Abstract
Infections caused by New Delhi metallo-β-lactamase (NDM)–producing strains of multidrug-resistant Klebsiella pneumoniae are a global public health threat lacking reliable therapies. NDM is impervious to all existing β-lactamase inhibitor (BLI) drugs, including the non–β-lactam BLI avibactam (AVI). Though lacking direct activity against NDMs, AVI can interact with penicillin-binding protein 2 in a manner that may influence cell wall dynamics. We found that exposure of NDM-1–producing K. pneumoniae to AVI led to striking bactericidal interactions with human cathelicidin antimicrobial peptide LL-37, a frontline component of host innate immunity. Moreover, AVI markedly sensitized NDM-1–producing K. pneumoniae to killing by freshly isolated human neutrophils, platelets, and serum when complement was active. Finally, AVI monotherapy reduced lung counts of NDM-1–producing K. pneumoniae in a murine pulmonary challenge model. AVI sensitizes NDM-1–producing K. pneumoniae to innate immune clearance in ways that are not appreciated by standard antibiotic testing and that merit further study.
Keywords: New Delhi metallo-β-lactamase (NDM), Klebsiella pneumoniae, non–β-lactam β-lactamase inhibitors, avibactam, innate immunity, platelet, neutrophil, human serum
Klebsiella pneumoniae has recently gained notoriety as a major culprit of antibiotic-resistant nosocomial infections across the globe [1]. The difficulty in treating this pathogen is increasing because of heterogeneous antibiotic resistance mechanisms, particularly the production of β-lactamases, which hydrolyze and inactivate first-line β-lactam antibiotics. An individual K. pneumoniae strain can harbor multiple β-lactamases encoded on plasmids or mobile genetic elements [2]. Among these, New Delhi metallo-β-lactamases (NDMs) are particularly worrisome because of their strikingly efficient hydrolysis of a broad range of β-lactam antibiotics [3] and rapid dissemination through horizontal gene transfer and clonal expansion [4]. Whereas β-lactamase inhibitors (BLIs) are the cornerstone of modern pharmaceutical therapy in terms of restoring the usefulness of β-lactam agents against such enzymatic resistance mechanisms [5], to date no BLIs with reported neutralizing activity against metallo-β-lactamases (including NDMs) have entered clinical trials [6]. Thus, infections with multidrug-resistant NDM–producing K. pneumoniae strains lack effective therapeutic options.
Classical BLIs possessing a 4-membered, nitrogen-containing β-lactam ring, such as sulbactam (SLB), clavulanic acid (CLV), or tazobactam (TAZ), were first introduced into the clinic in the 1980s and 1990s. These agents have always been paired with an extended-spectrum β-lactam in antibiotic formulations, such as ampicillin-SLB, amoxicillin-CLV, or piperacillin-TAZ. The structure of BLIs confers some measure of intrinsic antimicrobial action, a fact often overlooked because of their use as enzymatic inhibitors. For example, SLB has useful in vitro and clinical activity against strains of Acinetobacter baumannii [7, 8] and Burkholderia cepacia [9], while TAZ has direct activity against the Lyme disease spirochete Borrelia burgdorferi [10]. Best studied for SLB, these antimicrobial activities are mediated by direct binding to penicillin-binding proteins (PBPs), essential transpeptidases involved in cell wall biosynthesis, leading to morphologic effects such as filamentation, spherocyte formation, or bacterial lysis [11, 12]. Recently, we observed that even if standard susceptibility testing reveals negligible direct growth inhibition of a target pathogen by BLIs alone, significant synergy can be seen in combination with cell wall–active peptide antibiotics, such as SLB or TAZ in combination with daptomycin, to kill methicillin-resistant Staphylococcus aureus (MRSA); or TAZ in combination with colistin, to kill A. baumannii [13]. Together, the above studies suggest significant value in exploring bioactivities of BLIs beyond their enzymatic inhibition of bacterial β-lactamases.
In recent years, “non–β-lactam BLIs,” a variety of novel chemical entities with expanded spectra of enzyme inhibition, have begun to join the therapeutic arsenal for resistant pathogens. These include the diazabicyclooctanes such as avibactam (AVI), approved in 2015 for use in combination with ceftazidime (Avycaz; Allergan, Dublin, Ireland), and zidebactam (ZID), which is undergoing clinical trials for use in combination with cefepime (WCK 5222; Wockhardt, Mubai, India). The absence of the 4-membered β-lactam ring structure makes such agents less intuitive candidates for direct activities on the bacterial cell wall. However, it is important to recognize that sequence-based analyses of evolutionary relationships among important bacterial enzymes indicate that β-lactamases are actually descendants of integral cell wall biosynthetic enzymes, namely PBPs [14]. Indeed, PBPs of diverse pathogenic species, including Escherichia coli, Pseudomonas aeruginosa, Haemophilus influenzae, S. aureus, and Streptococcus pneumoniae, may be bound by AVI [15]. With regard to K. pneumoniae, AVI selectively binds PBP2 [16], a transpeptidase required for cell elongation and maintenance of the rod shape of gram-negative bacteria [17–19]. Further, reminiscent of TAZ sensitization of A. baumannii to colistin [13], AVI potentiates the activity of the membrane-active peptide antibiotic polymyxin B against K. pneumoniae and other gram-negative species [20]. In short, non–β-lactam BLIs also possess intriguing bioactivities independent of enzymatic blockage of bacterial β-lactamases.
For a successful treatment outcome in any deep-seated infection, administered antibiotics must work in concert with clearance mechanisms of the host innate immune system, including endogenous peptide antibiotics, serum complement, and phagocytic cells, such as neutrophils and macrophages. We have previously observed that β-lactam antibiotics lacking direct activity against multidrug-resistant pathogens in standard minimum inhibitory concentration (MIC) testing can sometimes potentiate the activity of human cathelicidin LL-37, a critical cationic host defense peptide made by myeloid cells and epithelium [21], against those same organisms. By example, sensitization to LL-37 killing is achieved in MRSA, using nafcillin [22], ceftaroline [23], or TAZ [13]; in vancomycin-resistant Enterococcus faecium, using ampicillin [24]; in S. pneumoniae, using ceftaroline [25]; and in Salmonella enterica, using ceftriaxone [26].
Given the selective binding of AVI to PBP2 in K. pneumoniae [16] and a precedent for certain PBPs in modulating bacterial LL-37 susceptibility [27], we explored the influence of this Food and Drug Administration (FDA)–approved non–β-lactam BLI on K. pneumoniae interactions with the human defense peptide and other soluble and cellular effectors of innate immunity. As an exemplar of urgent public health concern, we performed our studies by using an NDM-1–expressing K. pneumoniae strain for which prevailing logic would predict BLI monotherapy to be fruitless.
METHODS
Antibiotics, Antimicrobial Peptides, Human Serum, and Reagents
AVI (MedChemExpress), ZID (Medkoo Biosciences), TAZ (Chem-Impex International), and ceftazidime-AVI (CZA; GlaxoSmithKilne) stock solutions were prepared in molecular-grade water (Corning Cellgro) and stored at −20°C. For in vivo studies, AVI was reconstituted in phosphate-buffered saline (PBS). LL-37 and TAMRA-tagged LL-37 stock solutions (American Peptide) were prepared in molecular-grade water and stored at −80°C. Under a protocol approved by the University of California-San Diego (UCSD) Human Subjects Institutional Review Board (IRB), serum was obtained from at least four healthy, consenting volunteers and immediately pooled and aliquoted prior to storing at −80°C. Pooled serum specimens were heated at 55°C for 20 minutes in a water bath on the day of each experiment, to create heat-inactivated serum. Müller-Hinton broth (MHB; Spectrum Chemicals) was supplemented with 20–25 mg/L Ca2+ and 10–12.5 mg/L Mg2+. Tissue culture medium, Roswell Park Memorial Institute 1640 (RPMI; Thermo Fisher Scientific), was supplemented with 5% Luria broth (LB; Hardy Diagnostics). A physiological relevant concentration of LL-37 (4–8 μΜ) was used for experiments; the concentration of LL-37 in neutrophil granules is 40 μΜ [28], and the peptide is strongly upregulated in inflamed or infected tissue [29, 30]. In antimicrobial susceptibility testing protocols from the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing, the concentration of AVI is fixed at 4 mg/L when determining the MIC of CZA. Therefore, all in vitro studies were performed with an AVI concentration of 4 mg/L, which is well below the pharmacologically attainable AVI concentration of 14 mg/L in human tissue [31].
Bacterial Strains and In Vitro Susceptibility Tests
Principal experiments were conducted with the well-characterized and commercially available NDM-1–producing K. pneumoniae clinical isolate CDC 1100192 (Microbiologics) [32], originally isolated from a sputum specimen collected from a 13-month-old boy with chronic reactive airway disease and pneumonia at the University of California–Los Angeles Medical Center [33]. Complementary studies were conducted in two NDM-1–producing K. pneumoniae strains obtained from the Centers for Disease Control and Prevention and FDA Antibiotic Resistance Isolate Bank, CDC 0049 (sequence accession # SAMN04014890) and CDC 0158 (sequence accession # SAMN04014999); in the K. pneumoniae carbapenemase (KPC)–producing clinical isolates KP1088 and KP1004, collected by JMI Laboratories (North Liberty, IA) as a part of the SENTRY Antimicrobial Surveillance Program [34]; and in KP1100, collected by a tertiary-care hospital in the New York metropolitan area and described in earlier publications from our laboratory [35, 36]. Bacteria were grown overnight in LB, and isolates were stored with 50% glycerol at −80°C. Fresh colonies were streaked onto LB plates each week for all experiments. Broth microdilution antimicrobial susceptibility testing was performed in 5% LB–RPMI and cation-adjusted MHB (CA-MHB) according to CLSI guidelines.
Time-Kill Curves and Serum Killing Assays
Assays were conducted in 14-mL Falcon plastic tubes (Thermo-Fisher Scientific) in a final volume of 4 mL, with or without 20% pooled human serum specimens or heat-inactivated serum specimens. Bacteria were grown overnight in LB at 37°C with shaking to the stationary-growth phase and diluted to an OD600 of 0.40. Cultures were diluted in 5% LB–RPMI to an initial inoculum of 5 × 105 colony-forming units (CFU)/mL, antibiotics and/or LL-37 stocks were added to the final concentrations indicated, and tubes were placed in a shaking incubator at 37°C. Aliquots were collected at the indicated times and serially diluted for CFU enumeration.
Neutrophil and Platelet Killing Assays
Venous blood specimens were obtained after receipt of informed consent from healthy human subjects, under a protocol approved by the UCSD IRB. Human neutrophils were freshly isolated from heparinized blood, using Polymorphprep (Axis-Shield) per the manufacturer’s instructions, and erythrocytes were lysed with sterile water as previously described [37]. Platelets were isolated from blood specimens that were anticoagulated by using acid-citrate-dextrose buffer (Sigma). After centrifugation of whole-blood specimens (at 200 ×g for 15 minutes, without a break), the upper two thirds of the platelet-rich plasma was transferred to a nonsiliconized Eppendorf tube. The platelet-rich plasma was centrifuged (at 450 ×g for 10 minutes), and the platelet-poor serum was removed. The remaining pellet was resuspended in RPMI prior to seeding a 96-well plate with 1 × 107 cells/well. Cells were infected at a multiplicity of infection of 10 (neutrophils) or 0.01 (platelets) with NDM-1–producing K. pneumoniae untreated or grown overnight in a sub-bacteriostatic concentration of AVI (4 mg/L). Bacteria were otherwise prepared identically to those used in the killing assays described above. Only the neutrophil-containing plates were centrifuged (at 300 ×g for 5 minutes), to improve contact with bacteria. All plates were incubated at 37°C in 5% CO2. Aliquots were collected at the indicated times and serially diluted for CFU enumeration.
Fluorescence Microscopy
Single colonies of NDM-1–producing K. pneumoniae were picked from LB plates and grown in LB overnight. Overnight cultures were diluted 1:100 in 5% LB–RPMI and grown to an OD600 of 0.20 prior to seeding in a 96-well plate with 100 μL of culture medium per well. The antibiotic was added to exponentially growing bacteria (OD600 of 0.20), placed in shaker at 37°C, and collected after 2 hours. TAMRA-tagged LL-37 was added, and cultures were incubated for an additional 30 minutes. Cultures were subsequently stained with 2 mg/L of Hoechst (nucleic acid) and 0.5 μM of SYTOX Green (Life Technologies), and 6 μL was transferred onto a 1.2% agarose pad containing 10% LB–20% RPMI, for microscopy. Imaging analyses were performed using FIJI (ImageJ-1.51 w) and CellProfiler 3.0. The microscopist was not blinded.
Murine Lung Infection
Animal experiments were approved by the UCSD Institutional Animal Care and Use Committee.
Cultures of NDM-1–producing K. pneumoniae were grown overnight in LB at 37°C with shaking. Overnight cultures were diluted 1:100 in fresh LB and grown to an OD600 of 0.40. Bacteria were washed thrice with PBS via centrifugation at 3220 ×g at room temperature and resuspended in PBS to yield 2.5 × 1010 CFU/mL. Female (8-week-old) C57Bl/6J mice (Jackson Laboratories) were divided randomly into treatment and control groups. Mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. Once sedated, mice were intratracheally infected with 40 μL (1 × 109 or 5 × 108 CFU/mL) bacteria, using a plastic gel loading pipette after the vocal chords were visualized using an operating otoscope (Welch Allyn). Mice recovered on a warmed pad for 1 hour prior to receiving a 100-µL intraperitoneal dose of approximately 80 mg/kg AVI. AVI was dosed every 8 hours, for a total of 3 doses. Mice were euthanized with CO2 30 hours after infection and then underwent cervical dislocation. To enumerate total surviving bacteria in the lungs, all 5 lung lobes were removed and placed in a 2-mL sterile microtube (Sarstedt) containing 1 mL of PBS and 1-mm-diameter silica beads (Biospec). Lungs were weighed and then homogenized by shaking twice at 6000 rpm for 60 seconds, using a MagNA Lyser (Roche). Specimens were placed on ice as soon as they were harvested. Aliquots from each tube were serially diluted in PBS for CFU enumeration on LA plates.
Murine AVI Dosing
AVI is only approved by the FDA for use in combination with ceftazidime (Avycaz). Standard human dosing of Avycaz [31] is 2.5 g (2 g of ceftazidime and 0.5 g of AVI) every 8 hours by intravenous infusion over 2 hours. Therefore, daily dosing of AVI is 1.5 g/day, or 25 mg/kg/day for an average adult. The elimination half-life of AVI in mice is 10 times faster than that in humans [31, 38]. Accounting for this and the high bacterial inoculum used in our pneumonia model, mice received approximately 80 mg/kg intraperiteoneally every 8 hours, or approximately 5 mg/day for a mouse weighing 20 g.
Statistical Analyses
All data were collected from at least 3 biological replicates performed in at least technical triplicate. Statistical analyses were performed using GraphPad Prism, version 7.0d. P values < .05 were considered statistically significant.
RESULTS
Bactericidal Synergy of AVI With Human LL-37 Against NDM-1–producing K. pneumoniae
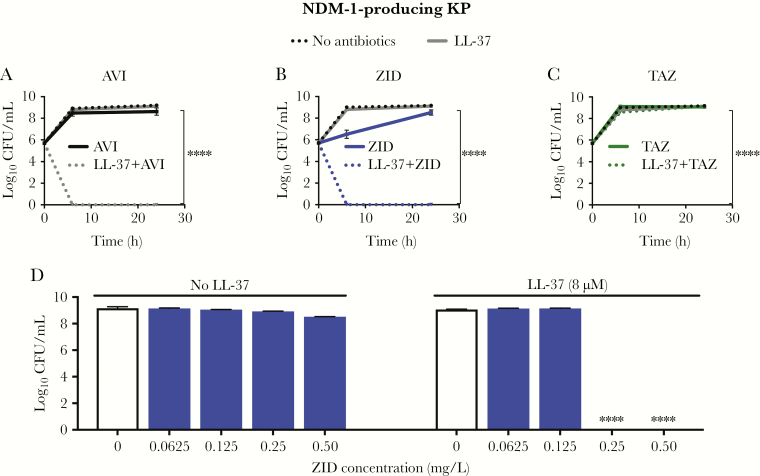
Human AMPs play an important role in innate immunity by restricting microbial proliferation. Pore formation or membrane destabilization can occur when a critical concentration of positively charged AMPs accumulate on the negatively charged surface of microbes, leading to microbial death through hypoosmotic lysis. Given structural and charge similarities between AMPs and synthetic polymyxin antibiotics that synergize with AVI [20], we hypothesized that AVI could enhance the activity of human cathelicidin LL-37. The NDM-1–producing K. pneumoniae clinical isolate is resistant to BLIs, BLI/β-lactam combinations, and LL-37 as single agents in the standard cation-adjusted MHB bacteriologic testing media or tissue culture-based media (eg, 5% LB–RPMI) often used for AMP testing (Table 1). However, marked synergistic killing of NDM-1–producing K. pneumoniae by 8 μM LL-37 was observed with AVI starting at 2 mg/L (an 8-log10 reduction), with no recovered bacteria at 24 hours (Figure 1A). Similar results were seen across KPC-producing and NDM-1–producing K. pneumoniae strains (Figure 1B–D), despite resistance to BLIs and LL-37 as single agents at the concentrations tested (Supplementary Table 1). Likewise, the FDA-approved drug CZA demonstrated profound killing of NDM-1–producing K. pneumoniae only in the presence of LL-37 (Supplementary Figure 1). ZID, a non–β-lactam BLI control without activity against metallo-β-lactamases and high-affinity binding for PBP2 in gram-negative bacteria [39, 40], also showed marked synergistic killing (an 8-log10 reduction) at doses as low as 0.25 mg/L at 24 hours (Figure 2B and 2D). No bactericidal activity was seen in NDM-1–producing K. pneumoniae treated solely with AVI, ZID, or LL-37 with or without TAZ, a BLI control with a β-lactam structure that binds PBP2 and is hydrolyzed by NDM [12] (Figure 2A–C).
Table 1.
Minimum Inhibitory Concentration (MICs) of Various Antibiotics Against New Delhi Metallo-β-Lactamase (NDM)-1–Producing Klebsiella pneumoniae, by Media
| Antibiotic | MIC, mg/L | |
|---|---|---|
| CA-MHBa | 5% LB–RPMIb | |
| Avibactam | 128 | 64 |
| Ceftazidime | >64 | >64 |
| Ceftazidime-avibactam (Avycaz) | 64 | 64 |
| Meropenem | 128 | 512 |
| Meropenem-vaborbactam (Vabomere) | 32 | 256 |
| Tazobactam | >64 | >64 |
| Ceftolozane-tazobactam | >64 | >64 |
| Aztreonam | 512 | 32 |
| LL-37 | 64 | 64 |
K. pneumoniae clinical isolate CDC 1100192, obtained from a sputum specimen, was used in this study.
aCation-adjusted Müller-Hinton broth (CA-MHB).
b5% Luria broth in Roswell Park Memorial Institute (RPMI) 1640 tissue culture medium.
Figure 1.
Avibactam (AVI) sensitizes New Delhi metallo-β-lactamase (NDM)-1–producing Klebsiella pneumoniae (KP) to killing by LL-37. A, AVI (4 mg/L) synergizes with LL-37 (8 μM) to kill NDM-1–producing KP in a dose-dependent fashion without bacterial regrowth 24 hours after treatment. B–D, Similar findings are seen across additional carbapenemase-producing clinical strains in the presence of 4 mg/L AVI and either 8 μM LL-37 (B and C) or 4 μM LL-37 (D). CFU, colony-forming units; KPC, KP carbapenemase. ****P < .0001, by Kruskal-Wallis 1-way analysis of variance.
Figure 2.
Zidebactam (ZID) sensitizes New Delhi metallo-β-lactamase (NDM)-1–producing Klebsiella pneumoniae (KP) to killing by LL-37. A, As seen with avibactam (AVI; 4 mg/L), ZID (4 mg/L), a diazabicyclooctane β-lactamase inhibitor (BLI) without a β-lactam ring, synergizes with LL-37 (8 μM) to kill NDM-1–producing KP in a dose-dependent fashion (B and D), whereas TAZ (20 mg/L), a BLI with a 4-membered β-lactam ring that is hydrolyzed by β-lactamases, does not demonstrate synergy (C). CFU, colony-forming units. ****P < .0001, by Kruskal-Wallis 1-way analysis of variance.
AVI Induces Morphologic Changes and Boosts LL-37–Mediated Membrane Permeability in NDM-1–producing K. pneumoniae
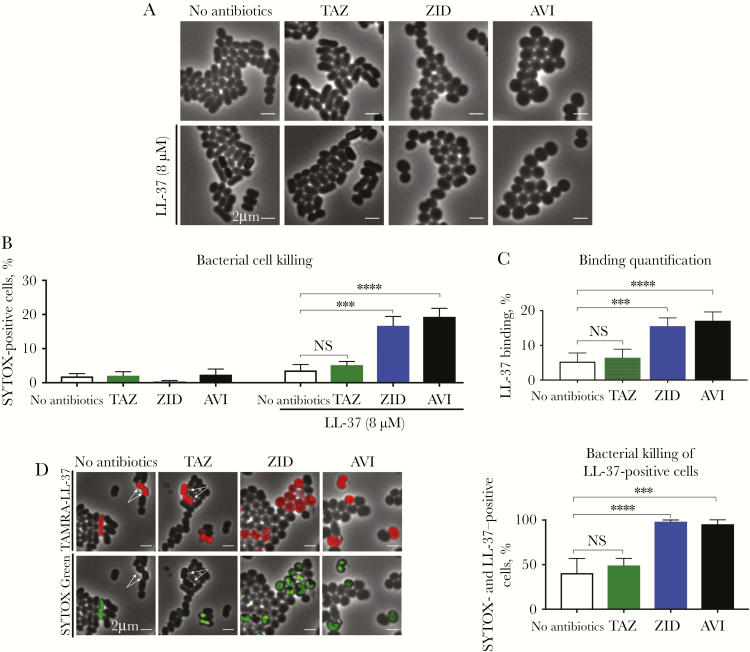
To gain insight into the mechanism of AVI and LL-37 synergy, we used fluorescence microscopy to examine cell morphology, LL-37 binding, and cell viability after 3 hours of monotherapy or combination therapy with LL-37 with or without AVI, ZID, or TAZ. In the presence of AVI or ZID, the normally rod-shaped NDM-1–producing K. pneumoniae became spherical (100%; n = 262 and n = 315, respectively; Figure 3A), an effect previously reported in E. coli secondary to PBP2 inhibition [17–19]. While TAZ (20 mg/L) binds PBP2 at high concentrations [12, 13], no morphologic change was observed in the presence of this BLI (Figure 2A), which is expected to be hydrolyzed by NDM because of its underlying β-lactam structure. Despite a strong effect on cell shape, only 2.4% of cells (n = 262) treated with AVI alone and 0.5% (n = 315) treated with ZID alone became nonviable, similar to the percentage of nonviable cells (3.6%; n = 352) for LL-37 treatment alone (Figure 3B). In contrast, 19.3% of cells (n = 167) treated with AVI plus LL-37 were nonviable (Figure 3B). Similarly, 15% (n = 266) treated with ZID plus LL-37 were nonviable (Figure 3B). This synergistic increase in killing correlated with a proportional increase in LL-37 binding (Figure 3C); that is, AVI (4 mg/L) or ZID (4 mg/L) pretreatment of NDM-1–producing K. pneumoniae enhanced binding of TAMRA-tagged LL-37 to the bacterial outer membrane by approximately 4-fold (Figure 3C), a phenotype not observed with TAZ (20 mg/L) pretreatment. Among cells that stained positive for LL-37, SYTOX Green permeability increased 2-fold with AVI or ZID cotreatment as compared to LL-37 alone or TAZ plus LL-37 (Figure 3D). Overall, these studies suggest that BLIs that confer a rod-to-sphere morphologic change render NDM-1–producing K. pneumoniae more susceptible to LL-37 binding, which subsequently increases the frequency of cell death.
Figure 3.
Treatment of New Delhi metallo-β-lactamase (NDM)-1–producing Klebsiella pneumoniae (KP) with sub–minimum inhibitory concentrations (MICs) of avibactam (AVI) or zidebactam (ZID) results in a rod-to-sphere morphologic change that increases the frequency of cell death only in the presence of LL-37. A–C, AVI (4 mg/L) or ZID (4 mg/L) induce a rod-to-coccus shape change (A) that, while nonlethal to the organism (B), results in increased binding of fluorescence-labeled LL-37 (8 μM) to the NDM-1–producing KP cell wall (C). D, Upon AVI (4 mg/L) or ZID (4 mg/L) treatment, nearly 100% of cells bound by fluorescence-labeled LL-37 (8 μM) were also SYTOX Green positive, a proxy for compromised membranes characteristic of dead cells, yet only approximately 50% of cells in the control conditions had concomitant staining. Arrows denote LL-37–positive cells that were SYTOX Green negative. NS, not significant.***P < .001 and ****P < .0001, by Kruskal-Wallis 1-way analysis of variance.
AVI Sensitized NDM-1–producing K. pneumoniae to Killing by Human Serum
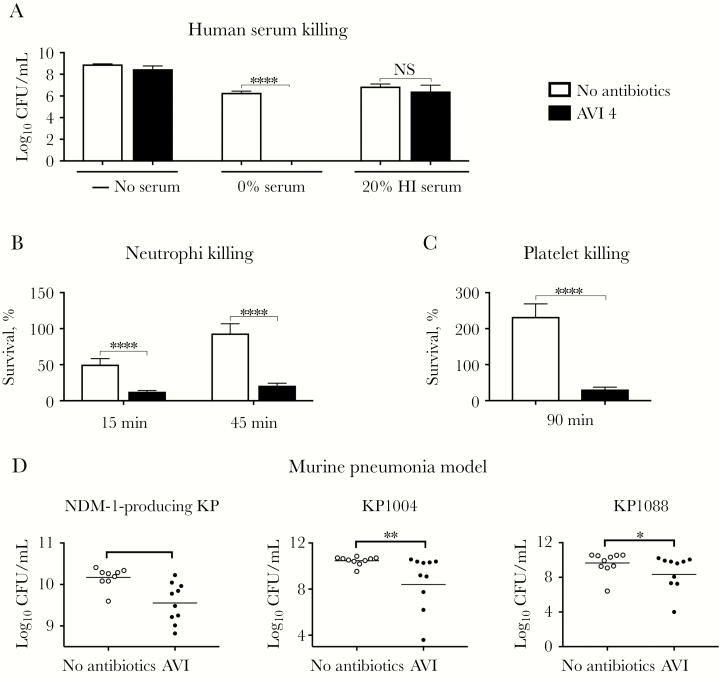
The complement system, a network of proteins found in high concentrations in human serum, is a key first-line element of host defense. Upon recognition of microbial patterns, the complement cascade is activated on bacterial surfaces, and the C5-C9 membrane attack complex can assemble to form pores in the gram-negative outer membrane, leading to cell lysis and death. Prior studies have demonstrated enhanced complement-mediated immunity in the presence of cell wall–active antibiotics that bind PBPs [41]. Since AVI binds PBP2 [16], we hypothesized that AVI could increase complement-mediated killing. Indeed, AVI treatment in the presence of 20% human serum resulted in a 6-log10 reduction in recoverable NDM-1–producing K. pneumoniae CFUs within 6 hours as compared to controls treated with 20% human serum alone. This effect was blocked by heat treatment to inactivate complement (Figure 4A).
Figure 4.
Avibactam (AVI) synergizes with frontline components of innate immunity to kill New Delhi metallo-β-lactamase (NDM)-1–producing Klebsiella pneumoniae (KP) in vitro and in vivo. A–C, AVI (4 mg/L) increases sensitivity of NDM-1–producing KP to complement-dependent killing in human serum (A), to neutrophil killing (B), and to platelet killing (C). D, AVI (240 mg/kg/day, administered via 3 doses) modestly but significantly reduces NDM-1– and KP carbapenemase–producing KP counts at 30 hours in a murine lung infection model (n = 10). CFU, colony-forming units; HI, heat inactivated; NS, not significant. *P < .05, **P < .01, and ****P < .0001, by the 2-tailed Student t test.
AVI Sensitizes NDM-1–producing K. pneumoniae to Killing by Neutrophils and Platelets
AVI synergy with LL-37 against NDM-1–producing K. pneumoniae suggests that AVI could also sensitize this drug-resistant pathogen to killing by neutrophils and platelets, which release cationic AMPs in response to bacterial stimuli [42, 43]. Indeed, pretreatment of NDM-1–producing K. pneumoniae with a sub-bacteriostatic concentration of AVI (4 mg/L) markedly sensitized NDM-1–producing K. pneumoniae to killing by freshly isolated human neutrophils (Figure 4B) and platelets (Figure 4C).
Activity of AVI Monotherapy in NDM-1–producing K. pneumoniae Lung Infection
AVI sensitization of NDM-1–producing K. pneumoniae to killing by cathelicidin, serum, neutrophils and platelets, suggested potential utility in vivo despite absent activity as a sole agent in the standard MIC testing paradigm. Although our mouse model was relatively resistant to lung infection by our NDM-1–producing K. pneumoniae isolate, we found in pilot studies that a high bacterial inoculum (1 × 109 CFU/mL) resulted in >50% mortality within 72 hours (Supplementary Figure 2). Using this challenge dose, we used a humanized dosing regimen corrected for the increased murine elimination half-life of AVI (ie, 80 mg/kg administered 1 hour after infection and then every 8 hours thereafter for a total of 3 doses). AVI monotherapy led to a modest (ie, 0.8-log) but statistically significant reduction in the number of NDM-1–producing K. pneumoniae CFU recovered from lungs 30 hours after challenge (Figure 4D). Similar findings were seen across KPC-producing K. pneumoniae strains at lower bacterial inoculums (5 × 108 CFU/mL; Figure 4D).
DISCUSSION
The increasing prevalence of drug-resistant pathogens threatens our ability to manage life-threatening bacterial infections and poses significant challenges to balancing the efficacy and toxicity of potential antimicrobial therapies. K. pneumoniae is particularly problematic because of its tendency to harbor metallo-β-lactamases, including NDMs, for which we have a paucity of reliable treatments. Innovative treatment strategies are central to addressing this critical and growing problem. To that end, the goal of this study was to reexamine the action of AVI, which has no appreciable direct antibacterial activity in standard testing paradigm on its own, in the context of the innate immune system. We found striking bactericidal activity against highly drug-resistant NDM-1– and KPC-producing clinical strains treated with AVI in the presence of LL-37, a human AMP abundantly expressed in neutrophils and at epithelial surfaces during infection. The concentrations of AVI used to achieve this sensitizing effect were well below pharmacologically attainable serum concentrations in patients receiving standard CZA dosing [31]. We also found marked synergy of CZA with LL-37, suggesting that this FDA-approved drug may possess a clinically applicable off-label use in treatment of NDM-producing K. pneumoniae infections through interactions with the innate immune system. This remarkable finding provides a potential mechanistic underpinning for prior studies that reported susceptibility of NDM-producing K. pneumoniae to CZA in vivo but not in vitro in the absence of immunomodulators [44].
While the precise molecular mechanism of this synergistic interaction remains to be discovered, subinhibitory concentrations of AVI appear to influence bacterial cell wall mechanics. AVI is a non–β-lactam BLI with no activity against NDMs. However, AVI binds selectively to PBP2 [16], an enzyme critical for maintaining the cellular morphology in gram-negative bacteria [17–19]. Microscopy demonstrated a K. pneumoniae morphologic transition from rod to sphere in the presence of AVI, reminiscent of morphologic changes in E. coli in the presence of mecillinam, a β-lactam that specifically inactivates PBP2 [17–19]. Similar findings were seen in the presence of ZID, a non–β-lactam BLI with high-affinity binding to PBP2. High but pharmacologically attainable doses of TAZ, a β-lactam BLI known to bind PBP2 [45], did not result in K. pneumoniae rod-to-sphere transition, presumably because of its inability to withstand hydrolysis by K. pneumoniae BLIs. Unlike TAZ-treated cells, AVI- or ZID-treated cells demonstrated increased LL-37 binding, leading to widespread bacterial cell death, detected via a SYTOX Green viability assay. These findings parallel our time-kill assay results, in which combination therapy with LL-37 and AVI or ZID (but not TAZ) reduced bacterial growth to below the assay’s detection limit within 6 hours. Overall, our findings underscore a heretofore undescribed synergistic relationship between the innate immune system and diazabicyclooctane BLIs with high-affinity binding to PBP2, such as AVI and ZID.
The presence of cell wall–active antibiotics that bind PBPs may also enhance complement microbicidal activity [41] by facilitating permeabilization of bacterial membranes. AVI sensitized NDM-1–producing K. pneumoniae to 20% human serum killing, an effect blocked when heat treatment was used to inactivate complement. Additionally, AVI enhanced killing of NDM-1–producing K. pneumoniae by neutrophils and platelets, cells that can exert their antimicrobial effects in part through the release of AMPs. In the absence of AVI, NDM-1–producing K. pneumoniae strains were unaffected by platelets, resulting in a doubling of the bacterial burden at 90 minutes. Moreover, AVI monotherapy resulted in a modest but statistically significant reduction in the NDM-1–producing K. pneumoniae bacterial burden in a murine pneumonia model. AVI thus provides a benefit beyond inactivation of β-lactamases by enhancing killing by the innate immune system. This effect, counterintuitive to current antibiotic assessment, is demonstrated here for an NDM-1–expressing K. pneumoniae strain whose β-lactamase enzymatic activity yields resistance to AVI and all other current BLIs.
Our in vivo study was limited because mice are not considered natural hosts of K. pneumoniae and because a high inoculum was required to create a disease phenotype. Furthermore, although AVI was administered every 8 hours, pharmacodynamic studies of AVI in neutropenic mice with lung infections indicate that dosing every 2 hours is significantly more efficacious than dosing every 8 hours [46], owing to the time-dependent nature of this drug. Nonetheless, we provide the first report of synergy between the host innate immune system and an FDA-approved antibiotic component with proven safety and tolerability. These findings provide an experimental rationale to further explore AVI as adjunctive therapy for highly drug-resistant NDM-1–producing bacterial strains, with the potential for translation to human clinical investigation close at hand.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH; Pediatric Scientist Development Program Fellowship K12-HD000850 to E. R. U. and grant U54-HD090259 to V. N. and G. S.), and the National Institute of Allergy and Infectious Diseases, NIH (grant U01-AI124316 to V. N., G. S., and J. P.).
Potential conflicts of interest. V. N. has received research grant support from Roche Pharma and is on the Scientific Advisory Board of Cidara Therapeutics. G. S. has received speaking honoraria from Allergan, Sunovion, and The Medicines Company; has received consulting fees from Allergan and Paratek Pharmaceuticals; and is on the scientific advisory boards of Cidara Therapeutics and Arsanis Pharmaceuticals. J. P. has an equity interest in Linnaeus Bioscience. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: ID Week, San Francisco, California, 3–7 October 2018
References
- 1. Chong Y, Shimoda S, Shimono N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Genet Evol 2018; 61:185–8. [DOI] [PubMed] [Google Scholar]
- 2. Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 2015; 59:5873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim Y, Cunningham MA, Mire J, Tesar C, Sacchettini J, Joachimiak A. NDM-1, the ultimate promiscuous enzyme: substrate recognition and catalytic mechanism. FASEB J 2013; 27:1917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017; 8:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Docquier JD, Mangani S. An update on β-lactamase inhibitor discovery and development. Drug Resist Updat 2018; 36:13–29. [DOI] [PubMed] [Google Scholar]
- 6. Somboro AM, Osei Sekyere J, Amoako DG, Essack SY, Bester LA. Diversity and proliferation of metallo-beta-lactamases: a clarion call for clinically effective metallo-beta-lactamase inhibitors. Appl Environ Microbiol 2018; 84:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin AS. Multiresistant Acinetobacter infections: a role for sulbactam combinations in overcoming an emerging worldwide problem. Clin Microbiol Infect 2002; 8:144–53. [DOI] [PubMed] [Google Scholar]
- 8. Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis 2010; 51:79–84. [DOI] [PubMed] [Google Scholar]
- 9. Jacoby GA, Sutton L. Pseudomonas cepacia susceptibility to sulbactam. Antimicrob Agents Chemother 1989; 33:583–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Urban C, Rahal JJ, Luft B. Effect of a beta-lactamase inhibitor, tazobactam, on growth and penicillin-binding proteins of Borrelia burgdorferi. FEMS Microbiol Lett 1991; 66:113–6. [DOI] [PubMed] [Google Scholar]
- 11. Kazmierczak A, Pechinot A, Siebor E, Cordin X, Labia R. Sulbactam: secondary mechanisms of action. Diagn Microbiol Infect Dis 1989; 12:139–46S. [DOI] [PubMed] [Google Scholar]
- 12. Urban C, Go E, Mariano N, Rahal JJ. Interaction of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and -susceptible Acinetobacter baumannii. FEMS Microbiol Lett 1995; 125:193–7. [Google Scholar]
- 13. Sakoulas G, Rose W, Berti A, et al. Classical beta-lactamase inhibitors potentiate the activity of daptomycin against methicillin-resistant Staphylococcus aureus and colistin against Acinetobacter baumannii. Antimicrob Agents Chemother 2017; 61:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massova I, Mobashery S. Kinship and diversification of bacterial penicillin-binding proteins and beta-lactamases. Antimicrob Agents Chemother 1998; 42:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asli A, Brouillette E, Krause KM, Nichols WW, Malouin F. Distinctive binding of avibactam to penicillin-binding proteins of Gram-negative and Gram-positive bacteria. Antimicrob Agents Chemother 2016; 60:752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutaria DS, Moya B, Green KB, et al. First penicillin-binding protein occupancy patterns of beta-lactams and beta-lactamase inhibitors in Klebsiella pneumoniae. Antimicrob Agents Chemother 2018; 62:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vinella D, Joseleau-Petit D, Thévenet D, Bouloc P, D’Ari R. Penicillin-binding protein 2 inactivation in Escherichia coli results in cell division inhibition, which is relieved by FtsZ overexpression. J Bacteriol 1993; 175:6704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spratt BG, Pardee AB. Penicillin-binding proteins and cell shape in E. coli. Nature 1975; 254:516–7. [DOI] [PubMed] [Google Scholar]
- 19. Legaree BA, Daniels K, Weadge JT, Cockburn D, Clarke AJ. Function of penicillin-binding protein 2 in viability and morphology of Pseudomonas aeruginosa. J Antimicrob Chemother 2007; 59:411–24. [DOI] [PubMed] [Google Scholar]
- 20. Pagès JM, Peslier S, Keating TA, Lavigne JP, Nichols WW. Role of the outer membrane and porins in susceptibility of β-lactamase-producing Enterobacteriaceae to ceftazidime-avibactam. Antimicrob Agents Chemother 2015; 60:1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front Biosci 2008; 13:3760–7. [DOI] [PubMed] [Google Scholar]
- 22. Sakoulas G, Okumura CY, Thienphrapa W, et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl) 2014; 92:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014; 36:1317–33. [DOI] [PubMed] [Google Scholar]
- 24. Sakoulas G, Bayer AS, Pogliano J, et al. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2012; 56:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakoulas G, Nonejuie P, Kullar R, Pogliano J, Rybak MJ, Nizet V. Examining the use of ceftaroline in the treatment of Streptococcus pneumoniae meningitis with reference to human cathelicidin LL-37. Antimicrob Agents Chemother 2015; 59:2428–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakoulas G, Kumaraswamy M, Kousha A, Nizet V. Interaction of antibiotics with innate host defense factors against Salmonella enterica serotype newport. mSphere 2017; 2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamilton A, Popham DL, Carl DJ, Lauth X, Nizet V, Jones AL. Penicillin-binding protein 1a promotes resistance of group B streptococcus to antimicrobial peptides. Infect Immun 2006; 74:6179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sørensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods 1997; 206:53–9. [DOI] [PubMed] [Google Scholar]
- 29. Johansson L, Thulin P, Sendi P, et al. Cathelicidin LL-37 in severe Streptococcus pyogenes soft tissue infections in humans. Infect Immun 2008; 76:3399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fabisiak A, Murawska N, Fichna J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol Rep 2016; 68:802–8. [DOI] [PubMed] [Google Scholar]
- 31. Avycaz (ceftazidime-avibactam) for injection (2.5 g) [package insert]. Irvine, CA: Allergen USA, Inc., 2018:1349–59. [Google Scholar]
- 32. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis 2013; 19:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mochon AB, Garner OB, Hindler JA, et al. New Delhi metallo-β-lactamase (NDM-1)-producing Klebsiella pneumoniae: case report and laboratory detection strategies. J Clin Microbiol 2011; 49:1667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2017; 61:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin L, Nonejuie P, Munguia J, et al. Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant Gram-negative bacterial pathogens. EBioMedicine 2015; 2:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fair RJ, Hensler ME, Thienphrapa W, Dam QN, Nizet V, Tor Y. Selectively guanidinylated aminoglycosides as antibiotics. ChemMedChem 2012; 7:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kristian SA, Datta V, Weidenmaier C, et al. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol 2005; 187:6719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berkhout J, Melchers MJ, van Mil AC, et al. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 2015; 59:2299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 2017; 61:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moya B, Barcelo IM, Bhagwat S, et al. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “beta-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-beta-lactamase-producing high-risk clones. Antimicrob Agents Chemother 2017; 61:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dutcher BS, Reynard AM, Beck ME, Cunningham RK. Potentiation of antibiotic bactericidal activity by normal human serum. Antimicrob Agents Chemother 1978; 13:820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cole JN, Nizet V. Bacterial evasion of host antimicrobial peptide defenses. Microbiol Spectr 2016; 4:1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol 2014; 12:426–37. [DOI] [PubMed] [Google Scholar]
- 44. Monogue ML, Abbo LM, Rosa R, et al. In vitro discordance with in vivo activity: humanized exposures of ceftazidime-avibactam, aztreonam, and tigecycline alone and in combination against New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 2017; 61:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Urban C, Go E, Mariano N, Rahal JJ. Interaction of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and -susceptible Acinetobacter baumannii. FEMS Microbiology Letters 1995; 125:193–7. [Google Scholar]
- 46. Berkhout J, Melchers MJ, van Mil AC, et al. Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother 2016; 60:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.