Abstract
Background
Tafenoquine (TQ) was recently approved by the US Food and Drug Administration for prophylaxis of malaria and, in addition, for eradication of the hepatic phase of the relevant Plasmodium species. In this study, we evaluated the efficacy of TQ for treatment of Babesia microti infection in mice with severe combined immunodeficiency (SCID).
Methods
SCID mice were infected with 1.1–1.5 × 108 B. microti–infected red blood cells by intraperitoneal injection. On day 3 or 4 postinfection, when parasitemia levels typically exceeded 10%, mice were treated with TQ vs vehicle alone, both administered by oral gavage.
Results
A single dose of TQ completely eliminated detectable parasites, with a >90% reduction in the level of parasitemia within just 4 days. Before elimination, a conspicuous phenotypic change in the parasite was observed. Although parasitologic cure was not achieved, there was no evidence for the development of drug resistance.
Conclusions
This study suggests that TQ may be a highly useful drug to treat B. microti infection in patients. If further animal studies establish that a marked reduction in B. microti parasitemia can be reliably achieved with peak blood levels of TQ known to be well tolerated in humans, a clinical trial in patients should be considered.
Keywords: Babesia microti, babesiosis, tafenoquine, SCID mice, malaria, protozoa
Tafenoquine, a drug approved in 2018 by the US Food and Drug Administration for malaria prophylaxis and for eradication of the hepatic phase of relevant Plasmodium species, may be a revolutionary new treatment for Babesia microti infections.
Babesia microti is an emerging pathogen in the United States that causes a malaria-like illness [1, 2]. Although it is most commonly acquired from the bite of an infected Ixodes scapularis tick, B. microti infection can also occur as a result of receiving a transfusion of an infected blood product or by vertical transmission from an infected pregnant woman to the fetus [1–4].
Symptomatic B. microti infections are typically treated with a 7- to 10-day course of a combination of 2 drugs: either azithromycin plus atovaquone, or clindamycin plus quinine [1, 2, 5, 6]. No single drug has been recognized to be clinically effective. Furthermore, since these combination therapies do not appear to rapidly reduce the level of parasitemia, red blood cell (RBC) exchange transfusions may be required in patients with severe illness and high parasitemia levels [1, 2]. In addition, the clindamycin plus quinine combination regimen is frequently associated with adverse effects, occurring in 72% of patients in one treatment trial [5]. Furthermore, highly immunocompromised patients typically require at least 6 weeks of treatment with these drug regimens, potentially extending to >2 years in patients who have received rituximab [2, 7, 8].
A well-tolerated, highly effective single drug, which may be administered for <7 days, is, to date, unheard of for the treatment of B. microti infections. In this study, we provide highly encouraging data on the efficacy of a single dose of the recently US Food and Drug Administration (FDA)–approved drug tafenoquine (TQ), for the treatment of B. microti infection in mice with severe combined immunodeficiency (SCID).
METHODS
Mouse Strains
CB17/lcr-Prkdcscid/lcrlcoCrl (SCID) female mice were obtained from Charles River Laboratories and used at 8–12 weeks of age. SCID mice are homozygous for the SCID spontaneous mutation Prkdcscid and are characterized by the absence of functional T and B cells and a normal hematopoietic microenvironment. All animal experimental protocols followed New York Medical College institutional guidelines for care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committees at New York Medical College.
Infection and Parasitemia
Babesia microti was obtained from the American Type Culture Collection (ATCC 30221 Gray strain isolated from a patient [9]). Mice were fed standard laboratory food with water available ad libitum. Mice were infected with 100 μL of blood intraperitoneally that was transferred from B. microti–infected CD1 outbred mice (Charles River) 5–8 days postinfection when parasitemia levels were 30%–50%. The challenge dose, therefore, was approximately 1.1–1.5 × 108B. microti–infected RBCs.
Parasitemia in mice was determined using standard methods to collect a drop of blood from the tail vein to create slide blood smears. Blood smears, fixed with 100% ice-cold methanol for 5 minutes followed by Giemsa stain for 30 minutes, were examined by bright field microscopy using a ×100 oil objective. To quantify the level of parasitemia, the mean of at least 3 counts of 100 RBCs was determined. Parasitemia was determined for 5 mice per time point unless otherwise stated. Parasitemia in the figures is shown as the mean and standard error for each group of mice with a line connecting the means for each group of mice on each day that data were available posttreatment. Parasites were considered undetectable if none were seen based on a scan of 1000 RBCs per slide. Statistical analyses for parasitemia levels in TQ-treated and untreated mice per day posttreatment were calculated in GraphPad Prism 8 software using 2-way repeated-measures analysis of variance, linear regression analysis, or 2-sample paired t test, as appropriate.
Tafenoquine
Tafenoquine succinate salt (tafenoquine succinate) was obtained from Sigma-Aldrich (>95% pure based on high-performance liquid chromatography). TQ was administered and dosed as the TQ succinate salt. Thus, the active TQ base delivered to mice at a dose of 20 mg/kg TQ succinate salt was 15.9 mg/kg. Stock solutions of TQ were prepared immediately prior to use and maintained at 4°C. The desired amount of TQ was resuspended with 0.5% w/v hydroxyethyl cellulose and 0.2% v/v Tween-80, as previously described [10–13].
In the initial experiments, a single dose of 20 mg/kg TQ was administered via oral gavage (18-gauge) on day 3 postinfection. A 200-μL volume was used in the first experiment. In subsequent experiments, however, 100 μL was used to decrease the possibility of adverse effects from the vehicle carrier.
An oral dose of 20 mg/kg of TQ succinate salt (15.9 mg/kg free base) was chosen for this experiment based on previous murine studies that evaluated the safety and efficacy of oral TQ against Plasmodium species (5–25 mg/kg) [10–13]. In murine studies that evaluated the efficacy of TQ for Plasmodium infections and in which it was stated whether dosing was based on the TQ succinate salt vs free base, dosing was based on the free base [10–13]. In previous murine studies, oral delivery of 20 mg/kg TQ required approximately 5–11 hours to reach maximum blood levels of TQ [10–13].
RESULTS
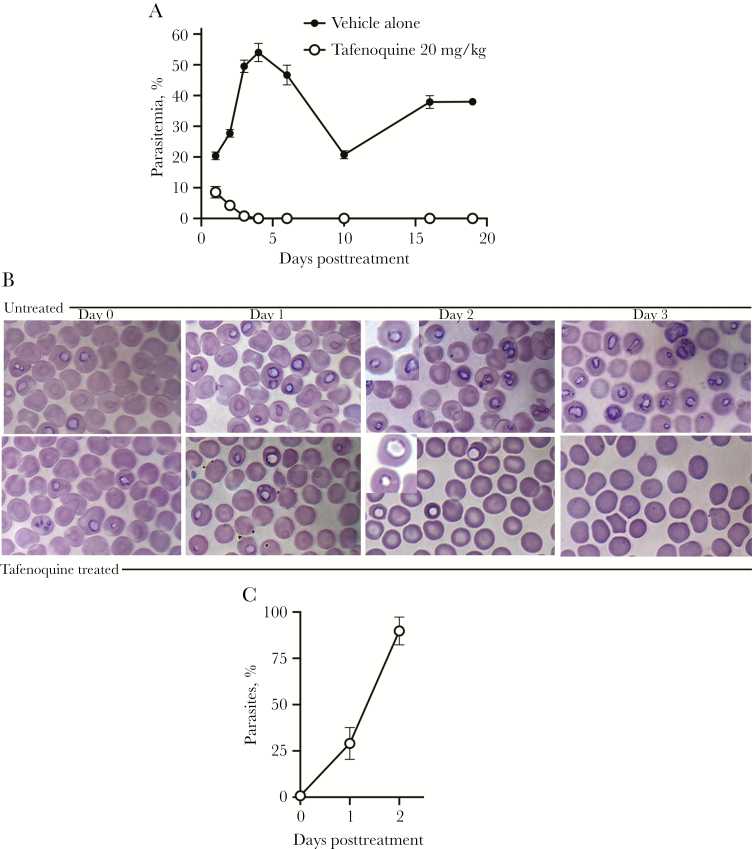
To establish B. microti infection, SCID mice were infected intraperitoneally with approximately 1.1–1.5 × 108B. microti–infected RBCs. On day 3 postinfection, when parasitemia was typically >10%, a single oral dose of 20 mg/kg TQ was administered (experiment 1). By 24 hours after treatment, the mean level of parasitemia in mice treated with vehicle alone was 20% compared to <10% in mice treated with TQ. By 48 hours the mean level of parasitemia had fallen to 4.3% and by 72 hours the mean level of parasitemia had fallen to 0.82% in TQ-treated mice. By 4 days following treatment with TQ, parasites were undetectable and remained undetectable throughout this experiment (18 days) (Figure 1A). Treated mice never developed visible symptoms of disease or discomfort such as ruffled fur. In contrast, mice treated with vehicle alone developed high parasitemia levels that peaked at approximately 50%, and they exhibited ruffled fur. Also, 2 of the 5 mice treated with vehicle alone died; one on day 8 posttreatment and one on day 9 posttreatment. The mean percentage of parasitemia in TQ-treated vs nontreated mice was significantly different at all time points assessed after day 0 when treatment was initiated (P < .0001). Linear regression analysis indicated that the reduction in percentage parasitemia per day was significant in TQ-treated mice (P < .0001).
Figure 1.
A, Percentage of parasitemia in mice treated with vehicle alone (control) or 20 mg/kg oral tafenoquine (TQ) on day 3 postinfection in experiment 1. Parasitemia percentages are shown as the mean and standard error (SE) for each group. The x-axis shows days after administration of a single TQ dose. TQ was administered on day 0; thus, day 1 after initiation of treatment is 24 hours. No parasites were detectable in blood smears by day 4 posttreatment. Two of the 5 mice treated with vehicle alone died; one on day 8 posttreatment and one on day 9 posttreatment. Thus, on day 10 and thereafter, the percentage of parasitemia values for the mice treated with vehicle alone are based on the 3 surviving mice. Below detection represents a scan of 1000 red blood cells per slide. The mean percentage of parasitemia in TQ-treated mice was statistically different from mice given vehicle alone at all of the time points evaluated (P < .0001; 2-way analysis of variance). B, Giemsa-stained blood smears from treated and untreated mice (vehicle alone) prior to treatment (day 0) and on days 1, 2, and 3 posttreatment. Blood smears from TQ-treated mice showed aberrant parasites characterized phenotypically by decreased chromatin staining. Imaging was performed with a Nikon ECLIPSE Ti-E Inverted Microscope System, ×100 Plan Apo oil objective (1.40). The inserts shown on day 2 posttreatment were magnified an additional 1.5 times using an Optivar for better visualization. Pictures were taken with a 16.25-megapixel monochrome DS-Qi2 digital camera capable of taking color pictures. C, Mean percentages and SE of parasites with the TQ-associated parasite phenotype prior to TQ treatment (day 0) and on days 1 and 2 post–TQ treatment are shown.
Figure 1B shows Giemsa-stained blood smears on day 0 prior to TQ treatment and days 1, 2, and 3 posttreatment compared to untreated mice. TQ treatment was associated with both a reduction in the level of parasitemia (Figure 1A) and an aberrant parasite phenotype, visible within 48 hours of treatment and characterized by a ghost-like appearance with only faint chromatin staining (Figure 1B and 1C). This phenotype was not observed in untreated mice (Figure 1B).
To assess whether the 20 mg/kg single-dose TQ treatment achieved complete clearance of parasites, 300 μL of blood from each treated mouse (approximately one-fourth of the total blood volume) was adoptively transferred to naive SCID recipient mice on day 18 posttreatment (when TQ should have been absent from the mice, as this time interval exceeded 7 serum half-lives of TQ, based on half-life values determined in prior mouse studies [10–12]) (experiment 2). Parasitemia was detected in SCID mice approximately 1 week after receiving these blood samples, indicating that the single oral dose of TQ of 20 mg/kg did not eliminate infection (data not shown).
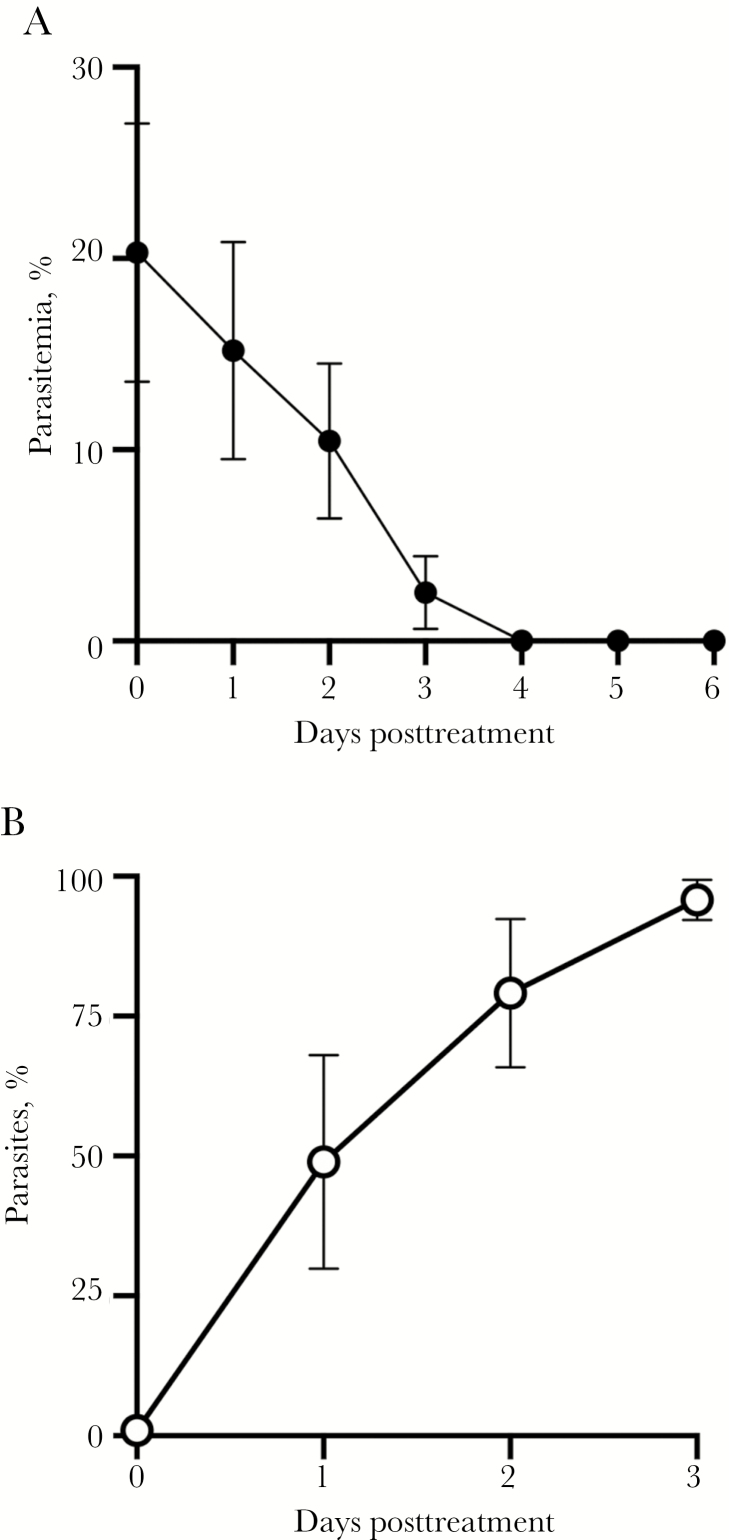
The SCID mice that developed parasitemia after adoptive transfer of blood from TQ-treated mice showed parasite levels ranging from 10% to 48% twelve days after transfer of blood. These mice were then treated with a single oral dose of 20 mg/kg of TQ on day 12 after the adoptive transfer to assess whether the residual B. microti parasites that persisted after TQ treatment had developed resistance to TQ (experiment 3). As shown in Figure 2A, the B. microti parasites remained susceptible to the drug. By 24 hours after treatment, the level of parasitemia fell from a mean value of 20.3% to 15.2% (an approximately 25% reduction), by 48 hours the mean level of parasitemia had fallen to 10.5% (a 52% reduction), and by 72 hours the mean level of parasitemia had fallen to 2.5% (an 88% reduction). By 4 days following treatment with TQ, parasites were undetectable, even in the mouse that had a parasitemia level of 46% prior to treatment. Linear regression analysis indicated that the reduction in percentage of parasitemia per day was significant in TQ-treated mice (P < .0001). As shown in Figure 2B, prior to parasite clearance, an increasing proportion of the remaining parasites had the aberrant TQ-associated phenotype that had been found in experiment 1 (Figure 1B).
Figure 2.
Recrudescent parasites after adoptive transfer to naive mice with severe combined immunodeficiency (SCID) remain susceptible to tafenoquine (TQ). Twelve days after blood was transferred from infected TQ-treated mice into naive SCID mice, oral TQ (20 mg/kg) was administered to these naive mice. A, Percentage parasitemia after administration of TQ is shown as the mean and standard error (SE) for each group of mice. Parasitemia was undetectable by day 4 after administration of TQ. Below detection represents a scan of 1000 red blood cells per slide. Linear regression analysis indicated that the reduction in percentage of parasitemia per day was significant in TQ-treated mice (P < .0001). B, The TQ treatment-associated phenotype was evident, and in some cases, predominant, within 24 hours of TQ treatment; nearly all of the visible parasites exhibited the altered phenotype by 72 hours after initiation of TQ treatment. The mean percentages and SE of Babesia microti parasites with the TQ-induced phenotype prior to and 1 day, 2 days, and 3 days after TQ administration compared to the total number of visualized parasites are shown.
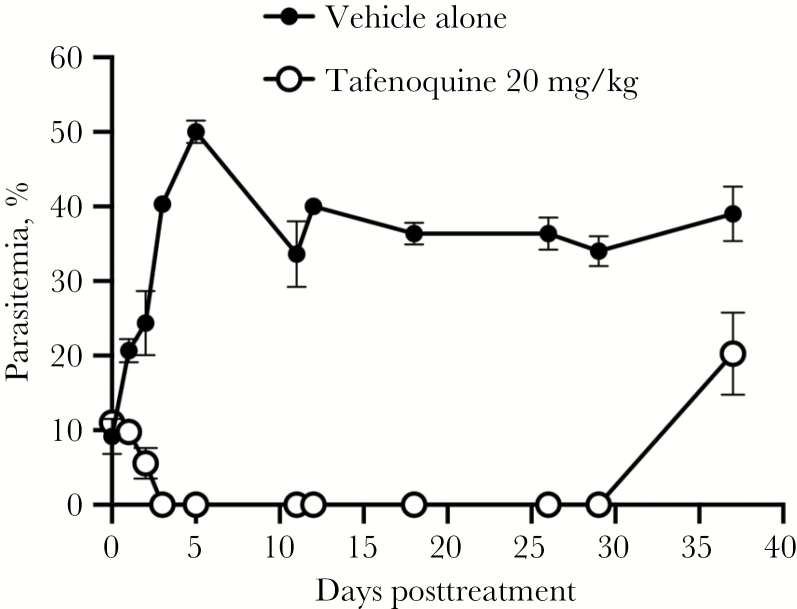
To determine if detectable parasitemia would reappear after TQ treatment in treated mice without adoptive transfer to other SCID mice, the original experiment (ie, experiment 1) was repeated, but the mice were observed over a longer period of time (experiment 4). In this experiment, TQ treatment resulted in a 50% decrease in parasitemia within the first 48 hours of treatment and parasites were undetectable by day 4 posttreatment (Figure 3). Parasite recrudescence, however, was evident in all TQ-treated mice by day 37 posttreatment (smears were not performed on days 30–36) (Figure 3).
Figure 3.
Parasite recrudescence in mice treated with a single dose of oral tafenoquine (TQ; 20 mg/kg). Parasite recrudescence was evident in all TQ-treated mice by day 37 posttreatment (smears were not performed on days 30–36). The percentage parasitemia after administration of TQ is shown as the mean and standard error for each group of mice.
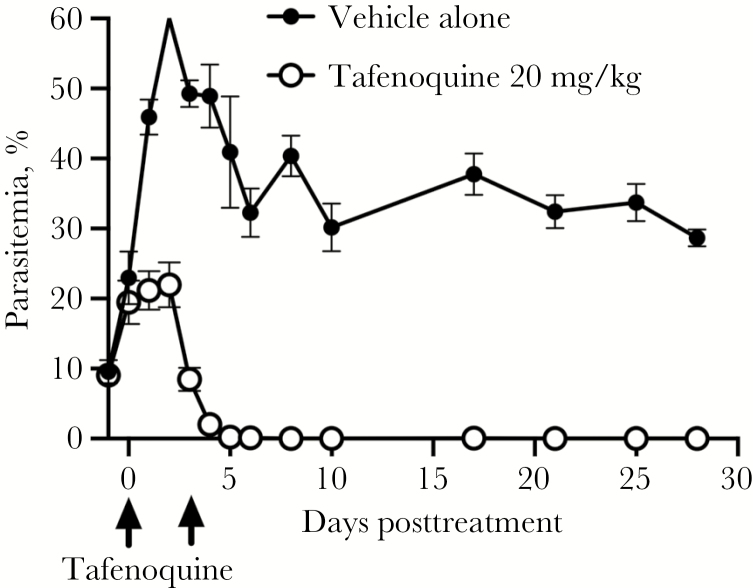
The elimination half-life of TQ in mice is approximately 1.6–2.3 days [10–13]. In contrast, the mean terminal half-life of TQ in humans is approximately 13–19 days [14–16]. To more accurately reflect the longer exposure to drug in humans, infected SCID mice were treated with 25 mg/kg TQ orally on day 4 postinfection (19.9 mg/kg dosage of the TQ active base) and given a second dose of 12.5 mg/kg (9.95 mg/kg of the TQ active base) orally 3 days later to compensate for the decrease in TQ blood levels over approximately one half-life in mice (experiment 5). TQ treatment was started on day 4 postinfection instead of day 3 to allow patent parasitemia levels to reach approximately 20% prior to treatment. Twenty-four hours (1 day) after the first dose of TQ in this experiment, the mean parasitemia level was 20% in treated mice compared with 45% in untreated mice (Figure 4). The mean parasitemia levels decreased to approximately 8% within 72 hours of the first dose of TQ. At this time point, mice were given an additional dose of 12.5 mg/kg of oral TQ. Five days after the first dose of TQ (2 days after the second dose of TQ), parasites were below the level of detection by blood smear. Parasites remained below the level of detection by blood smear at 28 days after the initiation of TQ treatment. The mean percentage parasitemia in TQ-treated vs nontreated mice was significantly different at all time points after day 0 when treatment was initiated, P < .0001. Linear regression analysis indicated that the reduction in percentage parasitemia per day was significant in TQ-treated mice (P < .0001).
Figure 4.
Oral tafenoquine (TQ) treatment on day 4 postinfection (25 mg/kg) with a repeat dose on day 7 postinfection (12.5 mg/kg) effectively eliminated parasitemia through day 28 posttreatment. Arrows mark the days the 2 doses of TQ were administered. Values for the mean percentage of parasitemia plus standard error are shown for each group of mice by day, pre- and posttreatment. Parasites were below the level of detection by day 5 after initiation of TQ treatment. Below detection represents a scan of 1000 red blood cells per slide. In contrast, parasitemia was detected in mice treated with vehicle throughout the experiment. There was a significant difference between the mean levels of parasitemia in TQ-treated and nontreated mice at all time points after day 0 when treatment was initiated (P < .0001, 2-way analysis of variance). Linear regression analysis indicated that the reduction in percentage of parasitemia post–TQ treatment was significant (P < .0001).
To assess whether there was complete clearance of parasites in mice receiving this 2-dose schedule of TQ, 300 μL of blood from each treated mouse was adoptively transferred to individual naive SCID mice 28 days after the first dose of TQ (experiment 6). Mice that received blood from TQ-treated mice exhibited detectable parasitemia starting on day 9 posttransfer (data not shown). Thus, extending TQ blood levels by approximately 1 additional half-life was also not sufficient to eliminate viable parasites from infected SCID mice.
The minimum effective dose of TQ was not determined in any of these experiments. However, it was observed that an oral dose of 5 mg/kg of TQ (3.8 mg/kg of TQ free base) administered on day 3 postinfection had no detectable effect on the level of parasitemia or on the parasite phenotype (data not shown) (experiment 7).
DISCUSSION
Tafenoquine is an 8-aminoquinoline discovered by scientists at the Walter Reed Army Institute of Research in 1978 (compound WR 238605) [17–20]. In 2018, this drug was approved by the FDA for prophylaxis of malaria and, in addition, for eradication of the hepatic phase of the relevant Plasmodium species [18, 19]. A single oral dose of 300 mg is approved to eradicate residual Plasmodium infection in the liver. The serum half-life in humans of TQ is approximately 2 weeks [18, 19]. The exact mechanism of action and molecular target of TQ for Plasmodium is unknown, particularly for blood-stage parasites. However, TQ has been shown to interfere with mitochondrial function and induce an apoptotic-like cell death in diverse protozoa including Plasmodium [19, 21].
In 1997, 2- and 4-day courses of TQ administered intramuscularly (totaling 4 and 8 doses, respectively) were tested for efficacy against B. microti in a hamster system [17]. Tafenoquine was the most effective drug of the 17 drugs or drug combinations that were tested. Tafenoquine 52 mg/kg given twice a day intramuscularly for 4 days was able to completely eradicate the parasite based on the inability to transfer infection from animals posttreatment to naive hamsters. The drug was much more effective than clindamycin plus quinine in this animal system. Despite these promising findings, to our knowledge, no other study besides the current one has attempted to confirm these results. Also, there are no published studies evaluating the efficacy of TQ in immunocompromised animals infected with B. microti parasites. In the hamster system study, blood levels of TQ were not reported for any of the dosages evaluated [17]. In the present study, just a single oral dose of TQ was highly effective in rapidly reducing parasitemia in highly immunocompromised SCID mice infected with B. microti, although parasitologic cure was not achieved. A single oral dose of TQ achieved a >90% reduction in the level of parasitemia within 4 days of TQ treatment, even in mice with pretreatment levels of parasitemia that exceeded 20%. In contrast, limited data in infected humans receiving combination therapies have shown only a 4.1%–12.9% reduction per day in the DNA copy numbers of B. microti [22].
Although a single dose of TQ effectively and dramatically decreased parasitemia in the current study, it was unable to prevent parasite recrudescence in SCID mice. However, single-dose therapy with TQ did not result in the development of resistance, as recrudescent parasites remained susceptible to TQ.
Limitations of the current study include that blood levels of TQ were not determined at any time point and only the percentage of RBCs that contained parasites was assessed, rather than the parasite density per microliter of blood. Also, a dose of 5 mg/kg was ineffective even though the dose of 20 mg/kg was highly effective.
This observation, in conjunction with the observation that extending the exposure to TQ in mice by approximately one half-life, through delivery of an additional dose of the drug, did not result in complete elimination of parasites, suggests that the effectiveness of single-dose TQ for treatment of B. microti infection in this animal system may be directly related to the peak blood level of the drug. Therefore, to better determine if the promising findings could potentially be extrapolated to patients, the peak blood level for the minimum effective dosage of TQ would need to be determined. In addition, future studies should consider including a less immunocompromised mouse system to align more closely with human infections, including mice that are asplenic but are otherwise not immunocompromised.
In conclusion, this study, along with a prior one in immunocompetent hamsters [17], suggests that TQ may be a highly useful drug to treat B. microti infections in patients, and indeed could potentially be the most effective agent available. The majority of infected patients should be able to tolerate TQ well, but those who are glucose-6-phosphate dehydrogenase deficient should not be treated with TQ [18]. If further animal studies of TQ treatment establish that a marked reduction in B. microti parasitemia can be reliably achieved with peak blood levels of drug known to be well tolerated in humans, a clinical trial of TQ in patients with babesiosis would be justified.
Notes
Acknowledgments. The authors thank Julia Singer, Shana Warner, and Lisa Giarratano for their assistance.
Financial support. This work was supported by the National Institutes of Health (grant number AI 142523 to D. G. M. and G. P. W.) and a New York Medical College/Touro Bridge Funding Program grant (to D. G. M.).
Potential conflicts of interest. G. P. W. has received research grants from Immunetics, Inc, the Institute for Systems Biology, Rarecyte, Inc, and Quidel Corporation; owns equity in Abbott/AbbVie; has been an expert witness in malpractice cases involving Lyme disease and babesiosis; and is an unpaid board member of the American Lyme Disease Foundation. D. G. M. reports no potential conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 2. Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 2016; 315:1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph JT, Purtill K, Wong SJ, et al. Vertical transmission of Babesia microti, United States. Emerg Infect Dis 2012; 18:1318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villatoro T, Karp JK. Transfusion-transmitted babesiosis. Arch Pathol Lab Med 2019; 143:130–4. [DOI] [PubMed] [Google Scholar]
- 5. Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343:1454–8. [DOI] [PubMed] [Google Scholar]
- 6. Lawres LA, Garg A, Kumar V, et al. Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J Exp Med 2016; 213:1307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raffalli J, Wormser GP. Persistence of babesiosis for >2 years in a patient on rituximab for rheumatoid arthritis. Diagn Microbiol Infect Dis 2016; 85:231–2. [DOI] [PubMed] [Google Scholar]
- 8. Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–6. [DOI] [PubMed] [Google Scholar]
- 9. Gleason NN, Healy GR, Western KA, Benson GD, Schultz MG. The “Gray” strain of Babesia microti from a human case established in laboratory animals. J Parasitol 1970; 56:1256–7. [PubMed] [Google Scholar]
- 10. Li Q, O’Neil M, Xie L, et al. Assessment of the prophylactic activity and pharmacokinetic profile of oral tafenoquine compared to primaquine for inhibition of liver stage malaria infections. Malar J 2014; 13:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vuong C, Xie LH, Potter BM, et al. Differential cytochrome P450 2D metabolism alters tafenoquine pharmacokinetics. Antimicrob Agents Chemother 2015; 59:3864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melariri P, Kalombo L, Nkuna P, et al. Oral lipid-based nanoformulation of tafenoquine enhanced bioavailability and blood stage antimalarial efficacy and led to a reduction in human red blood cell loss in mice. Int J Nanomedicine 2015; 10:1493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milner EE, Berman J, Caridha D, et al. Cytochrome P450 2D-mediated metabolism is not necessary for tafenoquine and primaquine to eradicate the erythrocytic stages of Plasmodium berghei. Malar J 2016; 15:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edstein MD, Kocisko DA, Walsh DS, Eamsila C, Charles BG, Rieckmann KH. Plasma concentrations of tafenoquine, a new long-acting antimalarial agent, in Thai soldiers receiving monthly prophylaxis. Clin Infect Dis 2003; 37:1654–8. [DOI] [PubMed] [Google Scholar]
- 15. McCarthy JS, Smith B, Reid M, et al. Blood schizonticidal activity and safety of tafenoquine when administered as chemoprophylaxis to healthy, non-immune participants followed by blood stage Plasmodium falciparum challenge: a randomized, double-blinded, placebo-controlled phase 1b study [manuscript published online ahead of print 1 November 2018]. Clin Infect Dis 2018. doi: 10.1093/cid/ciy939. [DOI] [PubMed] [Google Scholar]
- 16. Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. Am J Trop Med Hyg 1998; 58:645–9. [DOI] [PubMed] [Google Scholar]
- 17. Marley SE, Eberhard ML, Steurer FJ, Ellis WL, McGreevy PB, Ruebush TK 2nd. Evaluation of selected antiprotozoal drugs in the Babesia microti–hamster model. Antimicrob Agents Chemother 1997; 41:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baird JK. Tafenoquine for travelers’ malaria: evidence, rationale and recommendations. J Travel Med 2018; 1–13. doi: 10.1093/jtm/tay110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frampton JE. Tafenoquine: first global approval. Drugs 2018; 78:1517–23. [DOI] [PubMed] [Google Scholar]
- 20. Ebstie YA, Abay SM, Tadesse WT, Ejigu DA. Tafenoquine and its potential in the treatment and relapse prevention of Plasmodium vivax malaria: the evidence to date. Drug Des Devel Ther 2016; 10:2387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carvalho L, Luque-Ortega JR, Manzano JI, Castanys S, Rivas L, Gamarro F. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets leishmania respiratory complex III and induces apoptosis. Antimicrob Agents Chemother 2011; 54:5356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang G, Villafuerte P, Zhuge J, Visintainer P, Wormser GP. Comparison of a quantitative PCR assay with peripheral blood smear examination for detection and quantitation of Babesia microti infection in humans. Diagn Microbiol Infect Dis 2015; 6:376–82. [DOI] [PubMed] [Google Scholar]