. On the other hand, the diagnosis of Barrett’s-related neoplasia in younger patients is becoming more common in daily clinical practice. In fact, the incidence rate of EAC for the young has been steadily increasing in recent years[9]. However, the clinical characteristics of these younger EAC patients are poorly known. Even though some studies have evaluated the prognosis of EAC among young patients[10-12], few articles have identified the baseline clinical characteristics of this patient group with EAC[13,14]. Moreover, features and outcomes of young patients with early-stage EAC are poorly described. Yet detecting early-stage neoplasia holds the opportunity for curative endoscopic resection with excellent long-term outcomes. Thus, if this younger cohort differs significantly with respect to specific clinical characteristics from the more typical age category of BE neoplasia, these features could help to improve screening recommendations. Hence, we conducted a retrospective analysis of a prospectively maintained database of patients diagnosed with BE and early-stage EAC/high-grade dysplasia (HGD) to identify factors associated with the development of Barrett’s-related neoplasia occurring in younger patients. Additionally, we examined for any correlation between age groups and treatment outcomes.
MATERIALS AND METHODS
Study participants
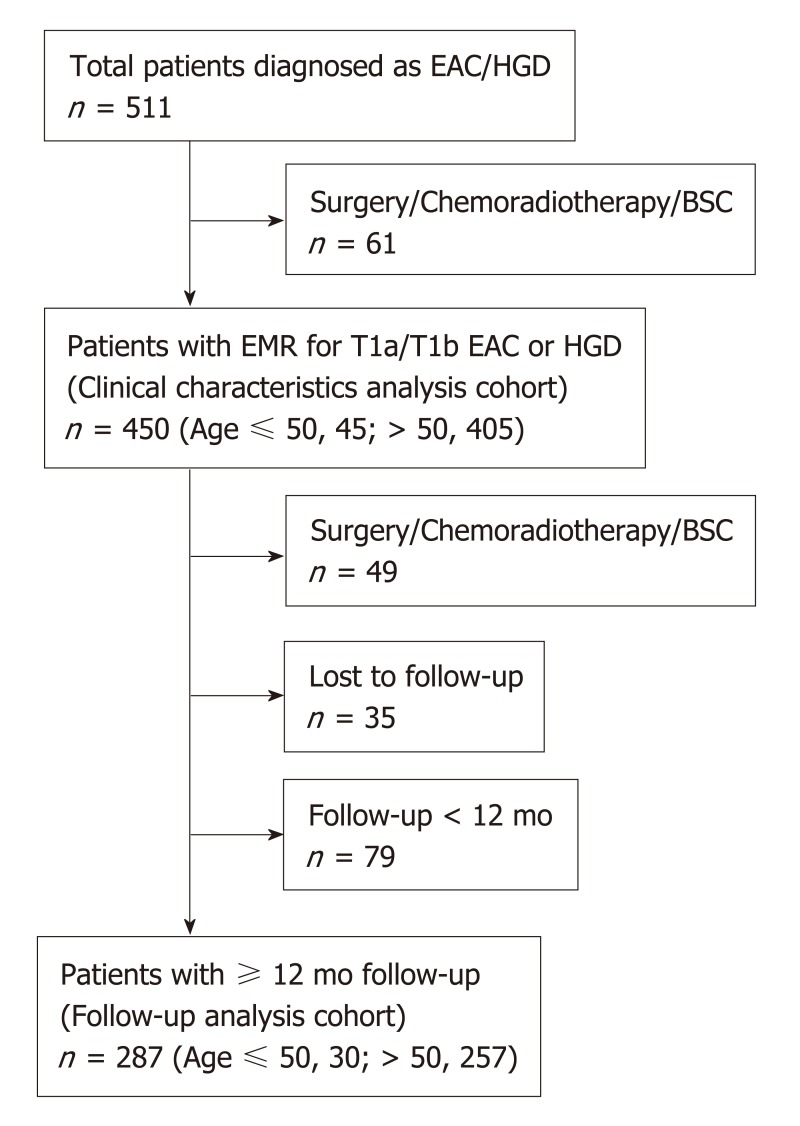
We conducted a retrospective analysis of a prospective database comprised of consecutive patients with early-stage EAC (T1a and T1b) and HGD in BE at a single, tertiary-referral center (St Michael’s Hospital, Toronto, Canada) between May 2001 and May 2017. For cases that occurred prior the establishment of the Prague criteria, we only included cases in which the circumferential and maximum BE length were documented at their index endoscopy[15]. Exclusion criteria were: (1) Patients who did not undergo endoscopic mucosal resection (EMR) (because of the absence of precise histopathological staging); (2) Patients who underwent esophagectomy or chemo/radiotherapy due to unfavorable features of the first EMR specimen such as submucosal invasion, poorly differentiated cancer (G3) and lympho-vascular invasion (Figure 1). All patients provided written informed consent for their inclusion in our database. The study was carried out in accordance with the Declaration of Helsinki and was approved by the St. Michael’s Hospital research ethics committee (#08-265).
Figure 1.
Flow diagram for the study patients. EAC: Esophageal adenocarcinoma; HGD: High-grade dysplasia; BSC: Best supportive care; EMR: Endoscopic mucosal resection.
Data collection and procedures
All patients provided detailed information via a demographic, medical history, and lifestyle questionnaire that ascertained the following characteristics: age at diagnosis for EAC or HGD, sex, ethnicity, height, weight, body mass index (BMI), comorbidites, family history of malignancy including EAC, tobacco and alcohol consumption, ongoing GERD-related symptoms (defined as having at least two episodes of reflux symptoms within the most recent three months) and medication [including proton pump inhibitors (PPIs), low-dose aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), and statins].
Regarding endoscopic findings, the circumferential (C) and maximum (M) length of BE based on the Prague criteria were systematically recorded[15]. The recorded distance from the diaphragm to the gastroesophageal junction, defined as the oral end of the gastric folds, was used to determine the presence or absence of a hiatus hernia. According to the Seattle protocol, multiple, 4-quadrant biopsies were obtained every 1 to 2 cm to identify intestinal metaplasia or dysplasia. EMR was performed when a visible lesion was found or to completely eradicate BE via radical EMR, particularly for short-segment BE. Most patients underwent radiofrequency ablation (RFA) of the remaining BE segments for complete eradication of BE, as described elsewhere[16]. Some patients underwent other eradication techniques such as bipolar electro-coagulation (BiCAP), photodynamic therapy (PDT), cryotherapy and hot avulsion due to the evolution of treatment strategies during the study period. Hot avulsion is our previously described technique used to eradicate small persistent BE areas 1 cm or less using hot biopsy forceps with cauterization[17].
All specimens were fixed in 10% neutral buffered formalin, embedded in paraffin and stained with hematoxylin and eosin staining. At least two experienced gastrointestinal pathologists analyzed all specimens. A diagnosis of BE was made based on the presence of intestinal metaplasia with goblet cells. The final T-staging diagnosis was based on pathology from the EMR specimen. Pathological reports were recorded in accordance with the Vienna classification[18] and EMR reports included the information such as grade of differentiation (G), depth of invasion according to the Vieth and Stolte system (M1-4, SM)[19], vertical margin and the presence or absence of lymphovascular invasion (LVI).
For assessment of endoscopic treatment-related outcomes, we included only patients followed up for more than 12 mo after initial EMR. Patients who underwent EMR were followed-up by endoscopy in 3- to 6-mo intervals with surveillance biopsies and additional endoscopic therapies (ablative methods or additional EMR), at the discretion of the endoscopist, until all visible BE was eradicated. Complete eradication of neoplasia (CE-N) was defined as the absence of endoscopic and pathologic evidence of adenocarcinoma or any dysplasia after endoscopic treatment. Complete eradication of intestinal metaplasia (CE-IM) was defined as complete absence of endoscopic evidence of BE and pathologic evidence of intestinal metaplasia on all follow-up biopsies. Recurrence of neoplasia and intestinal metaplasia were defined as any biopsy-confirmed dysplastic lesion and intestinal metaplasia detected on subsequent endoscopies following CE-N and CE-IM, respectively.
Statistical analysis
The mean ± standard deviation (SD) was used for variables with a normal distribution, and the median and interquartile range was used for variables with a skewed distribution. Differences between groups were analyzed using the Chi-square test and Fisher’s exact for categorical data, the Student t-test for comparing means and the Mann-Whitney U test for comparing medians for continuous data. Multivariable logistic regression models were performed to identify clinical and pathologic differences between younger (≤ 50 years) and older patients. We included the following variables in the multivariate logistic regression analysis: BMI (> 30 or < 30), history of smoking, ongoing GERD symptoms, family history of EAC, ethnicity (white) and sex (male); as these factors are well known to be associated with EAC and used to guide screening endoscopy in most guidelines[3-6]. We calculated adjusted odds ratios (ORs) and 95% confidence intervals (CIs) by multivariate logistic regression analysis using StatFlex software (Artech Co., Osaka, Japan). Two-sided P values < 0.05 were considered statistically significant.
RESULTS
Study population and clinical characteristics
We identified 450 patients diagnosed with Barrett’s-related EAC (T1a or T1b) or HGD during the study period. Of these, 45 patients (10%) were 50 years of age or younger [39 men (87%)] and 405 patients (90%) > 50 years [342 men (84%)].
Patient clinical characteristics are summarized in Table 1. Male and white patients were predominant in both groups with no significant differences. Young patients were more likely to be obese (BMI > 30, 48% vs 32%, P = 0.04) and to have ongoing GERD symptoms (55% vs 38%, P = 0.04), and less likely to have diabetes and hypertension, and to have ever been smokers on univariate analysis. There were no significant differences between the groups with regards to family history of EAC and alcohol consumption. Regarding medication use, we found that the older group was more likely to have used low-dose aspirin and statins compared to the younger patients, while there were no significant differences regarding use of PPIs and NSAIDs. With regard to endoscopic findings, the median circumferential extent of BE (Prague C) in the young and old groups were 2 cm and 1cm, respectively (P = 0.52) and the median maximal extent of BE (Prague M) was 4 cm and 4cm, respectively (P = 0.43). The prevalence of a hiatus hernia was not significantly different between groups (P = 0.12).
Table 1.
Patient characteristics
|
Young (≤ 50 yr) |
Old (> 50 yr) |
P value | |
| (n = 45) | (n = 405) | ||
| Sex (male) | 39/45 (87%) | 342/405 (84%) | 0.86 |
| Ethnicity (white) | 43/44 (98%) | 377/387 (97%) | 0.70 |
| BMI, kg/m2 (mean ± SD) | 29.69 ± 6.05 | 28.53 ± 5.72 | 0.09 |
| BMI > 30 kg/m2 | 21/44 (48%) | 124/389 (32%) | 0.04 |
| Diabetes | 5/45 (11%) | 91/390 (23%) | 0.01 |
| Hypertension | 13/45 (29%) | 211/382 (55%) | < 0.001 |
| Family history of malignancy | 21/44 (48%) | 234/382 (61%) | 0.08 |
| Family history of esophageal adenocarcinoma | 1/44 (2%) | 17/382 (5%) | 0.78 |
| GERD symptoms | 24/44 (55%) | 148/386 (38%) | 0.04 |
| Smoking | |||
| Ever smoking | 25/44 (57%) | 297/393 (76%) | 0.01 |
| Current smoking | 4/44 (9%) | 47/392 (11%) | 0.75 |
| Pack-years (mean ± SD) | 13.97 ± 15.56 | 23.46 ± 27.21 | 0.02 |
| Alcohol | |||
| Ever alcohol | 37/45 (82%) | 297/387 (77%) | 0.52 |
| Current alcohol | 31/45 (69%) | 241/375 (64%) | 0.54 |
| Medication use | |||
| Proton pump inhibitors | 38/43 (88%) | 335/374 (90%) | 0.98 |
| Low-dose aspirin | 5/45 (11%) | 130/388 (34%) | < 0.01 |
| NSAIDs | 4/45 (9%) | 34/384 (9%) | 0.79 |
| Statins | 10/45 (22%) | 189/388 (49%) | < 0.001 |
| Endoscopy | |||
| Prague C, median (IQR), cm | 2 (0-5) | 1 (0-5) | 0.52 |
| Prague M, median (IQR), cm | 4 (2-7) | 4 (2-7) | 0.43 |
| Hiatus hernia | 35/45 (78%) | 350/405 (86%) | 0.12 |
BMI: Body mass index; GERD: Gastroesophageal reflux disease; NSAIDs: Nonsteroidal anti-inflammatory drugs; SD: Standard deviation; IQR: Interquartile range.
Multivariate modeling (Table 2) showed that young patients were significantly more likely to be obese (BMI > 30, OR 2.06, 95%CI 1.07-3.98, P = 0.03) and to have ongoing GERD symptoms (OR 2.00, 95%CI 1.04-3.85, P = 0.04), whereas the young group was less likely to have a smoking history (OR 0.39, 95%CI 0.20-0.75, P < 0.01) compared to the old group.
Table 2.
Adenocarcinoma/high-grade dysplasia risk according to multivariate analysis: Young vs old
| Adjusted Odds ratio | 95% confidence interval | P value | |
| BMI > 30 | 2.06 | 1.07-3.98 | 0.03 |
| Ever smoking | 0.39 | 0.20-0.75 | < 0.01 |
| Ongoing GERD symptoms | 2.00 | 1.04-3.85 | 0.04 |
| Family history of esophageal adenocarcinoma | 0.73 | 0.09-5.85 | 0.77 |
| Ethnicity (white) | 0.77 | 0.09-6.42 | 0.81 |
| Sex (Male) | 1.44 | 0.56-3.70 | 0.45 |
BMI: Body mass index; GERD: Gastroesophageal reflux disease.
Pathological features
A comparison of the pathological features between young and old patients is summarized in Table 3. Thirty-one (69%) and 317 (78%) patients had EAC among young and old groups, respectively (P = 0.15). There were no significant differences between the groups in terms of depth of EAC invasion, tumor differentiation, LVI and rate of positive vertical (deep) margin.
Table 3.
Pathological features of esophageal adenocarcinoma, n (%)
|
Young (≤ 50 yr) |
Old (> 50 yr) |
P value | ||
| (n = 45) | (n = 405) | |||
| Histology | EAC | 31 (69) | 317 (78) | 0.15 |
| HGD | 14 (31) | 88 (22) | ||
| EAC depth | M1 | 7 (23) | 63 (20) | |
| M2 | 8 (26) | 75 (24) | ||
| M3 | 8 (26) | 47 (15) | ||
| M4 | 4 (13) | 96 (30) | ||
| SM | 4 (13) | 36 (11) | 0.41 | |
| Differentiation | G1 | 21 (68) | 212 (67) | |
| G2 | 6 (19) | 91 (29) | ||
| G3 | 4 (13) | 14 (4) | 0.22 | |
| Lympho-vascular invasion | 1 (3) | 32 (10) | 0.36 | |
| Vertical margin positive rate | 3 (10) | 32 (10) | 0.81 | |
EAC: Esophageal adenocarcinoma; HGD: High-grade dysplasia; M: Mucosa; SM: Submucosa.
Endoscopic treatment-related outcomes and follow-up
Clinical outcomes following endoscopic treatment were available for 287 patients who met inclusion criteria (Figure 1, young, n = 30; old, n = 257). Mean follow-up duration was 44.3 ± SD: 30.2 mo. All patients underwent EMR and 176 patients had additional one or more ablative therapies following the first EMR; RFA (n = 114), BiCAP (n = 17), PDT (n = 14), cryotherapy (n = 3) and hot avulsion (n = 107). The overall rates of CE-N and CE-IM were 86% and 63%, respectively. There were no significant differences between young and old groups in terms of CE-N (93% vs 86%, P = 0.38) and recurrence rates of neoplasia after CE-N (14.3% vs 18%, P = 0.81) (Table 4). Similarly, no differences were found regarding CE-IM (77% vs 62%, P = 0.16) and recurrence rates of intestinal metaplasia after CE-IM (30% vs 26%, P = 0.83).
Table 4.
Clinical outcomes following endoscopic treatment
|
Young (≤ 50 yr) |
Old (> 50 yr) |
P value | |
| (n = 30) | (n = 257) | ||
| Complete eradication of neoplasia | 28/30 (93%) | 220/257 (86%) | 0.38 |
| Recurrence of neoplasia | 4/28 (14%) | 40/220 (18%) | 0.81 |
| Complete eradication of intestinal metaplasia | 23/30 (77%) | 159/257 (62%) | 0.16 |
| Recurrence of intestinal metaplasia | 7/23 (30%) | 41/159 (26%) | 0.83 |
DISCUSSION
The clinical and pathologic characteristics of early-stage EAC in young patients has been poorly documented because of its low incidence. Our current study, based on a large prospective cohort of patients diagnosed with early-stage BE neoplasia, suggests that younger patients (≤ 50 years) with early-stage EAC or HGD were more likely to have ongoing GERD symptoms and to be obese compared to their older counterparts. Furthermore, we found that there were no significant differences in terms of endoscopic treatment-related outcomes between groups.
The incidence of EAC in young patients has been generally considered to be very low. Consequently, most guidelines recommend screening endoscopy only for patients > 50 years who have multiple risk factors. Murphy et al[9] demonstrated that the incidence of EAC amongst younger patients has been increasing just as the total number of EAC patients has increased. Thrift et al[20] speculated that declining infection rates of Helicobacter pylori may lead to higher rates of EAC in young cohorts in the near future, given the theory that this infection may reduce the risk for EAC. Indeed, our data revealed that the percentage of young early-stage EAC/HGD patients was significant (45/450, 10%). Another recent study using a large cohort demonstrated that the proportion of young (≤ 50 years) EAC patients was 9% (125/1363)[14] which is similar to our results, underlining that a significant number of Barrett’s-related neoplasms appear before the age of 50.
The prognosis of young EAC patients is still controversial. Some studies have reported that young patients with EAC presented with a more advanced stage of the disease and had poorer survival than older EAC patients[11,12,21]. In our cohort, the outcomes of endoscopic treatment for T1 EAC or HGD between both groups were similar. Therefore, it becomes of utmost importance to elucidate the risk factors that may facilitate the early detection of EAC in this younger population.
GERD symptoms are the most important risk factor for BE and EAC[3,5,6]. Cook et al[22], using a large pooled analysis of five population-based case-control studies, showed that the risk of EAC in patients with GERD symptoms for at least 30 years was 6.2-fold higher than in patients without GERD symptoms. On the other hand, another analysis comprising three additional studies revealed that this association was stronger in patients under the age of 50[14]. Similarly, we found that 92% of younger patients had ongoing GERD symptoms despite taking PPIs in our cohort (data not shown), which suggests that refractory GERD is strongly related to EAC risk in younger patients. However, Becher et al[23] demonstrated that GERD symptoms are more severe among younger than older patients, while aging is associated with more severe patterns of acid reflux and reflux esophagitis. Therefore, the mere presence of GERD symptoms is not sufficient to perform endoscopy. Additional risk factors should be considered in order to increase the efficiency of screening endoscopy especially among young patients.
Obesity is another important risk factor for BE and EAC[24]. Central adiposity is thought to be more critical for esophageal carcinogenesis than merely increased BMI, since central adiposity contributes not only to increasing acid reflux due to mechanical disruption of the gastroesophageal junction, but also promotes a proinflammatory state by releasing adipocytokines from visceral adipose tissue[25]. Adipocytokine-mediated carcinogenesis is thought to play an important role in other gastrointestinal malignancies such as colon[26] and pancreas[27] cancers. However, few studies have investigated the relationship between obesity and gastrointestinal carcinogenesis specifically in young patients. How obesity and central adiposity affect EAC risk, especially in young patients, remains unclear. Chak et al[13] demonstrated that obesity is associated with the development of EAC at an earlier age. We also found that obesity was more common in young patients with early-stage Barrett-related neoplasia. Altogether, our results suggest that screening endoscopy should be strongly considered in obese young patients with ongoing GERD symptoms.
Additionally, our results showed that any history of smoking had a stronger association with the older EAC/HGD group (> 50 years). Although cigarette smoking is a well-known risk factor for the development of both BE and EAC[28,29], the precise role of smoking in EAC carcinogenesis remains unclear. We have to emphasize that this result does not suggest smoking has a protective role for Barrett’s carcinogenesis in young cohort. In our cohort, the number of pack-years in older EAC group was significantly higher. We hypothesized that this association could be related to the accumulation of toxicity for a longer time span. We also thought why our results showed older patients was more likely to have hypertension and diabetes, and to take low-dose aspirin and statins were just due to aging.
It should be highlighted that our study population was based exclusively on patients diagnosed with early stage neoplasia. To the best of our knowledge, this is the first study to compare the baseline clinical characteristics and treatment outcomes between the young and old onset groups using a relatively large cohort including only early stage Barrett’s-related neoplasia. On the other hand, including only HGD and T1 tumor might be also interpreted as a limitation. However, focusing on early Barrett’s-related neoplasia amenable to endoscopic treatment allowed us to develop a suggestion that if we could detect the young patients with early stage Barrett’s neoplasia using the risk factors of ongoing GERD symptoms and obesity, their prognosis may compare favorably with older patients. We believe that this can complement the current guidelines for Barrett’s screening.
This study has some limitations. First, we did not have control groups such as young and old patients without Barrett’s-related neoplasia. Because of this point and retrospective nature of our study, we cannot say that GERD symptoms and obesity are predictive factors for EAC in young patients. Therefore, the risk factors that we extracted should be proved by a prospectively designed study. Second, as our institution is a tertiary referral center, our cohort may not be representative of the general population or community practice BE population. Third, due to the retrospective nature of the study, some patients were lost to follow-up. Therefore, we could not assess long-term morbidity and mortality.
In conclusion, we identified that patients ≤ 50 years old with early-stage EAC or HGD had greater odds of having ongoing GERD symptoms and to be obese than older patients. Our results may serve to improve the selection of younger patients who would most benefit from screening endoscopy. Further prospective studies are needed to clarify the risk factors specific to young patients with Barrett’s-related neoplasia.
ARTICLE HIGHLIGHTS
Research background
Older age is one of the most important risk factors for Barrett’s esophagus. Most guidelines set the cut-off at age 50. On the other hand, the diagnosis of Barrett’s neoplasia in younger patients is becoming more common in daily clinical practice.
Research motivation
The clinical characteristics of these younger esophageal adenocarcinoma (EAC) and high-grade dysplasia (HGD) patients are poorly known. If this younger cohort differs significantly with respect to specific clinical characteristics from the more typical age category of Barrett’s neoplasia, these features could help to improve screening recommendations.
Research objectives
To identify factors associated with the development of Barrett’s neoplasia occurring in younger patients.
Research methods
A retrospective analysis of a prospectively maintained database comprised of consecutive patients with early-stage EAC (pT1) and HGD at a tertiary-referral center between 2001 and 2017 was conducted. Baseline characteristics, drug and risk factor exposures, clinicopathological staging of EAC/HGD and treatment outcomes [complete eradication of neoplasia (CE-N), complete eradication of intestinal metaplasia (CE-IM), recurrence of neoplasia and recurrence of intestinal metaplasia) were retrieved. Multivariate analyses were performed to identify factors that differed significantly between older and younger (≤ 50 years) patients.
Research results
Four hundred fifty patients with T1 EAC and HGD were enrolled in this study. Forty-five patients (10%) were ≤ 50 years. Compared to the older group, young patients were more likely to have ongoing gastroesophageal reflux disease (GERD) symptoms and to be obese. The same pattern of differences was maintained with an even greater magnitude of effects on multivariate analysis. However, there were no significant differences regarding tumor histology, CE-N, CE-IM, recurrence of neoplasia and recurrence of intestinal metaplasia (mean follow-up, 44.3 mo).
Research conclusions
We identified that patients ≤ 50 years old with early-stage EAC or HGD had greater odds of having ongoing GERD symptoms and to be obese than older patients. Our results may serve to improve the selection of younger patients who would most benefit from screening endoscopy.
Research perspectives
Further prospective studies are needed to clarify the risk factors specific to young patients with Barrett’s-related neoplasia.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of St. Michael’s Hospital, University of Toronto.
Informed consent statement: Written informed consents for the upper GI endoscopy and their inclusion in our database were obtained from all participants.
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Data sharing statement: No additional data are available.
Peer-review started: April 1, 2019
First decision: April 30, 2019
Article in press: May 18, 2019
P-Reviewer: Abdulnour-Nakhoul S, Floria M, Friedrich, Hillman LC, Yücel O S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
Contributor Information
Yugo Iwaya, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada. yiwaya@shinshu-u.ac.jp.
Yuto Shimamura, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
Kenichi Goda, Department of Gastroenterology, Dokkyo Medical University, Tochigi 321-0293, Japan.
Enrique Rodríguez de Santiago, Department of Gastroenterology, Ramón y Cajal university hospital, Madrid 28034, Spain.
John Gerard Coneys, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
Jeffrey D Mosko, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
Gabor Kandel, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
Paul Kortan, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
Gary May, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
Norman Marcon, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
Christopher Teshima, Advanced Therapeutic Endoscopy Centre, St Michael’s Hospital, University of Toronto, Toronto M5B 1W8, Ontario, Canada.
References
- 1.Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett's esophagus: Development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER Cancer Stat Facts: Esophageal Cancer. Bethesda, MD: National Cancer Institute, 2016. Available from: https://seer.cancer.gov/statfacts/html/esoph.html. [Google Scholar]
- 3.Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, Hassan C, Pech O, Repici A, Bergman J, di Pietro M. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191–198. doi: 10.1055/s-0042-122140. [DOI] [PubMed] [Google Scholar]
- 4.ASGE Standards of Practice Committee. Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ, Hwang JH, Jain R, Jue TL, Khan KM, Lightdale J, Malpas PM, Maple JT, Pasha SF, Saltzman JR, Sharaf RN, Shergill A, Dominitz JA, Cash BD; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–1094. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Falk GW, Iyer PG, Gerson LB American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30–50; quiz 51. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, O'Donovan M, Bird-Lieberman E, Bhandari P, Jankowski JA, Attwood S, Parsons SL, Loft D, Lagergren J, Moayyedi P, Lyratzopoulos G, de Caestecker J British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 7.Edelstein ZR, Bronner MP, Rosen SN, Vaughan TL. Risk factors for Barrett's esophagus among patients with gastroesophageal reflux disease: A community clinic-based case-control study. Am J Gastroenterol. 2009;104:834–842. doi: 10.1038/ajg.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Provenzale D. Clinical and demographic predictors of Barrett's esophagus among patients with gastroesophageal reflux disease: A multivariable analysis in veterans. J Clin Gastroenterol. 2001;33:306–309. doi: 10.1097/00004836-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Murphy CC, Yang YC, Shaheen NJ, Hofstetter WL, Sandler RS. An age-period-cohort analysis of obesity and incident esophageal adenocarcinoma among white males. Dis Esophagus. 2017;30:1–8. doi: 10.1111/dote.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott Bolton J, Wu TT, Yeo CJ, Cameron JL, Heitmiller RF. Esophagectomy for adenocarcinoma in patients 45 years of age and younger. J Gastrointest Surg. 2001;5:620–625. doi: 10.1016/s1091-255x(01)80104-9. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Li H, Jia C, Ma X, Guo W, Li H. Clinicopathological features and prognosis of patients <45 years old with esophageal adenocarcinoma comparing to other age groups. J Thorac Dis. 2016;8:2724–2729. doi: 10.21037/jtd.2016.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oezcelik A, Ayazi S, DeMeester SR, Zehetner J, Abate E, Dunn J, Grant KS, Lipham JC, Hagen JA, DeMeester TR. Adenocarcinoma of the esophagus in the young. J Gastrointest Surg. 2013;17:1032–1035. doi: 10.1007/s11605-013-2177-6. [DOI] [PubMed] [Google Scholar]
- 13.Chak A, Falk G, Grady WM, Kinnard M, Elston R, Mittal S, King JF, Willis JE, Kondru A, Brock W, Barnholtz-Sloan J. Assessment of familiality, obesity, and other risk factors for early age of cancer diagnosis in adenocarcinomas of the esophagus and gastroesophageal junction. Am J Gastroenterol. 2009;104:1913–1921. doi: 10.1038/ajg.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drahos J, Xiao Q, Risch HA, Freedman ND, Abnet CC, Anderson LA, Bernstein L, Brown L, Chow WH, Gammon MD, Kamangar F, Liao LM, Murray LJ, Ward MH, Ye W, Wu AH, Vaughan TL, Whiteman DC, Cook MB. Age-specific risk factor profiles of adenocarcinomas of the esophagus: A pooled analysis from the international BEACON consortium. Int J Cancer. 2016;138:55–64. doi: 10.1002/ijc.29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN, Vieth M. The development and validation of an endoscopic grading system for Barrett's esophagus: The Prague C & M criteria. Gastroenterology. 2006;131:1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Dulai PS, Pohl H, Levenick JM, Gordon SR, MacKenzie TA, Rothstein RI. Radiofrequency ablation for long- and ultralong-segment Barrett's esophagus: A comparative long-term follow-up study. Gastrointest Endosc. 2013;77:534–541. doi: 10.1016/j.gie.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Aranda-Hernández J, Shimamura Y, Grin A, Iwaya Y, Cirocco M, Kandel G, May G, Kortan P, Raftopoulos S, Marcon N. Hot avulsion may be effective as salvage treatment for focal Barrett's esophagus remaining after endoscopic therapy for dysplasia or early cancer: A preliminary study. Endoscopy. 2018;50:8–13. doi: 10.1055/s-0043-119986. [DOI] [PubMed] [Google Scholar]
- 18.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieth M, Stolte M. Pathology of early upper GI cancers. Best Pract Res Clin Gastroenterol. 2005;19:857–869. doi: 10.1016/j.bpg.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: Analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 21.Portale G, Peters JH, Hsieh CC, Tamhankar AP, Almogy G, Hagen JA, Demeester SR, Bremner CG, Demeester TR. Esophageal adenocarcinoma in patients ≤ 50 years old: Delayed diagnosis and advanced disease at presentation. Am Surg. 2004;70:954–958. [PubMed] [Google Scholar]
- 22.Cook MB, Corley DA, Murray LJ, Liao LM, Kamangar F, Ye W, Gammon MD, Risch HA, Casson AG, Freedman ND, Chow WH, Wu AH, Bernstein L, Nyrén O, Pandeya N, Whiteman DC, Vaughan TL. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: A pooled analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON) PLoS One. 2014;9:e103508. doi: 10.1371/journal.pone.0103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becher A, Dent J. Systematic review: Ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol Ther. 2011;33:442–454. doi: 10.1111/j.1365-2036.2010.04542.x. [DOI] [PubMed] [Google Scholar]
- 24.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Sharma AN, Murad MH, Buttar NS, El-Serag HB, Katzka DA, Iyer PG. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1399–1412.e7. doi: 10.1016/j.cgh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 27.Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 28.Cook MB, Shaheen NJ, Anderson LA, Giffen C, Chow WH, Vaughan TL, Whiteman DC, Corley DA. Cigarette smoking increases risk of Barrett's esophagus: An analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142:744–753. doi: 10.1053/j.gastro.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman HG, Bhat S, Johnston BT, McManus D, Gavin AT, Murray LJ. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett's esophagus. Gastroenterology. 2012;142:233–240. doi: 10.1053/j.gastro.2011.10.034. [DOI] [PubMed] [Google Scholar]