Abstract
Objective
The sacroiliac joint can be a primary source of pain or part of multifactorial syndromes. As there is no single historical, physical examination-based, or radiological feature that definitively establishes a diagnosis of sacroiliac joint pain, diagnostic blocks are regarded as the gold standard. The primary aim of this randomized trial was to compare the posteroanterior approach with the classic oblique approach for sacroiliac joint injection based on an assessment of procedure times and patient-reported pain outcomes in subjects scheduled for fluoroscopically-guided sacroiliac joint injections.
Methods
Thirty patients were randomized into 2 groups of 15 patients each. The endpoints measured included the total length of procedure time, fluoroscopic time, needling time (length of time the needle was maneuvered), and pre- and postprocedure visual analogue scale pain scores.
Results
The posteroanterior approach was significantly shorter in terms of procedure time (p=0.03) and needling time (p=0.01) than the oblique approach. Adjusting for body mass index, the mean procedure and needling times were significantly shorter in the posteroanterior group than in the oblique group.
Conclusion
This study of the posteroanterior approach for fluoroscopic-guided sacroiliac joint injection observed shorter times for fluoroscopy, needling, and the overall procedure than were recorded for the widely prevalent oblique approach. This may translate to lower radiation exposure, lower procedural costs, and enhanced ergonomics of fluoroscopicallyguided sacroiliac joint injections.
Keywords: Sacroiliac joint pain, Sacroiliac joint injection, Intra-articular injections, Fluoroscopy, Anteroposterior view, Oblique view
INTRODUCTION
Low back pain is responsible for the loss of 149 million work days per year in the United States and accounts for about $100–$200 billion of the annual U.S. health care budget [1,2]. Low back pain is considered the second most common cause of disability with a steady increase in incidence from 3.9% in 1992 to 10.6% in 2006. Approximately 80% of the population will have an episode of low back pain during their lifetime [3]. About 25%–27% of patients with persistent mechanical low back pain below L5 have pain secondary to sacroiliac (SI) joint pathology [4]. The SI joint can be a primary source of pain or part of multifactorial syndromes, such as ankylosing spondylitis, psoriatic arthropathy, gouty arthritis, osteoarthritis, etc.
SI joint-related pain can be challenging to diagnose as it mimics pain originating from lumbar disc herniation, facet syndrome, gluteal tendinopathies, and hip joints, which is why the diagnosis of SI joint disease is frequently overlooked by clinicians. Numerous provocative physical examination maneuvers can help elucidate the pain of SI joint origin, but none are considered pathognomonic [5-7]. Imaging may help rule out other disorders that may simulate SI joint pain, but it fails to provide a specific marker that can successfully diagnose SI joint dysfunction or pain. There is a poor correlation between symptoms and radiological examinations, and it is hypothesized that the low epidemiological values (sensitivity 19%) of conventional X-rays can delay the diagnosis of sacroiliitis [8]. Computed tomography has a sensitivity of 57.5% and specificity of 69% in diagnosing pain from SI joint pathology, although a retrospective study reported that computed tomography was negative in 42% of patients with symptomatic SI joint inflammation [9]. Radionuclide bone scanning is also reported to be a poor diagnostic tool with a high specificity ranging from 89.5% to 100%, but a very low sensitivity of 13% to 46% [10-12]. Imaging modalities offer no significant diagnostic value in chronic SI joint pain without inflammatory arthritis, because the SI joint can be a source of pain despite normal radiological findings [13]. As there is no single historical, physical examination, or radiological feature to definitively establish a diagnosis of SI joint pain, diagnostic blocks are regarded as the gold standard [14,15].
A thorough knowledge of the unique anatomy and inherent challenges of performing diagnostic or therapeutic injections of the SI joint is vital for treating clinicians. The SI joint is classified anatomically as a diarthrodial synovial joint, but cadaveric studies have confirmed that the synovial part is limited to the anterior one-third and absence of a posterior joint capsule. The remainder of the SI joint is composed of an intricate set of muscles, ligaments, and fibrocartilaginous tissue (instead of hyaline cartilage), which along with topographical variations in the joint surface imparts morphologic heterogeneity to the joint [16-22]. Cadaveric studies have reported a marked variation in nerve innervation of the SI joint, making an anatomical nerve block ineffective and necessitating an intra-articular injection [5,23-26]. The current literature reveals a good level of evidence to support the use of SI joint infiltration with local anesthetic agents as a diagnostic test. Clinically guided SI joint injections have been reported to have a poor success rate, as the disparate anatomy of the joint compounds the subjective errors of injecting the drug at unintended locations [4,14,27-29]. As a result, the use of fluoroscopically guided SI joint injection of local anesthetic agents is widely advocated and has emerged as the standard diagnostic modality. The use of fluoroscopy enhances spatial visualization of the joint and the needle but also improves the precision, accuracy, and clinical outcomes dramatically [27,28]. Current evidence shows that fluoroscopically guided intra-articular SI joint injections provide symptomatic relief for up to 6 months [29]. This modality is cost-effective, as evidenced by the cost per quality-adjusted life year falling well below the threshold cost of 1 quality-adjusted life years [30]. Analogous to the varied anatomy of the SI joint, a multitude of fluoroscopic techniques have been designed based on enhanced joint space visualization, procedural ease, and operator comfort, each claiming better outcomes. Incorrect needle placement may lead to a false-positive or falsenegative response to injection as well as cause vascular, ligamentous, or osseous injuries [28,31]. Therefore, pain physicians are highly invested in devising newer techniques to enhance precision, accuracy, and safety of fluoroscopic-guided SI joint injections.
The purpose of our pilot study was to compare the newly designed posteroanterior approach, a modified posteroanterior (PA) technique for SI joint injection, with the classic oblique technique. The authors hypothesized that the newly designed posteroanterior approach developed in the pain clinic, will be associated with less procedure time, fluoroscopic time, needling time (length of time the needle was maneuvered), and pre- and postprocedure visual analog scale as compared to the classic oblique technique.
MATERIALS AND METHODS
This randomized double-blinded controlled trial control trial aimed to compare the posteroanterior approach with the classic oblique approach for SI joint injection based on assessment of procedure times and patient-reported pain outcomes. This study was approved by the hospital’s Institutional Review Board and was conducted from June 2015 till April 2016. Each patient’s history, physical examination, and radiologic findings were used to rule out other potential causes for their pain. We performed this technique on selected patients who presented with SI joint pain and were in the age range of 18–80 years, had no diabetes or hypertension, had a positive Patrick test (FABER [Flexion+ABduction+External Rotation] test) or Gaenslen test, and had no pain above the posterior superior iliac spine level. We excluded those patients with signs of local infection, coagulopathy, allergy to local anesthetics, and those currently on anticoagulation therapy. We assessed percentage pain relief using a visual analogue scale (VAS) after the procedure. Study patients consisted of 2 groups, each with 15 patients, referred to the same pain clinic for diagnostic and therapeutic SI joint injection. After obtaining a written informed consent, the patients were randomly assigned by the pain medicine specialist to receive SI joint injections either through posteroanterior approach or the classic oblique approach. The study subjects and the investigator (Anesthesiology resident) recording and reporting the study variables were blinded to the type of fluoroscopic procedure performed. In both groups, a solution of 2 mL of local anesthetic (bupivacaine hydrochloride; Abbott Laboratories, North Chicago, IL, USA) mixed with 40-mg methylprednisolone (Depo-Medrol; Pfizer, New York, NY, USA) was injected. SI joint injections were performed with or without sedation (sedation consisted of midazolam only). Fluoroscopic guidance was achieved via a C-arm, placed in a pulsed, low-dose fluoroscopic mode. All injections were performed by a single, experienced, board certified anesthesiology pain physician (AU) utilizing the same clinic site, a single fluoroscopy unit, and single radiation technologist.
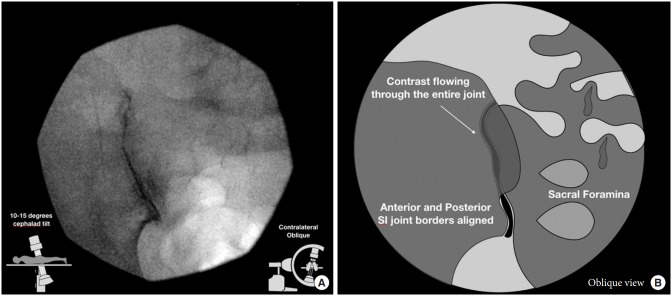
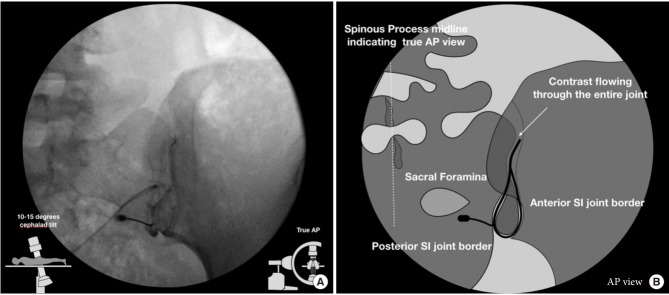
The patients were placed in the prone position on the fluoroscopic table. All procedures were performed with strict sterile technique. For the classic oblique approach, the C-arm was initially placed in a direct anteroposterior view, and a contralateral oblique of 5–15 degrees was used to obtain alignment of the anterior and posterior joint lines (Fig. 1). For the posteroanterior approach, the C-arm was initially placed in an anteroposterior view, allowing for visualization of both the anterior and posterior joint lines, then the C-arm was tilted in 5–15 degree cephalad direction to better expose the lower pole of the posterior joint line (Fig. 2). To gain access to the joint, we followed the beam of the C-arm to a target site just medial to the medial joint line along the lower one-third of the joint. Once the sacrum was contacted, we slipped the needle laterally into the joint space. The target for injection in both groups was the lower one-third of the visualized joint. Arthrography was completed with a small amount of ISOVUE contrast (ISOVUE-M 200 Contrast Media Injection Iopamidol 41% 20 mL vial; Bracco Diagnostic Inc., Monroe Township, NJ, USA). The endpoints measured included total length of procedure time, fluoroscopic time, needling time (length of time the needle was maneuvered), and pre- and postprocedure VAS pain scores (at 5, 10, 15, and 30 minutes). Statistical analyses of variables between groups used t-tests, Wilcoxon, and chi-square tests. To account for body mass index (BMI), a multiple regression was performed, with time as the outcome variable and the treatment and BMI as explanatory variables. The coefficient on the treatment variable revealed the average difference in time between the 2 groups, holding the BMI of the patient constant.
Fig. 1.
Oblique technique fluoroscopic view (A) and graphical illustration (B). In the oblique approach, the C-arm is rotated in a contralateral manner until the 2 joint lines become superimposed. Then one would target the inferior segment of this superimposed image, as the superior sacroiliac (SI) joint space is composed of interosseous ligaments.
Fig. 2.
Anteroposterior technique fluoroscopic view (A) and graphical illustration (B). In the anteriorposterior (AP) approach, image is taken with 5–15 degree cephalad tilt from the vertical of the fluoroscopy machine reveals a joint with 2 separately visible anterior and posterior joint lines. The anterior and posterior parts of the sacroiliac (SI) joint were delineated as lateral and medial joint spaces, respectively.
RESULTS
The posteroanterior approach group included 15 patients and the oblique group had 14 subjects, with 1 patient excluded from analysis to avoid bias. This excluded patient’s oblique procedure turned into a PA approach because of extraneous factors. Without adjusting for any other factors, the posteroanterior approach was significantly shorter in procedure time (p=0.03) and needling time (p=0.01) than the oblique approach (Table 1). Analysis of the fluoroscopy time, which represents how long a patient is exposed to radiation, is shown in Fig. 3. While overall the time parameters of posteroanterior procedure were shorter than the oblique, the PA group had 2 outliers and the oblique group had 1 outlier when fluoroscopy times were greater than 10 seconds. A t-test showed that the mean difference of 1.8 seconds between the 2 groups was not statistically significant (Table 1). Therefore, we performed a Wilcoxon test on the difference in medians of the 2 groups. The Wilcoxon test was preferred since the median does not account for the outliers. The median fluoroscopy time resulted in a significant median difference between the groups (Table 1). The mean BMI was similar for the 2 groups (31.0±12.2 kg/m2 for posteroanterior versus 31.5±7.1 kg/m2 for the oblique). To interpret the mean difference, data analyses were adjusted for BMI. That means if a patient with a BMI fixed at 30 (or any other arbitrary BMI) had both the posteroanterior and the oblique methods performed on them, the average difference in their time (procedure, needling, or fluoroscopy) for the 2 procedures would be the mean difference in time as shown. When adjusting for BMI, mean procedure time and needling time were significantly less in the posteroanterior group than the oblique group, but no difference was seen in fluoroscopy time using median regression analysis (Table 2). VAS pain scores for the 2 groups are shown in Table 3. The only significant difference was lower average VAS at 5-minute postprocedure (t=2.15, p=0.04) for the oblique group than for the posteroanterior group.
Table 1.
Comparison of groups by procedure times
| Variable | Posteroanterior (n = 15) | Oblique (n = 14) | Mean difference (sec) | t-test | p-value |
|---|---|---|---|---|---|
| Procedure time (sec) | 176.9 ± 83.4 | 288.7 ± 170.5 | 111.8 | 2.22 | 0.03 |
| Needling time (sec) | 19.5 ± 8.6 | 66.6 ± 58.3 | 47.0 | 2.99 | 0.01 |
| Fluoroscopy time (sec) | |||||
| Mean± SD | 7.0 ± 3.8 | 5.2 ± 3.9 | 1.8 | 1.25 | 0.22 |
| Median difference | 4.0 | 6.5 | 2.5 | 2.46* | 0.01 |
| Body mass index (kg/m2) | 31.0 ± 12.2 | 31.5 ± 7.1 | - | - | > 0.5 |
Values are presented as mean±standard deviation (SD).
Comparison of the average times (procedure, needle, and fluoroscopy) between the 2 groups without adjusting for any other factors. The total procedure time, measured in seconds, is 111.8 seconds less for posteroanterior procedures, on average, than for oblique procedures. The difference in total procedure time between the 2 groups is statistically significant.
Z statistic, Wilcoxon test.
Fig. 3.
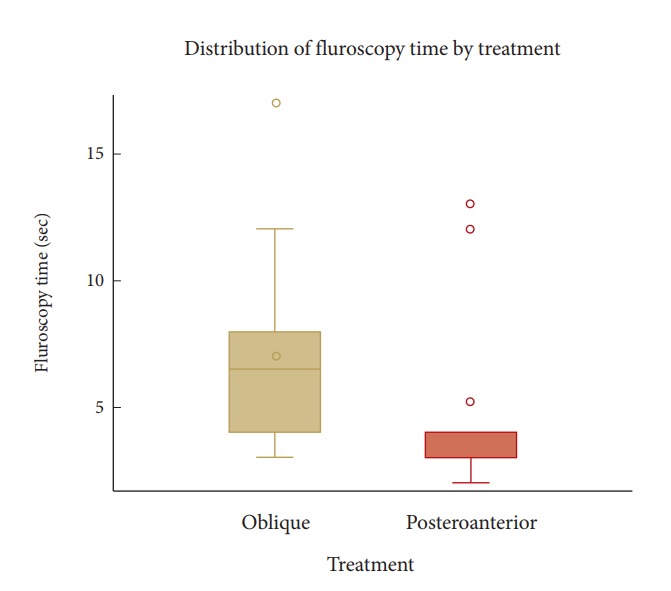
The boxplots show that posteroanterior times are lower than oblique for the most part. There are 2 outliers with fluoroscopy times greater than 10 seconds in the posteroanterior group as well as 1 outlier in the oblique group. A simple t-test would be biased due to these outliers. As proof, a t-test showed that the mean difference of 1.8 seconds, as shown in Table 1, between the 2 groups was not statistically significant (t = 1.25, p = 0.22). Therefore, we performed a Wilcoxon test, a test for the difference in medians of the 2 groups. The median Fluoroscopy time (in seconds) is 6.5 for the oblique group and 4.0 for the posteroanterior group, resulting in a median difference of 2.5 seconds between the groups.
Table 2.
Comparison of groups after adjusting for body mass index (BMI)
| Variable | Mean difference between posteroanterior and oblique time | T-statistic | p-value |
|---|---|---|---|
| Procedure time (sec) | 109.5 | 2.33 | 0.03 |
| Needling time (sec) | 46.6 | 3.08 | 0.001 |
| Fluoroscopy time (sec) | 1.2* | 1.24† | 0.26 |
When adjusting for BMI, mean procedure time and needle time were significantly less in the posteroanterior group than the oblique group, but no difference was seen in fluoroscopy time using median regression analysis. Unlike the first 2 times (procedure and needling times), we used median time for fluoroscopy time. We followed it with median regression, which simply models the median instead of the mean. We tried to employ the mean regression for fluoroscopy time, but there was no statistically significant mean difference when adjusting for BMI. Again, the outliers appear to bias modeling the mean. Therefore, for fluoroscopy time we reported the results from the median regression.
Median time.
Chi-square statistic.
Table 3.
Group comparison by visual analogue scale (VAS)
| VAS measure | Posteroanterior (n = 15) | Oblique (n = 14) | p-value |
|---|---|---|---|
| Preprocedure | 8.1 ± 1.0 | 8.1 ± 1.3 | 0.99 |
| Postprocedure | |||
| 5 Minutes | 3.3 ± 3.6 | 1.0 ± 2.1 | 0.04 |
| 10 Minutes | 2.6 ± 2.7 | 1.6 ± 2.2 | 0.09 |
| 15 Minutes | 1.9 ± 2.5 | 1.7 ± 2.7 | 0.56 |
| 30 Minutes | 1.8 ± 2.6 | 1.6 ± 2.6 | 0.78 |
Values are presented as mean±standard deviation.
The only significant difference between the 2 groups is that the average VAS 5 minutes postprocedure score is significantly lower (t=2.15, p=0.04) for the oblique group than for the posteroanterior group.
DISCUSSION
This single-site randomized trial showed that the newly designed, modified posteroanterior approach for SI joint injections was significantly shorter in procedure time and needling time and similar in VAS pain outcomes within 30-minute postprocedure in comparison to the classic oblique approach. The complex anatomical schematic of the SI joint has led to the development of various fluoroscopic-guided injection techniques, with an endpoint to delineate the anterior and posterior portions of the SI joint to facilitate ideal needle positioning and precise medication injection [32]. Based on a review of the literature, the 2 most common techniques are the direct PA technique and the oblique projection technique. In the PA approach, the more medial joint is posterior while the lateral joint is the anterior aspect. The target is the posterior one-third of the medial joint line [5,13,28]. Most practitioners will target the medial joint line, hitting the sacrum to gain perspective on depth and then carefully slipping laterally into the joint space. In the oblique approach, the C-arm is rotated in a contralateral manner until the 2 joint lines become superimposed. Then one would target the inferior segment of this superimposed image, as the superior SI joint space is composed of interosseous ligaments [32-34].
There are many modifications to this technique in current practice [13,35]. Dussault et al. [36] describe a modification to the posteroanterior approach. In this method, the C-arm is initially placed posteroanterior to the patient and the lowest 1 cm of the joint is marked on the skin, followed by a cephalad tilt of 20–25 degrees. A needle is then introduced into the original site marked on the skin in a posteroanterior manner until it gains access to the SI joint. The key to this technique is that the cephalic angulation of the C-arm displaces the inferior portion of the SI joint in a caudal direction. Our posteroanterior method uses a modification of the classic PA technique described by Dussault et al. [36] Instead of 20°–25° from the vertical, we used only 5–15 degree cephalad tilt from the vertical of the fluoroscopy machine. Our approach yielded much lower mean fluoroscopic time (7.0±3.8 seconds) compared to Dussault et al. [36] (108 seconds; range, 36–328 seconds). They reported that 64% (22 of 31) of subjects achieved a greater than 50% decrease in VAS compared to 100% of the subjects having the same outcome in our study [14]. Dreyfuss et al. [37] reported a modification to the oblique technique that utilizes dynamic fluoroscopy. They proposed contralateral oblique rotation of the C-arm to profile the joint, followed by rotation in an ipsilateral direction between 5–20 degrees to attain ideal separation of the joints. Interestingly, others have proposed using both the posteroanterior and oblique techniques simultaneously. Kasliwal and Kasliwal [38] reported that they used a PA view, angled cephalad, focusing on the lower part of the SI joint, followed by the contralateral oblique view, ranging from 0–30 degrees, until the widest space at the inferior portion of the SI joint was visible. They advanced the curved needle towards the inferior margin of the SI joint and used contralateral and ipsilateral oblique views to facilitate needle advancement in the appropriate plane. They then used lateral fluoroscopic tilt to confirm needle placement and visualize the spread of contrast. Of their 30 cases, needle position was satisfactory in 28 and contrast spread was satisfactory in 27 cases. They did not include fluoroscopy times although they commented that enhanced visualization of the depth of the needle improves safety by reducing the incidence of injuries to other structures in the pelvis. Gupta [39] described a novel 2-needle technique for difficult-toaccess SI joints. Gupta [39] describes a technique that uses a combination of oblique projection followed by a return to a PA projection to confirm proper placement of the needle tip before the injection of contrast. Rana et al. [35] analyzed computed tomography reconstructions of the pelvis from 100 SI joints in 50 patients who had clinically indicated pelvic scans. They reported that with a patient lying prone, the data supports using a tilt of the X-ray image intensifier 10 degrees caudal past the vertical anteroposterior view for the optimal approach of the SI joint’s inferior limb, which is the intended spot for needle placement. This observation by Rana et al. [35] supports our hypothesis that fluoroscopic-guided SI joint injections via a PA technique is anatomically superior and provides enhanced visualization of the needle tip in comparison to the oblique approach.
A further argument in support of our technique comes from an observational study conducted by Khuba et al. [40], who performed fluoroscopic-guided SI joint injections in 58 subjects via the anteroposterior approach. They reported that in 42 patients the anterior and posterior parts of the SI joint were delineated as lateral and medial joint spaces, respectively. In 18 subjects the SI joint was viewed as a single line, and they had to improvise by tilting the fluoroscope cranially, leading to the caudal displacement of the posterior joint line. They concluded that alignment of anterior and posterior aspects of the SI joint, i.e., the oblique technique, not only is time-consuming but also is associated with increased failure rates. Their findings recapitulate that the oblique view impedes the execution of an SI joint injection and that the PA approach not only offers a better view of anatomy but also is associated with less procedure time and has an inherently improved safety profile as there are no vital structures to avoid. The study is limited by the small sample size. Further multicenter studies with a large cohort should be conducted to provide further affirmation of our reported observations.
CONCLUSION
This study of our newly designed posteroanterior approach for fluoroscopic-guided SI joint injection observed lower times for fluoroscopy, needling, and overall procedure compared to the widely prevalent oblique approach. We were able to achieve similar levels of pain mitigation with less time via our technique. This may translate to lower radiation exposure, lower procedural costs, and enhanced ergonomics of fluoroscopic-guided SI joint injections. Future studies using this new approach may provide further evidence of its utility and serve to refine our views.
Acknowledgments
This study was presented at American Society of Regional Anesthesia and Pain Medicine (ASRA), Nov 2017.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Guo HR, Tanaka S, Halperin WE, et al. Back pain prevalence in US industry and estimates of lost workdays. Am J Public Health. 1999;89:1029–35. doi: 10.2105/ajph.89.7.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88 Suppl 2:21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25:353–71. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Rupert MP, Lee M, Manchikanti L, et al. Evaluation of sacroiliac joint interventions: a systematic appraisal of the literature. Pain Physician. 2009;12:399–418. [PubMed] [Google Scholar]
- 5.Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976) 1995;20:31–7. doi: 10.1097/00007632-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976) 1996;21:2594–602. doi: 10.1097/00007632-199611150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine (Phila Pa 1976) 1996;21:1889–92. doi: 10.1097/00007632-199608150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Vanelderen P, Szadek K, Cohen SP, et al. 13. Sacroiliac joint pain. Pain Pract. 2010;10:470–8. doi: 10.1111/j.1533-2500.2010.00394.x. [DOI] [PubMed] [Google Scholar]
- 9.Elgafy H, Semaan HB, Ebraheim NA, et al. Computed tomography findings in patients with sacroiliac pain. Clin Orthop Relat Res. 2001;(10):112–8. doi: 10.1097/00003086-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Slipman CW, Sterenfeld EB, Chou LH, et al. The value of radionuclide imaging in the diagnosis of sacroiliac joint syndrome. Spine (Phila Pa 1976) 1996;21:2251–4. doi: 10.1097/00007632-199610010-00013. [DOI] [PubMed] [Google Scholar]
- 11.Maigne JY, Boulahdour H, Chatellier G. Value of quantitative radionuclide bone scanning in the diagnosis of sacroiliac joint syndrome in 32 patients with low back pain. Eur Spine J. 1998;7:328–31. doi: 10.1007/s005860050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song IH, Carrasco-Fernández J, Rudwaleit M, et al. The diagnostic value of scintigraphy in assessing sacroiliitis in ankylosing spondylitis: a systematic literature research. Ann Rheum Dis. 2008;67:1535–40. doi: 10.1136/ard.2007.083089. [DOI] [PubMed] [Google Scholar]
- 13.Simopoulos TT, Manchikanti L, Singh V, et al. A systematic evaluation of prevalence and diagnostic accuracy of sacroiliac joint interventions. Pain Physician. 2012;15:E305–44. [PubMed] [Google Scholar]
- 14.Puhakka KB, Melsen F, Jurik AG, et al. MR imaging of the normal sacroiliac joint with correlation to histology. Skeletal Radiol. 2004;33:15–28. doi: 10.1007/s00256-003-0691-4. [DOI] [PubMed] [Google Scholar]
- 15.Jurik AG. Technique and radiation dose of conventional Xrays and computed tomography of the sacroiliac joint. Radiologe. 2004;44:229–33. doi: 10.1007/s00117-003-1016-2. [DOI] [PubMed] [Google Scholar]
- 16.Fortin JD, Sehgal N. Lennard TA. Pain procedures in clinical practice. Philadelphia (PA): Hanley & Belfus; 2000. Sacroiliac joint injection and arthrography with imaging correlation; pp. 265–75. [Google Scholar]
- 17.Smidt GL, Wei SH, McQuade K, et al. Sacroiliac motion for extreme hip positions. A fresh cadaver study. Spine (Phila Pa 1976) 1997;22:2073–82. doi: 10.1097/00007632-199709150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Bowen V, Cassidy JD. Macroscopic and microscopic anatomy of the sacroiliac joint from embryonic life until the eighth decade. Spine (Phila Pa 1976) 1981;6:620–8. doi: 10.1097/00007632-198111000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Brooke R. The sacro-iliac joint. J Anat. 1924;58(Pt 4):299–305. [PMC free article] [PubMed] [Google Scholar]
- 20.Gunterberg B, Romanus B, Stener B. Pelvic strength after major amputation of the sacrum. An exerimental study. Acta Orthop Scand. 1976;47:635–42. doi: 10.3109/17453677608988751. [DOI] [PubMed] [Google Scholar]
- 21.Miller JA, Schultz AB, Andersson GB. Load-displacement behavior of sacroiliac joints. J Orthop Res. 1987;5:92–101. doi: 10.1002/jor.1100050112. [DOI] [PubMed] [Google Scholar]
- 22.Robinson A. Cunningham’s text-book of anatomy. 6th ed. New York: Oxford University Press; 1931. [Google Scholar]
- 23.Vilensky JA, O'Connor BL, Fortin JD, et al. Histologic analysis of neural elements in the human sacroiliac joint. Spine (Phila Pa 1976) 2002;27:1202–7. doi: 10.1097/00007632-200206010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda R. Innervation of the sacroiliac joint. Macroscopical and histological studies. Nihon Ika Daigaku Zasshi. 1991;58:587–96. doi: 10.1272/jnms1923.58.587. [DOI] [PubMed] [Google Scholar]
- 25.Murata Y, Takahashi K, Yamagata M, et al. Sensory innervation of the sacroiliac joint in rats. Spine (Phila Pa 1976) 2000;25:2015–9. doi: 10.1097/00007632-200008150-00003. [DOI] [PubMed] [Google Scholar]
- 26.Szadek KM, Hoogland PV, Zuurmond WW, et al. Nociceptive nerve fibers in the sacroiliac joint in humans. Reg Anesth Pain Med. 2008;33:36–43. doi: 10.1016/j.rapm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101:1440–53. doi: 10.1213/01.ANE.0000180831.60169.EA. [DOI] [PubMed] [Google Scholar]
- 28.Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(2 Suppl):S49–283. [PubMed] [Google Scholar]
- 29.Althoff CE, Bollow M, Feist E, et al. CT-guided corticosteroid injection of the sacroiliac joints: quality assurance and standardized prospective evaluation of long-term effectiveness over six months. Clin Rheumatol. 2015;34:1079–84. doi: 10.1007/s10067-015-2937-7. [DOI] [PubMed] [Google Scholar]
- 30.Bydon M, Macki M, De la Garza-Ramos R, et al. The costeffectiveness of CT-guided sacroiliac joint injections: a measure of QALY gained. Neurol Res. 2014;36:915–20. doi: 10.1179/1743132814Y.0000000372. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg JM, Quint TJ, de Rosayro AM. Computerized tomographic localization of clinically-guided sacroiliac joint injections. Clin J Pain. 2000;16:18–21. doi: 10.1097/00002508-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Zou YC, Li YK, Yu CF, et al. A cadaveric study on sacroiliac joint injection. Int Surg. 2015;100:320–7. doi: 10.9738/INTSURG-D-13-00194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976) 1995;20:31–7. doi: 10.1097/00007632-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Kirkaldy-Willis WH, Burton CV, Cassidy JD. Kirkaldy-Willis WH. Managing low back pain. 3rd ed. New York: Churchill-Livingstone; 1992. The site and nature of the lesion; pp. 121–48. [Google Scholar]
- 35.Rana SH, Farjoodi P, Haloman S, et al. Anatomic evaluation of the sacroiliac joint: a radiographic study with implications for procedures. Pain Physician. 2015;18:583–92. [PubMed] [Google Scholar]
- 36.Dussault RG, Kaplan PA, Anderson MW. Fluoroscopy-guided sacroiliac joint injections. Radiology. 2000;214:273–7. doi: 10.1148/radiology.214.1.r00ja28273. [DOI] [PubMed] [Google Scholar]
- 37.Dreyfuss P, Cole AJ, Pauza K. Sacroiliac joint injection techniques. Phys Med Rehabil Clin N Am. 1995;6:785–813. [Google Scholar]
- 38.Kasliwal PJ, Kasliwal S. Fluoroscopy-guided sacroiliac joint injection: description of a modified technique. Pain Physician. 2016;19:E329–38. [PubMed] [Google Scholar]
- 39.Gupta S. Double needle technique: an alternative method for performing difficult sacroiliac joint injections. Pain Physician. 2011;14:281–4. [PubMed] [Google Scholar]
- 40.Khuba S, Agarwal A, Gautam S, et al. Fluoroscopic sacroiliac joint injection: is oblique angulation really necessary? Pain Physician. 2016;19:E1135–8. [PubMed] [Google Scholar]