Abstract
Few human immunodeficiency virus (HIV)–infected persons can maintain low viral levels without therapeutic intervention. We evaluate predictors of spontaneous control of the viral load (hereafter, “viral control”) in a prospective cohort of African adults shortly after HIV infection. Viral control was defined as ≥2 consecutively measured viral loads (VLs) of ≤10 000 copies/mL after the estimated date of infection, followed by at least 4 subsequent measurements for which the VL in at least 75% was ≤10 000 copies/mL in the absence of ART. Multivariable logistic regression characterized predictors of viral control. Of 590 eligible volunteers, 107 (18.1%) experienced viral control, of whom 25 (4.2%) maintained a VL of 51–2000 copies/mL, and 5 (0.8%) sustained a VL of ≤50 copies/mL. The median ART-free follow-up time was 3.3 years (range, 0.3–9.7 years). Factors independently associated with control were HIV-1 subtype A (reference, subtype C; adjusted odds ratio [aOR], 2.1 [95% confidence interval {CI}, 1.3–3.5]), female sex (reference, male sex; aOR, 1.8 [95% CI, 1.1–2.8]), and having HLA class I variant allele B*57 (reference, not having this allele; aOR, 1.9 [95% CI, 1.0–3.6]) in a multivariable model that also controlled for age at the time of infection and baseline CD4+ T-cell count. We observed strong associations between infecting HIV-1 subtype, HLA type, and sex on viral control in this cohort. HIV-1 subtype is important to consider when testing and designing new therapeutic and prevention technologies, including vaccines.
Keywords: HIV, AIDS, Africa, epidemiology, HIV subtype
Human immunodeficiency virus type 1 (HIV-1) subtype is important. We show that HIV subtype A is associated with viral control, using subtype C as a reference. Data from this and other studies suggest that HIV-1 subtype should be considered when designing new HIV therapeutic agents, prevention modalities, and vaccines.
Effective control of human immunodeficiency virus (HIV) infection is associated with improved clinical outcomes, including delayed onset of AIDS [1, 2], and studies indicate that the risk of sexually transmitting HIV is reduced for individuals with low plasma HIV loads [3, 4]. Spontaneous control of viral replication is rare, typically occurring in fewer than 5%–10% of all persons with HIV infection [1, 5]; it is also not well understood, particularly in the African context. A better understanding of spontaneous viral control could aid in development of new HIV treatment and cure options, prevention technologies, and vaccine design [6, 7].
The definition of HIV load control varies. All definitions include persons who are HIV seropositive by antibody testing (such as enzyme-linked immunosorbent assay [ELISA], rapid test, or Western blot) but who have a low or nondetectable HIV load in the absence of ART. A recent systematic review found that viremic controllers are most often defined as those with viral loads of <2000 copies/mL, but there was no clear consensus on the definition of control, and thresholds varied by up to 15 000 copies/mL [8]. Though our understanding of viral control is improving, a broader understanding of the role of the infecting viral subtype remains lacking. Here, we evaluate predictors of viral control in a cohort of volunteers from large, well-defined HIV-incidence cohorts in Africa enrolled within 1 year of their estimated date of infection.
METHODS
Ethical Considerations
This study was approved by local ethics review (Supplementary Materials). All volunteers provided informed consent before the collection of any study-related data.
Recruitment and Study Procedures
Volunteers were primarily recruited from HIV-1 epidemiology studies at 9 clinical research centers in Kenya, Rwanda, South Africa, Uganda, and Zambia, as described elsewhere [9]; additional details are in the Supplementary Materials.
At enrollment, each volunteer’s medical history was recorded and a physical examination was conducted. Volunteers were followed monthly for the first 3 months after the estimated date of HIV infection, quarterly through 2 years after the estimated date of infection, and every 6 months thereafter. At each follow-up visit, volunteers were asked whether they had started ART or taken antiretroviral drugs for prevention of mother-to-child transmission of HIV. A symptoms-directed physical examination was performed. All volunteers were assessed for ART eligibility per the national guidelines and referred for treatment as appropriate. Blood specimens were collected for syphilis-specific serologic analysis (performed annually), measurement of CD4+ and CD8+ T-cell counts (by FACSCount or FACSCalibur; Becton-Dickinson Biosciences, Rapid plasma reagin, Biotec Laboratories, Inc., UK), and, if required, confirmatory HIV testing (eg, for volunteers who tested positive for HIV p24 antigen but negative for HIV antibodies at enrollment). Peripheral blood mononuclear cells were collected and stored; plasma was stored for viral load testing and subtype determination. The latter was derived from an amplified 1.7-kb segment of the pol gene, using the REGA HIV-1 subtyping tool and the Stanford database (available at: http://hivdb.stanford.edu/). If the subtype could not be assigned by using the REGA tool, additional phylogenetic analysis was done [10]. Genomic DNA samples derived from peripheral blood mononuclear cells were used for genotyping 3 HLA class I loci (HLA-A, HLA-B, and HLA-C), with individual alleles resolved to 4-digit specificities by using polymerase chain reaction–based techniques and current HLA nomenclatures [11].
Definition of Viral Control
Viral control included aviremic control (viral load threshold, ≤ 50 copies/mL), viremic control (viral load threshold, 51–2000 copies/mL), and weak control (viral load threshold, 2001–10 000 copies/mL), collectively referred to as sustained viral control. Sustained viral control was defined as ≥2 consecutive viral load measurements ≤10 000 copies/mL (for weak control), ≤2000 copies/mL (for viremic control), and ≤50 copies/mL (for aviremic control) within 3–36 months after the estimated date of infection, plus at least 4 subsequent measurements for which the viral load in at least 75% was ≤10 000, ≤2000, and ≤50 copies/mL, respectively. All visits after the initiation of ART were excluded from analysis. No viral load data were recorded during short-term use of antiretroviral drugs for prevention of mother-to-child transmission of HIV. Plasma from participants was tested for the presence of antiretroviral drugs at the time their viral load dropped to ≤2000 copies/mL (Supplementary Materials).
Data Analysis
Multivariable logistic regression analysis with the ART-free follow-up duration as an offset variable was used to characterize predictors of viral control. Missing data on HIV-1 subtype were imputed using the subtype of the participant’s suspected transmitting partner, if known. For participants in South Africa or Zambia, subtype C was assumed if no partner data were available.
All analyses were performed using R, version 3.4.3 (available at: https://www.R-project.org), using the package “glmulti,” to determine the best regression model among models with up to 6 covariates from a set of 26 covariates that included year of the estimated date of infection, age group at estimated date of infection (<25 years vs ≥25 years), sex, risk group, HIV-1 subtype, baseline CD4+ T-cell count, and HLA alleles present in ≥50 volunteers. Baseline CD4+ T-cell count was defined as the month 3 measurement or the average of measurements that fell within the window of the month 3 visit. Missing baseline CD4+ T-cell counts were imputed using chained equations in which the month 6 and month 12 CD4+ T-cell counts, along with age group (<25 years vs ≥25 years), sex, subtype, and geographic region, were used to model the missing data. No interaction terms were included, except for sex by A*03 when prior data [12] suggested women with this allele were more likely to control whereas men were not. The best model is defined as the model with the smallest value for the Akaike information criterion, a measure that balances a model’s simplicity with its fit of the data [13].
Six sensitivity analyses were performed (Supplementary Materials). The first limited the analysis to participants with at least 1 year of ART-free follow-up, to assess the effect of participants who withdrew from the study early. The second analysis adjusted for ART guidelines that varied over time and by country. For this analysis, the time to recommended ART initiation was calculated for each participant, based on their CD4+ T-cell count over time, and used an approximation to Rwanda’s ART guidelines (ie, the most-conservative guidelines). Follow-up time was then calculated as the time from the estimated date of infection to the earliest occurrence of the following: actual ART initiation, recommended ART initiation, or last study visit. The third sensitivity analysis replaced HIV-1 subtype with a subtype-by-geographic-region covariate in which sites were categorized as belonging to either eastern or southern Africa. While subtype C is predominant (nearly 100%) in southern Africa, there was greater subtype diversity among eastern African participants. Fourth, we limited our analysis to women only, to allow a better comparison to the study by Venner et al [14]. Fifth, we excluded volunteers for whom we had to impute the baseline CD4+ T-cell count. Finally, we performed a multivariable analysis with a more conservative definition of controller, using a threshold of 2000 copies/mL.
RESULTS
Study Population and Viral Control
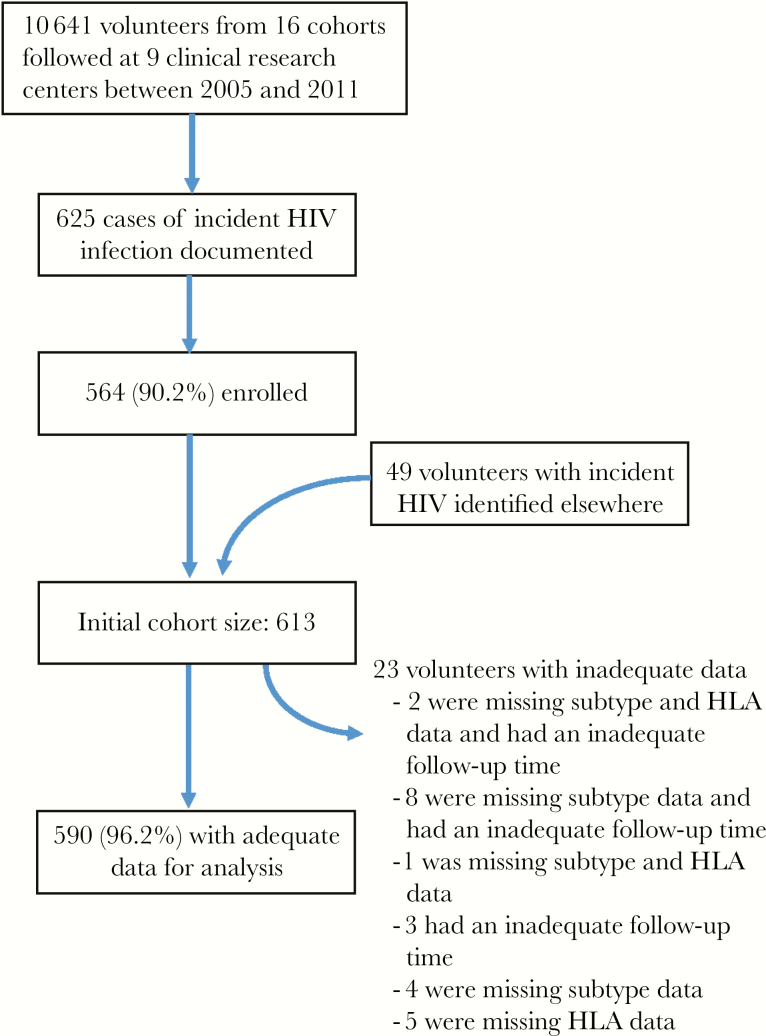
Between February 2006 and December 2011, 613 volunteers with incident HIV infection were identified and enrolled, including 113 from Kenya, 143 from Uganda, 94 from Rwanda, 234 from Zambia, and 29 from South Africa. Nearly all volunteers (564 [92%]) were identified during prospective follow-up in HIV incidence studies of key populations (Figure 1 and Table 1). Study follow-up data reported here were collected through April 2016.
Figure 1.
Flow of volunteers through the study. HIV, human immunodeficiency virus.
Table 1.
Description of Early Human Immunodeficiency Virus (HIV) Infection Cohort, by Location of Participating Clinical Research Center
| Location | Study Start | Enrollment Complete | Source Population(s) | Enrolled, No. | Included in Analysis, No. (%) |
|---|---|---|---|---|---|
| Kigali, Rwanda | Feb 2006 | May 2011 | Discordant couplesa | 94 | 92 (97.9) |
| Masaka, Uganda | Jun 2006 | Nov 2011 | Rural communities, discordant couplesa | 97 | 95 (97.9) |
| Kilifi, Kenya | Jun 2006 | Oct 2011 | Walk-in VCT clients, FSW, MSM | 88 | 84 (95.5) |
| Lusaka, Zambia | Jun 2006 | Jul 2011 | Discordant couplesa | 151 | 150 (99.3) |
| Kangemi, Kenya | Aug 2006 | Jul 2010 | FSW, clients of FSW, MSM | 25 | 25 (100) |
| Entebbe, Uganda | Aug 2006 | Oct 2010 | Discordant couples,a walk-in VCT clients | 46 | 39 (84.8) |
| Ndola and Kitwe, Zambia | Oct 2006 | Dec 2011 | Discordant couplesa | 83 | 79 (95.2) |
| Cape Town, RSA | Dec 2006 | Nov 2007 | At-risk community membersb | 7 | 5 (71.4) |
| Rustenburg, RSA | Oct 2009 | Dec 2011 | At-risk community members,b MSM | 22 | 21 (95.5) |
| Summary | Feb 2006 | Dec 2011 | … | 613 | 590 (96.2) |
Abbreviations: FSW, female sex workers; MSM, men who report sex with men; RSA, Republic of South Africa; VCT, voluntary counseling and testing for HIV.
aHeterosexual cohabiting couples of discordant HIV status (one infected, one not).
bSelf-reported heterosexual risk for HIV acquisition (see Methods).
Overall, 590 volunteers (96.2%) had sufficient data to evaluate viral control (Figure 1). Two hundred twenty-four volunteers (38.0%) achieved initial control (viral load, ≤10 000 copies/mL), but most later lost control of virus. In the cohort, 107 (18.1%) met our criteria for sustained viral control, with 25 (4.2%) and 5 (0.8%) exhibiting viremic and aviremic control, respectively. For the 107 controllers, the median time to control was 124 days (interquartile range, 84–302 days) after the estimated date of infection. The median follow-up time in the absence of ART was 3.3 years (range, 0.25–9.7 years). Over the course of follow-up, a similar proportion of controllers started ART, with 56.1% of viral controllers starting therapy in comparison to 59.4% of noncontrollers (P = .53). However, controllers had a longer median ART-free follow-up time than noncontrollers (5.1 years vs 3.2 years; P < .001, by the Wilcoxon rank sum test).
Predictors of Viral Control
Table 2 presents baseline cohort characteristics by control status. The best multivariable model for viral control (Table 3) included baseline CD4+ T-cell count, sex, subtype, age at the estimated date of infection, and HLA alleles B*45:01, B*57, and B*58:02. Infection with HIV-1 subtype A as compared to subtype C was associated with control (adjusted odds ratio [aOR], 2.1 [95% confidence interval {CI}, 1.3–3.5]), as was having the HLA class I variant B*57 (aOR, 1.9 [95% CI, 1.0–3.6]). Women (aOR, 1.8 [95% CI, 1.1–2.8]) were also more likely to control than men (Table 3). Although not significant at the .05 level, the presence of a B*58:02 allele (aOR, 0.5 [95% CI, .3–1.0]; P = .08) appeared unfavorable with respect to control. Being older was also suggestive of a lower odds of control (aOR, 0.6 [95% CI, .4–1.0]; P = .07), and each 100-cell increase in a volunteer’s baseline CD4+ T-cell count was suggestive of an increased odds of control (aOR, 1.1 [95% CI, 1.0–1.2]; P = .06). The presence of a B*45:01 allele (aOR, 0.7 [95% CI, .3–1.3]; P = .22) was not associated with control status, although its inclusion in the model improved the fit of the data.
Table 2.
Baseline Characteristics, by Human Immunodeficiency Virus (HIV) Control Status
| Characteristic | Volunteers, No. (%) | Sustained Control, No. (%)a | Viremic Control, No. (%)b | Aviremic Control, No. (%)c |
|---|---|---|---|---|
| Overall | 590 (100) | 107 (18.4) | 25 (4.2) | 5 (0.9) |
| Study site | ||||
| Kigali, Rwanda | 92 (100) | 25 (27.2) | 8 (8.7) | 2 (2.2) |
| Masaka, Uganda | 95 (100) | 16 (16.8) | 6 (6.3) | 1 (1.1) |
| Kilifi, Kenya | 84 (100) | 18 (21.4) | 3 (3.6) | 0 (0) |
| Lusaka, Zambia | 150 (100) | 11 (7.3) | 0 (0) | 2 (1.3) |
| Kangemi, Kenya | 25 (100) | 7 (28.0) | 5 (20.0) | 0 (0) |
| Entebbe, Uganda | 39 (100) | 10 (25.6) | 9 (23.1) | 0 (0) |
| Ndola and Kitwe, Zambia | 79 (100) | 14 (17.7) | 3 (3.8) | 0 (0) |
| Cape Town, RSA | 5 (100) | 0 (0) | 0 (0) | 0 (0) |
| Rustenburg, RSA | 21 (100) | 6 (28.6) | 2 (9.5) | 0 (0) |
| Estimated time of infection | ||||
| 2005–2006 | 170 (100) | 25 (14.7) | 10 (5.9) | 1 (0.6) |
| 2007–2008 | 218 (100) | 41 (18.8) | 7 (3.2) | 3 (1.4) |
| 2009–2011 | 202 (100) | 41 (20.3) | 8 (4.0) | 1 (0.5) |
| Age at estimated time of infection | ||||
| <25 y | 160 (100) | 38 (23.8) | 9 (5.6) | 0 (0) |
| ≥25 y | 430 (100) | 69 (16.1) | 16 (3.7) | 5 (1.2) |
| Sex and risk group | ||||
| Heterosexual male | 261 (100) | 33 (12.6) | 8 (3.1) | 2 (0.8) |
| MSM | 90 (100) | 17 (18.9) | 2 (2.2) | 0 (0) |
| Female | 239 (100) | 57 (23.9) | 15 (6.3) | 3 (1.3) |
| HLA-A*03, by sex | ||||
| Female | ||||
| No | 213 (100) | 49 (23.0) | 12 (5.6) | 1 (0.5) |
| Yes | 26 (100) | 8 (30.8) | 3 (11.5) | 2 (7.7) |
| Male | ||||
| No | 325 (100) | 46 (14.2) | 9 (2.8) | 2 (0.6) |
| Yes | 26 (100) | 4 (15.4) | 1 (3.9) | 0 (0) |
| HLA-B*45 allele | ||||
| No | 499 (100) | 96 (19.2) | 22 (4.4) | 5 (1.0) |
| Yes | 91 (100) | 11 (12.1) | 3 (3.3) | 0 (0) |
| HLA-B*58:02 | ||||
| No | 502 (100) | 96 (19.1) | 21 (4.2) | 4 (0.8) |
| Yes | 88 (100) | 11 (12.5) | 4 (4.6) | 1 (1.1) |
| HLA-B*57 | ||||
| No | 535 (100) | 90 (16.8) | 19 (3.6) | 4 (0.8) |
| Yes | 55 (100) | 17 (30.9) | 6 (10.9) | 1 (1.8) |
| HLA-B*81 | ||||
| No | 562 (100) | 101 (18.0) | 24 (4.3) | 5 (0.9) |
| Yes | 28 (100) | 6 (21.4) | 1 (3.6) | 0 (0) |
| HIV-1 subtype | ||||
| C | 273 (100) | 38 (13.9) | 6 (2.2) | 2 (0.7) |
| A | 207 (100) | 52 (25.12) | 15 (7.3) | 3 (1.5) |
| D | 81 (100) | 15 (18.5) | 4 (4.9) | 0 (0) |
| Otherd | 29 (100) | 2 (6.9) | 0 (0) | 0 (0) |
| Baseline CD4+ T-cell counte | 533 (435–695) | 646 (492–760) | 712 (560–803) | 483 (381–492) |
| ART-free follow-up duration, ye | 3.3 (3.2–5.1) | 5.1 (3.2–6.9) | 6.0 (3.7–7.7) | 6.9 (5.1, 6.9) |
Data are no. of volunteers (% with characteristic), unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; MSM, men who report sex with men; RSA, Republic of South Africa.
aVolunteers who maintain a viral load of ≤10 000 copies/mL (ie, all viral controllers).
bSubset of sustained controllers who maintain a viral load of 51–2000 copies/mL.
cSubset of sustained controllers who maintain a viral load of ≤50 copies/mL.
dTwelve volunteers had subtype A1/D virus, 6 had subtype A1/C, 2 had subtype A1/A2/D, 2 had subtype CRF02_AG, 2 had subtype G, and 1 each had subtype A1/C/D, B, C/K, CRF11_CPX, or D/C.
eData are median values (interquartile ranges) for 590 volunteers, 107 in the sustained control group, 25 in the viremic control group, and 5 in the aviremic control group.
Table 3.
Association Between Baseline Covariates and Sustained Control of Human Immunodeficiency Virus (HIV) Load in an Early Infection Cohort
| Covariate | Volunteers,a No. | ART-Free Follow-Up, PY | Viral Controllers, No. | Unadjusted Analysis | Adjusted Analysis | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||||
| Estimated time of infection | |||||||
| 2005–2006 | 170 | 760.5 | 25 | Reference | … | ||
| 2007–2008 | 218 | 812.4 | 41 | 1.48 (.86–2.58) | .163 | … | |
| 2009–2011 | 202 | 570.2 | 41 | 1.82 (1.06–3.19) | .032 | … | |
| Age at estimated time of infection | |||||||
| <25 y | 160 | 544.8 | 38 | Reference | Reference | ||
| ≥25 y | 430 | 1598.4 | 69 | 0.58 (.37–.91) |
.016 | 0.64 (.39–1.06) |
.068 |
| Sex | |||||||
| Male | 351 | 1270.9 | 50 | Reference | Reference | ||
| Female | 239 | 872.4 | 57 | 1.89 (1.24–2.90) |
.003 | 1.78 (1.12–2.84) |
.015 |
| Risk group | |||||||
| Discordant couple | 427 | 1522.8 | 71 | Reference | |||
| MSM | 90 | 311.7 | 17 | 1.19 (.64–2.12) |
.556 | ||
| Other heterosexual | 65 | 268 | 17 | 1.68 (.89–3.07) |
.097 | ||
| Unknown | 8 | 40.8 | 2 | 1.41 (.20–6.35) |
.681 | ||
| HLA-A*03, by sex | |||||||
| Female | |||||||
| No | 213 | 754.1 | 49 | Reference | |||
| Yes | 26 | 118.3 | 8 | 1.33 (.51–3.18) |
.539 | ||
| Male | |||||||
| No | 325 | 1166.7 | 46 | 0.55 (.35–.85) |
.008 | ||
| Yes | 26 | 104.2 | 4 | 0.57 (.16–1.59) |
.326 | ||
| HLA-B*45:01 | |||||||
| No | 499 | 1857.8 | 96 | Reference | Reference | ||
| Yes | 91 | 285.5 | 11 | 0.62 (.3–1.16) |
.160 | 0.65 (.31–1.25) |
.222 |
| HLA-B*58:02 | |||||||
| No | 504 | 1819.2 | 96 | Reference | Reference | ||
| Yes | 86 | 324 | 11 | 0.61 (.29–1.15) |
.148 | 0.53 (.25–1.04) |
.081 |
| HLA-B*57 | |||||||
| No | 535 | 1901.6 | 90 | Reference | Reference | ||
| Yes | 55 | 241.6 | 17 | 2.02 (1.07–3.72) |
.026 | 1.90 (.98–3.58) |
.051 |
| HLA-A*02:02 | |||||||
| No | 535 | 1946.9 | 95 | Reference | |||
| Yes | 55 | 196.3 | 12 | 1.32 (.64–2.55) |
.424 | ||
| Baseline CD4+ T-cell count | 590 | 2143.2 | 107 | 1.13b (1.04–1.23) |
.002 | 1.09b (1.00–1.18) |
.058 |
| HIV-1 subtype | |||||||
| C | 273 | 929.7 | 38 | Reference | Reference | ||
| A | 207 | 832.1 | 52 | 1.97 (1.24–3.17) |
.004 | 2.09 (1.27–3.49) |
.004 |
| D | 81 | 278.4 | 15 | 1.43 (.72–2.74) |
.287 | 1.44 (.71–2.80) |
.292 |
| Otherc | 29 | 102.9 | 2 | 0.46 (.07–1.64) |
.306 | 0.51 (.08–1.88) |
.383 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; MSM, men who report sex with men; OR, odds ratio; PY, person-years.
aData are for 590 volunteers with viral load, subtype, and HLA data available.
bData are the odds of viral control for every 100-cell increase in counts (ie, in the adjusted analysis, the odds of control increases 9% for every 100-cell increase in baseline CD4+ T-cell count).
cTwelve volunteers had subtype A1/D virus, 6 had subtype A1/C, 2 had subtype A1/A2/D, 2 had subtype CRF02_AG, 2 had subtype G, and 1 each had subtype A1/C/D, B, C/K, CRF11_CPX, or D/C.
Aviremic and Viremic Viral Control
We observed too few aviremic and viremic viral controllers for a systematic multivariable analysis. However, after combining the aviremic with the viremic controllers, we found a significant association with HIV-1 subtype after adjustment for age group, sex, baseline CD4+ T-cell count, and presence of HLA allele B*57. With subtype C as the reference group, we observed an aOR of 3.31 (95% CI, 1.37–8.65) for subtype A and viral control (Supplementary Table 6).
Sensitivity Analyses
While 5 of 6 analyses confirmed results of our primary analysis (Supplementary Materials), our analysis comparing controllers with subtype C infection in southern Africa to those in eastern Africa showed that East African volunteers with subtype C were more likely to control (aOR, 3.3 [95% CI, 1.1–9.1]) than southern African volunteers with subtype C infection. Because geography was significantly correlated with HIV subtype (98% of volunteers [249 of 255] in Zambia and South Africa were infected with subtype C), we were unable to initially control for it. The prevalence of control varied by site within southern Africa, ranging from no controllers in Cape Town to a prevalence of 7% in Lusaka, 18% in Copperbelt, and 29% in Rustenburg (P = .01, by the Fisher exact test; Table 2). This difference may be explained in part by the fact that Rustenburg volunteers were younger than volunteers from Zambia (median ages at the estimated date of infection, 25 and 33 years; P < .001, by the Wilcoxon rank sum test).
DISCUSSION
In this adult, African cohort, we observed that 5% of volunteers demonstrated viral control to a level of ≤2000 viral copies/mL, including a small number (<1%) of aviremic controllers who maintained undetectable or nearly undetectable viral loads, while an additional 13% maintained weak control, with viral loads ranging from 2001 to 10 000 copies/mL. Subtype A–infected volunteers were more likely to control than subtype C–infected volunteers (aOR, 2.1 [95% CI, 1.3–3.5]), even when we controlled for multiple factors.
The definition of HIV load control varies across studies, making direct comparisons challenging. While we have presented our data according to the most common definitions observed in a systematic review of studies of viral control [8], owing to sample size limitations our multivariable analysis required the adoption of a broader definition of control. Most studies that report on viral control have done so in relatively homogeneous cohorts and rarely mention the infecting HIV-1 subtype. Okulicz et al, in a US military cohort, using the same viral thresholds as us, observed aviremic control in <1% of subjects and viremic control in 3% [1]. Madec et al found that 7% of seroconverters enrolled in CASCADE (comprising 22 cohorts across Canada, Europe, and Australia) also controlled virus to a level of <400/500 copies/mL at 2 consecutive visits, and this was more common among women than men [15]. Lambotte et al, in France, also used a different definition of control (<400 copies/mL in >90% of tested samples from individuals known to have been infected with HIV for >10 years) and found that control was exhibited in <1% of HIV-infected patients [5]. In an Australian cohort, the authors defined controllers as people with viral loads of <400 copies/mL for at least 2 years and observed a prevalence of 1.5% [2]. A recent prevalence study from Uganda defined controllers as persons who had a viral load of <2000 copies/mL, had a care duration of ≥5 years, were ART naive, and had a serial CD4+ T-cell count of ≥500 cells/µL; the authors reported a prevalence of 0.26%, although because limited data on their source population were shown, this is likely an underestimate [16].
In another recent study, Venner et al described a cohort of 286 African women in Uganda and Zimbabwe in which they observed a similar overall prevalence of viral control (ie, 7%, with control defined as a viral load of <2000 viral copies/mL and a CD4+ T-cell count >350 for >3 years). However, they observed the opposite correlation between subtype and control, noting that control was more common among women with HIV-1 subtype C infection as compared to those with subtype A infection [14]. While these results are contrary to ours, their cohort comprised only women, was smaller than ours, and did not control for HLA typing, which we found to be significantly associated with viral control and, as we have previously published, disease progression [17]. If we limited our analysis to the 239 women with HLA and subtype data (Supplementary Materials), we obtained results similar to our primary analysis when comparing subtype A to subtype C (aOR, 2.4 [95% CI, 1.1–5.2]). Furthermore, additional sensitivity analyses suggest that our observed relationship between infecting HIV-1 subtype and viral control persists under varying conditions. While Venner et al suggest that a hypothesized “milder” subtype C infection may be associated with a longer latency period, which in turn may allow for increased opportunities for transmission and thus greater spread, others have argued that the rapid spread of subtype C is due to higher replicative fitness and infectiousness [18, 19]. Our own work in this cohort has shown higher viral loads among persons infected with subtype C as compared to subtype A, supporting the hypothesis of subtype C’s increased infectiousness [20]. Results of epidemiologic studies have also been inconclusive. A report among African couples with a discordant HIV status (hereafter, HIV-discordant couples) reported no evidence of an increased risk of transmission associated with HIV-1 subtype C [21], while another study, from Uganda, found that HIV-1 subtype A has a higher rate of transmission than subtype D among HIV-discordant couples, but they were not able to assess subtype C [22]. Given that early treatment is important for improving clinical outcomes [23] and is increasingly common, observational studies such as these are no longer possible, and this question may never be answered definitively. However, as infecting subtype has been shown to be associated with clinical outcomes in multiple studies [14, 17, 24, 25] and now with viral control, it would seem prudent to consider how infecting subtype may affect new prevention technologies, including vaccines.
We evaluated a broad range of HLA alleles and found that HLA-B*57 was predictive of viremic control, although this association was of borderline significance (P = .051). This is in agreement with the literature on disease progression and viral control [26–29], although the relationships between B*57 and disease control often appear to be more robust, suggesting that this allele may play a stronger role in clinical outcomes than viral suppression. Previous studies have reported B*45 to be associated with poor clinical outcomes of HIV infection [30] or higher HIV loads [31], but here we found that volunteers with B*45:01 were not associated with control; however, the allele remained in our final model, suggesting that it may play some role in characterizing viral control. Some studies have suggested the presence of the B*58:02 allele to be unfavorable [32]; similarly, our data suggested that persons with the allele may be less likely to control HIV (aOR, 0.5 [95% CI, .2–1.0]). Previously, we observed in this cohort a sex-allele interaction with HLA-A*03, where women with A*03 were shown to have positive outcomes and men were not [12]. Although we observed a higher prevalence of viral control in women with A*03, with multivariable analysis we observed that this interaction was not predictive of viral control.
We found that women were more likely to be controllers than men. The CASCADE cohort found a similar result [15]. Route of exposure has been shown to affect viral fitness in the new host, with male-to-female transmission associated with lower viral fitness [33]. We have also previously shown that the sex of the seroconverter was associated with early set-point viral loads in HIV-discordant couples [34]. Less fit viruses may be easier to control, supporting our findings.
A limitation of this study is the relatively short follow-up duration for volunteers. Follow-up in many of the reports of viral control is measured in decades [1, 2, 5], and follow-up our study ranged from <1 year to nearly 10 years. We observed that some volunteers controlled virus only to lose control later (eg, see sensitivity analysis 2); with an increased follow-up duration, we may have observed more cases like this, lowering our estimated prevalence of control. Access to ART varied across country and region, introducing a bias in the length of ART-free follow-up by enrollment center. To control for this, we undertook a sensitivity analysis, using Rwandan government ART guidelines (Rwanda was typically the earliest adopter of new World Health Organization guidelines on ART initiation); standardizing our cohort to these guidelines, we still observed that control was more common among volunteers infected with subtype A. Our main finding of viral control being associated with HIV-1 subtype was driven in part by the low prevalence of viral control among volunteers from Lusaka, our largest recruitment center (Table 1). Our sensitivity analysis in which subtype was replaced by geographic region, suggested that eastern Africans infected with subtype C are more likely to control their virus than southern Africans and that the frequency of viral control between those with subtype A and those with subtype C in eastern Africa is similar (Supplementary Table 3). However, the number of eastern Africans infected with subtype C is small (n = 24), and our observed regional difference in viral control may be due to other unmeasured covariates.
Nearly one third of volunteers were enrolled too late to have a blood specimen collected at baseline (defined as 3 months after estimated date of HIV acquisition) for determination of the CD4+ T-cell count, and for these volunteers we imputed this data point. In another sensitivity analysis, we limited our analysis to the 392 volunteers with baseline CD4+ T-cell count data and found that the relationship between subtype A and subtype C persisted, although with the smaller sample size, the statistical significance was attenuated. Perhaps more importantly, we did not have CD4+ T-cell count estimates for volunteers before they acquired HIV infection. In a study conducted by these teams and in many of these clinical research centers, we observed a significant difference in CD4+ T-cell count, with lower counts observed among healthy, HIV-uninfected persons living in Zambia as compared to East Africa [35], similar to what Venner et al also noted: Zimbabwean women tended to have lower CD4+ T-cell counts than their Ugandan counterparts [14]. Although the CD4+ T-cell count approximately 3 months after infection was correlated with viral control in this cohort, it would have been informative to describe any immunological discrepancies prior to HIV acquisition.
Also, 23 volunteers (4%) from the cohort were missing infecting HIV-1 subtype data, missing HLA data, or did not have an adequate amount ART-free follow-up time to contribute to the study (Figure 1). While this represents only a small portion of our overall cohort, early ART initiation or loss to follow-up may be associated with disease progression, and our estimate of viral control may thus be a modest overestimate were these volunteers to be included. Additionally, our subtypes are derived from the pol sequence, and we may have missed some viral recombination. The clinical significance of this is unclear, and our derivation of subtype still proved to be significantly associated with viral control.
A main strength of this cohort is the large, well-defined source populations across regions where multiple HIV subtypes are prevalent. These vaccine-preparedness cohorts focused on persons at elevated risk of HIV acquisition (eg, men who report sex with men and female sex workers), but in many cases, an individual was considered to have an elevated risk because they were married to a person with HIV infection. Because we enrolled >90% of consecutively identified volunteers with incident HIV infection from these vaccine-preparedness studies of HIV transmission, our estimates of the prevalence of viral control are less likely to be further affected by selection bias beyond being a member of these key populations. Because HIV testing for these volunteers was done quarterly or monthly and included p24 antigen testing and polymerase chain reaction analysis, the between-test period during which HIV infection occurred was narrow, allowing us to estimate the date of HIV infection with relative precision. Previously, we noted that symptoms of acute retroviral syndrome were more common among volunteers with subtype A infection than among those with subtype C or D infection—highlighting potential subtype-mediated differences prior to the onset of viral control [36].
Infecting HIV-1 subtype is important. Controlling for volunteer HLA type, sex, and other characteristics, we observed that persons infected with HIV subtype A were significantly more likely to control virus than were persons with subtype C. Previous research in this cohort and others has shown that subtype is also associated with disease progression. While this question may never be answered conclusively, our data suggest that infecting HIV-1 subtype should be considered when designing new HIV therapeutic agents, prevention modalities, or vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. A. P. was responsible for conceptualization, methodology, formal analysis, data curation, original draft preparation, review, and editing. W. R. was responsible for methodology, formal analysis, data curation, original draft preparation, review, and editing. W. K., E. K., M. I., E. R., A. K., E. J. S., O. A, V. E., J. T., S. L., G. M., L. G. B., G. A.-B., P. C., M. H. L., P. M., H. M., and F. K. were responsible for investigation, data curation, review, and editing. E. H., S. A., and F. P. were responsible for conceptualization, investigation, data curation, review, and editing. K. M. W. was responsible for formal analysis, data curation, original draft preparation, review, and editing. P. F., P. K., and J. G. were responsible for conceptualization, methodology, investigation, data curation, review, and editing. A. T. was responsible for investigation, data curation and analysis, review, and editing.
Acknowledgments. We thank all of the volunteers who enrolled into the Early Infection Cohort study, without whom this work would have been impossible; the staff of each clinical research center and the International AIDS Vaccine Initiative (IAVI) staff in Johannesburg and in London at IAVI’s Human Immunology laboratory, based at Imperial College; the teams at Clinical Laboratory Services and University of the Witwatersrand in Johannesburg, South Africa, responsible for viral load testing and HIV sequence generation and analysis; the clinical data base management team at the Perinatal HIV Research Center in Johannesburg, for their hard work; Dr Eduard Acosta and his laboratory team at the University of Alabama–Birmingham and Dr Raymond Schinazi and his laboratory team at Emory University, for accommodating our requests to screen for the presence of antiretroviral drugs; and the Fogarty AIDS International Training in Research Program and the Emory Center for AIDS Research for support of selected authors.
Disclaimer. The contents of this article are the responsibility of the authors and do not necessarily reflect the views of the US Agency for International Development or the US government. This article is published with permission of the Director of the Kenya Medical Research Institute. IAVI donors (ie, the study funders) played no role in study design, conduct, analysis, or manuscript preparation.
Financial support. This study was sponsored by the International AIDS Vaccine Initiative (IAVI), which in turn is funded by many donors, including the Bill and Melinda Gates Foundation, the Ministry of Foreign Affairs of Denmark, Irish Aid, the Ministry of Finance of Japan in partnership with The World Bank, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation, the United Kingdom Department for International Development, and the US Agency for International Development (the full list of IAVI donors is available at: http://www.iavi.org).
Potential conflicts of interest. In the role of sponsor, International AIDS Vaccine Initiative (IAVI) staff in collaboration with research partners wrote the study protocol, monitored study progress, conducted study analysis, and the primary author of this article (M. A. P.) is an IAVI employee. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Okulicz JF, Marconi VC, Landrum ML, et al. ; Infectious Disease Clinical Research Program (IDCRP) HIV Working Group Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis 2009; 200:1714–23. [DOI] [PubMed] [Google Scholar]
- 2. Sajadi MM, Constantine NT, Mann DL, et al. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr 2009; 50:403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses 2001; 17:901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–404. [DOI] [PubMed] [Google Scholar]
- 5. Lambotte O, Delfraissy JF. HIV controllers: a homogeneous group of HIV-1 infected patients with a spontaneous control of viral replication. Pathol Biol (Paris) 2006; 54:566–71. [DOI] [PubMed] [Google Scholar]
- 6. Buckheit RW 3rd, Salgado M, Martins KO, Blankson JN. The implications of viral reservoirs on the elite control of HIV-1 infection. Cell Mol Life Sci 2013; 70:1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koofhethile CK, Ndhlovu ZM, Thobakgale-Tshabalala C, et al. CD8+ T cell breadth and ex vivo virus inhibition capacity distinguish between viremic controllers with and without protective HLA class I alleles. J Virol 2016; 90:6818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gurdasani D, Iles L, Dillon DG, et al. A systematic review of definitions of extreme phenotypes of HIV control and progression. AIDS 2014; 28:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamali A, Price MA, Lakhi S, et al. ; IAVI Africa HIV Prevention Partnership Creating an African HIV clinical research and prevention trials network: HIV prevalence, incidence and transmission. PLoS One 2015; 10:e0116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Price MA, Wallis CL, Lakhi S, et al. ; IAVI Early Infection Cohort Study Group Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses 2011; 27:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang J, Cormier E, Gilmour J, et al. ; IAVI African HIV Research Network Human leukocyte antigen variants B*44 and B*57 are consistently favorable during two distinct phases of primary HIV-1 infection in sub-Saharan Africans with several viral subtypes. J Virol 2011; 85:8894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Price MA, He D, et al. ; IAVI Africa HIV Prevention Partnership Host genetics and viral load in primary HIV-1 infection: clear evidence for gene by sex interactions. Hum Genet 2014; 133:1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bedrick EJ, Tsai C-L. Model selection for multivariate regression in small samples. Biometrics 1994; 50:226–31. [Google Scholar]
- 14. Venner CM, Nankya I, Kyeyune F, et al. Infecting HIV-1 subtype predicts disease progression in women of Sub-Saharan Africa. EBioMedicine 2016; 13:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madec Y, Boufassa F, Porter K, Meyer L; CASCADE Collaboration Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS 2005; 19:2001–7. [DOI] [PubMed] [Google Scholar]
- 16. Kayongo A, Gonzalo-Gil E, Gümüşgöz E, et al. Brief report: identification of elite and viremic controllers from a large urban HIV ambulatory center in Kampala, Uganda. J Acquir Immune Defic Syndr 2018; 79:394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amornkul PN, Karita E, Kamali A, et al. ; IAVI Africa HIV Prevention Partnership Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS 2013; 27:2775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Essex M. Human immunodeficiency viruses in the developing world. Adv Virus Res 1999; 53:71–88. [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez MA, Ding M, Ratner D, et al. High replication fitness and transmission efficiency of HIV-1 subtype C from India: Implications for subtype C predominance. Virology 2009; 385:416–24. [DOI] [PubMed] [Google Scholar]
- 20. Prentice HA, Price MA, Porter TR, et al. ; IAVI Africa HIV Prevention Partnership Dynamics of viremia in primary HIV-1 infection in Africans: insights from analyses of host and viral correlates. Virology 2014; 449:254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kahle E, Campbell M, Lingappa J, et al. ; Partners in Prevention HSVHIV Transmission Study Team HIV-1 subtype C is not associated with higher risk of heterosexual HIV-1 transmission: a multinational study among HIV-1 serodiscordant couples. AIDS 2014; 28:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiwanuka N, Laeyendecker O, Quinn TC, et al. HIV-1 subtypes and differences in heterosexual HIV transmission among HIV-discordant couples in Rakai, Uganda. AIDS 2009; 23:2479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. World Health Organization, Switzerland, 2015. [PubMed] [Google Scholar]
- 24. Kaleebu P, French N, Mahe C, et al. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J Infect Dis 2002; 185:1244–50. [DOI] [PubMed] [Google Scholar]
- 25. Kanki PJ, Hamel DJ, Sankalé JL, et al. Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis 1999; 179:68–73. [DOI] [PubMed] [Google Scholar]
- 26. Chang CH, Kist NC, Stuart Chester TL, et al. HIV-infected sex workers with beneficial HLA-variants are potential hubs for selection of HIV-1 recombinants that may affect disease progression. Sci Rep 2015; 5:11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costello C, Tang J, Rivers C, et al. HLA-B*5703 independently associated with slower HIV-1 disease progression in Rwandan women. AIDS 1999; 13:1990–1. [DOI] [PubMed] [Google Scholar]
- 28. Carrington M, Walker BD. Immunogenetics of spontaneous control of HIV. Annu Rev Med 2012; 63:131–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010; 330:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sampathkumar R, Peters HO, Mendoza L, et al. Influence of HLA class I haplotypes on HIV-1 seroconversion and disease progression in Pumwani sex worker cohort. PLoS One 2014; 9:e101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang J, Tang S, Lobashevsky E, et al. ; Zambia-UAB HIV Research Project Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J Virol 2002; 76:8276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valenzuela-Ponce H, Alva-Hernández S, Garrido-Rodríguez D, et al. ; Mesoamerican HIV Project Group Novel HLA class I associations with HIV-1 control in a unique genetically admixed population. Sci Rep 2018; 8:6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carlson JM, Schaefer M, Monaco DC, et al. HIV transmission. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science 2014; 345:1254031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yue L, Prentice HA, Farmer P, et al. Cumulative impact of host and viral factors on HIV-1 viral-load control during early infection. J Virol 2013; 87:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karita E, Ketter N, Price MA, et al. CLSI-derived hematology and biochemistry reference intervals for healthy adults in eastern and southern Africa. PLoS One 2009; 4:e4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanders EJ, Price MA, Karita E, et al. Differences in acute retroviral syndrome by HIV-1 subtype in a multicentre cohort study in Africa. AIDS 2017; 31:2541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.