Abstract
Background
BK virus (BKV) is a significant cause of nephropathy in kidney transplantation. The goal of this study was to characterize the course and source of BKV in kidney transplant recipients.
Methods
We prospectively collected pretransplant plasma and urine samples from living and deceased kidney donors and performed BKV polymerase chain reaction (PCR) and immunoglobulin G (IgG) testing on pretransplant and serially collected posttransplant samples in kidney transplant recipients.
Results
Among deceased donors, 8.1% (17/208) had detectable BKV DNA in urine prior to organ procurement. BK viruria was observed in 15.4% (6/39) of living donors and 8.5% (4/47) of deceased donors of recipients at our institution (P = .50). BKV VP1 sequencing revealed identical virus between donor–recipient pairs to suggest donor transmission of virus. Recipients of BK viruric donors were more likely to develop BK viruria (66.6% vs 7.8%; P < .001) and viremia (66.6% vs 8.9%; P < .001) with a shorter time to onset (log-rank test, P < .001). Though donor BKV IgG titers were higher in recipients who developed BK viremia, pretransplant donor, recipient, and combined donor/recipient serology status was not associated with BK viremia (P = .31, P = .75, and P = .51, respectively).
Conclusions
Donor BK viruria is associated with early BK viruria and viremia in kidney transplant recipients. BKV PCR testing of donor urine may be useful in identifying recipients at risk for BKV complications.
Keywords: BK virus, kidney transplantation, pretransplant, donor, serology, PCR
Donor BK viruria is associated with early BK viruria and viremia in kidney transplant recipients. BK virus polymerase chain reaction testing of donor urine may be useful in identifying recipients at risk for BK virus complications.
BK virus (BKV) is the most common viral cause of nephropathy in kidney transplant recipients. Unlike other common viruses that affect transplant outcomes (eg, cytomegalovirus and Epstein–Barr virus), pretransplant donor and recipient screening for BKV is not performed in practice. BKV is a pervasive virus that infects 90% of the population by adulthood and can reactivate to high levels in immunocompromised hosts. However, degree of immunosuppression itself does not fully explain the higher prevalence of BKV nephropathy seen in kidney transplant recipients compared to other organ recipients who receive higher levels of immunosuppression [1–3]. Given the persistence of BKV in uroepithelial cells after initial infection, the kidney donor may be a significant source of infection. Thus, an important consideration in the utility of pretransplant screening is the significance of donor BKV on posttransplant recipient BKV outcomes.
This is a single-center prospective study in which we performed BKV polymerase chain reaction (PCR) and serology in donors and recipients in a cohort of kidney transplant recipients. This study also includes evaluation of BKV in deceased kidney donors through collaboration with our local organ procurement organization (OPO), Donor Network West. Specifically, we aimed to determine the rate of active shedding in kidney donors (deceased and living) and recipients, evaluate for donor transmission of BKV, and determine associations of pretransplant BKV status with posttransplant BKV outcomes.
MATERIALS AND METHODS
Study Design and Population
During August 2016–December 2017, plasma and urine samples were prospectively collected from deceased and living kidney donors as well as recipients prior to kidney transplantation. Deceased donor specimens were collected prior to kidney donation through Donor Network West, the third-largest OPO in the United States that serves northern California and Nevada, during the same time period and included specimens from donors whose kidney recipients were located at other institutions. In addition, we collected serial posttransplant urine samples every 2–4 weeks for the first 3 months after transplant and plasma samples every 3 months for 1 year in kidney transplant recipients at our institution. Nucleic acid stabilizer was added to urine samples in 1:1 proportion in DNA/RNA Shield (Zymo Research) within 48 hours of collection and all samples were stored at –80°C. Kidney transplant recipients received induction therapy with an interleukin 2 receptor blocker (calculated panel reactive antibody [cPRA] <20%) or antithymocyte globulin (cPRA >20%) and maintenance immunosuppression with steroids, tacrolimus, and mycophenolate mofetil with steroid taper over 2 weeks to 6 months in unsensitized patients. This study was reviewed and approved by the institutional review board (IRB) of Stanford University (IRB-36663).
BKV Quantitative PCR
Total nucleic acid was extracted from 200 μL plasma or 400 μL urine using the EZ1 Virus Minikit version 2.0 on the EZ1 Advanced XL instrument (Qiagen) and eluted into a final volume of 60 μL. BKV DNA was quantitated by real-time PCR by amplifying a 75-bp region of VP1 using forward primer 5′-CCTTACCCAATTTCCTTTTTGCT-3′ and reverse primer 5′-ATACATAGGCTGCCCATCCAC-3′ and probe FAM-TGGTTCTCCTGTTTATA-BHQ1). Total reaction volume was 25 μL, including 10 μL eluate, 4 μL Quantifast 5x master mix, 400 nm primer and 100 nm probe, and 1 μL of internal control. PCR was performed on the Rotor-Gene Q (Qiagen) real-time PCR instrument. Cycling conditions were as follows: 94°C for 5 minutes, and 45 cycles of 94°C for 10 seconds, 52°C for 30 seconds, and 72°C for 20 seconds. The linear range of the assay was 2.3–7.3 log10 IU/mL and the lower limit of detection was 106 IU/mL. BK viremia and viruria were defined as detectable virus by quantitative PCR (qPCR).
BKV VP1 Sequencing
DNA sequencing of the BKV VP1 region was performed using forward primer HF (5′-TGGACTTAAGAAATCAACAAA GTGTACATTCAGGA-3′) and reverse primer NR (5′-CTGCTGAA GATTCCCAAAGGTCAGAC-3′) in a 25-μL reaction using 10 μL of eluate, 12.5 μL of NEB LongAmp Hot Start 2x master mix and 400 nM primer. The following reactions were run in a DNA engine PTC-200 thermal cycler (Bio-Rad) with the following cycling conditions: 94°C for 2 minutes, 40 cycles of 94°C for 30 seconds, 60°C for 30 seconds, 68°C for 1 minute 40 seconds, and 72°C for 10 minutes. The 1415-bp amplicon was visualized in ethidium bromide–stained agarose gel. Dideoxynucleotide chain-termination sequencing was performed by Elim Biopharm.
BKV Antibody Testing
BKV immunoglobulin G (IgG) antibody testing was performed using a virus-like particle–based indirect enzyme-linked immunosorbent assay to detect human IgG antibodies to the VP1 viral capsid protein (Viracor) [4]. The assay antibody titers range from <1:40 to >1:163 840 based on the specimen dilution that produced signal greater than the assay background level.
Statistical Analysis
Categorical data were reported as counts and percentages and continuous data as medians with ranges, and significance was tested by Fisher exact and Mann–Whitney tests, respectively. P values < .05 were considered statistically significant. Multiple sequence alignment and a phylogenetic tree were constructed by neighbor-joining methods using ClustalX and Mega 7.0 software [5, 6]. Kaplan–Meier survival curves representing time to BK viruria and BK viremia were compared using the log-rank test. Patients were censored for graft failure or end of follow-up. None of the patients died during the follow-up period. Patients were followed for 1 year after transplant for BKV outcomes. Analyses were performed with R version 3.3.3 software.
RESULTS
Plasma and urine samples from 208 deceased kidney donors from our OPO were prospectively collected and tested for BKV DNA by qPCR. Of the 208 deceased donors evaluated through our OPO, 47 donated kidneys to recipients at our institution and were included in subsequent analysis. Eighty-six kidney transplant recipients (Table 1) at our institution with both pretransplant donor (47 deceased and 39 living) and recipient samples during the study timeframe were identified, and paired plasma and urine specimens were tested by BKV qPCR (plasma and urine) and BKV IgG (plasma).
Table 1.
Clinical Characteristics of Kidney Transplant Recipients
| Characteristic | No. (%) |
|---|---|
| Age, y, median (IQR) | 49.0 (36.9–60.1) |
| Sex | |
| Male | 59 (68.6) |
| Female | 27 (31.4) |
| Race | |
| White | 35 (40.7) |
| Hispanic | 24 (27.9) |
| Asian | 22 (25.6) |
| Black | 5 (5.8) |
| Donor status | |
| Deceased | 47 (54.6) |
| Living | 39 (45.3) |
| Indication | |
| Inherited | 15 (17.4) |
| Diabetes | 14 (16.3) |
| Vascular | 13 (15.1) |
| Glomerular | 25 (29.1) |
| Other (eg, drug) | 6 (6.9) |
| Unknown | 13 (15.1) |
| cPRA score, median (IQR) | 7.0 (0–49.0) |
| HLA mismatch | |
| 0–3 mismatch | 35 (41.3) |
| 4–6 mismatch | 51 (58.6) |
| Induction regimen | |
| ATG | 76 (88.3) |
| Basiliximab | 3 (3.5) |
| No induction | 8 (9.3) |
| ATG dose, mg/kg | |
| 0 | 11 (12.7) |
| <3 | 14 (16.3) |
| 3 to <5 | 46 (53.8) |
| ≥5 | 14 (16.3) |
| Multiorgan transplant | |
| No | 81 (94.3) |
| Yes | 5 (5.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ATG, antithymocyte globulin; cPRA, calculated panel reactive antibody; HLA, human leukocyte antigen; IQR, interquartile range.
BK Viruria in Deceased and Living Donors
Among deceased donors, 8.1% (17/208) had detectable BKV DNA in urine prior to organ procurement. Median viral load was 228 IU/mL (range, <200–15 415 IU/mL). Forty-seven of these donors donated kidneys to recipients at our institution, including 4 donors with BK viruria (8.5%). Among living kidney donors, 15.4% (6/39) had detectable BKV DNA in urine prior to transplant, with a median viral load of 1676 IU/mL (range, <200–22 611 IU/mL). Presence of BK viruria was not significantly different between deceased and living kidney donors (P = .502). Among nonanuric recipients, 6.1% (2/33) were BK viruric pretransplant. No donor (deceased or living) or recipient had detectable BKV DNA in plasma prior to transplant.
Early BKV Kinetics in Recipients of BK Viruric Donors
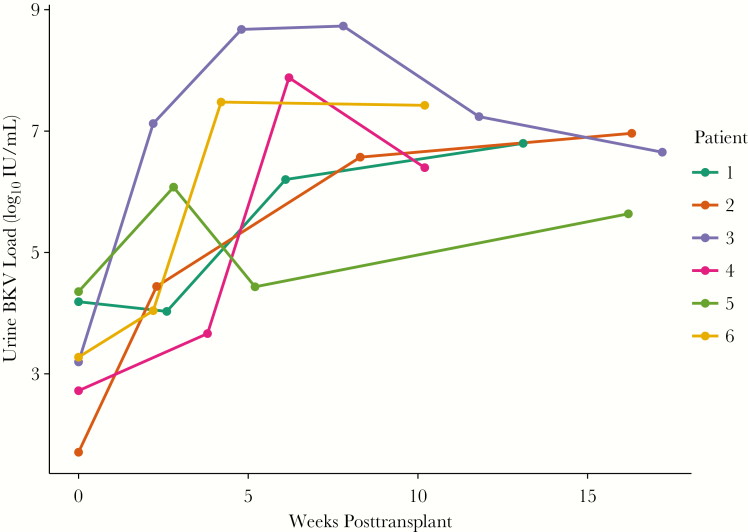
In total, 10 of the 86 (11.6%) kidney transplant recipients at our institution had detectable BKV DNA in a pretransplant donor or recipient urine specimen: 8 donor only, 1 donor and recipient, and 1 recipient only. Next, we evaluated urine samples from recipients collected every 2–4 weeks for the first 3 months posttransplantation for BK viruria. Among recipients who received a kidney from a donor with detectable BKV DNA in the urine prior to transplant, 66.6% (6/9) developed BK viruria posttransplant, all within 2–4 weeks posttransplant. BKV viral titers in urine increased 3–4 log IU/mL and persisted at high levels in the first weeks after transplant (Figure 1). The one recipient who was BK viruric pretransplant whose donor was not BK viruric did not develop BK viruria posttransplant.
Figure 1.
Posttransplant urine BK virus (BKV) DNA levels in recipients of BK viruric donors who developed BK viruria posttransplant. In the 6 recipients of donors who were BK viruric at time of transplant, posttransplant urine BKV DNA levels increased several log-fold during the first 2–4 weeks of transplant that sustained for at least the first 3 months after transplant. The other 3 transplant recipients of BK viruric donors did not develop posttransplant BK viruria or viremia (not shown).
Donor Transmission of BKV
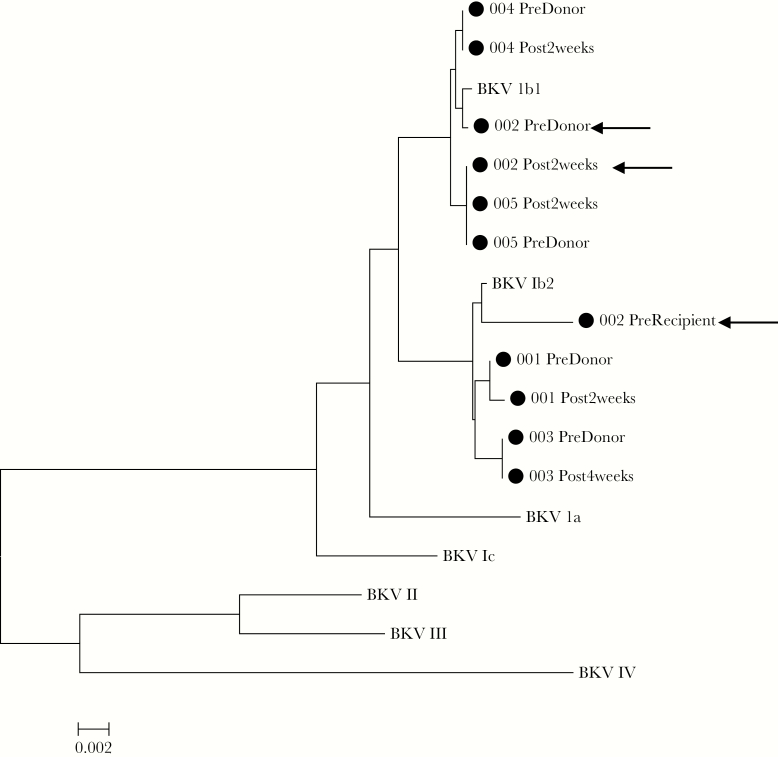
To identify donor transmission of BKV, we sequenced the VP1 region of donor and posttransplant recipients’ BK virus. BKV VP1 sequencing was successful in 5 of 6 recipients with donor BKV viruria and posttransplant BKV viruria and revealed nearly genetically identical virus (<10 bp, <1% difference) between donor and recipient (Figure 2). BKV identified in these patients were predominantly genotype 1b1 and 1b2 virus. Notably, in the 1 kidney transplant recipient with both pretransplant donor (1b1) and recipient (1b2) BK viruria, posttransplant BK virus in the recipient was identical to the donor (1b1).
Figure 2.
BK virus (BKV) VP1 sequencing of donors and posttransplant recipient. Virus found in paired donors and recipients were nearly genetically identical (<10 bp difference) and more similar than virus found in other pairs. Recipient 002 (arrows) was BK viruric with a genotype 1b2 virus and received a kidney from a donor who was viruric with a 1b1 virus. Virus identified in the recipient after transplant was a 1b1 virus similar to that in the donor. Reference accession numbers were as follows: BKV Ia: NC_001538; 1b1: AB211371; 1b2: AB260032; 1c: AB211377; II: AB263920; III: M23122; and IV: AB269826.
BKV Outcomes According to Donor BK Viruria Status
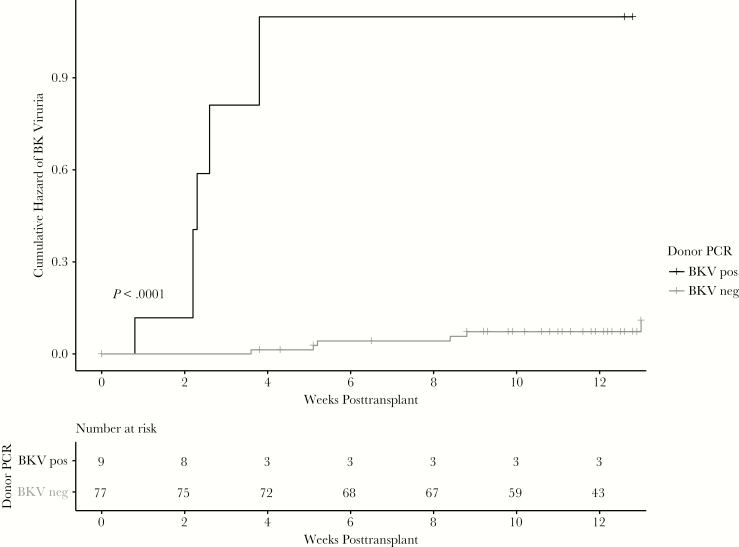
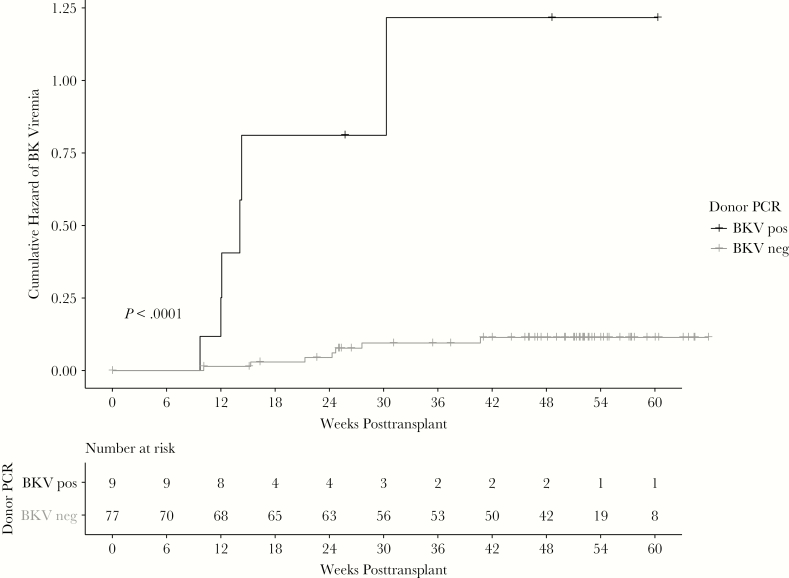
Recipients of non-BK viruric donors were less likely to develop BK viruria at 3 months compared to recipients of BK viruric donors (7.8% vs 66.6%; P < .001). Kaplan–Meier survival curves representing time to BK viruria were significantly different between groups (log-rank test, P < .001; Figure 3) with recipients of BK viruric donors having a higher risk and a shorter time to onset for BK viruria. Median time to BK viruria in recipients of viruric donors was 2.3 (range, 0.8–2.3) weeks compared to 5.2 (range, 3.6–13) weeks. By 1 year, all 6 recipients had developed BK viremia compared to 7 of the 78 (8.9%) recipients of non-BK viruric donors (P < .001). Kaplan–Meier survival curves for time to BK viremia were also significantly different (log-rank test, P < .001; Figure 4). Median time to BK viremia in recipients of viruric donors was 12.0 (range, 9.7–30.3) weeks compared to 24.3 (range, 10.1–40.7) weeks in recipients of non-BK viruric donors. Despite onset of BK viremia, most patients in both groups resolved BK viremia quickly, either spontaneously or with reduction in immunosuppression. Peak viral load ranged from <200 to 3788 IU/mL. No recipient developed BK virus nephropathy by 1 year.
Figure 3.
Pretransplant donor BK viruria is associated with early posttransplant recipient BK viruria. Recipients of BK viruric donors were more likely to develop BK viruria at 3 months (66.6% vs 7.8%; P < .001) and develop BK viruria earlier (log-rank test, P < .001) compared with kidney recipients from non-BK viruric donors. Abbreviations: BKV, BK virus; neg, negative; PCR, polymerase chain reaction; pos, positive.
Figure 4.
Pretransplant donor BK viruria is associated with early posttransplant recipient BK viremia. Recipients of BK viruric donors were also more likely to develop BK viruria at 3 months (66.6% vs 8.9%; P < .001) and develop BK viruria earlier (log-rank test, P < .001) compared with kidney recipients from non-BK viruric donors. Abbreviations: BKV, BK virus; neg, negative; PCR, polymerase chain reaction; pos, positive.
Influence of Pretransplant BKV Antibody Titers
All pretransplant donor and recipient samples from the 86 kidney transplant recipients were seropositive for BKV. Titers ranged from 1:2560 and 1:163 840. When BKV IgG titers were analyzed as a continuous variable, pretransplant donor titers were higher in kidney transplant recipients who had BK viruria compared with those who did not (median, 1:40 960 vs 1:10 240; P = .390). Higher donor BKV IgG titers were also seen in recipients who developed BK viremia, though findings were not statistically significant (median, 1:10 240 vs 1:40 960; P = .054). Pretransplant recipient BKV IgG titers were not significantly associated with BK viruria (1:10 240 vs 1:10 240; P = .54) or viremia (median, 1:10 240 vs 1:10 240; P = .61). Using a 1:40 960 threshold to define low- and high-titer groups as determined by the distribution of data that optimized discrimination between groups, pretransplant donor (7.8% vs 20.0%; P = .16), recipient (10.0% vs 16.1%; P = .49), and combined donor (D)/recipient (R) BKV IgG status (Dlow/Rlow: 10.8%; Dlow/Rhigh: 0%; Dhigh/Rhigh: 29.4%; Dhigh/Rlow: 23.1%; P = .093) were not associated with BK viruria. Similarly, pretransplant donor (Dlow: 9.8% vs Dhigh: 20%; P = .31), recipient (Dlow: 12.5% vs Dhigh: 15.2%; P = .75), or combined donor/recipient BKV IgG status (Dlow/Rlow: 8.6%; Dlow/Rhigh: 12.5%; Dhigh/Rhigh: 12.5%; Dhigh/Rlow: 23.1%; P = .51) were also not significantly associated with BK viremia. In time-to-event analysis, BKV IgG titers of donor, recipient, and combined donor/recipient status were also not associated with the development of BK viruria (P = .09, P = .40, and P = .07, respectively) or BK viremia (P = .20, P = .80, and P = .60, respectively) by log-rank testing.
DISCUSSION
In this study, we evaluated BKV shedding rates among deceased donors, donor transmission of BKV, and association between pretransplant donor/recipient BKV status defined by qPCR and serology with posttransplant BKV outcomes. BK viruria among deceased kidney donors just prior to transplant was 8.1%, which was not significantly different from BK viruria among living donors at our institution (15.1%). These rates are comparable to the 8%–27% rate of BK viruria previously reported in living donors [7–11] and the 7%–12% rate of BK viruria in asymptomatic healthy individuals [12, 13], indicating that BKV replication among deceased donors at time of organ procurement is similar to that in living donors and healthy individuals.
Findings from this study provide further support for donor transmission of BKV [10, 14]. Sequencing of the BKV VP1 gene revealed that the virus found in the posttransplant recipient was nearly genetically identical to the virus found in the donor. After introduction of donor virus, kidney transplant recipients demonstrated a rapid and persistent several logarithmic viral load increase beginning as early as 2 weeks after transplantation. Such early BK viruria after kidney transplant can predispose to significant pathology [15], particularly in the context of the heightened immunosuppression used in early transplantation. Indeed, the majority of kidney transplant recipients whose donors were BK viruric at time of transplant developed early BK viruria and viremia. However, most patients in our cohort did not develop persistent BK viremia and none developed BKV nephropathy at 1 year of follow-up. Thus, additional factors, such as older age, human leukocyte antigen mismatch, steroid exposure, rejection treatment, and overall level of immunosuppression [2, 16, 17], are also likely important determinants for prolonged BK viremia and nephropathy.
Nevertheless, donor BKV shedding identifies a group at high risk for BKV complications and may be more useful than BKV serology in discriminating individuals who develop early BK complications. In our adult cohort, 100% of donors and recipients were BKV IgG positive. While higher donor BKV IgG titers were seen in kidney transplant recipients who developed BK viremia, the finding was not statistically significant. These results are in contrast to BKV DNA results in urine, which were significantly associated with BK viremia (P < .001). Differing results have been previously reported regarding pretransplant BKV IgG screening, from questionable utility [11, 18] to strong associations with BK viremia in multivariate analysis [19]. This work provides support for pretransplant BKV PCR screening in donors [8, 9, 11] over serology, though more work is needed to understand the utility of both testing modalities in the context of current screening protocols.
Strengths of this study include evaluation of pretransplant donor and recipient pairs, including testing of urine samples that allowed for sequencing of virus between donor–recipient pairs, which enabled analysis for donor-transmitted virus. In addition, this study identified BKV shedding rates in urine among deceased donors through prospective evaluation of donors from our local organ procurement organization. Limitations of this study include the small number of viruric donors and limited power for robust multivariate analysis and no paired kidney recipients from a single BKV viruric donor to perform paired recipient virus analyses to further confirm findings.
These findings add to a growing body of literature highlighting the role of donor-transmitted BKV and utility of pretransplant donor BKV PCR screening. Donor urine BKV PCR testing may impact risk-stratification algorithms that affect frequency of posttransplant BKV surveillance and/or personalization of immunosuppression management, donor selection in highly sensitized kidney transplant, and identification of at-risk patients for enrollment in clinical trials related to BKV. In summary, donor BK viruria predisposes to recipient BK viruria very early after transplantation, creating an environment that, in the context of multiple host-related factors, can result in the development of early BKV-related complications in kidney transplant recipients.
Notes
Acknowledgments.We thank the Stanford Translational Research Integrated Database Environment (STRIDE), the Donor Network West team, Jake Krong, and Megan Fife for their dedicated contribution to this project.
Financial support.This work was supported by the National Institutes of Health, National Center for Advancing Translational Sciences (Clinical and Translational Science Award numbers TL1TR001084 and UL1TR001085 to S. K. T.); and the Intermountain-Stanford Collaboration Grant (to S. K. T., B. A. P., T. S., and D. A.).
Potential conflicts of interest.All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Scadden JR, Sharif A, Skordilis K, Borrows R. Polyoma virus nephropathy in kidney transplantation. World J Transplant 2017; 7:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trofe-Clark J, Sawinski D. BK and other polyomaviruses in kidney transplantation. Semin Nephrol 2016; 36:372–85. [DOI] [PubMed] [Google Scholar]
- 3. Demey B, Tinez C, François C, et al. Risk factors for BK virus viremia and nephropathy after kidney transplantation: a systematic review. J Clin Virol 2018; 109:6–12. [DOI] [PubMed] [Google Scholar]
- 4. Laskin BL, Sullivan KE, Hester J, Goebel J, Davies SM, Jodele S. Antibodies to BK virus in children prior to allogeneic hematopoietic cell transplant. Pediatr Blood Cancer 2015; 62:1670–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics 2002; Chapter 2, Unit 2.3. doi: 10.1002/0471250953.bi0203s00. [DOI] [PubMed] [Google Scholar]
- 7. Vera-Sempere FJ, Rubio L, Felipe-Ponce V, et al. Renal donor implication in the origin of BK infection: analysis of genomic viral subtypes. Transplant Proc 2006; 38:2378–81. [DOI] [PubMed] [Google Scholar]
- 8. Schwarz A, Linnenweber-Held S, Heim A, Framke T, Haller H, Schmitt C. Viral origin, clinical course, and renal outcomes in patients with BK virus infection after living-donor renal transplantation. Transplantation 2016; 100:844–53. [DOI] [PubMed] [Google Scholar]
- 9. Verghese PS, Schmeling DO, Knight JA, Matas AJ, Balfour HH Jr. The impact of donor viral replication at transplant on recipient infections posttransplant: a prospective study. Transplantation 2015; 99:602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmitt C, Raggub L, Linnenweber-Held S, Adams O, Schwarz A, Heim A. Donor origin of BKV replication after kidney transplantation. J Clin Virol 2014; 59:120–5. [DOI] [PubMed] [Google Scholar]
- 11. Grellier J, Hirsch HH, Mengelle C, et al. Impact of donor BK polyomavirus replication on recipient infections in living donor transplantation. Transpl Infect Dis 2018; 20:e12917. [DOI] [PubMed] [Google Scholar]
- 12. Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 2009; 199:837–46. [DOI] [PubMed] [Google Scholar]
- 13. Kling CL, Wright AT, Katz SE, et al. Dynamics of urinary polyomavirus shedding in healthy adult women. J Med Virol 2012; 84:1459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant 2005; 5:2213–21. [DOI] [PubMed] [Google Scholar]
- 15. Saundh BK, Baker R, Harris M, Welberry Smith MP, Cherukuri A, Hale A. Early BK polyomavirus (BKV) reactivation in donor kidney is a risk factor for development of BKV-associated nephropathy. J Infect Dis 2013; 207:137–41. [DOI] [PubMed] [Google Scholar]
- 16. Elfadawy N, Yamada M, Sarabu N. Management of BK polyomavirus infection in kidney and kidney-pancreas transplant recipients: a review article. Infect Dis Clin North Am 2018; 32:599–613. [DOI] [PubMed] [Google Scholar]
- 17. Pham PT, Schaenman J, Pham PC. BK virus infection following kidney transplantation: an overview of risk factors, screening strategies, and therapeutic interventions. Curr Opin Organ Transplant 2014; 19:401–12. [DOI] [PubMed] [Google Scholar]
- 18. Bijol V, Cimic A, Viscidi RP, Hymes LC. Pretransplant IgG antibodies to polyoma BK virus in pediatric renal transplants. Pediatr Transplant 2010; 14:224–7. [DOI] [PubMed] [Google Scholar]
- 19. Wunderink HF, van der Meijden E, van der Blij-de Brouwer CS, et al. Pretransplantation donor-recipient pair seroreactivity against BK polyomavirus predicts viremia and nephropathy after kidney transplantation. Am J Transplant 2017; 17:161–72. [DOI] [PubMed] [Google Scholar]