Summary
Smells can arise from a source external to the body, and stimulate the olfactory epithelium upon inhalation through the nares (orthonasal olfaction). Alternatively, smells may arise from inside the mouth during consumption, stimulating the epithelium upon exhalation (retronasal olfaction). Both ortho- and retronasal olfaction produce highly salient percepts, but the two percepts have very different behavioral implications. Here, we use optogenetic manipulation in the context of a flavor preference learning paradigm to investigate differences in the neural circuits that process information in these two sub-modalities of olfaction. Our findings support a view in which retronasal, but not orthonasal odors share processing circuitry commonly associated with taste. First, our behavioral results reveal that retronasal odors induce rapid preference learning, and have a potentiating effect on orthonasal preference learning. Second, we demonstrate that inactivation of the insular gustatory cortex selectively impairs expression of retronasal preferences. Thus, orally-sourced (retronasal) olfactory input is processed by a brain region responsible for taste processing, whereas externally-sourced (orthonasal) olfactory input is not.
Introduction
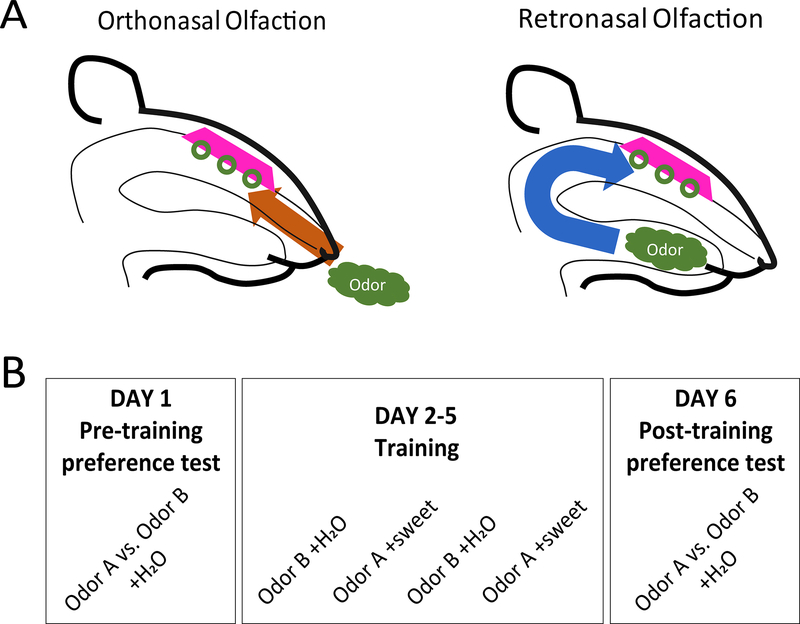
In mammals, smells can reach the main olfactory epithelium via either of two paths that can be thought of as representing distinct sub-modalities of olfaction. In orthonasal olfaction (hereafter “ortho”), odors in the external environment reach the epithelium through inhalation via the nostrils, whereas in retronasal olfaction (“retro”), odorous stimuli present in the mouth are sampled during exhalation via the back of the throat (Fig 1A). Ortho experience typifies what we naïvely think of as our sense of smell, but retro experience, despite being somewhat covert, exerts a powerful influence on perception and behavior: it is often colloquially conflated with taste in everyday language, and more technically considered to be a component of flavor [1].
Figure 1. Conceptual and experimental design.
(A) Odors enter the nasal cavity via the external nares (orthonasal) or through the internal nares via the back of the throat when food is consumed (retronasal). (B) Odor preference was assessed before and after training sessions consisting of alternating days with exposure to sweet-paired odor A and plain water-paired odor B. Exact duration of training varied between the different conditions (2, 4 or 6-day training), as indicated in the text and Figures.
The route to the nasal epithelium is not all that distinguishes retro from ortho stimuli. Another difference has to do with behavioral context, and specifically with the fact that retro is inherently linked to food and consumption. Ortho, meanwhile, is more general purpose—while it does signal the external presence of foods available for consumption, it is equally involved in signaling the presence of predators, potential mates, familiar locations, and environmental hazards. The two modes thus provide an animal with very different kinds of information [2], and it follows that animals may use the two differently [1, 3–7]. Specifically, one could hypothesize that the use of retro stimuli might be similar to, and even linked to, that of tastes. One important way that this similarity might be expressed is with regard to the learning of consumption preferences. Taste preferences or aversions can be learned in a single trial, a fact that likely reflects an urgency that comes of the stimulus being internal when sensed [8]. Retronasal odors, which are similarly internally-sourced, might therefore be predicted to be particularly effective for rapid preference learning [9, 10]. In parallel with similarities in how taste and retro information is used to drive behavior, it is reasonable (if speculative) to predict that the neural circuits involved in retro and taste preference learning might overlap.
These are the two central predictions tested in the current work. Experiment 1 tests how well (in terms of speed) freely behaving rodents learn odor preferences based on ortho, retro, and combined ortho and retro delivery. Next, we use optogenetic inhibition to test implications of the differences observed in Experiment 1 for the cortical processing of ortho and retro odors. Based on the above, and on published evidence that gustatory cortical (GC) neurons respond to both tastes [11–13] and odors [14–16], and is necessary for the expression of olfactory preferences [17, 18], we predict that GC is uniquely involved in the processing of retro preference memory.
Our results confirm our hypotheses: retro learning is faster than, and a potentiating force on, ortho learning. Furthermore, GC inactivation disrupts retro odor preference learning while leaving ortho unaffected. These findings reveal that GC plays a crucial cross-modal role in flavor processing [4, 16, 17, 19–21] that depends on the behavioral relevance of a non-gustatory sensory experience. At a broader level, our results suggest that chemosensory neural processing to some degree reflects stimulus sourcing rather than epithelium.
Results
All of the experiments described below make use of a simple preference learning task in which one of two odors is paired with a non-caloric sweet-taste reward and the other with plain water. The basic paradigm is illustrated in Figure 1B. Similar procedures, which causes an increase in preference for the sweet-paired odor [22], have been used previously to study the hedonic qualities of odors [23–25]. In the current setup, rats initiated stimulus delivery by entering nose poke ports. In retro conditions, odors were dissolved in water, and delivered via surgically-implanted cannulae (see Methods). In ortho conditions, odorized air was delivered in front of the nose via olfactometry, and accompanied by intra-oral delivery of plain water. During pre-training and post-training preference testing, animals chose freely between two odors (available through two side-by-side nose pokes). During training, only a single odor was available through a single nose poke. On training days where odor A was available, the intra-oral fluid stimulus contained a non-caloric sweet taste (0.2% saccharin); on training days where odor B was available, the intra-oral fluid stimulus did not contain taste. We quantified pre- and post-training odor preferences as the ratio of nose pokes to odors A and B. Preference learning was assessed in terms of the change in preference for odor A from before to after training. We present two sets of experiments, the first of which tests whether the speed and strength of olfactory preference learning depends on the route of odor delivery (i.e., retro versus ortho); the second set of experiments tests whether the previously-described involvement of gustatory cortex (GC) in olfactory preference learning [17, 18] depends upon the route of odor delivery.
Experiment 1. The speed of retro preference learning is distinct from that of ortho preference learning
Retro training supports rapid preference learning
To assess the effects of olfactory modality on preference learning, we first compared pre- and post-training preferences in the retro condition (for details on the experimental conditions, see Table 1). Based on the fact that retro odors, like tastes, are orally-sourced, we predicted that odor preference learning would occur rapidly with retro experience, similarly to taste learning [8–10, 26, 27]. Previous work has shown that rodents readily perceive and discriminate between odors delivered intra-orally [23, 28].
Table 1.
Odor modalities used during Training and Testing in the different conditions.
| Condition | Odor modality during Training | Odor modality during Preference Testing |
|---|---|---|
| Retro | Retro | Retro |
| Ortho | Ortho | Ortho |
| Retro/Ortho | Retro | Ortho |
| Cmbd/Ortho | Retro+Ortho | Ortho |
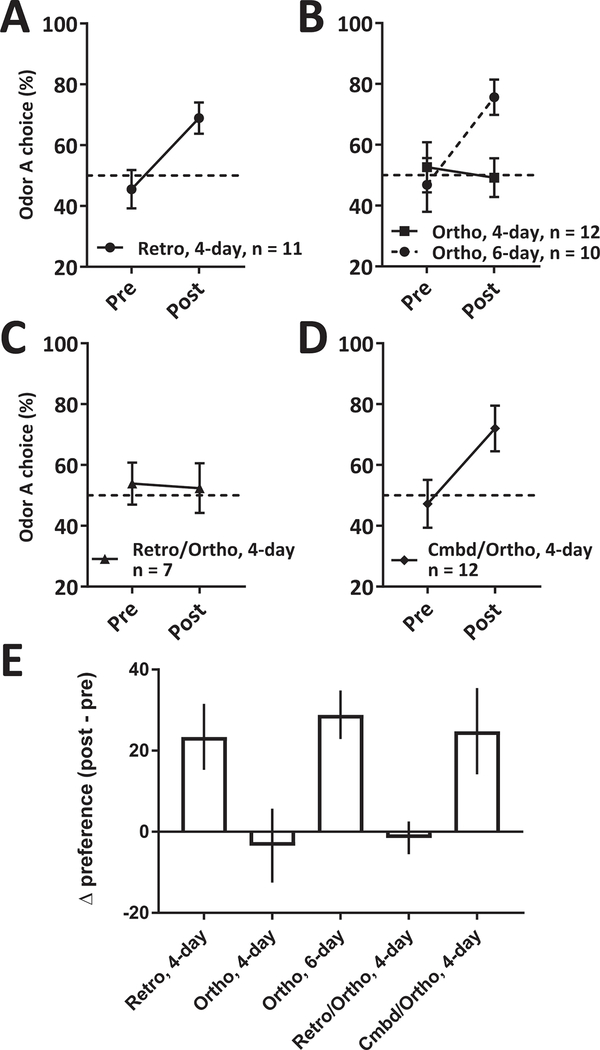
We first tested the effectiveness of retro conditioning using a 4-day conditioning paradigm, as described in Figure 1B. Figure 2A shows preferences before and after training, revealing that rats effectively learn preferences, as indicated by higher preference for the sweet-paired odor after training than before (paired t(10)=2.88, p<0.01). We also confirmed that even a single presentation of each retro odor pairing during training (i.e., 2-day training) caused an increase in preference for the sweet-paired odor (paired t(9)=2.33, p<0.05, data not shown). That is, preferences for retro odors are learned with as little as a single exposure.
Figure 2. Learning and expression of ortho and retronasal odor preferences.
(A-D) Mean (±SEM) preference for odor A (the odor paired with sweet taste during training) before and after training in the Retro (A), Ortho (B), Retro/Ortho (C) and Cmbd/Ortho (D) conditions. (E) Mean (±SEM) change in preference (post–pre) for all conditions.
Note that even though retro odor stimuli were presented intra-orally in solution, the odor molecules used here are not known to bind to gustatory receptors in the lingual epithelium. While in humans retro stimulation is typically misperceived as “taste” (a referred sensation that is technically considered a part of the “flavor” percept [1]), retro stimuli can be distinguished solely on the basis of their olfactory qualities (i.e., by their ability to differentially bind olfactory receptors in the nasal epithelium). Furthermore, even if retro stimuli did have distinct attendant gustatory and/or mouth feel properties, previous work makes it clear that preferences for those properties are unlikely to be rapidly learned through association with saccharin (see STAR Methods and Discussion for more details on this argument). Thus, the observed learning was olfactory in nature—the learning of a retro preference.
Preferences are learned more slowly using ortho delivery
Next, we asked if the same rapid learning is observed using ortho odors. We applied the 4-day training procedure described above, changing only the route of odor delivery, but found that sweet-paired odor preference does not increase following two exposures to each odor (Figure 2B, “Ortho, 4-day”).
This result goes against naïve expectation. It seems intuitive that ortho odor presentation, which rodents (and humans) use to perceive the nearness of food, should support preference learning. We considered two hypotheses to explain rats’ failure to learn ortho preferences: 1) Perhaps our rats were simply unable to detect the ortho odor stream; or 2) Perhaps ortho training is not ineffective, but simply less effective than retro. To test these two contrasting possibilities, we ran another group of rats that received ortho training, but with an additional pair of training sessions. This 6-day training regimen proved to support preference learning for ortho odors (Figure 2B, “Ortho, 6-day”), with rats showing a greater preference for the sweet-paired odor following training than before.
A statistical comparison of the two ortho groups revealed that ortho training enhanced preference for the sweet-paired odor only after 6 days of training (Training [pre, post] x Group [4-day, 6-day] interaction: F(1, 20)=8.03, p<0.05; post-hoc test comparing pre and post preference for Ortho, 6-day: p<0.01; Ortho, 4-day: p=NS; see STAR Methods for a more detailed description of the analytic approach). These results support the hypothesis that olfactory preference learning is stronger (in that it is learned more quickly) using retro as compared to ortho odors. Note, however, that the possibility remains that the ortho stimuli were perceived at a lower intensity than the same stimuli presented in the retro modality, and that they were thus harder to learn about—or, alternatively, that they were perceived to be particularly aversive. These hypotheses will be taken up below.
Preference learning does not transfer from retro to ortho presentation
While the above data demonstrate that rats do learn ortho preferences, the conclusion that ortho delivery is less effective for preference learning than retro delivery is counterintuitive and hard to explain. Perhaps this finding reflects the fact that most real-world ortho preference learning occurs only following feeding experiences—perhaps only after an orally-sourced pairing of taste with retro odor does an animal know that an ortho odor is associated with a good/bad taste. We tested this possibility, asking whether orally-sourced olfactory preferences directly generalize to a preference for the same stimulus sniffed from an external air stream (i.e., whether preferences learned retronasally are expressed orthonasally). While we predicted that they would, differences between the modalities—different peripheral processing mechanisms [29–32], different central systems activated [4–6, 33–35], and different perceptual properties [5, 36–41]—provide ample reason to hypothesize otherwise.
To perform this test, we ran another group of rats in the 4-day retro training paradigm, but presented ortho odors in both the pre- and post-training preference tests (Retro/Ortho condition in Table 1). The result is shown in Figure 2C: rats undergoing the same retro training protocol that induced a preference for the sweet-paired retro odor, showed no increase in preference for sweet-paired odor presented in the orthonasal modality. Thus, quickly retro-trained preferences cannot be expressed orthonasally. Statistical comparison between this group and the other groups that underwent 4-day training regimens (“Retro” and “Ortho, 4-day” in Figures 2A and 2B, respectively) confirmed appearances. Only rats in the Retro group learned to prefer the sweet-paired odor (Training x Group interaction F(1,27)=3.41; post-hoc test comparing pre and post preference for Retro: p<0.05; Ortho, 4-day: p=NS; Retro/Ortho: p=NS). Thus, sturdy preference learning in the retro olfactory modality failed to generalize to the same odor when presented in the ortho modality.
Combined retro and ortho odor presentation potentiates ortho learning
The results presented above confirm the distinction between ortho and retro olfactory modalities, but leave us still needing to explain how ortho-delivered food aromas that engender strong preferences in our normal experience do not do so in the lab, even when preferences have been formed to the same stimulus delivered retro. We therefore reconsidered our earlier argument, hypothesizing that the answer might lie in the fact that real-world olfactory stimulation in the context of consumption is actually neither ortho nor retro, but both. As foods naturally stimulate gustatory, ortho, and retro input pathways during consumption, retro odors might potentiate ortho learning (here we are defining “potentiating” as causing an ortho preference to form earlier than it otherwise would, much as the inclusion of a taste stimulus enhances odor learning in the paradigm known as “taste-potentiated odor aversion” [3, 9, 10]).
To test this prediction, we ran another group of rats in the following condition: during training sessions, odors were presented via both retro and ortho modalities (delivering via IOC and in the airstream, see STAR Methods). Pre- and post-training preference were tested by delivering odors in the ortho modality only (Cmbd/Ortho condition in Table 1). Figure 2D shows the result from this experiment, revealing that combined ortho and retro odor delivery during training results in the learning and expression of ortho preferences for the sweet-paired odor. A comparison of these data to those from the Retro condition (Figure 2A) shows no difference in preference (Training x Group interaction F(1,21)<1, p=NS). Thus, pairing ortho and retro delivery during training results in ortho preferences that do not differ significantly from learned retro preferences. Since training using only ortho odors did not induce ortho preferences, this result suggest that the presence of retro odors does in fact potentiate ortho odor preferences.
Figure 2E replots the results from Experiment 1 in terms of learning-related change in preference. This presentation reveals that preference learning in the different conditions fell neatly into two subgroups: animals either learned a preference or not. Preferences were learned when using: 1) Retro training and retro testing of any duration; 2) 6-Day training using ortho odors and ortho testing; and 3) Combined retro and ortho training and ortho testing. Learned preferences for ortho odors failed to appear when training was performed using retro only odors. These results demonstrate basic differences between ortho and retro olfaction, and in fact suggest a coherent framework for understanding the functional relationship between the two. This framework considers chemosensation in terms of sourcing (internal versus external), grouping retro with gustation (i.e., how they are used, and why retro seems more potent for preference learning, see Discussion). Next, we consider a direct implication of this framework with regard to neural mechanism.
Experiment 2. Primary gustatory cortex (GC) is uniquely involved in retro preference learning
Differences between the processing of ortho and retro odors could conceivably arise from multiple sources, including (but not limited to) central sources. Specifically, it is reasonable to hypothesize that the same odor delivered retro and ortho could activate a non-identical set of neural structures [4–6, 33, 42].
Here, we specifically hypothesized that the taste system, and in particular primary gustatory cortex (GC), might be specifically crucial for the processing of retro odors. To test this hypothesis, we optogenetically inhibited GC (GCx) during testing sessions (i.e., after preference learning had taken place; Figure 3A), and examined whether trained odor preferences were expressed normally during GCx (note that preference testing was done with unimodal odor stimuli, and that GC was intact during training where odors were paired with saccharin, ensuring that any impact of GCx was not on taste processing).
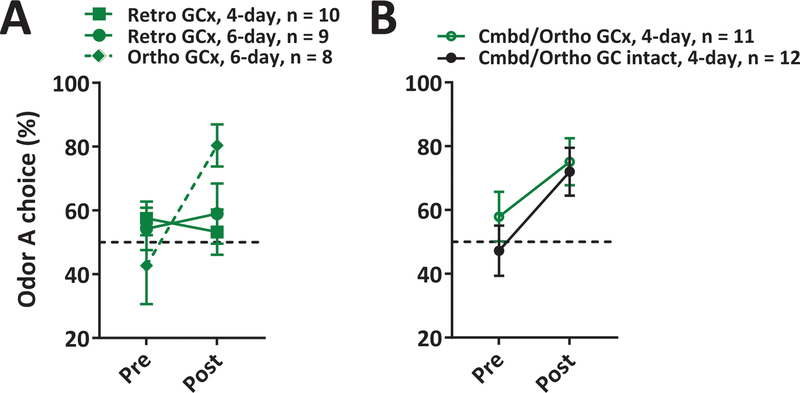
Figure 3. Effect of GCx on the expression of ortho- and retronasal preferences.
(A-B) Mean (±SEM) preference for the odor A before and after training in the Retro GCx, Ortho GCx (A), and Cmbd/Ortho GCx and GC intact conditions (B). Note: Retro, 4-day GCx data are from [17] and included here for new analysis. See also Figure S1.
GCx inhibits expression of retro but not ortho preferences
We compared the impact of GCx on preferences expressed during post-training preference testing in three groups of rats: 1) Ortho, 6-day (see Figure 2B); 2) Retro, 4-day (see Figure 2A); and 3) Retro, 6-day (to control for the possibility that any effect of GCx might be traceable to training duration). Critically, rats in the first two groups express similar preferences when GC is intact. However, a comparison of preferences displayed under GCx (Figure 3A) confirms our hypothesis that GC is involved in expressing retro-trained preferences only. GCx blocked expression of preferences in both retro groups, but not in the ortho group (Training x Group interaction F(2,24)=3.50, p<0.05; post-hoc test comparing pre and post preference for Ortho: p<0.01; Retro, 4-day: p=NS; Retro, 6-day: p=NS).
Note that this result is inconsistent with the idea that the relative ineffectiveness of ortho preference learning in Experiment 1 reflects some relative weakness of ortho odor intensity. Had the two methods of delivery merely activated the same circuit to differing degrees as a result of different intensities, the weaker learning would have been the more sensitive to neural perturbation. Instead, the opposite pattern was observed here: the delivery condition which we have established as most effective for preference learning was the condition disrupted by GCx.
Inactivation of GC does not affect retro-potentiated ortho preferences
As these data answer one question (GC is involved in expressing retro- but not ortho-learned preferences), another presents itself: if simultaneous retro and ortho presentation during training potentiates ortho preference learning, does this training method also render learned ortho preferences sensitive to GCx? It could be expected that GC might be recruited into the circuit processing an ortho odor, if the retro component of training causes the ortho odor to be labeled as food-related. Alternatively, retro and ortho odors might simply recruit structurally distinct networks independent of learning itself. We assessed these different predictions by inactivating GC during the testing phase of the combined retro and ortho training, ortho only testing condition. The results from this experiment are shown in Figure 3B, demonstrating that GCx did not inhibit expression of ortho preferences when rats were trained with combined ortho and retro odors. Comparing preference learning in the combined training condition under GCx and GC intact conditions reveals that GCx failed to have a significant effect on ortho-tested preferences (Training x Group interaction: F<1, p=NS). That is, GCx caused no significant loss of the ortho preference learned through combined retro and ortho training, confirming that GC is a part of the circuit required for processing retro but not ortho olfactory preferences.
Discussion
Our results confirm, first and foremost, that rats learn olfactory preferences in the retro modality. The idea that rats perceive (and use) retro odors at all was, until recently, a point of controversy. Some have argued that the epiglottis in the rat is positioned in such a way as to shunt airflow away from the nasal cavity during consumption [29–32]. Our data joins those using go/no-go [28, 43] and taste potentiated odor aversion [9, 27, 44] and conditioning [3, 4] tasks in showing that rats can make use of retro olfaction.
Somewhat more surprising, perhaps, is the fact that preference formation requires more sweet-ortho pairings than sweet-retro pairings. While this finding echoes a commonly described anecdotal finding that odor learning is more effective if the odorant is suspended in fluid and consumed than if it is sniffed [9, 17, 25, 45], it is not what the intuitively recognized strength of preferences for ortho odors would have led us to predict. Furthermore, our prediction that retro training might generalize to ortho preferences was disproven. That is, rats trained with effective retro-taste pairings showed no preference for those same odors delivered orthonasally. Preference learning is submodality-specific.
But whereas neither taste-ortho pairing nor taste-retro pairing was sufficient to drive quickly-forming ortho odor preferences, a training regimen that paired taste with combined retro and ortho odor presentation did do so. That is, the experience of an odor in both retro and ortho modalities during the same consumption event appears to be a critical component enabling quick development of an ortho preference, consistent with prior reports regarding odor aversions [3].
The full pattern of results supports our contention that at least one basic, important difference between ortho and retro has to do with the status of retro as an orally-sourced chemosensory input, a property that shared between retro and taste. Like retro, taste is orally sourced; like retro, robust taste memory is achieved in as little as a single trial [8, 46, 47]; and like retro, taste can potentiate ortho preference conditioning [9, 27, 44]. It becomes reasonable to propose, reminiscent of the arguments of earlier theorists [1], that retronasal olfaction leads to the engagement of circuitry also used in taste processing. Given our recent data suggesting that performance on olfaction tasks involving retro odors is dependent on activity in GC [17, 18], it made sense to hypothesize that GC might impact retro-trained preferences more than ortho.
In fact, our results suggest that retro expression of retro-trained olfactory preferences are uniquely sensitive to GC perturbation. Retro processing has been previously linked to a number of non-olfactory sensory circuits via MRI studies using human subjects [5, 21] and electrophysiological studies in rodents [4], but our data represent unprecedentedly direct evidence of retro specificity in extra-olfactory circuitry. While previous work demonstrated GC and olfactory cortex to be functionally connected [17, 48], the present results show that this connectivity takes on special significance in the context of the expression of retro preferences.
How an animal identifies retro odors as food-related remains to be studied. While the route of olfactory stimulation is typically regarded as the distinguishing characteristic of retro versus ortho olfaction (see Figure 1A), this distinction implies a host of contextual variables that distinguish the submodalities. Retro is associated with exhalation, chewing, licking, swallowing, and post-ingestive effects; any or all of these could contribute to the animal identifying a retro odor as orally-sourced [30, 36]. Our results, and in particular the fact that combined ortho and etro training potentiates ortho preferences without bringing GC into the network coding ortho odors, suggests that these contextual variables are a necessary part of the online recognition that an odor is orally-sourced: combined training clearly renders the rat capable of recognizing that an ortho odor is food-associated, but does not “fool” the system into thinking that the ortho odor is orally-sourced; for GC to be called into play in processing an odor, the rat must be performing at least some aspects of the feeding act.
Of course, it is possible that some critical but as-of-yet unplumbed subset of contextual factors (caloric content of a stimulus in the mouth, perhaps) would be sufficient to recruit GC into the processing of orthonasally presented odor preference. Of particular interest in this regard might be the specifics of stimulus timing: in our combined presentation experiments, we delivered the ortho stimulus simultaneously with the retro stimulus—a decision that successfully potentiated the ortho preference learning, but that did not render that ortho preference GC-dependent. Earlier work [49] has suggested that GC inactivation does perturb behavioral responses to audiovisual cues that precede foods. The different temporal relationships between oral and external stimulus presentation could well affect how the pairings impact the central nervous system. The fact that naturalistic stimulus sampling probably involves a highly complex pattern of ortho and retro stimulation implies that there is likely much left to learn about the interaction between the submodalities.
Our earlier behavioral work suggested that GC is not a part of the circuit that codes olfactory stimuli per se, but is an important modulator of that circuit [18]. The current work greatly enriches this understanding, demonstrating that GC is not necessary for expressing olfactory preferences generally—ortho preferences and even ortho preferences induced via retro potentiation were preserved during GCx. Instead, the role of GC in the potentiation process is likely felt during learning itself, rather than directly supporting processing of the odor that was potentiated. Such an interpretation is consistent with the sensory-and-gate hypothesis of taste-potentiated odor aversion (TPOA) learning, whereby an internally-sourced stimulus (in the case of TPOA, a taste) serves to direct the processing of an external sense (an ortho odor) as if it were internal [46]. To the degree that this description holds, one would predict that internally- and externally-sourced senses should be independent. Disrupting the hedonic value of the gating stimulus should have no impact on that of the external sense after learning [50]. This is exactly what we observe here: GCx disrupts the expression of learned preference for retro (internal) odors, while preserving preferences ortho odors, even when it was originally retro-potentiated.
Of course, there remain potential confounds to discuss. Most centrally, the possibility must be considered that our retro stimuli also carried non-olfactory properties, the most obvious of which is taste. If our retro solutions also had a detectable taste, it is possible that quick retro learning was in fact taste learning. We consider this explanation to be of vanishingly low likelihood for several reasons: 1) We specifically selected odor concentrations that were determined in prior work to be below non-olfactory behavioral detection thresholds [10, 43]; 2) For this argument to explain our combined retro and ortho results, one must posit that a near-threshold non-olfactory component of the retro stimulus was a stronger driver of learning in the combined training than sweet taste (which failed to drive rapid development of ortho preferences) itself; and 3) The suggestion that rapid retro learning was actually a conditioned taste preference requires that sweet taste somehow potentiated the palatability of the (at strongest, near-threshold) taste of the retro stimulus in the same fluid mixture. A direct examination of this last possibility revealed that there are very few circumstances in which high-concentration sucrose can change the palatability of other tastes in mixture, and no circumstances in which the saccharin used here does so. In fact, in the exact same experiments that taste fails to enhance the palatability of other tastes, the same taste easily enhances the palatability of retro odors [51]. The likelihood that mouth feel of the retro solution confounded our conclusions is, if anything, even lower than the likelihood of taste confounding, as that would also require that the different low-concentration solutions somehow had distinct somatosensory properties. Thus we conclude that our retro learning was genuine olfactory learning.
Finally, we consider the unlikely possibility that difference between ortho and retro learning can be accounted for in terms of intensity differences (i.e., the explanation that ortho odor was either too weak or too aversive to support fast learning). We did in fact induce preferences that were expressed quickly and orthonasally (Figure 2D), which suggests that our ortho presentations are easily detected. Our data also discard the possibility that ortho odors were more aversive than retro odors. If this had been the case, rats in ortho experiments would have poked less often, which we did not observe. Even if ortho and retro delivery were different in intensity, the impact of that ortho presentation was clearly potentiated by simultaneous retro presentation, a fact that highlights a distinction between the two modalities that must extend beyond intensity.
In showing the depth of the difference between ortho and retro olfaction, the current results add to the growing body of evidence that neural regions classically thought to be dedicated to the processing of particular stimulus modalities are in fact not—that the labels “gustatory” and “olfactory” cortex oversimplify at best, and mischaracterize at worst. In fact, our work suggests that a distinction of “internal vs external” may in some cases (as suggested previously, see [1, 2]) be a more meaningful basis of neural system delineation than “taste versus smell.” Such a distinction intrinsically incorporates both the importance of context and the primacy of behavior [52] in determining the nature of neural systems.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Requests for information about reagents and resources should be directed to the Lead Contact, Don Katz (dbkatz@brandeis.edu). There are no restrictions on the availability or sharing of resources.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Naïve adult female Long-Evans rats (www.criver.com), weighing between 250 and 300 g at the time of surgery served as subjects. All subjects were individually housed and kept on a 12/12 hour light/dark cycle. Experiments were performed during the light cycle in a sound-proof testing room. All procedures complied with the Brandeis University Institutional Animal Care and Use Committee guidelines. Animals that exhibited weight loss below 80% of their baseline weight (measured before the start of water deprivation), complications during recovery from surgery or other signs of distress were excluded from further experimental procedures.
METHOD DETAILS
Surgery
Stereotaxic surgery was performed under ketamine/xylazine anesthesia. Optic fibers (rats in Experiment 2 only) were implanted bilaterally in gustatory insular cortex (GC, 1.4 mm anterior to Bregma, 5 mm lateral to the midline, 4.7 mm ventral from the surface of the brain). Intra-oral cannulae (IOC, all rats) were implanted into the oral cavity [53].
Adeno-associated virus (serotype 9) coding for ArchT (AAV-CAG-ArchT-GFP; www.genetherapy.unc.edu) was injected into GC bilaterally (5 μl/hemisphere) using a Nanoject III (https://www.drummondsci.com/) at a rate of ~5 nl/s, three weeks before implantation of optic fibers and IOC (rats in Experiment 2 only). AAV serotype 9 is known to spread well across the tissue and infect all cell types [54].
GC was defined as the region of insular cortex where we [11, 13, 55, 56] and others [57–59] have repeatedly found a high density of taste responses. This region corresponds with the region previously identified as receiving projections from the gustatory thalamus [60].
Stimuli and Stimulus Delivery
Monomolecular odorants amyl acetate and methyl valerate [10, 17, 43] served as olfactory stimuli. Retro stimulation consisted of 30 μl drops of aqueous solution infused directly into the oral cavity through intra-oral cannulae (IOC) via a syringe pump. Retro odor intensity were held constant below mouthfeel thresholds for (0.025% of pure odor in distilled water). Ortho stimulation of odors was achieved through a closed-system olfactometer that provides high temporal and concentration control [17, 61]. Pure odorant solution was volatilized under N2 (10 ml/min) mixed with medical grade air (190 ml/min), propelled through teflon-coated tubing fitted into nose poke ports, and removed from the port using a vacuum pump, all via a MATLAB controlled solenoid system. Odor concentration, as measured by a photo-ionization detector was highly consistent between sessions and between presentations within a session. During preference testing sessions (Figure 1B), ortho odors were accompanied by a 30 μl drop of plain distilled water presented through IOC (timing aligned with onset of ortho delivery). During training sessions, presentations of one odor (odor A) were accompanied by saccharin (0.2%, either in plain distilled water [for ortho deliveries] or in mixture with the odor [for retro deliveries], 30 μl drops through IOC).
During all sessions, rats initiated stimulus delivery by entering an infra-red-armed nose poke port, which triggered instantaneous infusion of fluid, and (in case of conditions involving ortho odor delivery) odorized air to diffuse into of the nose poke port for a total of 0.5 s. Water, saccharin, and retro odors (dissolved in, depending on condition, either water or the water/saccharin solution) were infused via syringe pump through the IOC. In conditions involving ortho odor stimulation, fluid delivery occurred at onset of odor delivery.
In the combined retro and ortho condition, onset of ortho delivery (as described above) was aligned on retro delivery of the same odor. While this highly controlled combined stimulation protocol does not fully resemble natural consumption-related ortho and retro odor stimulation, it does allow a direct comparison with the conditions that use ortho or retro odors in isolation.
Optical Illumination
GC was illuminated bilaterally with 532 nm light from a laser (30–40 mW) through multimode optic fibers (200 μm diameter) connected via a stainless steel ferrule (www.thorlabs.com) [62]. For the 6-day training Ortho and Retro GCx conditions, illumination dynamics were identical, allowing direct comparison. Laser onset was aligned on the time the animal triggered one of the nose pokes, followed after 0.3 s by stimulus onset, and after 1.3 s by laser offset. Although the exact timing of olfactory stimulation in the retro condition is unknown, previous electrophysiological recordings from both GC and piriform olfactory cortex has shown that spiking responses to retro odors presented via IOC are most robust in the first second after stimulus delivery [14, 15]. Thus, our protocol ensured that GC was inactivated during the time olfactory stimulation occurred. In the 4-day training retro condition, both laser onset and stimulus delivery were aligned on the time the animal triggered one of the nose pokes, followed by laser offset after 2.5 s [17]. Optic fibers were implanted just above GC, identified as described above. Light strength was chosen to allow sufficient power to cause inactivation of cells at a depth of up to 1 mm from the tip of the fiber [63], thus covering a large portion of identified taste cortex while leaving unaffected cells outside of this region. Thus, we have high confidence that all of the impact of illumination was confined to the region of taste cortex (see Figure S1).
Note that we specifically avoided, as much as possible, complex network effects that arise when only one particular cell type is manipulated (e.g., the fact that inactivation of interneurons necessarily disinhibits the firing of other cortical neurons), by inactivating GC neurons in a cell type general manner (similar to pharmacological inactivation, but with vital temporal control).
Note also that overall consumption was not affected by GCx. Comparing total number of pokes during preference testing in GCx and corresponding GC intact conditions revealed no significant differences (independent samples t-test for Retro 4-day, Ortho 6-day and Cmbd/Ortho conditions: all t<1.9, all p=NS).
Histology
To verify the location and success of virus injections and fiber placements, rats were deeply anesthetized with ketamine/xylazine and perfused transcardially with saline followed by 10% formalin. Brains were post-fixed in a solution of 10% formalin and 30% sucrose for cryoprotection, and sectioned on a sliding microtome at 50um. Sections around the virus injection site were selected, washed 3×5 in PBS, and DAPI stained for cell visualization. DAPI, endogenous GFP and optical fiber tract location were imaged using epifluorescence microscopy on a Keyence microscope, and processed using ImageJ. Animals who demonstrated fiber placements outside of the GC area, or virus that failed to infect GC, were excluded from analysis.
Experimental Procedures
Rats were first trained to insert their nose into two side-by-side nose poke ports for at least 0.5 s through behavioral shaping for water reward, and then subjected to the general behavioral protocol outlined in Figure 1B.
During preference testing sessions (pre and post, each 30 minutes duration), subjects were free to approach a pair of nose pokes giving access to two different odors. In between preference testing sessions, rats underwent training sessions (2, 4 or 6 days, as indicated in the text and Figlures). During training sessions, subjects were free to approach a single nose poke giving access to, on alternating days, saccharin paired with odor A or plain water paired with odor B, on an AB / ABAB / ABABAB or BA / BABA / BABABA schedule (order of presentation and identity of odors A and B were counterbalanced across rats within each condition). The maximum number of nose pokes for each training day was limited to 250 pokes (or 7.5 ml) to avoid unequal exposure. In Experiment 2, optical illumination was applied only during preference testing after training.
In all training and testing sessions, stimulus delivery was initiated by the rat, with a minimum 3 s inter-trial interval (controlled by custom stimulus presentation software in MATLAB). Odors were delivered orthonasally, retronasally or both on different phases of the olfactory learning paradigm, depending on the experimental condition (the specific details of each condition are described in Results). To ensure sufficient consumption, access to water outside the context of the experiment was limited.
QUANTIFICATION AND STATISTICAL ANALYSIS
To assess the effect of sweet-taste pairing on odor preference, relative preference for the sweet-paired odor was calculated before and after training as: (pokes to odor A / ([pokes to odor A + pokes to odor B]) * 100). Throughout the Results section, effects are reported in terms of whether preference learning occurred (i.e., whether pre-post preference changes were significant) in individual conditions, and (just as importantly) whether learning in one condition differed from that in another (thus avoiding inference problems described in [64]). To instantiate this plan while avoiding undue proliferation of independent p-values, statistical tests are based upon the results of two-way analyses of variance (ANOVA), with one within-subject effect (Training: pre-training, post-training) and one between-subject effect (Group, with varying levels depending on the exact comparison); if and when these ANOVAs reveal significant Training x Group interactions, they can be legitimately supported by simple effects post-hoc tests, which then, within the aegis of a single analysis, allow us to validly determine whether preference learning differed in the different training conditions (all tests performed using SPSS). That is, the ANOVA interaction tells us that the groups are different, and the simple effects post-hoc tests tell us how they differ. Group n, means and standard error are indicated on Figures. In addition, a change in preference value was calculated (change in preference = post training preference − pre training preference) to provide purely visual comparison of the effectiveness of each olfactory learning condition. Any analyses that differed from this template are noted as such in the text. Note that we did not run (or analyze) virus-alone or laser-alone control conditions in Experiment 2. This was done to save time and rat use (the current study involved 89 rats across 10 experimental groups), because the methods have been extensively tested [17, 65], but most importantly because these controls were simply unnecessary in the context of our Experiment 2 hypotheses, which consisted of comparisons of GCx impact on ortho- and retro-trained rats: the fact that GCx inhibited learning in some groups (see Results) replicates [17] the effectiveness of the manipulation, and the fact that the precise same procedure failed to inhibit learning in other groups (see Results) reveals that the effect of GC manipulation on odor preference learning depends on training procedure—regardless of whether virus infusion or laser illumination have some minor impact on their own.
DATA AND SOFTWARE AVAILABILITY
The data and analysis scripts that support the findings of this study will be made available by the lead contact, Don Katz (dbkatz@brandeis.edu), upon reasonable request.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Virus Strains | ||
| AAV-CAG-ArchT-GFP (serotype 9) | University of North Carolina Vector Core | N/A |
| Chemicals | ||
| Isoamyl acetate | Sigma-Aldrich | W205532 |
| Methyl valerate | Sigma-Aldrich | W275204 |
| Saccharin sodium | Sigma-Aldrich | PHR1348 |
| Experimental Models: Organisms/Strains | ||
| Long Evans rat | Charles River Laboratories | Strain code: 006 |
Acknowledgments
This work was supported by NIH R03 DC14017 and R01 DC016063 (to JXM), and R01 DC7702 and DC6666 (to DBK).
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- 1.Rozin P (1982). “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys 31, 397–401. [DOI] [PubMed] [Google Scholar]
- 2.Gibson JJ (1966). The senses considered as perceptual systems, (Oxford, England: Houghton Mifflin; ). [Google Scholar]
- 3.Chapuis J, Messaoudi B, Ferreira G, and Ravel N (2007). Importance of retronasal and orthonasal olfaction for odor aversion memory in rats. Behav Neurosci 121, 1383–1392. [DOI] [PubMed] [Google Scholar]
- 4.Chapuis J, Garcia S, Messaoudi B, Thevenet M, Ferreira G, Gervais R, and Ravel N (2009). The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J Neurosci 29, 10287–10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small DM, Gerber JC, Mak YE, and Hummel T (2005). Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47, 593–605. [DOI] [PubMed] [Google Scholar]
- 6.Bender G, Hummel T, Negoias S, and Small DM (2009). Separate signals for orthonasal vs. retronasal perception of food but not nonfood odors. Behav Neurosci 123, 481–489. [DOI] [PubMed] [Google Scholar]
- 7.Rowe TB, and Shepherd GM (2016). Role of ortho-retronasal olfaction in mammalian cortical evolution. J Comp Neurol 524, 471–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GARCIA J, KIMELDORF DJ, and KOELLING RA (1955). Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122, 157–158. [PubMed] [Google Scholar]
- 9.Bouton ME, Jones DL, Mcphillips SA, and Swartzentruber D (1986). Potentiation and Overshadowing in Odor-Aversion Learning - Role of Method of Odor Presentation, the Distal-Proximal Cue Distinction, and the Conditionability of Odor. Learning and Motivation 17, 115–138. [Google Scholar]
- 10.Slotnick BM, Westbrook F, and Darling FMC (1997). What the rat’s nose tells the rat’s mouth: Long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Animal Learning and Behavior 25, 357–369. [Google Scholar]
- 11.Katz DB, Simon SA, and Nicolelis MA (2001). Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci 21, 4478–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T, Yuyama N, Kato T, and Kawamura Y (1984). Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol 51, 616–635. [DOI] [PubMed] [Google Scholar]
- 13.Fontanini A, and Katz D (2006). State-dependent modulation of time-varying gustatory responses. J Neurophysiol 96, 3183–3193. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsen CL, and Fontanini A (2017). Processing of Intraoral Olfactory and Gustatory Signals in the Gustatory Cortex of Awake Rats. J Neurosci 37, 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maier JX (2016). Single-neuron responses to intra-oral delivery of odor solutions in primary olfactory and gustatory cortex. J Neurophysiol, jn 00802 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincis R, and Fontanini A (2016). Associative learning changes cross-modal representations in the gustatory cortex. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maier JX, Blankenship ML, Li JX, and Katz DB (2015). A Multisensory Network for Olfactory Processing. Curr Biol 25, 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortis-Santiago Y, Rodwin BA, Neseliler S, Piette CE, and Katz DB (2010). State dependence of olfactory perception as a function of taste cortical inactivation. Nat Neurosci 13, 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasiter PS, Deems DA, and Garcia J (1985). Involvement of the anterior insular gustatory neocortex in taste-potentiated odor aversion learning. Physiol Behav 34, 71–77. [DOI] [PubMed] [Google Scholar]
- 20.Bult JH, de Wijk RA, and Hummel T (2007). Investigations on multimodal sensory integration: texture, taste, and ortho- and retronasal olfactory stimuli in concert. Neurosci Lett 411, 6–10. [DOI] [PubMed] [Google Scholar]
- 21.Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, and Small DM (2010). The insular taste cortex contributes to odor quality coding. Front Hum Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holman E (1975). Immediate and delayed reinforcers for flavor preferences in rats. Learn Motiv 6, 91–100. [Google Scholar]
- 23.Gautam SH, and Verhagen JV (2010). Evidence that the sweetness of odors depends on experience in rats. Chem Senses 35, 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green BG, Nachtigal D, Hammond S, and Lim J (2012). Enhancement of retronasal odors by taste. Chem Senses 37, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torquet N, Aime P, Messaoudi B, Garcia S, Ey E, Gervais R, Julliard AK, and Ravel N (2014). Olfactory preference conditioning changes the reward value of reinforced and non-reinforced odors. Front Behav Neurosci 8, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darling FM, and Slotnick BM (1994). Odor-cued taste avoidance: a simple and efficient method for assessing olfactory detection, discrimination and memory in the rat. Physiol Behav 55, 817–822. [DOI] [PubMed] [Google Scholar]
- 27.Batsell WR, Trost CA, Cochran SR, Blankenship AG, and Batson JD (2003). Effects of postconditioning inflation on odor + taste compound conditioning. Learn Behav 31, 173–184. [DOI] [PubMed] [Google Scholar]
- 28.Rebello MR, Kandukuru P, and Verhagen JV (2015). Direct behavioral and neurophysiological evidence for retronasal olfaction in mice. PLoS One 10, e0117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linforth R, Martin F, Carey M, Davidson J, and Taylor AJ (2002). Retronasal transport of aroma compounds. J Agric Food Chem 50, 1111–1117. [DOI] [PubMed] [Google Scholar]
- 30.Masaoka Y, Satoh H, Akai L, and Homma I (2010). Expiration: the moment we experience retronasal olfaction in flavor. Neurosci Lett 473, 92–96. [DOI] [PubMed] [Google Scholar]
- 31.Scott JW, Acevedo HP, Sherrill L, and Phan M (2007). Responses of the rat olfactory epithelium to retronasal air flow. J Neurophysiol 97, 1941–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni R, Michalski MH, Brown E, Doan N, Zinter J, Ouellette NT, and Shepherd GM (2015). Optimal directional volatile transport in retronasal olfaction. Proc Natl Acad Sci U S A 112, 14700–14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shepherd GM (2006). Smell images and the flavour system in the human brain. Nature 444, 316–321. [DOI] [PubMed] [Google Scholar]
- 34.Furudono Y, Cruz G, and Lowe G (2013). Glomerular input patterns in the mouse olfactory bulb evoked by retronasal odor stimuli. BMC Neurosci 14, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautam SH, Short SM, and Verhagen JV (2014). Retronasal odor concentration coding in glomeruli of the rat olfactory bulb. Front Integr Neurosci 8, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdach KJ, Kroeze JH, and Koster EP (1984). Nasal, retronasal, and gustatory perception: an experimental comparison. Percept Psychophys 36, 205–208. [DOI] [PubMed] [Google Scholar]
- 37.Koza BJ, Cilmi A, Dolese M, and Zellner DA (2005). Color enhances orthonasal olfactory intensity and reduces retronasal olfactory intensity. Chem Senses 30, 643–649. [DOI] [PubMed] [Google Scholar]
- 38.Welge-Lüssen A, Husner A, Wolfensberger M, and Hummel T (2009). Influence of simultaneous gustatory stimuli on orthonasal and retronasal olfaction. Neurosci Lett 454, 124–128. [DOI] [PubMed] [Google Scholar]
- 39.Hannum M, Stegman MA, Fryer JA, and Simons CT (2018). Different Olfactory Percepts Evoked by Orthonasal and Retronasal Odorant Delivery. Chem Senses 43, 515–521. [DOI] [PubMed] [Google Scholar]
- 40.Lim J, and Johnson MB (2011). Potential mechanisms of retronasal odor referral to the mouth. Chem Senses 36, 283–289. [DOI] [PubMed] [Google Scholar]
- 41.Lim J, and Johnson MB (2012). The role of congruency in retronasal odor referral to the mouth. Chem Senses 37, 515–522. [DOI] [PubMed] [Google Scholar]
- 42.Iannilli E, Bult JH, Roudnitzky N, Gerber J, de Wijk RA, and Hummel T (2014). Oral texture influences the neural processing of ortho- and retronasal odors in humans. Brain Res 1587, 77–87. [DOI] [PubMed] [Google Scholar]
- 43.Gautam SH, and Verhagen JV (2012). Direct behavioral evidence for retronasal olfaction in rats. PLoS One 7, e44781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inui T, Shimura T, and Yamamoto T (2006). Effects of brain lesions on taste-potentiated odor aversion in rats. Behav Neurosci 120, 590–599. [DOI] [PubMed] [Google Scholar]
- 45.Barnes DC, Chapuis J, Chaudhury D, and Wilson DA (2011). Odor fear conditioning modifies piriform cortex local field potentials both during conditioning and during post-conditioning sleep. PLoS One 6, e18130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia J, Lasiter PS, Bermudez-Rattoni F, and Deems DA (1985). A general theory of aversion learning. Ann N Y Acad Sci 443, 8–21. [DOI] [PubMed] [Google Scholar]
- 47.Welzl H, D’Adamo P, and Lipp HP (2001). Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res 125, 205–213. [DOI] [PubMed] [Google Scholar]
- 48.Sugai T, Yoshimura H, and Onoda N (2005). Functional reciprocal connections between olfactory and gustatory pathways. Chem Senses 30 Suppl 1, i166–167. [DOI] [PubMed] [Google Scholar]
- 49.Kusumoto-Yoshida I, Liu H, Chen B, Fontanini A, and Bonci A (2015). Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proceedings of the National Academy of Sciences of the United States of America 112, 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin JY, Roman C, and Reilly S (2009). Taste-potentiated odor aversion learning in rats with lesions of the insular cortex. Brain Res 1297, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capaldi ED, and Privitera GJ (2008). Potentiation of taste and extract stimuli in conditioned flavor preference learning. Learn Behav 36, 62–66. [DOI] [PubMed] [Google Scholar]
- 52.Krakauer J, Ghazanfar A, Gomez-Marin A, MacIver M, and Poeppel D (2017). Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 93, 480–490. [DOI] [PubMed] [Google Scholar]
- 53.Phillips MI, and Norgren RE (1970). A Rapid Method for Permanent Implantation of an Intraoral Fistula in Rats. Behav Res Meth Instr 2, 124–&. [Google Scholar]
- 54.Aschauer DF, Kreuz S, and Rumpel S (2013). Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PloS one 8, e76310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maier JX, and Katz DB (2013). Neural dynamics in response to binary taste mixtures. J Neurophysiol 109, 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadacca BF, Rothwax JT, and Katz DB (2012). Sodium concentration coding gives way to evaluative coding in cortex and amygdala. Journal of Neuroscience 32, 9999–10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kosar E, Grill HJ, and Norgren R (1986). Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain research 379, 329–341. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T, Matsuo R, Kiyomitsu Y, and Kitamura R (1989). Taste Responses of Cortical-Neurons in Freely Ingesting Rats. J Neurophysiol 61, 1244–1258. [DOI] [PubMed] [Google Scholar]
- 59.Samuelsen CL, Gardner MP, and Fontanini A (2012). Effects of cue-triggered expectation on cortical processing of taste. Neuron 74, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kosar E, Grill HJ, and Norgren R (1986). Gustatory cortex in the rat. II. Thalamocortical projections. Brain research 379, 342–352. [DOI] [PubMed] [Google Scholar]
- 61.Verhagen JV, Wesson DW, Netoff TI, White JA, and Wachowiak M (2007). Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci 10, 631–639. [DOI] [PubMed] [Google Scholar]
- 62.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, and Deisseroth K (2010). Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature protocols 5, 439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yizhar O, Fenno LE, Davidson TJ, Mogri M, and Deisseroth K (2011). Optogenetics in neural systems. Neuron 71, 9–34. [DOI] [PubMed] [Google Scholar]
- 64.Nieuwenhuis S, Forstmann BU, and Wagenmakers EJ (2011). Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14, 1105–1107. [DOI] [PubMed] [Google Scholar]
- 65.Li JX, Maier JX, Reid EE, and Katz DB (2016). Sensory Cortical Activity Is Related to the Selection of a Rhythmic Motor Action Pattern. J Neurosci 36, 5596–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analysis scripts that support the findings of this study will be made available by the lead contact, Don Katz (dbkatz@brandeis.edu), upon reasonable request.