Abstract
Abnormal cerebrospinal fluid (CSF) levels of β-amyloid peptides (Aβ42) and Tau and cognitive decline are typical characteristics of Alzheimer’s disease (AD). Since dysregulation in lipid metabolism accompanies abnormal amyloid formation, we quantified glycerophospholipids (GP) and sphingolipids (SP) in CSF fractions from participants with late-onset AD (LOAD, n = 29) or with Other Dementia (OD, n = 10) to determine if alterations in lipid metabolism account for pathological differences. Aβ42 and total Tau levels were determined using a sandwich ELISA. Liposomal-based fluorescent assays were used to measure phospholipase A2 (PLA2) and acid or neutral sphingomyelinase (aSMase, nSMase) activities. Supernatant fluid (SF) and nanoparticle (NP) lipids were quantified using LC-MS/MS. Although CSF Aβ42 and Tau levels are similar, phosphatidylserine (PS) in SF and ceramide (CM) levels in NP are significantly higher in OD compared with LOAD. The aSMase but not the nSMase activity is higher in OD. PLA2 activity in CSF from OD subjects positively correlates with several GP classes in SF and NP fractions but not in LOAD fractions. Our data indicate differences in CSF lipid metabolism between dementia variants. Higher levels of inflammatory and apoptotic lipids may induce faster neuronal death, resulting in the earlier cognitive decline in patients with OD phenotypes.
Keywords: amyloid, ceramide, cerebrospinal fluid, late-onset Alzheimer’s disease, lysophosphatidylcholine, other dementia, phosphatidylserine, phospholipase A2, sphingomyelinase, Tau proteins
1. Introduction
Late-onset Alzheimer’s disease (LOAD) is the most common neurodegenerative disorder characterized by progressive loss of cognitive function that interferes with daily activities [1]. The pathology involves neurofibrillary tangles, composed of hyperphosphorylated Tau proteins and β-amyloid (Aβ) plaques, which obstruct proper synapse function and lead to neuronal cell loss and atrophy [1]. The most significant risk factor is age: the risk doubles every five years after the age of 65, with a higher AD prevalence in females than males [2,3]. There is also a strong genetic component that indicates an increase in dementia risk in individuals expressing the E4 isoform of ApoE gene [4]. ApoE modulates Aβ metabolism and transport and thus prevents the formation of toxic Aβ fibrils [5]. Current biomarkers of AD include elevated CSF T-Tau, elevated P-Tau, and diminished Aβ42 levels [6,7,8]. However, these biomarkers are more readily used to distinguish AD patients from non-AD patients rather than differentiating between AD and other dementias.
In addition to LOAD, Frontotemporal Dementia (FTD), Dementia with Lewy Body (DLB), and vascular dementia (VaD) account for a substantial amount of cases of other dementias. FTD constitutes 5–15% of all dementia cases and is the second most common cause of dementia for patients under the age of 65 [9]. Unlike LOAD, FTD patients have relative preservation of memory; nonetheless, they experience progressive deterioration in language, behavior, and personality [9]. The genetic background of FTD lies on two mutations on the microtubules associated protein Tau gene (MAPT) and progranulin (GRN) gene, respectively [9]. DLB is yet another common type of dementia that is characterized by the presence of Lewy bodies in the brainstem, throughout the amygdala and the cortex [10]. Lewy bodies are composed of accumulated α-synuclein inside the nuclei of neurons. Symptoms are typically similar to that of AD, although DLB patients experience visual hallucinations, visuospatial deficits, fluctuating cognitive awareness, and parkinsonian symptoms [10,11,12]. Memory impairment is more pronounced in AD than DLB, whereas SPECT and PET scans have demonstrated hypometabolism and hypoperfusion in the primary visual cortex in DLB but not in AD [13].
Lipids are involved in critical neurobiological functions, such as membrane formation, cellular binding and recognition, transport, energy, and signaling [14,15]. In AD, lipid biochemistry is dysregulated, causing profound effects on signaling and Aβ plaque accumulation [16,17,18,19,20,21]. Glycerophospholipid (GP), a main component of membranes, are reduced in AD patients, which is attributable to abnormal phospholipid metabolism and turnover. More specifically, lipid characterizations have revealed decreased phosphatidylcholine (PC) and phosphatidylethanolamine (PE) in the frontal and parietal cortex of AD, while charged GPs on cellular membranes directly interact with and influence the release of Aβ peptides from cells [22]. In AD, changes in GPs in cerebrospinal fluid (CSF) accompany an increase in phospholipase activity [23]. There is significant stabilization of nonspecific β sheets through this interaction, and a thin polar dehydrated film is created by ganglioside GM1 to promote Aβ aggregation [24]. Sphingolipids (SPs) are essential for signal transmission through their roles as extracellular receptor ligands and intracellular secondary messengers [25,26]. SP metabolism is important in AD by disrupting protein-lipid interactions as well as membrane trafficking and signaling [27].
Several enzymes, as well as lipids, interact with Aβ metabolism [19,28,29,30,31,32]. Oxidative stress has been shown to stimulate sphingomyelinase (SMase), an enzyme that converts sphingomyelin (SM)to ceramide (CM) [33,34]. CM, a second messenger and regulator of apoptosis, stabilizes β secretase activity [31]. In AD, increased CM levels regulate β and γ secretases and amyloid precursor protein (APP) processing on lipid rafts [35]. Mislocalization of β and γ secretase to lipid rafts in post Golgi rather than lysosomes can promote Aβ accumulation [35]. Sphingosine is associated with apoptosis and is elevated in AD brains [36]. Sphingosine also plays a role in vesicle fusion and exocytosis [37]. Increased cholesterol increases the risk of AD, and more cholesterol in membranes has been associated with larger lipid raft size and accelerated α and β secretase activity [38,39,40].
The huge role of lipid metabolism in dementia and differences in clinical manifestation of diseases led us to hypothesize that lipid metabolism may differentiate dementias. This project aims to determine if lipid metabolism can differentiate LOAD from other dementias (OD), such as FTD and DLB. This characterization will allow for further research on early biomarkers of dementia subtypes. It would provide insight into any possible metabolic pathways, early diagnosis, and effective treatment of these diseases.
2. Materials and Methods
2.1. Study Participants
Human study participants were enlisted through newspaper advertising and visits to senior centers and assisted living residences. Criteria for inclusion required participants to be between the ages of 45 to 100 years old and diagnosed with LOAD [41], DLB, FTD, or vascular dementia (VaD). Criteria for exclusion comprised of patients taking anticoagulants or having contraindications against lumbar punctures. The local Internal Review Board of Huntington Memorial Hospital approved the protocol (FWA2338), and we obtained a signed informed consent form from each study participant. A Uniform Data Set was used to obtain all data sets within one month as described by the National Alzheimer’s Coordinating Center [42,43], and included structured clinical interviews; list of over the counter medications, nutritional supplements, and prescription drugs; physical examinations focusing on risk factors of dementia associated with the neurological and cardiovascular systems. We performed Clinical MRI on a 1.5T GE scanner to exclude significant vascular or neoplastic conditions. Clinical tests for study participants included the Mini-Mental State Exam [44], Montreal Cognitive Assessment [45], and Clinical Dementia Rating (CDR) [46]. A clinically probable diagnosis of the human participants was concluded after fulfillment of current criteria of LOAD [47] and other dementias based on consensus criteria of DLB [48] behavior variant FTD [49] and VaD [50]. The clinically probable dementia (LOAD, DLB, FTD, VaD, or mixed dementia) classification was established after the exam workup previously mentioned with triple scoring of neuropsychological test independently by research staff and clinical conferencing by at least three faculty clinicians [51].
2.2. ApoE Genotyping
ApoE genotyping was performed using a polymerase chain reaction mixture of primers specific for E2, E3, and E4, using the protocol described by Calero et al. [52]. We created a scale to quantify the participants’ risk from ApoE4: E2/E2 = 0, E2/E3 = 1, E3/E3 = 2, E2/E4 = 3, E3/E4 = 4, E4/E4 = 5.
2.3. Quantification of CSF Total Protein, Tau, and Aβ42
A clinician obtained lumbar CSF between 8:00 am and 10:00 am after an overnight fast, and laboratory personnel removed cellular debris by centrifugation at 1000 g for 2 min. We measured CSF levels of Aβ42 and total Tau using a sandwich ELISA kit (Innotest β-amyloid1-42 and Innotest hTAU-Ag, Innogenetics, Gent, Belgium) as previously described [51]. We determined total protein concentration in CSF using a fluorescent protein assay kit (QuantIT, Invitrogen/Molecular Probes, Eugene, OR, USA) using serum albumin (0–500 ng/mL) as a standard as previously described [51].
2.4. Enzyme Activity Assays
To measure phospholipase A2 (PLA2), acid sphingomyelinase (aSMase), or neutral sphingomyelinase (nSMase) activity in the CSF samples, we used a modified liposomal-based fluorescent assay with 7-hydroxycoumarinyl-arachidonate (0.3 mM), 7-hydroxycoumarinyl-linolenate (0.3 mM), hydroxycoumarinyl-6-hyptenoate (0.3 mM), 10 mM dioleoylphophatidylcholine (DOPC), and dioleoylphosphatidylglycerol (DOPG) (10 mM) as substrates [23]. To initiate PLA2 activity, we added CSF containing 10 µg total protein to 96 well plates containing 50 µL PLA2 substrates and 50 mM Tris HCl buffer (pH 8.9). PLA2 activity was measured using an excitation wavelength of 360 nm and emission at 460 nm, and specific activity was calculated as the relative fluorescent units (RFU)/µg protein/min) for CSF samples.
aSMase and nSMase activities in CSF were determined using an Amplex red fluorometric assay following the recommended protocol obtained from the kit’s manufacturer (Invitrogen/Molecular Probes, Eugene, OR, USA). Activities are presented as relative fluorescent units per minute for a 10 µg protein applied in duplicates on 96 well plates [53].
2.5. Lipid Extraction
We prepared lipid fractions consisting of supernatant fluid (SF) and nanoparticles (NP), according to Harrington et al. [54]. After the addition of internal standards and retention time calibrants (5 ng PC (11:0/11:0), 1 ng D4-PAF, 5 ng lysophosphatidylcholine (LPC) (11:0), and 5 ng D4-LPC) to 1 mL SF, lipids were extracted using a modified Bligh & Dyer protocol [55]. Briefly, we added 2 mL of methanol containing 0.2 mg/mL BHT and 1 mL of chloroform to 1 mL of CSF. After sonication of the resulting mixture for 10 min, we added 1 M NaCl (1 mL) to facilitate the separation of a lipid-rich chloroform layer. We performed a similar extraction procedure on the NP fraction after resuspension in 1 M NaCl solution. After drying under N2, we reconstituted lipid extracts in HPLC solvent before LC-MS/MS analyses.
2.6. LC-MS/MS of Lipids
We separated lipid extracts on a TSK-Gel Amide-80 Column (2.0 × 150 mm) using a binary solvent system as previously described [23,53]. After LC, lipids were positively ionized in electrospray ionization (ESI) and detected using several MS scanning modes in a triple quadrupole mass spectrometer (TSQ Classic, Thermo Fisher Scientific, San Jose, CA, USA) [23,53]. We optimized all GP and SP scans to their respective collision energies and collected data using three different scan windows from 0–6 min for CM, dihydroceramide (dhCM), PE, unknown lipid containing phosphoethanolamine (xPE), and N-acyl phosphatidylethanolamine (NAPE), 6–14.5 min for PC, SM, phosphatidylserine (PS), and platelet-activating factor (PAF), and 14.5–40 min for LPC.
2.7. LC-MS/MS Data Analysis
We used the Qual Browser module of the Xcalibur software (Thermo Fisher, San Jose, CA, USA) for peak integration of GP and SP classes. All integrated peaks were normalized to the internal standard (IS), PC (11:0/11:0) for quantification. For quantification of lipids classes in CSF fractions, known amounts of lipids with a fixed 5 ng IS were run separately through the LC-MS/MS. Standard curves of the lipid amounts versus lipid/PC (11:0/11:0) intensities were acquired and used to quantify the lipids in the SF and NP fractions (ng/mL CSF) [53].
2.8. Statistical Methods
All analyses were performed using GraphPad Prism software (Version 7, GraphPad Software, Inc., La Jolla, CA, USA) and 0.05 significance level. All lipid data are presented as mean ± SEM (ng/mL). Student paired t-tests were used to determine differences between Aβ, Tau, lipids, and proteins among the clinical groups. We used Fisher’s exact tests for categorical variables describing the presence or absence of risk factors in our study groups. We used Spearman ranked analyses to assess the correlation between GP and SP classes with Aβ42, Tau, PLA2, nSMase, or aSMase.
3. Results
3.1. Demographic Data and CSF Biomarkers
A total of 39 participants consented and were classified as having dementia. After clinical assessment, some participants met the criteria of LOAD (n = 29), while the rest were classified as other dementias (n = 10). The OD category was composed of DLB (n = 3), behavior variant FTD (n = 3), and vascular or mixed dementia (n = 4). Analyses of education and ApoE genotype numerical scale showed no group differences. Similarly, there was no significant difference in age, sex, body mass index (BMI), and neuropsychological classification between LOAD and OD (Table 1). Likewise, Aβ42 and Tau levels in the CSF were similar in LOAD compared with OD (Table 1).
Table 1.
Demographics and CSF biomarkers of LOAD and OD.
| Categories | LOAD | OD | 1 Difference (% LOAD) |
|---|---|---|---|
| Number of Subjects | 29 | 10 | |
| Female (%) | 53% | 40% | |
| Mean ± SEM | |||
| Age (years) | 77.4 ± 1.8 | 77.3 ± 4.0 | −0.1 |
| Education (years) | 14.5 ± 2.8 | 14.0 ± 3.1 | −3.5 |
| ApoE Genotype | 3.6 ± 0.2 | 4 ± 0 | 11.4 |
| BMI | 1.6 ± 0.2 | 1.3 ± 0.3 | −19.4 |
| 2 Neuropsych Classification | 9.1 ± 0.2 | 8.8 ± 0.3 | −3.72 |
| MMSE | 15 ± 1.5 | 16.0 ± 2.7 | 7.5 |
| CSF Tau (pg/mL) | 472.6 ± 41.1 | 387.2 ± 100.6 | −18.1 |
| CSF Aβ42 (pg/mL) | 506.2 ± 43.1 | 432.3 ± 59.4 | −13.8 |
| Aβ42/Tau | 1.4 ± 0.2 | 1.2 ± 0.3 | −12.1 |
| Dementia duration (years) | 5.7 ± 0.9 | 3.5 ± 0.6 | −39.0 |
1 Difference of means = (OD–LOAD)/LOAD × 100. 2 Neuropsychology scale > 8 indicates dementia. LOAD: Late-onset Alzheimer’s disease; OD: Other Dementia; BMI: body mass index; MMSE: Mini-Mental State Examination; CSF: cerebrospinal fluid.
Typical for dementias, both groups had reduced Aβ42 and elevated Tau levels in contrast to age-match cognitively normal individuals (722 ± 299, 273 ± 149 pg/mL, respectively) [51]. Dementia duration was 39% less in OD compared with LOAD, suggesting that OD participants (Table 1) have a higher rate of disease progression.
3.2. Dementia Risk Factors and Medication Use
We examined the frequencies of some AD risk factors in LOAD and OD to ascertain that these did not contribute to any differences in lipid metabolism. Our data show that the occurrence of the most common risk factors was similar in the LOAD and OD populations (Table 2).
Table 2.
Risk factors in late-onset Alzheimer’s disease (LOAD) and other dementia (OD) participants.
| Risk Factors | LOAD n (Frequency), % |
OD n (Frequency), % |
p Value 1 |
|---|---|---|---|
| Obesity # | 26 (3), 11.5 | 9 (0), 0 | 0.55 |
| Hyperlipidemia | 28 (9), 32.1 | 10 (4), 40 | 0.71 |
| Hypertension | 27 (15), 55.6 | 10 (4), 40 | 0.48 |
| Heart disease | 28 (11), 39.3 | 10 (4), 40 | 0.99 |
| Diabetes | 29 (4), 13.9 | 10 (1) 10 | 0.99 |
| Family history of dementia | 29 (14), 48.3 | 10 (2), 20 | 0.15 |
1 Fisher’s exact test. # BMI > 30.
We examined the number of prescription and over the counter medications in our study populations. The mean number of prescription medications in LOAD (mean ± SD, 4.2 ± 2.3) did not significantly (p > 0.4) differ from that of OD (mean ± SD, 3.8 ± 3.5). Similarly, the number of over the counter supplements were similar (p > 0.6), suggesting that differences in medication usage might not account for metabolic differences in LOAD compared with OD.
3.3. GPs in LOAD and OD
To determine if lipid metabolism is different in CSF from LOAD compared with OD, we measured the GP contents of the SF and NP fractions using LC-MS/MS. Of several GP classes in SF, only phosphatidylserine (PS) levels were significantly (p < 0.05) higher in OD compared with LOAD (Table 3). In general, there is a decrease in PC and PE levels in SF and an increase in LPC. Although not significantly different, PC and PE levels were 54 % and 20 % higher in NP from OD compared with LOAD (Table 3).
Table 3.
Glycerophospholipids (GPs) in supernatant fluid (SF) and nanoparticle (NP).
| GPs in SF (ng/mL) | LOAD | OD | 1 Difference (% LOAD) |
| LPC | 15.6 ± 1.1 | 16.0 ± 2.1 | 2.8 |
| PC | 8169.1 ± 915.1 | 7064.3 ± 1238.3 | −13.5 |
| LPC/PC | 0.0022 ± 0.0002 | 0.0023 ± 0.0002 | 4.6 |
| PS | 6.2 ± 1.0 | 16.01 ± 6.6 | 149.4 * |
| NAPE | 10.6 ± 2.3 | 15.09 ± 3.1 | 42.6 |
| PE | 27.8 ± 3.2 | 16.4 ± 2.5 | −41.2 |
| xPE | 1.3 ± 0.1 | 1.40± 0.2 | 11.2 |
| GPs in NP (ng/mL) | LOAD | OD | % LOAD |
| LPC | 3.05 ± 0.3 | 2.7 ± 0.2 | −11.2 |
| PC | 40.05 ± 4.8 | 61.9 ± 23.4 | 54.5 |
| LPC/PC | 0.09 ± 0.01 | 0.08 ± 0.01 | −10.1 |
| PAF | 0.08 ± 0.02 | 0.06 ± 0.01 | −28.3 |
| NAPE | 6.8 ± 1.1 | 6.9 ± 1.3 | 0.8 |
| PE | 7.9 ± 0.6 | 9.5 ± 1.3 | 20.5 |
| xPE | 1.8 ± 0.5 | 1.7 ± 0.2 | −7.3 |
The data are the mean ± SEM, ng/mL. The p values compare LOAD to OD and * denotes p < 0.05. 1 Difference of means as a percentage of LOAD = (OD–LOAD)/LOAD × 100. LPC: lysophosphatidylcholine; PC: phosphatidylcholine; LPC/PC: lysophosphatidylcholine to phosphatidylcholine ratio; PS: phosphatidylserine; NAPE: N-acyl phosphatidylethanolamine; PE: phosphatidylethanolamine; xPE: unknown lipid containing phosphoethanolamine; PAF, platelet-activating factor.
3.4. Sphingolipids in LOAD and OD
We compared levels of SP classes in different CSF fractions to determine if SP metabolism is altered in LOAD compared to OD. CM levels in NP was higher for OD compared with LOAD, while levels of other SP classes were similar in both clinical groups, although we noticed a tendency for higher levels in OD compared with LOAD (Table 4).
Table 4.
Sphingolipids (SP) in supernatant fluid (SF) and nanoparticle (NP) of late-onset Alzheimer’s disease (LOAD) and other dementia (OD).
| SP Classes in SF | LOAD (ng/mL) | OD (ng/mL) | 1 Difference (% LOAD) |
| SM | 1888.2 ± 138.2 | 1892.1 ± 400.1 | 2.1 |
| CM | 60.0 ± 6.9 | 73.2 ± 20.9 | 22.2 |
| dhCM | 179.2 ± 18.9 | 186.3 ± 63.7 | 4.0 |
| CM/SM | 0.0309 ± 0.0022 | 0.0369 ± 0.0041 | 19.4 |
| SP Classes in NP | LOAD (ng/mL) | OD (ng/mL) | % LOAD |
| SM | 95.2 ± 8.1 | 110.7 ± 20.6 | 16.3 |
| CM | 5.9 ± 0.6 | 6.5 ± 0.4 | 9.4 * |
| dhCM | 53.9 ± 3.0 | 55.6 ± 5.7 | 3.2 |
| CM/SM | 0.07 ± 0.01 | 0.08 ± 0.01 | 2.7 |
These data are the mean ± SEM, ng/mL. The p values compare sphingolipids in LOAD versus OD and * denotes p < 0.05. 1 Difference of means as the percentage of LOAD = (OD–LOAD)/LOAD × 100. SM: sphingomyelin; CM: ceramide; dhCM: dihydroceramide; CM/SM: ceramide to sphingomyelin ratio.
3.5. Enzyme Activities of LOAD and OD CSF Samples
To determine mechanisms that may account for significant changes in lipids, we analyzed the activities of three enzymes using fluorescent assays. PLA2 and nSMase activity assays revealed no differences between LOAD and OD (Table 5).
Table 5.
Phospholipase A2 (PLA2), neutral sphingomyelinase (nSMase), and acid sphingomyelinase (aSMase) activities in the cerebrospinal fluid (CSF) of late-onset Alzheimer’s disease (LOAD) and other dementia (OD) groups.
| Enzymes | LOAD (RFU/min) | OD (RFU/min) | 1 Difference (% LOAD) |
|---|---|---|---|
| PLA2 | 1008.1 ± 59.2 | 959.2 ± 60.1 | −4.9 |
| nSMase | 24.3 ± 2.2 | 20.4 ± 1.9 | −18.9 |
| aSMase | 15.0 ± 1.5 | 20.7 ± 1.7 | 37.9 * |
| aSMase/nSMase | 0.8 ± 0.7 | 1.1 ± 0.1 | 39.3 * |
1 Difference of means as a percentage of LOAD = (OD–LOAD)/LOAD × 100 and * denotes p < 0.05.
However, we observed significantly higher aSMase activity in CSF from OD participants compared with LOAD (p < 0.05). The ratio aSMase/nSMase was higher in OD compared to LOAD (p < 0.02).
3.6. Correlation of Glycerophospholipids and Aβ42 or Tau
To determine any link between lipid metabolism and AD pathology, we performed Spearman correlation analyses between GP classes and Aβ42 and Tau levels in CSF. We observed a negative relationship between the Aβ42 and LPC/PC in SF; Spearman ρ = −0.46 with p < 0.02 for LOAD samples. In addition, total Tau correlated with LPC in NP for LOAD participants (ρ = 0.45, p < 0.02). In contrast, only LPC in SF positively correlated with Tau in OD (ρ = 0.85, p < 0.01).
3.7. Correlation of Sphingolipids and Aβ42 or Tau
There were no statistically significant correlations noted between Aβ42 or Tau with SF or NP sphingolipids from LOAD study participants. In contrast to LOAD, Tau negatively correlated with CM in the SF fraction (ρ = −0.75, p < 0.03) in OD. Also, Tau levels negatively correlated to SM levels in supernatant fluids (ρ = −0.77, p < 0.03).
3.8. Correlation of Enzyme Activities with CSF Lipids
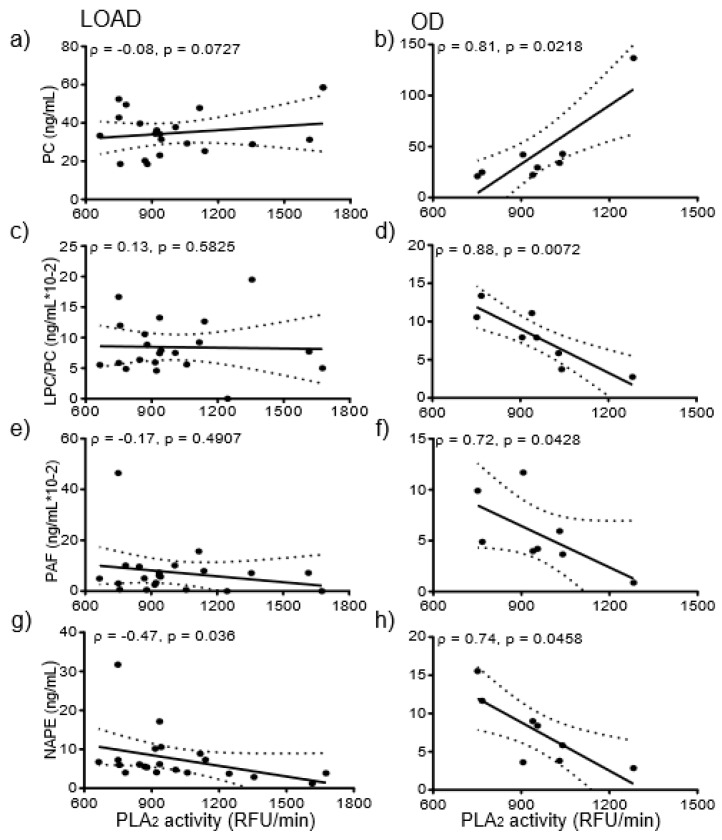
We next determined whether enhanced lipase activity accounted for metabolic differences between LOAD and OD. PLA2 activity correlated with several SF and NP lipids in CSF from OD participants. For OD samples, LPC in SF positively correlated with PLA2 activity in CSF (ρ = 0.81, p < 0.03), but no such correlation was evident for LOAD. Similarly, xPE levels in SF correlated with PLA2 activity (ρ = 0.86, p < 0.02) in OD but not in LOAD. PLA2 activity in LOAD did not correlate with NP PC (Figure 1a), while PLA2 activity positively correlated with NP PC in OD (Figure 1b).
Figure 1.
Correlations between PLA2 activity and GPs- LOAD demonstrated no significant correlation between PLA2 activity and PC in NP (a). However, PLA2 positively correlated with PC in NP in OD samples (ρ = 0.81, p < 0.03) (b). PLA2 activity in CSF from LOAD subjects did not correlate with NP LPC/PC (c), while PLA2 activity of OD negatively correlated (ρ = −0.88, p < 0.01) with NP LPC/PC (d). LOAD PLA2 activity did not correlate with NP PAF (e), while OD PLA2 activity negatively correlated (ρ = −0.74, p < 0.05) with NP PAF (f). PLA2 activity negatively correlated with NAPE in both LOAD (ρ = −0.47, p < 0.05) (g) and OD (ρ = −0.47, p < 0.05) (h). PLA2 activity was available for eight OD because of CSF limitation for two OD samples.
Similarly, PLA2 activity did not correlate with LPC/PC in LOAD (Figure 1c) but negatively correlated (ρ = −0.88 (p < 0.01) with LPC/PC of OD (Figure 1d). Finally, PLA2 activity in LOAD did not correlate with platelet-activating factor-like lipids (PAF-LL) (Figure 1e) but negatively correlated with NP PAF-LL in OD (Figure 1f). The only correlation that was significant across both LOAD and OD was between PLA2 activity and NAPE and only in the NP fractions. PLA2 activity in LOAD negatively correlated with NAPE in NP (ρ = −0.47, p < 0.04) (Figure 1g). NP NAPE levels negatively correlated (ρ = −0.74, p < 0.05) with the activity of PLA2 (Figure 1h).
We also determined the correlation of SP levels in CSF fractions from OD participants with aSMase or nSMase activities. Aβ42/Tau ratio was significantly related to nSMase activity (ρ = 0.68, p < 0.04). NP-SM was negatively related to the activity of aSMase (ρ = −0.68, p < 0.05), and NP dhCM was positively correlated with the activity of nSMase (ρ = 0.70, p < 0.03).
4. Discussion
Our objective is to determine if there are any differences in lipid metabolic pathways in CSF from subjects with LOAD compared with those with OD. In this study, the OD population is made up of participants with DLB, FTD, VaD, and mixed dementia that can often be clinically differentiated from LOAD by the earlier age of onset, step-wise progression, and shorter survival time [48]. OD subjects have a median survival time from symptom onset of 6 ± 1.1 years compared with LOAD subjects who have a survival time from presentation of 11.7 years [56]. In our age-matched study population, duration of dementia is shorter in OD than in LOAD (Table 1). Given the accelerated rate of progression and death in OD, the degree of lipid metabolic abnormality may be different between OD and LOAD. Our study confirms this by demonstrating changes in GP, SP, and enzymes that metabolize important inflammatory and apoptotic lipids.
Examining GP classes, the only significant variation was PS that almost tripled in the SF of OD compared with LOAD. Typically found in the inner leaflet of plasma membranes and mitochondrial-associated membranes, PS only constitutes 2–10% of total lipids [57,58]. Although present in small amounts, PS is crucial in the signaling apoptosis [59]; cells externalize PS to the outer leaflet as a recognition signal to phagocytic cells or to initiate the blood clotting cascade [60]. The increase in PS in SF may indicate accelerated neurodegeneration in OD compared with LOAD study participants: OD brain cells exposed to higher PS levels are undergoing apoptosis faster than LOAD. Higher PS may account for the earlier clinical manifestation and faster cognitive decline in participants with OD.
Our data also indicate that there is a disruption in GP metabolism in LOAD and OD that may impact Aβ42 and Tau metabolism. In regards to the correlation in LOAD between Aβ42 and LPC/PC in SF, alteration in lipid metabolism may influence the proper clearance of Aβ42, resulting in the pathological features associated with Aβ42 aggregation [61]. The indication, then, is that those with higher levels of Aβ42 in CSF have a lower disturbance of lipid metabolism. In contrast, highly perturbed lipid membranes do not only result in abnormal clearance of Aβ42 but increase the susceptibility of amyloid precursor protein (APP) to digestion by secretases [62,63]. We also found that higher levels of Tau, that indicate pathology found in dementia, positively correlated with LPC in both LOAD and OD CSF samples. Since LPC is derived from PC by PLA2-catalyzed lipolysis, induces myelination, and disrupts the blood-brain barrier [64], these data suggest that disruption of membrane structure is associated with dementia. Our data are in agreement with several studies showing that many PLA2 isoforms contribute to AD pathology [23,65,66,67].
SPs are another group of lipids essential in brain function and implicated in AD pathology [53,68]. Of the major SP classes detected in CSF, only the levels of CM was higher in NP of OD compared with LOAD. As a critical component for the initiation of apoptosis and autophagy, CM acts on several cells, including neurons [31,69,70]. Thus, higher levels of CM can cause increased rates of apoptosis in neural cells in OD compared with LOAD. This faster cell death would cause the clinical presentation to be identified earlier in OD compared to LOAD participants.
Further evidence for a role of SPs in OD is shown when Tau levels negatively correlate with CM and SM in SF. How Tau interacts with lipids has not been widely studied, but one can conclude that SP metabolism associates with Tau and this interaction is different in LOAD compared with OD.
Several enzymes modify GPs and SPs to form lipid mediators of inflammation or lipids that modify neuronal function. PLA2 is notable for its role in Alzheimer’s disease and other neurological disorders, due to the release of the inflammatory precursor arachidonic acid [67,71]. Once released, cyclooxygenase, lipoxygenase, and cytochrome p450 convert arachidonic acid to bioactive lipid mediators of inflammation. These PLA2- and COX-derived products are crucial in regulating an inflammatory response in LOAD [72]. Inflammatory cytokines can induce the synthesis of PLA2 and also stimulate the de novo synthesis of ceramide and PAF [73,74,75,76]. In our study, PLA2 activity in CSF from OD participants correlated with levels of several GPs; LPC and xPE in SF, and PC, PAF, NAPE, and LPC/PC in the NP fraction. PAF is an inflammatory GP produced and metabolized through two variations of PLA2—cytosolic and Lp-PLA2 [77]. LPC interacts with inflammatory cells to increase cytokine production, such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6 [78]. LPC appears to be a direct reflection on the intensity of reactive oxygen species present at the inflammatory site and can induce localized demyelination in rats [77,79]. A higher ratio of LPC/PC indicates that greater inflammatory markers are present in the CSF of OD patients. Correlation of PLA2 with these lipids suggests that hydrolysis may be linked with destructive neuro-inflammation in subjects with OD.
NAPE is a significant component of intracellular membranes and has been shown to accumulate under pathological conditions that involve the degradation of membranes [80,81]. NAPE maintains proper physiological membrane configurations [81]. As NAPE accumulates in the brain tissue of LOAD and OD, fewer NAPE molecules will be removed in the CSF. The negative correlation between NAPE and PLA2 observed in LOAD and OD reflects a disturbance in neuronal membrane lipids.
The activities of aSMAse and aSMase/nSMase ratio were higher in CSF from subjects with OD compared to LOAD. aSMase and nSMase are both involved in the degradation of SM to form CM, a known pro-apoptotic biolipid [31,82,83]. Functional consequences of SM degradation include apoptosis, growth arrest, and decreased protein secretion [84]. OD also demonstrated a significant negative correlation between aSMase activity and NP SM. The higher aSMase activity in OD compared with LOAD may account for elevated CM levels. Thus, higher aSMase activity in OD can result in faster apoptosis than in LOAD. A second positive correlation is noted between nSMase and dhCM. dhCM is a precursor to CM, and increased levels of dhCM are associated with oxidative stress [34,85]. Therefore, oxidative stress linked with lipid metabolic dysregulation may associate with AD pathophysiology.
5. Conclusions
This pilot cross-sectional study shows that lipid metabolism in CSF fractions differs between LOAD and OD. The small OD sample size limits our ability to examine previously described lipidomic variations between mixed dementia phenotypes [86]. These studies of lipid metabolism can provide insight into the dysfunction of metabolic pathways that can be used for diagnosis or to target AD therapy [87,88]. Replication with bigger sample size and consideration of different age of onset for defined dementia variants will be needed to associate known clinical phenotypes with specific lipid metabolic changes in CSF fractions.
Acknowledgments
Study participants generously donated their time and CSF samples. Fuller Seminary Neuropsychology graduate students assisted in the diagnosis of AD. Sherri Lee provided clerical help and managed the study participants.
Abbreviations
| CSF | cerebrospinal fluid |
| CM | ceramide |
| dhCM | dihydroceramide |
| LC | liquid chromatography |
| LOAD | late-onset Alzheimer’s Disease |
| LPC | lysophosphatidylcholine |
| PAF_LL | platelet-activating factor-like lipids |
| PE | phosphatidylethanolamine |
| PC | phosphatidylcholine |
| PS | phosphatidylserine |
| GP | glycerophospholipid |
| PLA2 | phospholipase A2 |
| NAPE | N-acyl phosphatidylethanolamine |
| NP | nanoparticles |
| SM | sphingomyelin |
| SMase | sphingomyelinase |
| SP | sphingolipids |
| SF | supernatant fluid |
| xPE | unknown lipid containing phosphoethanolamine |
Author Contributions
Conceptualization, A.F. and M.H.; Methodology, A.F., M.H., and J.C.; Validation, A.F. and J.C.; Formal analysis, S.S., C.O., and A.F.; Resources, A.F. and M.H.; Data curation, C.O. and A.F.; Writing—original draft preparation, S.S. and A.F.; Writing—review and editing, S.S., X.A., M.H. and A.F.; Supervision, A.F.; Project administration, A.F. and M.H.; Funding acquisition, A.F. and M.H.
Funding
The L.K. Whittier Foundation, the Jerry Dunlevy Family, and the Helen Posthuma Foundations.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Holtzman D.M., Morris J.C., Goate A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D., Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 3.Hirtz D., Thurman D.J., Gwinn-Hardy K., Mohamed M., Chaudhuri A.R., Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 4.Irie F., Fitzpatrick A.L., Lopez O.L., Kuller L.H., Peila R., Newman A.B., Launer L.J. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE ε4: The cardiovascular health study cognition study. Arch. Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verghese P.B., Castellano J.M., Garai K., Wang Y., Jiang H., Shah A., Bu G., Frieden C., Holtzman D.M. ApoE influences amyloid-beta (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc. Natl. Acad. Sci. USA. 2013;110:E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson N., Andreasson U., Persson S., Arai H., Batish S.D., Bernardini S., Bocchio-Chiavetto L., Blankenstein M.A., Carrillo M.C., Chalbot S., et al. The Alzheimer’s association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395.e6. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagan A.M., Holtzman D.M. Cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark. Med. 2010;4:51–63. doi: 10.2217/bmm.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blennow K., Vanmechelen E., Hampel H. Csf total tau, Aβ42 and phosphorylated tau protein as biomarkers for Alzheimer’s disease. Mol. Neurobiol. 2001;24:87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- 9.Rademakers R., Neumann M., Mackenzie I.R. Advances in understanding the molecular basis of frontotemporal dementia. Nat. Rev. Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C.K. Parkinson’s disease with dementia, lewy-body disorders and alpha-synuclein: Recent advances and a case report. Acta Neurol. Taiwan. 2011;20:4–14. [PubMed] [Google Scholar]
- 11.Lebedev A.V., Beyer M.K., Fritze F., Westman E., Ballard C., Aarsland D. Cortical changes associated with depression and antidepressant use in Alzheimer and lewy body dementia: An mri surface-based morphometric study. Am. J. Geriatr. Psychiatry. 2014;22:4–13. doi: 10.1016/j.jagp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Gomperts S.N., Locascio J.J., Marquie M., Santarlasci A.L., Rentz D.M., Maye J., Johnson K.A., Growdon J.H. Brain amyloid and cognition in lewy body diseases. Mov. Disord. 2012;27:965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampel H., Broich K., Hoessler Y., Pantel J. Biological markers for early detection and pharmacological treatment of Alzheimer’s disease. Dialogues Clin. Neurosci. 2009;11:141–157. doi: 10.31887/DCNS.2009.11.2/hhampel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piomelli D., Astarita G., Rapaka R. A neuroscientist’s guide to lipidomics. Nat. Rev. Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- 15.Emamzadeh F.N., Allsop D. Alpha-synuclein interacts with lipoproteins in plasma. J. Mol. Neurosci. 2017;63:165–172. doi: 10.1007/s12031-017-0967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooqui A.A., Liss L., Horrocks L.A. Neurochemical aspects of Alzheimer’s disease: Involvement of membrane phospholipids. Metab. Brain Dis. 1988;3:19–35. doi: 10.1007/BF01001351. [DOI] [PubMed] [Google Scholar]
- 17.Florent-Bechard S., Desbene C., Garcia P., Allouche A., Youssef I., Escanye M.C., Koziel V., Hanse M., Malaplate-Armand C., Stenger C., et al. The essential role of lipids in Alzheimer’s disease. Biochimie. 2009;91:804–809. doi: 10.1016/j.biochi.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Han X. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stage of Alzheimer’s disease: A tale of shotgun lipidomics. J. Neurochem. 2007;103:171–179. doi: 10.1111/j.1471-4159.2007.04708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosicek M., Hecimovic S. Phospholipids and Alzheimer’s disease: Alterations, mechanisms and potential biomarkers. Int. J. Mol. Sci. 2013;14:1310–1322. doi: 10.3390/ijms14011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Echten-Deckert G., Walter J. Sphingolipids: Critical players in Alzheimer’s disease. Prog. Lipid Res. 2012;51:378–393. doi: 10.1016/j.plipres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Penke B., Paragi G., Gera J., Berkecz R., Kovacs Z., Crul T., László V. The role of lipids and membranes in the pathogenesis of Alzheimer’s disease: A comprehensive view. Curr. Alzheimer Res. 2018;15:1191–1212. doi: 10.2174/1567205015666180911151716. [DOI] [PubMed] [Google Scholar]
- 22.Lemkul J.A., Bevan D.R. Lipid composition influences the release of Alzheimer’s amyloid beta-peptide from membranes. Protein Sci. 2011;20:1530–1545. doi: 10.1002/pro.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonteh A.N., Chiang J., Cipolla M., Hale J., Diallo F., Chirino A., Arakaki X., Harrington M.G. Alterations in cerebrospinal fluid glycerophospholipids and phospholipase A2 activity in Alzheimer’s disease. J. Lipid Res. 2013;54:2884–2897. doi: 10.1194/jlr.M037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu R.K., Tsai Y.T., Ariga T. Functional roles of gangliosides in neurodevelopment: An overview of recent advances. Neurochem. Res. 2012;37:1230–1244. doi: 10.1007/s11064-012-0744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz M., Fabelo N., Ferrer I., Marin R. “Lipid raft aging” in the human frontal cortex during nonpathological aging: Gender influences and potential implications in Alzheimer’s disease. Neurobiol. Aging. 2018;67:42–52. doi: 10.1016/j.neurobiolaging.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Bartke N., Hannun Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009;50:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haughey N.J., Bandaru V.V., Bae M., Mattson M.P. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim. Biophys. Acta. 2010;1801:878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi E.Y., Ege C., Winans A., Majewski J., Wu G., Kjaer K., Lee K.Y. Lipid membrane templates the ordering and induces the fibrillogenesis of Alzheimer’s disease amyloid-beta peptide. Proteins. 2008;72:1–24. doi: 10.1002/prot.21887. [DOI] [PubMed] [Google Scholar]
- 29.Davis C.H., Berkowitz M.L. Interaction between amyloid-beta (1–42) peptide and phospholipid bilayers: A molecular dynamics study. Biophys. J. 2009;96:785–797. doi: 10.1016/j.bpj.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan J., Donkin J., Wellington C. Greasing the wheels of abeta clearance in Alzheimer’s disease: The role of lipids and apolipoprotein e. Biofactors. 2009;35:239–248. doi: 10.1002/biof.37. [DOI] [PubMed] [Google Scholar]
- 31.He X., Huang Y., Li B., Gong C.X., Schuchman E.H. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol. Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheikh A.M., Michikawa M., Kim S.U., Nagai A. Lysophosphatidylcholine increases the neurotoxicity of Alzheimer’s amyloid beta1-42 peptide: Role of oligomer formation. Neuroscience. 2015;292:159–169. doi: 10.1016/j.neuroscience.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 33.Alessenko A.V., Bugrova A.E., Dudnik L.B. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer’s disease. Biochem. Soc. Trans. 2004;32:144–146. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- 34.Malaplate-Armand C., Florent-Bechard S., Youssef I., Koziel V., Sponne I., Kriem B., Leininger-Muller B., Olivier J.L., Oster T., Pillot T. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol. Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Vetrivel K.S., Cheng H., Lin W., Sakurai T., Li T., Nukina N., Wong P.C., Xu H., Thinakaran G. Association of gamma-secretase with lipid rafts in post-golgi and endosome membranes. J. Biol. Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagen-Euteneuer N., Lutjohann D., Park H., Merrill A.H., Jr., van Echten-Deckert G. Sphingosine 1-phosphate (S1P) lyase deficiency increases sphingolipid formation via recycling at the expense of de novo biosynthesis in neurons. J. Biol. Chem. 2012;287:9128–9136. doi: 10.1074/jbc.M111.302380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darios F., Wasser C., Shakirzyanova A., Giniatullin A., Goodman K., Munoz-Bravo J.L., Raingo J., Jorgacevski J., Kreft M., Zorec R., et al. Sphingosine facilitates snare complex assembly and activates synaptic vesicle exocytosis. Neuron. 2009;62:683–694. doi: 10.1016/j.neuron.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popp J., Lewczuk P., Kolsch H., Meichsner S., Maier W., Kornhuber J., Jessen F., Lutjohann D. Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. J. Neurochem. 2012;123:310–316. doi: 10.1111/j.1471-4159.2012.07893.x. [DOI] [PubMed] [Google Scholar]
- 39.Vetrivel K.S., Thinakaran G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim. Biophys. Acta. 2010;1801:860–867. doi: 10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood W.G., Schroeder F., Igbavboa U., Avdulov N.A., Chochina S.V. Brain membrane cholesterol domains, aging and amyloid beta-peptides. Neurobiol. Aging. 2002;23:685–694. doi: 10.1016/S0197-4580(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 41.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Jr., Kaye J., Montine T.J., et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weintraub S., Salmon D., Mercaldo N., Ferris S., Graff-Radford N.R., Chui H., Cummings J., DeCarli C., Foster N.L., Galasko D., et al. The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychologic test battery. Alzheimer Dis. Assoc. Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beekly D.L., Ramos E.M., Lee W.W., Deitrich W.D., Jacka M.E., Wu J., Hubbard J.L., Koepsell T.D., Morris J.C., Kukull W.A., et al. The national Alzheimer’s coordinating center (NACC) database: The uniform data set. Alzheimer Dis. Assoc. Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 44.Crum R.M., Anthony J.C., Bassett S.S., Folstein M.F. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;269:2386–2391. doi: 10.1001/jama.1993.03500180078038. [DOI] [PubMed] [Google Scholar]
- 45.Freitas S., Simoes M.R., Alves L., Santana I. Montreal cognitive assessment: Validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2013;27:37–43. doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- 46.Morris J.C. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the alzheimer type. Int. Psychogeriatr. 1997;9:173–176; discussion 177–178. doi: 10.1017/S1041610297004870. [DOI] [PubMed] [Google Scholar]
- 47.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKeith I.G., Dickson D.W., Lowe J., Emre M., O’Brien J.T., Feldman H., Cummings J., Duda J.E., Lippa C., Perry E.K., et al. Diagnosis and management of dementia with lewy bodies: Third report of the dlb consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 49.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chui H.C., Victoroff J.I., Margolin D., Jagust W., Shankle R., Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the state of california alzheimer’s disease diagnostic and treatment centers. Neurology. 1992;42:473–480. doi: 10.1212/WNL.42.3.473. [DOI] [PubMed] [Google Scholar]
- 51.Harrington M.G., Chiang J., Pogoda J.M., Gomez M., Thomas K., Marion S.D., Miller K.J., Siddarth P., Yi X., Zhou F., et al. Executive function changes before memory in preclinical Alzheimer’s pathology: A prospective, cross-sectional, case control study. PLoS ONE. 2013;8:e79378. doi: 10.1371/journal.pone.0079378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calero O., Hortiguela R., Bullido M.J., Calero M. Apolipoprotein e genotyping method by real time PCR, a fast and cost-effective alternative to the taqman and fret assays. J. Neurosci. Methods. 2009;183:238–240. doi: 10.1016/j.jneumeth.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 53.Fonteh A.N., Ormseth C., Chiang J., Cipolla M., Arakaki X., Harrington M.G. Sphingolipid metabolism correlates with cerebrospinal fluid beta amyloid levels in Alzheimer’s disease. PLoS ONE. 2015;10:e0125597. doi: 10.1371/journal.pone.0125597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrington M.G., Fonteh A.N., Oborina E., Liao P., Cowan R.P., McComb G., Chavez J.N., Rush J., Biringer R.G., Huhmer A.F. The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cerebrospinal Fluid Res. 2009;6:10. doi: 10.1186/1743-8454-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 56.Weder N.D., Aziz R., Wilkins K., Tampi R.R. Frontotemporal dementias: A review. Ann. Gen. Psychiatry. 2007;6:15. doi: 10.1186/1744-859X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vance J.E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008;49:1377–1387. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Kay J.G., Grinstein S. Sensing phosphatidylserine in cellular membranes. Sensors. 2011;11:1744–1755. doi: 10.3390/s110201744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenzer C., Simionato E., Largeau C., Scarcelli V., Lefebvre C., Legouis R. Autophagy mediates phosphatidylserine exposure and phagosome degradation during apoptosis through specific functions of GABARAP/LGG-1 and LC3/LGG-2. Autophagy. 2018:1–14. doi: 10.1080/15548627.2018.1512452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fadok V.A., de Cathelineau A., Daleke D.L., Henson P.M., Bratton D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 61.Morgado I., Garvey M. Lipids in amyloid-beta processing, aggregation, and toxicity. Adv. Exp. Med. Biol. 2015;855:67–94. doi: 10.1007/978-3-319-17344-3_3. [DOI] [PubMed] [Google Scholar]
- 62.Lahdo R., Coillet-Matillon S., Chauvet J.P., de La Fourniere-Bessueille L. The amyloid precursor protein interacts with neutral lipids. Eur. J. Biochem. 2002;269:2238–2246. doi: 10.1046/j.1432-1033.2002.02882.x. [DOI] [PubMed] [Google Scholar]
- 63.Urmoneit B., Turner J., Dyrks T. Cationic lipids (lipofectamine) and disturbance of cellular cholesterol and sphingomyelin distribution modulates gamma-secretase activity within amyloid precursor protein in vitro. Prostaglandins Other Lipid Mediat. 1998;55:331–343. doi: 10.1016/S0090-6980(98)00032-X. [DOI] [PubMed] [Google Scholar]
- 64.Muramatsu R., Kuroda M., Matoba K., Lin H., Takahashi C., Koyama Y., Yamashita T. Prostacyclin prevents pericyte loss and demyelination induced by lysophosphatidylcholine in the central nervous system. J. Biol. Chem. 2015;290:11515–11525. doi: 10.1074/jbc.M114.587253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stephenson D.T., Lemere C.A., Selkoe D.J., Clemens J.A. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in alzheimer’s disease brain. Neurobiol. Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 66.Farooqui A.A., Horrocks L.A. Plasmalogen-selective phospholipase A2 and its involvement in Alzheimer’s disease. Biochem. Soc. Trans. 1998;26:243–246. doi: 10.1042/bst0260243. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Mejia R.O., Mucke L. Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim. Biophys. Acta. 2010;1801:784–790. doi: 10.1016/j.bbalip.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosicek M., Zetterberg H., Andreasen N., Peter-Katalinic J., Hecimovic S. Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer’s disease. Neurosci. Lett. 2012;516:302–305. doi: 10.1016/j.neulet.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 69.Farooqui A.A., Horrocks L.A., Farooqui T. Interactions between neural membrane glycerophospholipid and sphingolipid mediators: A recipe for neural cell survival or suicide. J. Neurosci. Res. 2007;85:1834–1850. doi: 10.1002/jnr.21268. [DOI] [PubMed] [Google Scholar]
- 70.Mielke M.M., Lyketsos C.G. Alterations of the sphingolipid pathway in Alzheimer’s disease: New biomarkers and treatment targets? Neuromol. Med. 2010;12:331–340. doi: 10.1007/s12017-010-8121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ng C.Y., Kannan S., Chen Y.J., Tan F.C.K., Ong W.Y., Go M.L., Verma C.S., Low C.M., Lam Y. A new generation of arachidonic acid analogues as potential neurological agent targeting cytosolic phospholipase A2. Sci. Rep. 2017;7:13683. doi: 10.1038/s41598-017-13996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao J.S., Rapoport S.I., Kim H.W. Altered neuroinflammatory, arachidonic acid cascade and synaptic markers in postmortem Alzheimer’s disease brain. Transl. Psychiatry. 2017;7:e1127. doi: 10.1038/tp.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adibhatla R.M., Hatcher J.F. Altered lipid metabolism in brain injury and disorders. Subcell. Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bazan N.G., Colangelo V., Lukiw W.J. Prostaglandins and other lipid mediators in Alzheimer’s disease. Prostaglandins Other Lipid Mediat. 2002;68–69:197–210. doi: 10.1016/S0090-6980(02)00031-X. [DOI] [PubMed] [Google Scholar]
- 75.Combs C.K., Karlo J.C., Kao S.C., Landreth G.E. Beta-amyloid stimulation of microglia and monocytes results in tnfalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lukiw W.J., Bazan N.G. Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem. Res. 2000;25:1173–1184. doi: 10.1023/A:1007627725251. [DOI] [PubMed] [Google Scholar]
- 77.Silva I.T., Mello A.P., Damasceno N.R. Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A(2) (LP-PLA(2)): A review. Lipids Health Dis. 2011;10:170. doi: 10.1186/1476-511X-10-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang Y.H., Schafer-Elinder L., Wu R., Claesson H.E., Frostegard J. Lysophosphatidylcholine (LPC) induces proinflammatory cytokines by a platelet-activating factor (PAF) receptor-dependent mechanism. Clin. Exp. Immunol. 1999;116:326–331. doi: 10.1046/j.1365-2249.1999.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grin’kina N.M., Abdel-Baki S.G., Bergold P.J. Reversible behavioral deficits in rats during a cycle of demyelination-remyelination of the fimbria. PLoS ONE. 2013;8:e53775. doi: 10.1371/journal.pone.0053775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kilaru A., Isaac G., Tamura P., Baxter D., Duncan S.R., Venables B.J., Welti R., Koulen P., Chapman K.D. Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase. Lipids. 2010;45:863–875. doi: 10.1007/s11745-010-3457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wellner N., Diep T.A., Janfelt C., Hansen H.S. N-acylation of phosphatidylethanolamine and its biological functions in mammals. Biochim. Biophys. Acta. 2013;1831:652–662. doi: 10.1016/j.bbalip.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Kornhuber J., Muehlbacher M., Trapp S., Pechmann S., Friedl A., Reichel M., Muhle C., Terfloth L., Groemer T.W., Spitzer G.M., et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE. 2011;6:e23852. doi: 10.1371/journal.pone.0023852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang P., Liu B., Jenkins G.M., Hannun Y.A., Obeid L.M. Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis. J. Biol. Chem. 1997;272:9609–9612. doi: 10.1074/jbc.272.15.9609. [DOI] [PubMed] [Google Scholar]
- 84.Wang G., Dinkins M., He Q., Zhu G., Poirier C., Campbell A., Mayer-Proschel M., Bieberich E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): Potential mechanism of apoptosis induction in Alzheimer disease (AD) J. Biol. Chem. 2012;287:21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Apraiz A., Idkowiak-Baldys J., Nieto-Rementeria N., Boyano M.D., Hannun Y.A., Asumendi A. Dihydroceramide accumulation and reactive oxygen species are distinct and nonessential events in 4-HPR-mediated leukemia cell death. Biochem. Cell Biol. 2012;90:209–223. doi: 10.1139/o2012-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lam S.M., Wang Y., Duan X., Wenk M.R., Kalaria R.N., Chen C.P., Lai M.K., Shui G. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol. Aging. 2014;35:2369–2381. doi: 10.1016/j.neurobiolaging.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Canals D., Perry D.M., Jenkins R.W., Hannun Y.A. Drug targeting of sphingolipid metabolism: Sphingomyelinases and ceramidases. Br. J. Pharmacol. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohaibes R.J., Fiol-deRoque M.A., Torres M., Ordinas M., Lopez D.J., Castro J.A., Escriba P.V., Busquets X. The hydroxylated form of docosahexaenoic acid (DHA-H) modifies the brain lipid composition in a model of Alzheimer’s disease, improving behavioral motor function and survival. Biochim. Biophys. Acta Biomembr. 2017;1859:1596–1603. doi: 10.1016/j.bbamem.2017.02.020. [DOI] [PubMed] [Google Scholar]